Abstract
Vasodilator-stimulated phosphoprotein (VASP) is involved in multiple actin-mediated processes, including regulation of serum response factor (SRF) activity. We used the SRF transcriptional assay to define functional domains in VASP and to show that they coincide with those required for F-actin accumulation, as determined by a quantitative FACS assay. We identified inactive VASP mutants that can interfere both with F-actin assembly and with SRF activation by wild-type VASP. These VASP mutants also inhibit actin-based motility of Vaccinia virus and Shigella flexneri. VASP-induced F-actin accumulation and SRF activation require both functional Rho and its effector mDia, and conversely, mDia-mediated SRF activation is critically dependent on functional VASP. VASP and mDia also associate physically in vivo. These findings show that VASP and mDia function cooperatively downstream of Rho to control F-actin assembly and SRF activity.
Keywords: mDia1/RhoA/signal transduction/SRF/VASP
Introduction
Ena/VASP (vasodilator-stimulated phosphoprotein) family proteins are involved in numerous actin-regulated processes, including cell motility, epithelial cell adhesion and pathogen F-actin tail formation (for reviews, see Reinhard et al., 2001; Krause et al., 2002). The Ena/VASP proteins, VASP, Evl and Mena, which are expressed in many cell types (Reinhard et al., 1992; Gertler et al., 1996), are localized to actin bundles, focal adhesions and the leading edge (Reinhard et al., 1995). Each protein is composed of three conserved domains (Figure 1A). The N-terminal EVH1 (Ena-VASP homology 1) domain is structurally related to the PH and PTB domains (Fedorov et al., 1999; Prehoda et al., 1999) and binds the FP4 motif (D/EFPPPPXD), which is found in Zyxin, Vinculin and the Listeria monocytogenes ActA protein (Southwick and Purich, 1994; Pistor et al., 1995; Brindle et al., 1996; Gertler et al., 1996; Reinhard et al., 1996; Niebuhr et al., 1997). A central polyproline-rich region binds to profilin and to SH3- and WW-domain proteins (reviewed in Bear et al., 2001). At the C-terminus, the EVH2 domain contains binding sites for G- and F-actin and a coiled- coil motif required for oligomerization (Figure 1A) (Bachmann et al., 1999; Walders-Harbeck et al., 2002). Phosphorylation of Ena/VASP proteins may regulate their affinity for F-actin (Laurent et al., 1999; Harbeck et al., 2000) or SH3-domain proteins (Lambrechts et al., 2000; Howe et al., 2002). Ena/VASP proteins appear to play a role in F-actin assembly, although their exact function in actin dynamics remains unclear. VASP has been reported in various studies to facilitate ActA-mediated, Arp2/3-dependent actin polymerization (Loisel et al., 1999; Skoble et al., 2001), to nucleate F-actin assembly independently of Arp2/3 (Lambrechts et al., 2000; Fradelizi et al., 2001), and to promote actin filament elongation by antagonizing capping protein activity (Bear et al., 2002).
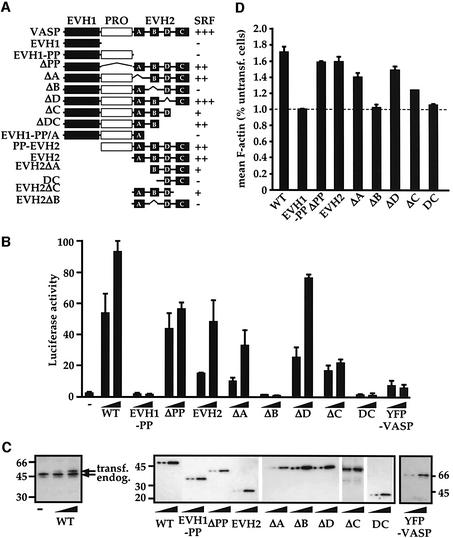
Fig. 1. VASP domains required for SRF activation and F-actin assembly coincide. (A) SRF activation by VASP mutants. The EVH1 and polyproline-rich regions are shown as filled and open boxes, and the four conserved sequence blocks constituting the EVH2 domain as black squares. (B) Representative SRF activation data. Cells expressed VASP mutants at inputs judged to give comparable protein expression levels (0.05 and 0.15 µg for VASP, VASPΔPP, VASPΔD and YFP–VASP; 0.15 and 0.5 µg for EVH1-PP, EVH2, VASPΔA, VASPΔB, VASPΔC and VASP–DC), together with the SRF reporter gene 3D.Aluc (0.05 µg) and Renilla luciferase transfection control (0.05 µg). Reporter activity is expressed relative to its activation by the constitutively active SRF derivative SRF-VP16. (C) VASP protein expression. Cells expressed VASP mutants as in (B). Cell lysates were analysed by immunoblot using polyclonal VASP antibody (left) or epitope-tag antibody (right). (D) F-actin content. Cells expressed VASP derivatives at levels corresponding to their maximal activity in the SRF reporter gene assay activation. Mean cellular F-actin content was determined relative to that of untransfected cells in the same population using FACS.
We showed previously that expression of VASP strongly induces the activity of the transcription factor SRF (serum response factor) in NIH 3T3 fibroblasts (Sotiropoulos et al., 1999). SRF, a MADS box protein, controls expression of immediate-early and muscle-specific genes, including the cytoskeletal proteins β-actin and vinculin (Arsenian et al., 1998). Stimulation of cells by serum or mitogenic phospholipids, such as LPA, regulates the activity of SRF via a Rho-controlled signalling pathway, which involves alterations in actin dynamics (Hill et al., 1995; Sotiropoulos et al., 1999). Two RhoA effector pathways control SRF activity (Tominaga et al., 2000; Copeland and Treisman, 2002; Geneste et al., 2002). The ROCK-LIMK pathway acts to stabilize F-actin by inhibiting the activity of the depolymerizing/severing factor cofilin (Maekawa et al., 1999), while the RhoA-Diaphanous pathway apparently stimulates F-actin assembly by promoting filament nucleation (Watanabe et al., 1999; Pruyne et al., 2002; Sagot et al., 2002). SRF activation appears to arise from depletion of the G-actin pool, since it is inhibited by overexpression of non-polymerizable actin derivatives (Posern et al., 2002).
In this paper we exploit the rapid and simple SRF activation assay to analyse functional domains in VASP. We demonstrate that VASP domains involved in F-actin assembly and in SRF activation are identical. We identify inactive VASP derivatives that interfere with VASP-stimulated SRF activity and F-actin assembly, as well as actin tail formation, in the pathogens Vaccinia and Shigella flexneri. The interfering VASP mutants also block serum- and Diaphanous-induced SRF activation. Our analysis provides evidence that VASP functions in Rho-dependent signalling to control actin assembly via the Diaphanous pathway.
Results
VASP domains mediating SRF activation and F-actin assembly coincide
To investigate the relationship between the ability of VASP to potentiate SRF activity and the assembly of F-actin, we analysed the activity of a set of N-terminally, epitope-tagged VASP derivatives (Figure 1A). To evaluate the effect on VASP on F-actin assembly we used a quantitative FACS assay, in which the mean F-actin content of cells expressing VASP is measured relative to that of untransfected cells in the same population (Copeland and Treisman, 2002; Geneste et al., 2002). Expression of intact VASP potently activated the SRF reporter gene 3D.ALuc (Figure 1B; Sotiropoulos et al., 1999) and substantially increased mean cellular F-actin content (Figure 1D). Immunoblot analysis revealed that maximal SRF activation was obtained at a level of transiently expressed VASP of ∼50% that of the endogenous protein (Figure 1C). Given a transfection efficiency of 25–30%, as determined by the FACS analysis, this indicates that expression of VASP at levels comparable to that of the endogenous protein is sufficient to robustly activate SRF and F-actin assembly (see Discussion).
Analysis of the VASP mutants is shown in Figure 1. Deletion of the C-terminal EVH2 domain abolished both SRF activation and F-actin assembly. In contrast, expression of the EVH2 domain alone, or VASP mutants lacking the polyproline region or EVH1 domain, exhibited substantial activity in both assays. Single alanine substitutions at each of the three phosphorylation sites, S157, S239 and T278, did not affect VASP- or EVH2-induced SRF activity (data not shown). Within the EVH2 domain, deletion of the B-block abolished both VASP-induced SRF activation and F-actin assembly. In contrast, deletion of the EVH2 A-block, or a further short region of homology (the ‘D-block’) had no significant effect on SRF activation or F-actin assembly. Deletion of the EVH2 C-block somewhat reduced VASP activity in both assays, but also inhibited the Renilla luciferase and SRF-VP16 transfection controls, suggesting that its expression has toxic effects (data not shown). These deletions had similar effects in the context of the isolated EVH2 domain (Figure 1; data not shown).
N-terminally YFP- or green fluorescent protein (GFP)-tagged VASP derivatives have been studied extensively in other systems (Rottner et al., 1999; Geese et al., 2002; Loureiro et al., 2002). YFP–VASP or GFP–VASP activated SRF only weakly (Figure 1B; data not shown). This did not reflect YFP interference with the reporter assay itself, since expression of YFP or GFP alone had no effect on either basal or VASP-induced SRF reporter activity (data not shown). For technical reasons we were unable to use the FACS assay to investigate F-actin assembly by YFP–VASP. We therefore tested the effect of VASP and YFP–VASP on the proportion of co-expressed actin recovered from cells by Triton X-100 detergent extraction. In this assay, F-actin is retained in the detergent-insoluble pellet fraction, while unpolymerized actin is recovered in the detergent-soluble supernatant (Posern et al., 2002). Expression of intact VASP substantially increased the amount of actin recovered in the pellet fraction, whereas YFP–VASP did not (see Supplementary figure S1, left panel, available at The EMBO Journal Online). Moreover, while in these experiments wild-type and endogenous VASP were recovered predominantly in the detergent-soluble fraction, YFP–VASP was recovered mainly in the pellet (see Supplementary figure S1, right panels).
Taken together, these data establish a close correlation between the ability of VASP derivatives to activate SRF assay and their ability to promote F-actin assembly, and identify three inactive VASP mutants, EVH1-PP, ΔB and DC. YFP-tagged VASP derivatives were not studied further owing to the weak activity of the YFP–VASP protein in our assays.
The B-block determines VASP localization to F-actin in NIH 3T3 cells
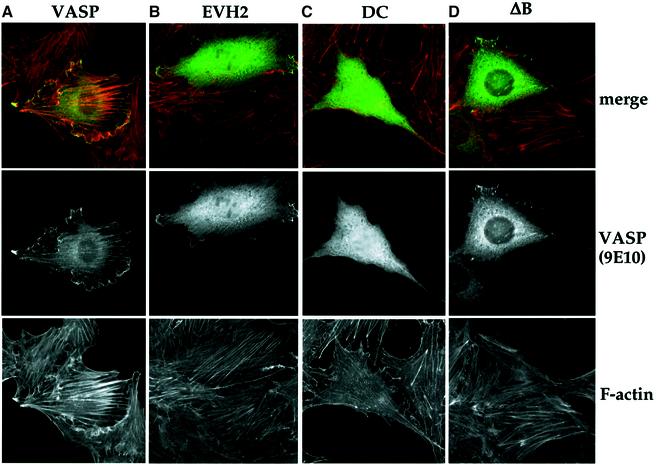
We compared the localization of intact VASP to that of the minimal active EVH2 domain, and the inactive VASPΔB and DC derivatives. We found that, as previously reported in BAE and baby hamster kidney (BHK) cells (Haffner et al., 1995; Price and Brindle, 2000), expression of full-length VASP in NIH 3T3 cells induced polarized and thickened F-actin bundles (Figure 2A). All the VASP derivatives were found to be distributed diffusely throughout the cytoplasm. The active VASP and EVH2 proteins were also able to localize to F-actin bundles and the cell periphery. The inactive VASPΔB protein did not colocalize with F-actin, but was recruited to focal adhesions, similar to the corresponding Mena derivative (Figure 2D) (Loureiro et al., 2002). The inactive EVH1, EVH1-PP and DC derivatives were not recruited to focal adhesions or actin filaments, nor did they accumulate at the cell periphery (Figure 2C; data not shown). Localization of VASP to F-actin structures including the cell periphery thus requires the EVH2 B-block, while localization to focal adhesions requires the EVH1 and EVH2 regions. These results are consistent with the view that localization of VASP at the cell periphery may be required for its activity. Such localization cannot be sufficient for efficient VASP activity, however, because although YFP–VASP exhibits a similar localization (Bear et al., 2002; Loureiro et al., 2002), it is only weakly active in our assays.
Fig. 2. Intracellular localization of intact VASP, its minimal active derivative EVH2, and the inactive mutants ΔB and DC. NIH 3T3 cells expressing the indicated VASP mutants were maintained in 0.5% FCS for 16 h before fixing for indirect immunofluorescence. Top: Merged image of F-actin (rhodamine–phalloidin; red) and VASP (9E10 epitope-tag; green). Middle: Flag-VASP (9E10 epitope-tag). Bottom: F-actin (rhodamine–phalloidin).
Interfering VASP derivatives
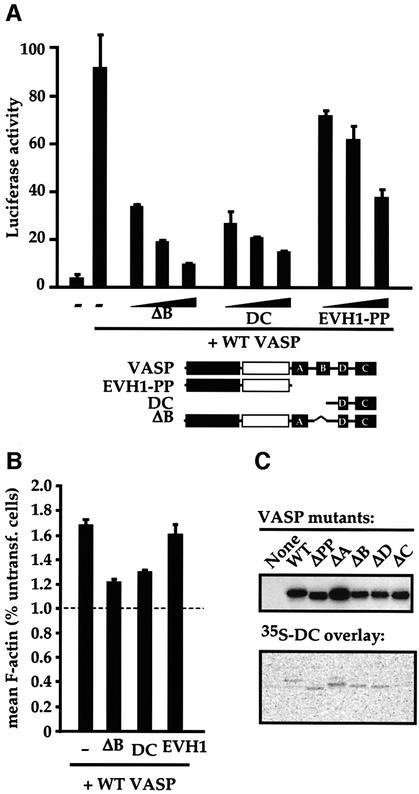
The quantitative nature of the SRF and F-actin assembly assays enabled us to readily identify VASP derivatives that are completely inert or that retain an ability to interfere with the function of intact VASP. Increasing amounts of the inactive VASP derivatives were cotransfected with a fixed amount of intact VASP and the effect on SRF reporter activity was measured. VASPΔB, DC and EVH1-PP all exhibited a dose-dependent ability to inhibit VASP-induced SRF activation, with maximal inhibition approaching background levels (Figure 3A). Similarly, both VASPΔB and DC proteins significantly inhibited VASP-induced F-actin accumulation, as measured in the FACS assay. An inactive VASP derivative comprising the EVH1 domain alone inhibited neither process (data not shown; Figure 3B). Since the short DC fragment contains the C-block required for VASP oligomerization, we tested whether the DC fragment is sufficient to interact with the intact protein. Protein overlay assays with [35S]methionine-labelled DC confirmed that this VASP fragment is indeed capable of direct interaction with VASP derivatives containing an intact EVH2 domain C-Block (Figure 3C). Similar results were obtained using full-length VASP or VASPΔB as a probe (data not shown).
Fig. 3. The inactive VASP mutants ΔB and DC are interfering mutants. (A) Interference with VASP-induced SRF activation. NIH 3T3 cells transfected with the SRF reporter expressed intact VASP (0.15 µg) or the indicated VASP mutants (0.1, 0.3 and 0.9 µg). The structure of the mutants is shown below. (B) Interference with VASP-induced F-actin accumulation. Cells expressing intact VASP (0.75 µg) and the indicated VASP mutants (4.5 µg) were analysed for mean cellular F-actin content relative to that of untransfected cells in the same population using FACS. (C) The VASP EVH2 block C is sufficient to bind intact VASP. Lysates from cells expressing the indicated VASP mutants were separated by SDS–PAGE (12.5% gel), transferred to a membrane, and probed either with anti-Flag antibodies (upper panel) or with 35S-labelled VASP DC (residues 278–380), produced by in vitro translation (lower panel).
Interfering VASP mutants inhibit Vaccinia and S.flexneri actin tail formation
The ability of the inactive VASP derivatives to interfere with VASP-induced SRF activation and F-actin accumulation prompted us to test whether expression of such proteins could block other VASP functions, such as their role in pathogen actin tail formation. Ena/VASP proteins are involved in intracellular motility of L.monocytogenes (reviewed by Bear et al., 2001; Frischknecht and Way, 2001) and are recruited to the actin tails of Vaccinia virus (Zeile et al., 1998; Frischknecht et al., 1999) as well as S.flexneri (Chakraborty et al., 1995; Laine et al., 1997).
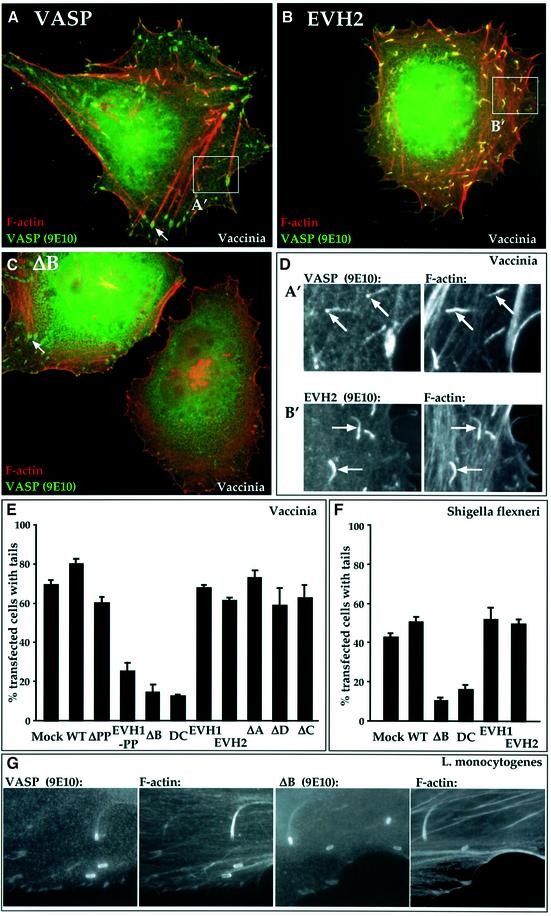
In Vaccinia-infected HeLa cells, expression of intact VASP did not affect the proportion of cells displaying actin tails (Figure 4A and E). VASP was concentrated at the virus itself and detectable along the F-actin tail, as reported previously for endogenous VASP (Figure 4D). Within the infected cells, VASP was associated with the viral factory in the perinuclear region, and also localized to F-actin bundles and focal adhesions (Figure 4A). VASPΔPP behaved similarly to the intact protein (Figure 4E). The isolated EVH2 domain also had no effect on Vaccinia actin tail formation (Figure 4B and E); however, EVH2 was more evenly distributed along the actin tail and did not accumulate at focal adhesions (Figure 4D). The inactive EVH1 domain, which does not interfere with VASP-induced F-actin accumulation, neither affected tail formation nor became localized to the virus particle (Figure 4E; data not shown). In contrast, expression of the dominant interfering VASPΔB substantially reduced both the proportion of cells with actin tails and the number of tails per cell (Figure 4C and E). The VASP derivatives EVH1-PP and DC also inhibited Vaccinia actin tail formation (Figure 4E; and data not shown). The VASP derivatives affected S.flexneri-associated actin tails in a similar fashion: expression of active VASP derivatives had no effect on tail formation, while VASP derivatives that interfered with VASP-induced SRF activation and F-actin assembly also interfered with actin tail formation (Figure 4F). In contrast, neither wild-type VASP nor VASPΔB affected actin tail formation by L.monocytogenes in our cells (Figure 4G), indicating that VASPΔB does not interfere non-specifically with F-actin assembly. Taken together, these results provide strong evidence that VASP facilitates the assembly of Vaccinia and S.flexneri actin tails.
Fig. 4. Interfering VASP mutants block Vaccinia and Shigella F-actin tail formation. Adherent HeLa cells expressing VASP (0.3 µg) or its derivatives (0.6 µg) were maintained in 10% FCS. Sixteen hours later, the cells were infected for 8 h with Vaccinia (A–D, F) or for 4 h with L.monocytogenes (E) or S.flexneri (F), and processed for immunofluorescence. Cells were stained for F-actin using Alexa 568-phalloidin (red) and for the VASP epitope tag using 9E10 antibody (green). (A–D) VASPΔB blocks Vaccinia actin tail formation. Merged images are shown. Arrows indicate instances of VASP localization to focal adhesions. DAPI staining indicated the presence of viral particles at the tail ends (not shown). Quantitation is shown in (F). (A) Wild-type VASP; similar results were obtained upon infection of cells expressing GFP. (B) VASP EVH2. (C) VASPΔB blocks tail formation. (D) VASP and F-actin localization in the tails of cells expressing intact VASP (A′) or VASP EVH2 (B′). (E and F) Data summaries. The proportion of transfected Vaccinia-infected cells with any viral tails in a given field is shown. Data are represented as means ± SEM (n = 3). (E) Vaccinia data. In control infected cells expressing GFP, cells exhibiting tails generally contained 30–60 virus particles with tails. Expression of the interfering mutants reduced the proportion of cells displaying tails, and decreased the number of virus particles with tails to five to 10 per cell. (F) Shigella flexneri data. In control infected cells expressing GFP alone, those cells displaying tails contained only two to eight bacteria with tails. (G) VASPΔB expression allows the formation of L.monocytogenes actin tails. Separate images of VASP and F-actin are shown for infected cells expressing intact VASP (left) or VASPΔB (right). VASP derivatives did not affect the number of bacteria per infected cell. In two independent experiments, 58% and 55% (intact VASP) and 54% and 52% (VASPΔB) of infected cells displayed tails.
Interfering VASP proteins block serum-induced SRF activation
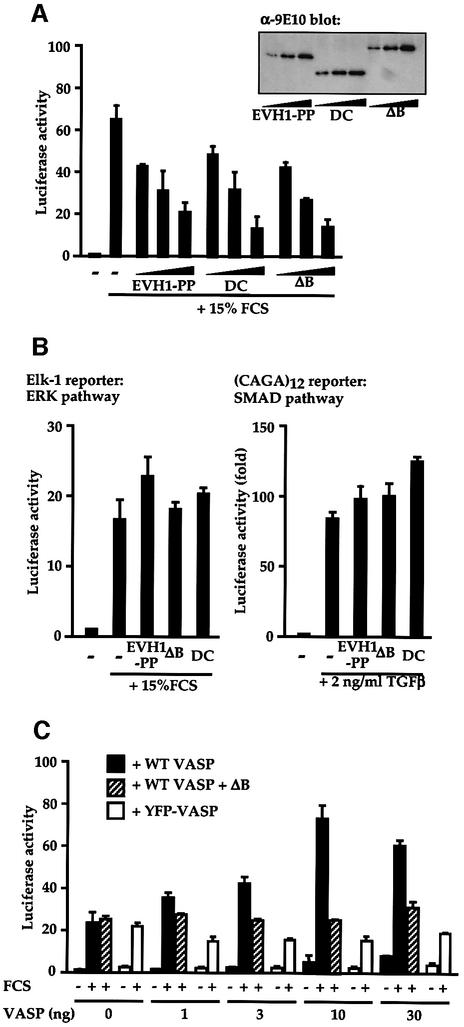
We next used the interfering VASP derivatives to test the involvement of VASP in serum induction of SRF activity, which is mediated by a pathway requiring functional RhoA and actin polymerization (Hill et al., 1995; Sotiropoulos et al., 1999). Expression of each of the interfering VASP derivatives, EVH1-PP, VASPΔB and DC, inhibited serum-induction of SRF activation in a dose-dependent manner (Figure 5A). To assess the specificity of this inhibition we examined activation of two other signal-regulated reporter systems. Expression of VASPΔB had no effect on either serum-induced activation of an ERK-dependent Elk-1 reporter (Figure 5B, left) (Marais et al., 1993), or on TGF-β induced activation of the Smad3-/4-dependent CAGA12 reporter (Figure 5B, right) (Dennler et al., 1998). Thus, VASPΔB specifically inhibits the SRF activation pathway in NIH 3T3 cells.
Fig. 5. Serum-induced SRF activation requires functional VASP. (A) Interference with serum-induced SRF activation. Cells transfected with the SRF reporter and plasmids expressing VASP mutants (0.1, 0.3 and 0.9 µg) were analysed for reporter activity before and after serum stimulation. Inset: VASP immunoblot. (B) Interference by the VASP mutants is signal pathway-specific. Cells transfected with NLex.ElkC and LexOP.Luc (left; ERK pathway) or CAGA12.Luc (right; SMAD pathway) together with control plasmid RLTK and expressed VASP mutants (0.9 µg). Luciferase activity was determined following stimulation with 15% FCS (left) or 2 ng/ml TGFβ (right). (C) Functional VASP is required for maximal serum-induced SRF activation. MVD7 cells, transfected with SRF reporter 3D.Aluc and RLTK control plasmid, expressed intact VASP or YFP–VASP, together with VASPΔB (0.9 µg). Reporter activity was determined before and after serum stimulation.
To test the involvement of VASP in signalling to SRF further we examined reporter activation in the MVD7 fibroblast cell line, which contains VASP and Mena null mutations and lacks detectable Evl protein (Bear et al., 2000). SRF reporter gene activation was inefficient in these cells, and in contrast to NIH 3T3 cells this response was not inhibited by expression of VASPΔB (Figure 5C, solid bars). Expression of wild-type VASP in MVD7 cells increased the maximal serum-induced level of reporter activity, and this could now be reduced to the level seen in untransfected cells by expression of VASPΔB (Figure 5C, hatched bars). In contrast, expression of YFP– or GFP–VASP, which only weakly activate SRF in NIH 3T3 cells, did not potentiate serum-induced SRF activation in MVD7 cells (Figure 5C, open bars; and data not shown). These results strongly suggest that VASP activity is required for maximal serum-induced SRF activation.
VASP-induced SRF activation and F-actin assembly is Rho dependent
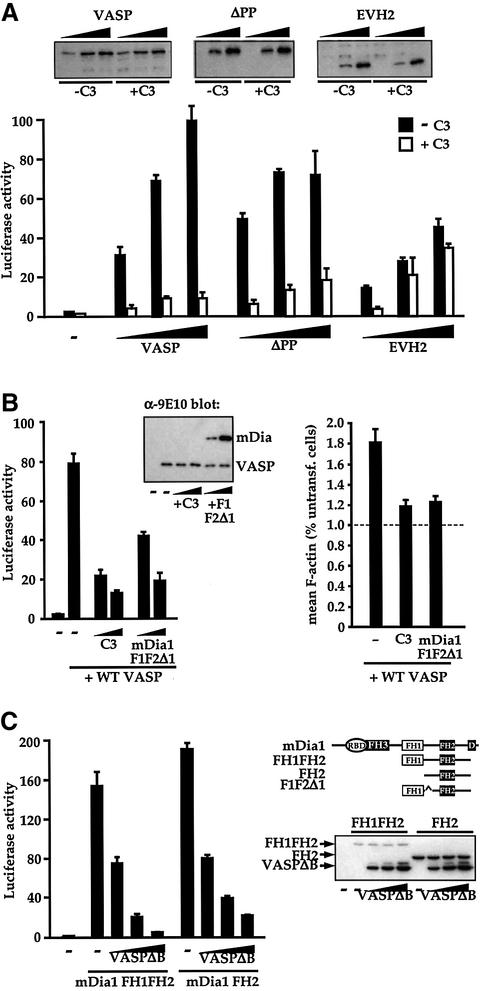
To gain further insight into how VASP might be involved in SRF regulation, we tested the role of RhoA in VASP-induced SRF activation. First, we measured SRF reporter activity upon expression of increasing amounts of intact VASP, VASPΔPP or EVH2 proteins in NIH 3T3 cells in the presence or absence of C3 exoenzyme, which inactivates RhoA. VASP-induced SRF activation was reduced by >85% by C3 expression (Figure 6A). A similar result was obtained when VASPΔPP was used to activate the reporter, but activation by the isolated EVH2 domain was largely independent of functional RhoA (Figure 6A). The inhibitory effect of C3 was not due to inhibition of focal adhesion assembly, since SRF activation by serum, active mDia1 or VASP was unaffected following focal adhesion disruption by 2,3-butanedione monoxime treatment (data not shown).
Fig. 6. VASP functions in the Rho–mDia–actin pathway to SRF activation. (A) Functional Rho is required for efficient VASP-induced SRF activation. Cells transfected with SRF reporter expressed intact VASP (0.02, 0.05, 0.15 µg) or VASP mutants (ΔPP: 0.02, 0.05, 0.15 µg; EVH2: 0.05, 0.2, 0.6 µg), together with C3 transferase (0.1 µg) as indicated. (Inset) Immunoblot analysis of VASP protein levels. (B) Functional Rho and mDia1 are required for VASP-induced SRF activation and F-actin accumulation. Left: SRF activation. Cells transfected with SRF reporter and expressing C3 transferase (0.1 µg) or interfering mDia1 mutant F1F2Δ1 (Copeland and Treisman, 2002) (0.9 µg) were processed as in (A). Inset: VASP immunoblot of cell lysates. Right: F-actin accumulation. Cells expressed intact VASP (0.75 µg) with either C3 transferase (0.5 µg) or mDia1 mutant F1F2Δ1 (4.5 µg). Mean cellular F-actin content of the transfected cells was determined relative to that of untransfected cells in the same population using FACS. (C) Functional VASP is required for efficient mDia1- induced SRF activation. Left: cells transfected with SRF reporter expressed activated mDia1 mutants FH1FH2 (0.03 µg) or FH2 (0.3 µg) (Copeland and Treisman, 2002), and VASPΔB (0.1, 0.3 and 0.9 µg). Right: mDia derivatives. Ellipse, Rho-binding domain; D, DAD domain. Immunoblot for mDia1 indicates that VASPΔB expression does not affect mDia1 protein levels.
The requirement for functional Rho in VASP-induced SRF activation prompted us to investigate the Rho effectors involved. The Diaphanous family of formin proteins regulate F-actin assembly in response to Rho activation, and are required for signalling to SRF in many cell types (Tominaga et al., 2000; Copeland and Treisman, 2002; Geneste et al., 2002). To test whether Diaphanous activity is required for VASP-induced SRF activation, we evaluated the effect of coexpression of the dominant interfering mDia1 derivative F1F2Δ1 (Copeland and Treisman, 2002). SRF reporter activation by intact VASP was inhibited by F1F2Δ1 in a dose-dependent manner; moreover, VASP-induced F-actin assembly was substantially inhibited by expression either of C3 exoenzyme or mDia1 F1F2Δ1 (Figure 6B). These results suggest that VASP functions in the Rho-dependent signalling pathway to SRF.
To examine whether VASP functions upstream of, or parallel with, mDia in the SRF signalling pathway we tested the effect of the interfering VASP derivatives on mDia1-induced SRF activation. Two activated mDia1 derivatives FH1FH2, which lacks the N-terminal Rho-binding and FH3 domains, and FH2, which comprises the minimal FH2-containing region required for F-actin assembly (Copeland and Treisman, 2002), both efficiently activated SRF, and this activation was blocked by expression of VASPΔB (Figure 6C).
Physical association between VASP and mDia1
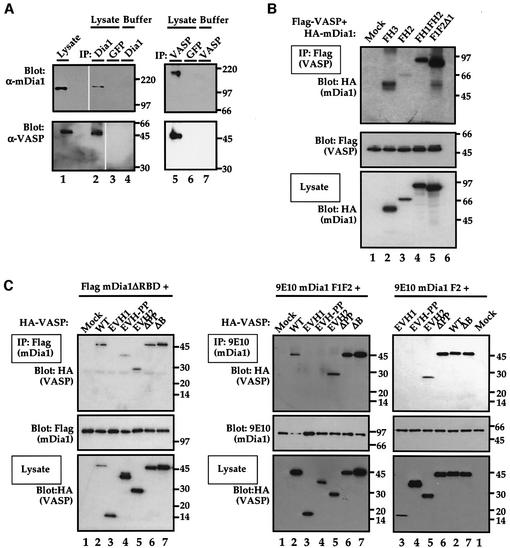
The experiments presented in the preceding section are consistent with the idea that VASP and Diaphanous function in concert, downstream of Rho, in SRF activation and F-actin assembly, but do not exclude the possibility that mDia1 and VASP act in different pathways that utilize a common cofactor. To address this issue we investigated whether mDia and VASP proteins physically associate in vivo by performing co-immunoprecipitation experiments. VASP was recovered in the anti-mDia1 immunoprecipitate, but not the control immunoprecipitate (Figure 7A, lanes 1–4). In a reciprocal experiment, we recovered endogenous mDia1 in anti-VASP immunoprecipitates (Figure 7A, lanes 5–7). No significant variation in association between the two proteins occurred upon serum stimulation (data not shown). The association of mDia1 with VASP is consistent with the notion that the two proteins function in the same pathway.
Fig. 7. Physical interaction between VASP and mDia1. (A) Association of endogenous VASP and mDia1. NIH 3T3 cell extracts or buffer were immunoprecipitated either with crosslinked anti-mDia1 or control anti-GFP beads (left panels), or with crosslinked anti-VASP or control anti-GFP beads (right panels). The precipitates were analysed by immunoblot, with protein detection by anti-mDia1 (upper panels) or anti-VASP (lower panels). Protein input in the lysate lanes (left) was 1/30 that used for the IP. Removal of irrelevant lanes from the scanned image of a single gel is indicated by gaps. (B) Two regions of mDia1 mediate VASP interaction. Lysates were prepared from cells expressing Flag-VASP and the indicated HA-mDia1 mutants, immunoprecipitated using Flag–agarose, and analysed by immunoblotting with HRP-coupled anti-HA. Bottom: mDia1 expression levels in the lysate. (C) Two regions of VASP mediate interactions with mDia1. Lysates were prepared from expressing HA-VASP derivatives and either Flag-mDia1 ΔRBD (left) or 9E10-tagged mDia1 F1F2 or F2 (right). Immunoprecipitates prepared using Flag–agarose (left) or myc–agarose (right) were analysed by immunoblotting with HRP-coupled anti-HA, or with 9E10 and secondary anti-mouse HRP-coupled antibodies as indicated. (Bottom) VASP expression levels in the lysate.
To gain insight into the regions of the two proteins required for their association, we performed co-immunoprecipitation assays on transiently expressed epitope-tagged mDia and VASP derivatives. Intact VASP associated both with the mDia FH3 domain and with the minimal active FH2 domain, although the latter interaction was enhanced by the presence of the proline-rich FH1 domain (Figure 7B). The interfering mDia1 mutant F1F2Δ1 could also associate with VASP (Figure 7B). We next investigated which VASP domains are required for mDia1 association. Interaction between intact VASP and the active mDia1 derivative mDia1ΔRBD, which lacks the Rho-binding domain, was readily detectable (Figure 7C, left). The dominant interfering derivative VASPΔB also associated with mDia1ΔRBD, as did VASPΔPP and EVH2 (Figure 7C, left). Association of the VASP EVH1 domain with mDia1ΔRBD was undetectable, while EVH1-PP interacted weakly (Figure 7C, left). In contrast, mDia1 FH1FH2 associated only with VASP derivatives containing the EVH2 domain (Figure 7B, right) suggesting that the mDia1 FH3 region may mediate interactions with the VASP polyproline-rich region. The mDia interfering derivative F1F2Δ1 exhibited similar interactions with these VASP derivatives (data not shown), as did the minimal active mDia1 derivative FH2 (Figure 7C). We were unable to detect actin in the immunoprecipitates using anti-actin antibodies, excluding the possibility that actin was mediating the VASP–mDia1 association; moreover, no VASP or mDia proteins were recovered in mock immunoprecipitations using protein-A or -G–Sepharose beads, or in the absence of cotransfected mDia1 proteins using either anti-myc or anti-M2 agarose conjugates (data not shown). We conclude that association between VASP and mDia1 is mediated by interactions involving multiple domains of each protein.
Discussion
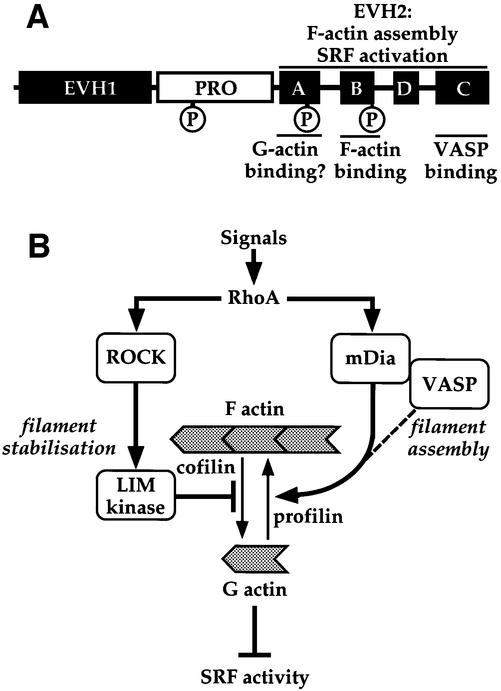
Proteins of the Ena/VASP family have been implicated in many processes that require dynamic actin remodelling (for review, see Reinhard et al., 2001; Krause et al., 2002). Here we show that VASP expression is sufficient to induce F-actin accumulation, and that the domains required for this coincide with those required for SRF activation (Figure 8A). To obtain more information on the cellular functions of VASP we used the SRF assay to identify its dominant-negative derivatives. These specifically block the formation of Vaccinia- and S.flexneri-induced actin tails, and inhibit both mDia1- and serum-induced SRF activation. VASP-induced SRF activation is dependent on RhoA and functional mDia, and VASP and mDia can be coprecipitated from cell extracts. Our data support a model in which VASP is required for efficient operation of the mDia effector pathway that controls F-actin assembly and SRF activation downstream of Rho (Figure 8B).
Fig. 8. Function of VASP in SRF activation. (A) Functional domains of VASP. The EVH1, polyproline-rich and EVH2 domains are shown as in Figure 1, with the three PKA phosphorylation sites indicated as circles. Functional roles of the EVH2 domain are indicated at the top and biochemical roles of the EVH2 subdomains at the bottom. (B) Proposed role for VASP in Rho-controlled F-actin assembly. Two Rho effector pathways control F-actin accumulation through its stabilization (ROCK → LIMK → cofilin pathway) or its assembly (mDia/VASP pathway). The dotted line indicates that the precise role of VASP in the mDia pathway remains unclear. SRF activity reflects depletion of the cellular G-actin pool, which may derepress an SRF coactivator.
In our assays, expression of VASP at levels comparable to those of the endogenous cellular protein suffices for strong stimulation of SRF activity and F-actin assembly. This might be expected if the function of VASP is normally antagonized by other cytoskeletal regulators, and indeed recent findings suggest that VASP competes with capping protein for the barbed ends of actin filaments (Bear et al., 2002). More surprisingly, we found that YFP– and GFP–VASP derivatives exhibited only weak activity in both the SRF and F-actin assembly assays, although such proteins are sufficient to restore Ena/VASP function in other assay systems (Rottner et al., 1999; Geese et al., 2002; Loureiro et al., 2002).
We found that the EVH2 region is necessary and sufficient for SRF activation or F-actin assembly (Figure 8A). This is unlikely merely to reflect its recruitment by endogenous VASP, since the EVH2 protein is not absolutely dependent on the C-block oligomerization domain for SRF activity (Figure 1A). Deletion of the EVH2 C-block, which we find is sufficient to mediate interaction with intact VASP, weakens both SRF activation and F-actin assembly, although this result must be treated with caution since this protein appears toxic to cells. The A-block, which contains a putative G-actin binding motif, and the PKA phosphorylation sites, do not appear important for VASP activity in our assays. However, removal of the EVH2 domain B-block (Bachmann et al., 1999) completely abolishes both SRF activation and F-actin assembly. Since the B-block mediates F-actin binding in vitro and is required for targeting of the VASP relative Mena to the leading edge (Bear et al., 2002), one or other of these functions must therefore be critical for VASP-induced F-actin assembly. In contrast, the VASP polyproline-rich region is not absolutely required for SRF activation or F-actin assembly. Ena/VASP proteins contain multiple domains involved in protein–protein interactions (Reinhard et al., 2001), and are likely to function as multiprotein complexes, so this result may reflect the recruitment of cofactors via other proteins in the complex.
The VASP sequences required for SRF activation and F-actin assembly in our assay are identical to those recently shown to mediate the inhibitory effects of Mena on cell movement, as determined by stable transfection of the VASP-/Mena-null cell line MVD7 with GFP–Mena derivatives (Loureiro et al., 2002). The congruence of the two assays suggests that the functional domains we have mapped are unlikely to reflect obligatory association of transfected VASP with the endogenous protein. The whole cell motility assay therefore probably reflects the function of Ena/VASP proteins in F-actin assembly. Presumably even the low activity of the GFP–VASP protein is sufficient in this assay. A potentially significant difference between the two studies, however, is the requirement for phosphorylation in the motility assays. Although not essential in our studies, phosphorylation of VASP proteins occurs in detached cells or platelets, and regulates their dynamic interactions with SH3-domain ligands (Lambrechts et al., 2000; Howe et al., 2002) and F-actin (Laurent et al., 1999; Harbeck et al., 2000).
We identified three inactive VASP mutants that effectively interfere with VASP-induced SRF activation and F-actin formation in NIH 3T3 cells. These mutants lack either the EVH2 domain or the EVH2 B-block, or consist of VASP C-terminal sequences including the oligomerization domain. Each mutant retains protein interaction domains such as the EVH1 domain, the polyproline-rich region or the EVH2 C-block, and it is therefore likely that their interfering properties arise from the formation of functionally defective VASP complexes. In contrast, the isolated EVH1 domain did not act as an interfering mutant, although it is inactive in our assays; nevertheless, a mutant of this type has been reported to disrupt epithelial cell adhesion (Vasioukhin et al., 2000).
The interfering mutants prevented the formation of Vaccinia- and Shigella-induced F-actin tails in HeLa cells, supporting the notion that VASP is involved in F-actin tail assembly by these pathogens. Studies in the VASP-/Mena-null MVD7 cell line, however, indicate that VASP is not absolutely required for tail formation, suggesting that it probably functions as an accessory protein (T.P.Newsome and M.Way, unpublished observations). Nevertheless, even if VASP only potentiates F-actin formation at the tail, it is unlikely that recruitment of the interfering mutants would be functionally neutral given their effects on F-actin assembly. Binding of VASP to ActA also potentiates Listeria movement in vivo (Pistor et al., 1995; Smith et al., 1996; Niebuhr et al., 1997) and in vitro (Loisel et al., 1999). In contrast to its inhibitory effect on Vaccinia or Shigella actin tail formation, however, the interfering mutant VASPΔB did not affect Listeria actin tail formation, in agreement with a recent study (Geese et al., 2002). This must reflect differences between the role of Ena/VASP proteins in the generation of actin tails by the different pathogens.
Our functional data provide several lines of evidence that VASP and mDia cooperate in the signalling pathway to F-actin assembly and SRF (Figure 8B). This view is supported by our finding that the VASP and mDia proteins associate physically in vivo. It appears that both the polyproline and EVH2 regions of VASP contribute to association with mDia, perhaps reflecting the multicomponent nature of the complex. Interfering derivatives of either protein can still associate with the other. This suggests that the VASP interfering mutants function by forming non-functional complexes with mDia1. The mDia1 relative Bni1 nucleates F-actin assembly in vitro through its FH2 region (Pruyne et al., 2002; Sagot et al., 2002), and the analogous domain of mDia1 suffices for SRF activation and F-actin assembly both in vivo and in vitro (Copeland and Treisman, 2002; J.W.Copeland, unpublished data). An attractive possibility, analogous to the recruitment of both the Arp2/3 complex and VASP by L.monocytogenes ActA, is that the ability of VASP to compete with capping protein allows it to extend actin filaments initiated by the F-actin nucleating function of mDia1. Future studies are needed to test this idea and to resolve the exact nature and stoichiometry of the VASP–Dia complex.
Materials and methods
Plasmids
The following plasmids were as described: SRF reporter 3D.ALuc (Geneste et al., 2002); LexOP.tkLuc and NLex.ElkC MAPK reporter system (Marais et al., 1993); CAGA12-Luc SMAD reporter (Dennler et al., 1998); pRL-TK Renilla luciferase control was from Promega; mDia1 ΔRBD1, F3, F2, F1/F2, F1/F2Δ1 (Copeland and Treisman, 2002). Derivatives of human VASP (Price and Brindle, 2000) expressed in EFplink (Sotiropoulos et al., 1999) were as follows: EVH1, codons 1–113; EVH-PP, codons 1–225; EVH2, codons 225–380. In VASP derivatives ΔA, ΔB and ΔD, codons 226–245, 258–277 and 300–315, respectively, are replaced by a NotI site, introducing three alanine codons. VASPΔC lacks residues 340–380. In the C-terminal fusion protein EYFP–VASP, the YFP and VASP open reading frames were separated by the linker sequence DPLVTAASVLEFCRYPSHW.
Transfections and reporter gene assays
Transient transfection of NIH 3T3 cells (1.5 × 105 cells per six-well plate) used Lipofectamine (Invitrogen). Mixes contained p3D.ALuc (50 ng), pRL-TK (50 ng), expression plasmid amounts as specified in the figure legends, and appropriate vector DNA to 1.2 µg per well. Cells were maintained in 0.5% fetal calf serum (FCS) for 24 h until lysed, or for 16 h before stimulation with 15% serum for 8 h. Firefly luciferase activity was normalized to Renilla luciferase activity and expressed relative to activation by constitutively active SRF derivative SRF.VP16 (100 ng) as described previously (Geneste et al., 2002). Figures show the means ± SEM of at least three independent experiments.
Pathogen infections
HeLa cells were transfected with VASP variants for 5 h, then were maintained in 10% FCS and infected the next day with Vaccinia (Western Reserve strain) for 8 h, or S.flexneri (strain SC301) or L.monocytogenes as described previously (Frischknecht et al., 1999) for 3–4 h before fixation as above. Actin tail forming efficiency was quantified as described previously (Frischknecht et al., 1999). The presence of a single actin tail within a cell was scored as positive. Actin was visualized with Alexa 568–phalloidin (Molecular Probes), and anti-9E10 (CRUK) was used to detect VASP expression.
Immunofluorescence and FACS analysis
Cells were fixed at 37°C in 4% paraformaldehyde in phosphate-buffered saline (PBS), and permeabilized in 0.2% Triton X-100 in PBS. Staining was in 5% FCS/PBS for 1 h at room temperature. Antibody dilutions were: M2 anti-Flag (Sigma F3165), 1:200; anti-9E10 (Cancer Research UK), 1:150; FITC– or rhodamin–phalloidin (Molecular Probes) 1:200; and FITC- or Cy3-conjugated anti-mouse (Dako A/S), 1:300. Images were generated using an Zeiss Axioplan II microscope with Plan-Neofluar 63× objective and appropriate filters, with a Quantix CCD camera (Photometrics) and SmartCapture 2 software (Applied Imaging). FACS quantitation of F-actin content was exactly as described previously (Copeland and Treisman, 2002; Geneste et al., 2002), and data are presented as the means ± SEM of three independent experiments.
Immunoprecipitation and immunoblot
Immunoprecipitations were as described previously (Grosse et al., 2000), with minor modifications. For immunoprecipitations of endogenous proteins, polyclonal anti-mDia1 or anti-VASP (Alexis) or control anti-GFP (Santa Cruz) antibodies were coupled to protein A beads using disuccinimidyl suberate (Pierce). NIH 3T3 cells (4.0 × 106 cells per 150 mm dish) were lysed in 1 ml of modified EBC buffer [50 mM Tris–HCl pH 8.0, 120 mM NaCl, 0.5% NP-40, 1 mM EGTA, 1 mM NaF, 500 µM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 µM leupeptin and 0.1 µM aprotinin] for 30 min on ice, cleared and immunoprecipitated for 2 h at 4°C (4.0 × 106 cell equivalents per reaction). Immunoprecipitated proteins resolved by SDS–PAGE were detected by immunoblot using anti-mDia1 or anti-VASP antibodies, employing standard methods. For studies of transfected proteins, 3.0 × 105 transfected NIH 3T3 cells (one 60 mm dish) were lysed in 300 µl of lysis buffer (50 mM Tris–HCl pH 7.5, 75 mM KCl, 2 mM EGTA, 1 mM NaF, 500 µM Na3VO4, 0.5% Triton X-100, 5% glycerol, 1 mM PMSF, 1 µM leupeptin, 0.1 µM aprotinin). Following immunoprecipitation with anti-M2 (Flag) or anti-myc agarose conjugates (Sigma Aldrich), proteins were resolved by SDS–PAGE and detected by horseradish peroxidase (HRP)-conjugated anti-hemagglutinin (HA), HRP-conjugated anti-M2 (flag), or anti-9E10 with HRP-coupled anti-mouse IgG secondary antibody. For far western blotting, [35S]methionine-labelled VASP DC synthesized by in vitro translation was used as probe.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
Gareth Inman generously performed the SMAD experiment for us. We thank Caroline Hill, Klemens Rottner and laboratory members for discussions and/or technical advice. Nick Brindle, Frank Gertler and Klemens Rottner supplied us with the human VASP cDNA, MVD7 cells, and GFP–VASP and –Mena fusion genes, respectively. We thank Derek Davies and Kathy Simpson of the LRI FACS facility for assistance and Fiona Williamson for the reference database. R.G. was funded by an EMBO long-term fellowship, and J.W.C. by a fellowship from the Hitchings-Elion Program of the Burroughs-Wellcome Fund.
Note added in proof
Our recent results indicate that changes in actin dynamics control the nuclear accumulation of MAL, an SRF coactivator. Consistent with the results presented in this paper, expression of intact VASP induces nuclear accumulation of MAL in serum-starved cells, while expression of the interfering VASP derivative VASPΔB inhibits nuclear accumulation of MAL in serum-stimulated cells [Miralles,F., Posern,G., Zaromytidou, A.-I. and Treisman,R. (2003) Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell, 113, 329–342].
References
- Arsenian S., Weinhold,B., Oelgeschlager,M., Ruther,U. and Nordheim,A. (1998) Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J., 17, 6289–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann C., Fischer,L., Walter,U. and Reinhard,M. (1999) The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding and actin bundle formation. J. Biol. Chem., 274, 23549–23557. [DOI] [PubMed] [Google Scholar]
- Bear J.E., Loureiro,J.J., Libova,I., Fassler,R., Wehland,J. and Gertler,F.B. (2000) Negative regulation of fibroblast motility by Ena/VASP proteins. Cell, 101, 717–728. [DOI] [PubMed] [Google Scholar]
- Bear J.E., Krause,M. and Gertler,F.B. (2001) Regulating cellular actin assembly. Curr. Opin. Cell Biol., 13, 158–166. [DOI] [PubMed] [Google Scholar]
- Bear J.E. et al. (2002) Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell, 109, 509–521. [DOI] [PubMed] [Google Scholar]
- Brindle N.P., Holt,M.R., Davies,J.E., Price,C.J. and Critchley,D.R. (1996) The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem. J., 318, 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T. et al. (1995) A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J., 14, 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. and Treisman,R. (2002) Activation of SRF by the Diaphanous Related Formin mDia1 is mediated by its effects on actin polymerisation. Mol. Biol. Cell, 13, 4088–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S., Itoh,S., Vivien,D., ten Dijke,P., Huet,S. and Gauthier,J.M. (1998) Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J., 17, 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A.A., Fedorov,E., Gertler,F. and Almo,S.C. (1999) Structure of EVH1, a novel proline-rich ligand-binding module involved in cytoskeletal dynamics and neural function. Nat. Struct. Biol., 6, 661–665. [DOI] [PubMed] [Google Scholar]
- Fradelizi J., Noireaux,V., Plastino,J., Menichi,B., Louvard,D., Sykes,C., Golsteyn,R.M. and Friederich,E. (2001) ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol., 3, 699–707. [DOI] [PubMed] [Google Scholar]
- Frischknecht F. and Way,M. (2001) Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol., 11, 30–38. [DOI] [PubMed] [Google Scholar]
- Frischknecht F., Cudmore,S., Moreau,V., Reckmann,I., Rottger,S. and Way,M. (1999) Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr. Biol., 9, 89–92. [DOI] [PubMed] [Google Scholar]
- Geese M., Loureiro,J.J., Bear,J.E., Wehland,J., Gertler,F.B. and Sechi,A.S. (2002) Contribution of Ena/VASP proteins to intracellular motility of listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization. Mol. Biol. Cell, 13, 2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneste O., Copeland,J. and Treisman,R. (2002) LIM kinase and diaphanous cooperate to regulate serum response factor and actin dynamics. J. Cell Biol., 157, 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler F.B., Niebuhr,K., Reinhard,M., Wehland,J. and Soriano,P. (1996) Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell, 87, 227–239. [DOI] [PubMed] [Google Scholar]
- Grosse R., Roelle,S., Herrlich,A., Hohn,J. and Gudermann,T. (2000) Epidermal growth factor receptor tyrosine kinase mediates Ras activation by gonadotropin-releasing hormone. J. Biol. Chem., 275, 12251–12260. [DOI] [PubMed] [Google Scholar]
- Haffner C., Jarchau,T., Reinhard,M., Hoppe,J., Lohmann,S.M. and Walter,U. (1995) Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. EMBO J., 14, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck B., Huttelmaier,S., Schluter,K., Jockusch,B.M. and Illenberger,S. (2000) Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem., 275, 30817–30825. [DOI] [PubMed] [Google Scholar]
- Hill C.S., Wynne,J. and Treisman,R. (1995) The Rho family GTPases RhoA, Rac1 and CDC42Hs regulate transcriptional activation by SRF. Cell, 81, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Howe A.K., Hogan,B.P. and Juliano,R.L. (2002) Regulation of vasodilator-stimulated phosphoprotein phosphorylation and interaction with Abl by protein kinase A and cell adhesion. J. Biol. Chem., 277, 38121–38126. [DOI] [PubMed] [Google Scholar]
- Krause M., Bear,J.E., Loureiro,J.J. and Gertler,F.B. (2002) The Ena/VASP enigma. J. Cell Sci., 115, 4721–4726. [DOI] [PubMed] [Google Scholar]
- Laine R.O., Zeile,W., Kang,F., Purich,D.L. and Southwick,F.S. (1997) Vinculin proteolysis unmasks an ActA homolog for actin-based Shigella motility. J. Cell Biol., 138, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts A., Kwiatkowski,A.V., Lanier,L.M., Bear,J.E., Vandekerckhove,J., Ampe,C. and Gertler,F.B. (2000) cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem., 275, 36143–36151. [DOI] [PubMed] [Google Scholar]
- Laurent V., Loisel,T.P., Harbeck,B., Wehman,A., Grobe,L., Jockusch,B.M., Wehland,J., Gertler,F.B. and Carlier,M.F. (1999) Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J. Cell Biol., 144, 1245–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel T.P., Boujemaa,R., Pantaloni,D. and Carlier,M.F. (1999) Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature, 401, 613–616. [DOI] [PubMed] [Google Scholar]
- Loureiro J.J., Rubinson,D.A., Bear,J.E., Baltus,G.A., Kwiatkowski,A.V. and Gertler,F.B. (2002) Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. Mol. Biol. Cell, 13, 2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M. et al. (1999) Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science, 285, 895–898. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne,J. and Treisman,R. (1993) The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell, 73, 381–393. [DOI] [PubMed] [Google Scholar]
- Niebuhr K. et al. (1997) A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J., 16, 5433–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistor S., Chakraborty,T., Walter,U. and Wehland,J. (1995) The bacterial actin nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins. Curr. Biol., 5, 517–525. [DOI] [PubMed] [Google Scholar]
- Posern G., Sotiropoulos,A. and Treisman,R. (2002) Mutant actins reveal a role for unpolymerised actin in control of transcription by Serum Response Factor. Mol. Biol. Cell, 13, 4167–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehoda K.E., Lee,D.J. and Lim,W.A. (1999) Structure of the enabled/VASP homology 1 domain-peptide complex: a key component in the spatial control of actin assembly. Cell, 97, 471–480. [DOI] [PubMed] [Google Scholar]
- Price C.J. and Brindle,N.P. (2000) Vasodilator-stimulated phosphoprotein is involved in stress-fiber and membrane ruffle formation in endothelial cells. Arterioscler. Thromb. Vasc. Biol., 20, 2051–2056. [DOI] [PubMed] [Google Scholar]
- Pruyne D., Evangelista,M., Yang,C., Bi,E., Zigmond,S., Bretscher,A. and Boone,C. (2002) Role of formins in actin assembly: nucleation and barbed-end association. Science, 297, 612–615. [DOI] [PubMed] [Google Scholar]
- Reinhard M., Halbrugge,M., Scheer,U., Wiegand,C., Jockusch,B.M. and Walter,U. (1992) The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J., 11, 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M., Jouvenal,K., Tripier,D. and Walter,U. (1995) Identification, purification and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein). Proc. Natl Acad. Sci. USA, 92, 7956–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M., Rudiger,M., Jockusch,B.M. and Walter,U. (1996) VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. FEBS Lett., 399, 103–107. [DOI] [PubMed] [Google Scholar]
- Reinhard M., Jarchau,T. and Walter,U. (2001) Actin-based motility: stop and go with Ena/VASP proteins. Trends Biochem. Sci., 26, 243–249. [DOI] [PubMed] [Google Scholar]
- Rottner K., Behrendt,B., Small,J.V. and Wehland,J. (1999) VASP dynamics during lamellipodia protrusion. Nat. Cell Biol., 1, 321–322. [DOI] [PubMed] [Google Scholar]
- Sagot I., Rodal,A.A., Moseley,J., Goode,B.L. and Pellman,D. (2002) An actin nucleation mechanism mediated by Bni1 and Profilin. Nat. Cell Biol., 4, 626–631. [DOI] [PubMed] [Google Scholar]
- Schratt G., Philippar,U., Berger,J., Schwarz,H., Heidenreich,O. and Nordheim,A. (2002) Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J. Cell Biol., 156, 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoble J., Auerbuch,V., Goley,E.D., Welch,M.D. and Portnoy,D.A. (2001) Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation and Listeria monocytogenes motility. J. Cell Biol., 155, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.A., Theriot,J.A. and Portnoy,D.A. (1996) The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria and the localization of vasodilator-stimulated phosphoprotein and profilin. J. Cell Biol., 135, 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos A., Gineitis,D., Copeland,J. and Treisman,R. (1999) Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell, 98, 159–169. [DOI] [PubMed] [Google Scholar]
- Southwick F.S. and Purich,D.L. (1994) Arrest of Listeria movement in host cells by a bacterial ActA analogue: implications for actin-based motility. Proc. Natl Acad. Sci. USA, 91, 5168–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T., Sahai,E., Chardin,P., McCormick,F., Courtneidge,S.A. and Alberts,A.S. (2000) Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell, 5, 13–25. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer,C., Yin,M. and Fuchs,E. (2000) Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell, 100, 209–219. [DOI] [PubMed] [Google Scholar]
- Walders-Harbeck B., Khaitlina,S., Hinssen,H., Jockusch,B. and Illenberger,S. (2002) The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett., 529, 275. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kato,T., Fujita,A., Ishizaki,T. and Narumiya,S. (1999) Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol., 1, 136–143. [DOI] [PubMed] [Google Scholar]
- Zeile W.L., Condit,R.C., Lewis,J.I., Purich,D.L. and Southwick,F.S. (1998) Vaccinia locomotion in host cells: evidence for the universal involvement of actin-based motility sequences ABM-1 and ABM-2. Proc. Natl Acad. Sci. USA, 95, 13917–13922. [DOI] [PMC free article] [PubMed] [Google Scholar]