Abstract
Recent evidence indicates that membrane microdomains, termed lipid rafts, have a role in B-cell activation as platforms for B-cell antigen receptor (BCR) signal initiation. To gain an insight into the possible functioning of lipid rafts in B cells, we applied liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) methodologies to the identification of proteins that co-purified with lipid rafts of Raji cells. Among these raft proteins, we characterized a novel protein termed Raftlin (raft-linking protein). Like the Src family kinase, Raftlin is localized exclusively in lipid rafts by fatty acylation of N-terminal Gly2 and Cys3, and is co-localized with BCR before and after BCR stimulation. Disruption of the Raftlin gene in the DT40 B-cell line resulted in a marked reduction in the quantity of lipid raft components, including Lyn and ganglioside GM1, while overexpression of Raftlin increased the content of raft protein. Moreover, BCR-mediated tyrosine phosphorylation and calcium mobilization were impaired by the lack of Raftlin and actually potentiated by overexpression of Raftlin. These data suggest that Raftlin plays a pivotal role in the formation and/or maintenance of lipid rafts, therefore regulating BCR-mediated signaling.
Keywords: B cell/BCR/lipid raft/phosphorylation
Introduction
Recent biochemical, pharmacological and imaging data indicate that cell plasma membranes contain microdomains, distinct from the bulk plasma membrane and caveolae, which are frequently referred to as lipid rafts. Lipid rafts are rich in cholesterol and sphingolipids (Fridriksson et al., 1999), therefore are insoluble in certain non-ionic detergents such as Triton X-100 at 4°C (Hooper, 1999; Brown and London, 2000). Due to their high lipid content, they can be isolated to low density during sucrose density ultracentrifugation (Brown and Rose, 1992). These properties have been used to separate lipid rafts from bulk plasma membrane (Hooper, 1999; Brown and London, 2000). Studies of lipid rafts isolated from various cells have suggested that they are enriched for certain protein species, including glycosylphosphatidylinositol (GPI)-linked proteins, fatty-acylated proteins, receptors and cytoskeletons, as well as many proteins known to be important for signal transduction (Brown and London, 1998; Hooper, 1999; Brown and London, 2000; von Haller et al., 2001).
In B cells, it is believed that lipid rafts aggregate at the site of B-cell antigen receptor (BCR) engagement and act as platforms for receptor signaling and trafficking. In resting B cells, BCR is excluded from rafts, which concentrate the Src-family kinase Lyn. Most other membrane proteins are also excluded from rafts, including the negative regulators of B-cell function CD22 and CD45 (Cheng et al., 1999; Weintraub et al., 2000). In the absence of antigen, the BCR monomer has a weak affinity for the rafts, but multivalent antigen binding oligomerizes the BCR, increasing such affinity (Pierce, 2002). Stable residency in the rafts results in association with Lyn, which phosphorylates the immunoreceptor tyrosine-based activation motifs (ITAMs) of the BCR, recruiting Syk and initiating signaling cascades.
BCR-induced tyrosine phosphorylation of phospholipase C (PLC)-γ2, which is mediated by Syk and Btk, is responsible for its increased activity, which allows the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messengers, inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) (Rigley et al., 1989; Takata et al., 1994; Takata and Kurosaki, 1996). DAG activates protein kinase C (PKC) (Nishizuka, 1988), and IP3 causes Ca2+ release from intracellular stores after binding to the IP3 receptor (IP3R). These signals lead to a variety of biological responses and depend on the developmental stage of B cells (Rajewsky, 1996).
Several fundamental questions remain concerning rafts and BCR. The molecular mechanism that governs how BCR oligomerization after antigen binding induces the association of BCR with rafts is still unclear. Moreover, it remains to be determined whether the immunological synapse that is observed in B cells has a central role in B-cell signaling, as has been established for the T-cell immunological synapse. Another issue is the composition of the rafts themselves, and the relationship between raft structure and function in BCR signaling. In addition, the ways in which the raft components change during B-cell development, including the alteration of the relationship between rafts and the BCR, are still unknown.
To answer these questions, raft-associated proteins need to be identified and the function of each molecule in lipid rafts must be defined. Recently, many integral and peripheral raft-resident proteins have been identified by proteomics analysis in various cell types (Garin et al., 2001; von Haller et al., 2001; Mairhofer et al., 2002; Nebl et al., 2002). However, raft proteins in B cells have not been characterized well for the function of rafts or BCR signal transduction. In this study, lipid rafts were isolated from Raji B cells, and the proteins were identified by liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). The focus was on a B-cell specific raft protein, termed Raftlin, which is a major component of the raft fraction. Its roles in raft formation and BCR-mediated signaling were characterized using the DT40 chicken B-cell line. Without Raftlin, the quantity of raft fraction components was significantly reduced and BCR signal transduction was severely impaired. Therefore, Raftlin was considered necessary for efficient BCR signaling by forming or maintaining lipid rafts.
Results
Identification of Raftlin, a novel protein in lipid rafts
Lipid rafts have been implicated in signal transduction of the BCR as well as the T-cell receptor (TCR). Although proteomic analysis of the T-cell raft has been reported (von Haller et al., 2001), a cyclopedic study of proteins concentrated in lipid rafts in B cells has not been performed. Thus, we prepared a raft fraction from Raji B cells using sucrose gradient ultracentrifugation and identified the proteins contained in the fraction. To confirm the separation of raft fractions from other fractions, each fraction was loaded onto SDS–PAGE and visualized by silver staining (Figure 1A). A considerable amount of protein existed at the interface between 35 and 5% sucrose (Figure 1A, lane 3), and the fraction contained many tyrosine-phosphorylated proteins (data not shown), as well as Lyn and GM1 detected by cholera toxin B subunit (CTB) binding. After being repeatedly washed to remove loosely associated proteins in this fraction, the proteins were separated by SDS–PAGE, and the main gel bands were excised (Figure 1B, a–o). The proteins contained in each gel band were determined by in-gel tryptic digestion followed by LC-ESI-MS/MS. Although most of the proteins were signaling molecules, including G-protein subunits or cytoskeletal proteins already known to localize in lipid rafts, several novel proteins were identified (Table I, KIAA1273, KIAA0143, KIAA0084; Supplementary table I, available at The EMBO Journal Online). Most of them were overlapped in T-cell rafts, but some, such as Fgr, CSK, protein phosphatase-1γ1 and BAP37, are not listed in the report by von Haller and coworkers (von Haller et al., 2001).
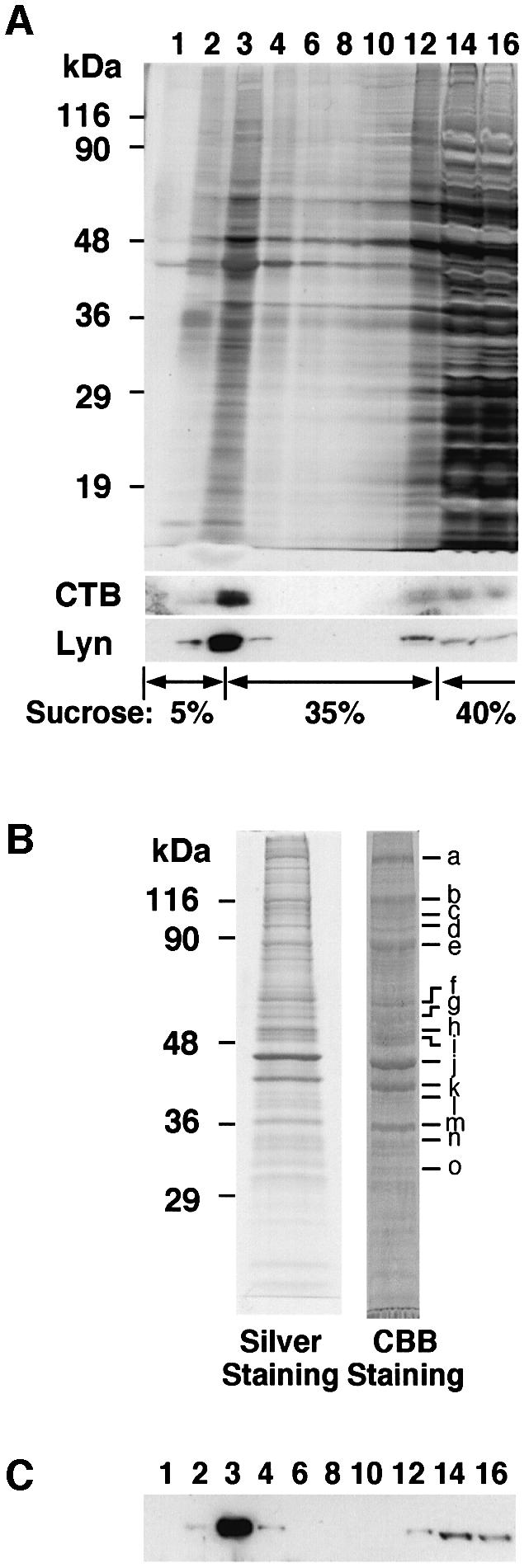
Fig. 1. Raft purification from Raji B cells. (A) Raji cells were lysed and fractionated using sucrose gradient ultracentrifugation. Each fraction from 6 × 105 cells was loaded onto SDS 12% polyacrylamide gels, and visualized by silver staining or blotted using CTB and anti-Lyn MAb. The numbers indicate the fractions from the top. (B) The raft fraction was loaded on SDS–PAGE (10% gel) and visualized by Coomassie Brilliant Blue staining (1.5 × 108 cells) or silver staining (1.5 × 107 cells). The main bands (a–o) were excised, and the proteins included in the gel were determined using LC-ESI-MS/MS. (C) As in (A), but the fractions were immunoblotted using anti-human Raftlin Ab.
Table I. Identified proteins co-purified with lipid rafts from Raji B cells.
| Identified proteins | Bands | No. of peptides(protein coverage)a | NCBI reference |
|---|---|---|---|
| Cellular myosin heavy chain | a | 55 (33.9) | 6166599 |
| Myosin Iβ | b | 35 (44.6) | 13431674 |
| Unconventional myosin 1G | b | 18 (39.8) | 14269502 |
| Neprilysin | c | 18 (29.3) | 67699 |
| KIAA1274 (similar to mouse paladin) | c | 5 (9.2) | 14738662 |
| KIAA0143 | d | 6 (14.3) | 14741682 |
| KIAA0084 | e | 15 (31.0) | 14731284 |
| Semaphorin 7A | e | 16 (30.8) | 4504237 |
| Ezrin (villin 2) | e | 8 (16.5) | 21614499 |
| Lyn (splice form A) | f | 20 (42.0) | 125480 |
| Fgr | f | 13 (27.6) | 4885235 |
| Lyn B | g | 15 (37.5) | 2117805 |
| Tubulin β-1 chain | g | 14 (48.6) | 135448 |
| Tubulin α-1 chain | g | 13 (40.8) | 135395 |
| RN-tre | g | 7 (12.2) | 7661864 |
| CSK | h | 17 (40.9) | 729887 |
| Casein kinase 1 | h | 7 (18.0) | 11177010 |
| Lymphocyte-specific protein 1 | h | 6 (28.6) | 106923 |
| Flotillin-2 | i | 19 (50.7) | 4758394 |
| Flotillin-1 | i | 12 (40.5) | 5031699 |
| β actin | j | 10 (39.5) | 16359158 |
| Similar to CD48 antigen | j | 5 (19.3) | 14727779 |
| Gi α-2 | k | 14 (48.2) | 15779126 |
| Gi α-3 | k | 13 (35.0) | 5729850 |
| Glyceraldehyde-3-phosphate dehydrogenase | l | 13 (47.5) | 7669492 |
| Protein phosphatase-1 γ1 | l | 7 (30.4) | 484316 |
| G β-2 | m | 11 (37.7) | 306785 |
| G β-1 | m | 9 (30.3) | 11321585 |
| HLA-DR α heavy chain | m | 3 (20.1) | 1335101 |
| BAP37 | m | 3 (14.9) | 6563274 |
| F-actin capping protein β subunit | n | 6 (23.9) | 4826659 |
| VDAC-1 | n | 4 (21.6) | 4507879 |
| VDAC-2 | n | 3 (13.0) | 1172554 |
| SNAP-23 | o | 11 (61.6) | 6685971 |
| RAL-A | o | 4 (29.6) | 131834 |
aPercentages of identified peptides in a whole protein.
Among the raft proteins in B cells, we focused on KIAA0084, since it has an N-terminal Met-Gly-Cys sequence that is a typical dual acylation motif and was concentrated in the raft fraction (Figure 1C). This protein was named Raftlin (raft-linking protein). The Raftlin cDNA encodes a protein of 578 amino acids (Figure 2A) with a calculated molecular weight of 63 145, although transient expression in human embryonic kidney (HEK)293T cells or endogenous expression in Raji cells yielded human Raftlin protein estimated to be ∼90 kDa by SDS–PAGE (Figure 1B). This discrepancy may be due to the acidic nature of the protein, which has a calculated isoelectric point of 5.3. Chicken Raftlin cDNA was also isolated by 5′ and 3′ rapid amplification of cDNA ends (RACE) using DT40 B-cell mRNA. The deduced primary amino acid sequence of human Raftlin is 56% identical to that of chicken Raftlin, and these have two highly conserved domains: the N-terminal region (amino acids 1–165 of human Raftlin, 80% identity) and the C-terminal region (amino acids 276–450 of human Raftlin, 79% identity) (Figure 2A). These domains might be important for the function of Raftlins. A comparison of the deduced amino acid sequence of human Raftlin with the NCBI protein database revealed that there is no homologous protein with a known function, but Raftlin has weak similarity to a hypothetical protein FLJ30574, which is named Raftlin-2 (Figure 2B). Raftlin and Raftlin-2 have only 29% identity in overall structure, but they have 36 and 39% identities in the N-terminal and the C-terminal conserved domains, respectively.
Fig. 2. Deduced amino acid sequences of Raftlins. (A) A comparison of the deduced amino acid sequences of human Raftlin and chicken Raftlin. (B) A comparison of the deduced amino acid sequences of human Raftlin and human Raftlin-2. (C) Northern blot analysis of mouse Raftlin expression in mouse tissues. Mouse G3PDH was a control for the amount of RNAs. (D) Western blot analysis of human Raftlin expression in several human cell lines. Lysates from 1 × 105 cells were immunoblotted with anti-human Raftlin Ab. Similar levels of protein loading were confirmed by western blotting with anti-STAT5b Ab.
The tissue distribution of mouse Raftlin mRNA was examined by northern blotting using poly(A)+ RNA. The Raftlin mRNA was expressed most abundantly in the spleen and thymus, suggesting that Raftlin is exclusively expressed in lymphocytes (Figure 2C). High expression of Raftlin mRNA in lymphoid tissues was also observed in human tissues using RT–PCR analysis (data not shown). Raftlin protein expression was investigated by western blotting using several human cell lines: HEK293T (epithelial-like kidney cell), Jurkat (T lymphocyte), and Raji and Daudi (B lymphocyte). Raftlin protein was expressed in B-cell lines but not in T-cell or epithelial-like cell lines (Figure 2D). Raftlin was not detected in Jurkat cells by our antibody, but the same protein was detected in the raft fraction of Jurkat cells by mass spectrometry (von Haller et al., 2001). On the basis of these data, it was assumed that Raftlin is expressed predominantly in lymphocytes.
Fatty acylation of human Raftlin is required for localization in rafts
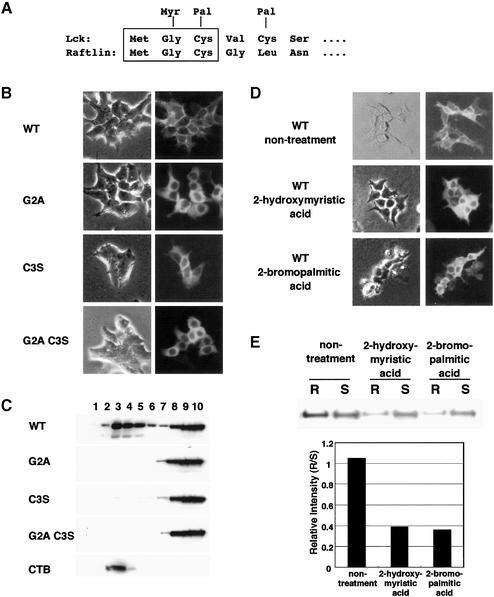
The N-terminal sequence of Raftlin was homologous to that of the Src family protein tyrosine kinase, including Lck and Fyn, known to be localized in lipid rafts (Figure 3A) (Resh, 1999). With respect to Lck, it has been reported that the N-myristoylation of Gly2 is required for membrane localization and that the S-palmitoylation of Cys3 is involved in the localization to the raft domain (Kosugi et al., 2001). To investigate whether Gly2 and Cys3 residues of Raftlin are important for localization to rafts, three mutants (G2A, C3S and G2A C3S) were prepared and expressed in HEK293T cells. Fluorescence microscopy revealed that: (i) wild-type Raftlin was localized in the plasma membrane; (ii) mutated Raftlins at the G2 residue (G2A and G2A C3S) were localized in cytosol; and (iii) mutated Raftlin at only the C3 residue (C3S) was localized in cytosol and the cell membrane (Figure 3B). Western blot analysis following fractionation using sucrose gradient centrifugation revealed that large amounts of wild-type Raftlin were localized in lipid rafts (Figure 3C, lanes 3–5) but that mutated Raftlins at the G2 or C3 residues were detected in the soluble fractions (Figure 3C, lanes 9 and 10). Furthermore, plasma membrane localization (Figure 3D) as well as accumulation in the raft fraction (Figure 3E) was significantly reduced after cells were treated with 2-hydroxymyristic acid and 2-bromopalmitic acid, inhibitors of myristoylation and palmitoylation, respectively (Webb et al., 2000). On the basis of these data, Raftlin was assumed to be myristoylated and palmitoylated at the G2 and C3 residues, respectively, both of which are necessary for residence in lipid rafts.
Fig. 3. The effect of fatty acylation on the cellular localization of Raftlin. (A) Comparison of the N-terminal amino acid sequences of human Lck and human Raftlin. S-palmitoylation and N-myristoylation sites of human Lck are shown as Pal and Myr. (B and C) Cellular localization of human Raftlin expressed in HEK293T cells. Wild-type and mutated Raftlins (WT, G2A, C3S and G2A C3S) expressed as GFP fusion proteins were observed by fluorescence microscopy (B), and the fractions using sucrose gradient ultracentrifugation were blotted with anti-GFP Ab and CTB (C). (D and E) Effect of fatty acylation inhibitors on the localization of human Raftlin in HEK293T cells. Wild-type Raftlin fused GFP was expressed in the absence or presence of 2-hydroxymyristic acid or 2-bromopalmitic acid, and observed by fluorescence microscopy (D). After sucrose gradient ultracentrifugation, combined raft fractions (R; fractions 1–5) and combined soluble fractions (S; fractions 9–13) were blotted with anti-GFP Ab (E). Relative intensity (R/S) was plotted.
Co-localization of Raftlin and BCR
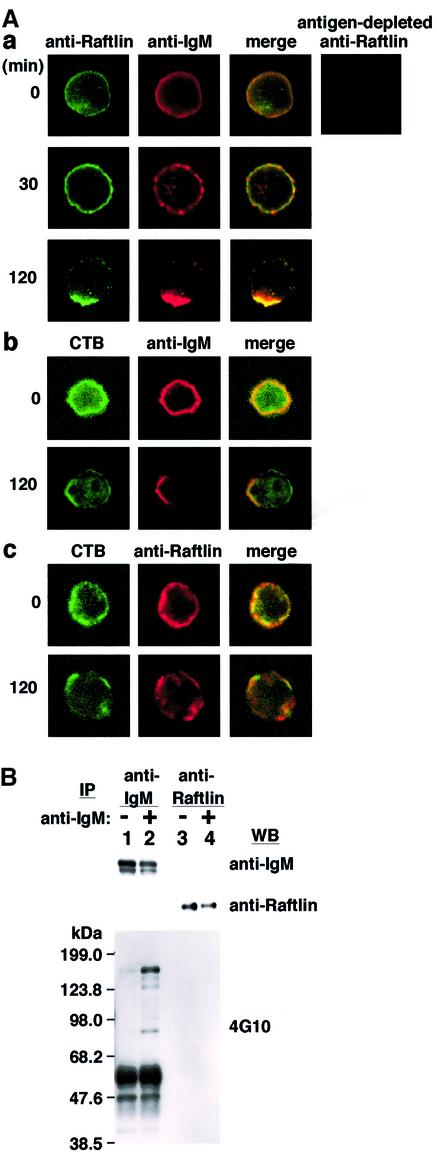
To investigate the localization of endogenous Raftlin in B cells before and after BCR stimulation, Raftlin was immunostained and observed by confocal microscopy using Daudi B cells. Raftlin and BCR were stained smoothly on the cell membrane in a non-stimulated state (Figure 4A, a, 0 min). Specific detection of Raftlin by our antibody was confirmed by the lack of staining with antiserum depleted with recombinant Raftlin protein (Figure 4A, a, antigen-depleted anti-Raftlin). BCR ligation with anti-immunoglobulin M antibody (IgM Ab) resulted in the clustering of Raftlin as patches like membrane IgM (Figure 4A, a, 30 min), and Raftlin and IgM were concentrated in the same capping region after 2 h stimulation (Figure 4A, a, 120 min). CTB-Alexa488 staining revealed co-localization of GM1 with both Raftlin and membrane IgM (Figure 4A, b and c). These data indicate that Raftlin co-localized with BCR in the lipid raft before and after BCR stimulation.
Fig. 4. Localization of Raftlin in Daudi cells. (A) Co-localization of BCR and Raftlin in Daudi cells. Cells were stimulated with anti-human IgM Ab for indicated periods and stained with a combination of anti-human Raftlin Ab and anti-human IgM Ab (a), or CTB and anti-human IgM Ab (b), or CTB and anti-human Raftlin Ab (c). Antiserum passed through a recombinant Raftlin protein column (antigen-depleted anti-Raftlin) was used as a negative control. (B) Immunoprecipitation of BCR and Raftlin using Daudi cells. Cells (6 × 107) stimulated with or without anti-human IgM (20 µg/ml) for 5 min at 37°C were lysed with digitonin-lysis buffer and immunoprecipitated with anti-human IgM Ab and protein-G–Sepharose 4FF (Amersham Bioscience) or anti-human Raftlin IgG-immobilized Sepharose. Immunoprecipitants were divided into three aliquots and blotted with anti-human IgM, anti-human Raftlin and anti-phosphotyrosine Abs (4G10).
Next, we examined whether Raftlin binds directly to BCR. To address this question, cells stimulated with anti-IgM Ab were lysed with digitonin, which has been shown to maintain BCR and src–kinase interactions (Burkhardt et al., 1991). As shown in Figure 4B, although several tyrosine-phosphorylated proteins were co-immunoprecipitated with anti-BCR Ab, Raftlin was not detected in the immunoprecipitates with anti-BCR Ab. Moreover, neither IgM nor tyrosine-phosphorylated proteins were co-precipitated with anti-Raftlin Ab. These data indicate that Raftlin localizes close to BCR, but that these two do not interact directly.
Disruption of the Raftlin gene resulted in reduced cell proliferation
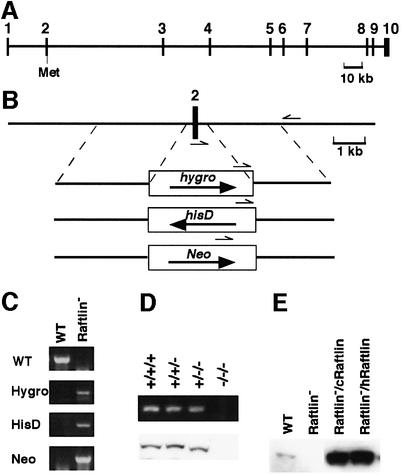
To evaluate the function of Raftlin in B cells, Raftlin-deficient DT40 B cells (Raftlin– cells) were generated by the gene targeting method. To do this, the human Raftlin gene structure was first defined. In the NCBI human genomic database, the human Raftlin gene was found in two contigs (accession numbers AC010727 and AC090948) that map to the human chromosome 3p, which consists of 10 exons (Figure 5A). The first methionine is included in the second exon, and the entire Raftlin gene covers ∼200 kb. Attempts were made to replace exon 2 with drug-resistant genes. The chicken genomic DNAs of 7- and 3-kb fragments upstream and downstream of exon 2 (encoding amino acids 1–49), respectively, were obtained using PCR, and the targeting vectors were constructed (Figure 5B). Because three rounds of recombinations were necessary to prepare complete Raftlin-deficient cells (Figure 5B–D), it was assumed that the Raftlin gene localizes in the second chromosome, known to be trisomy in DT40 cells. To exclude the possibility that the differences between wild-type and knockout cells were artifacts of drug screening, human Raftlin (hRaftlin) cDNA as well as chicken Raftlin (cRaftlin) cDNA was re-introduced into Raftlin-deficient cells (Raftlin–/cRaftlin or Raftlin–/hRaftlin cells). Because of a strong constitutive cytomegalovirus promoter, the hRaftlin and cRaftlin expression levels in these mutants were much higher than those of the endogenous protein (Figure 5E).
Fig. 5. Disruption of the Raftlin gene in chicken DT40 B cells. (A) The intron and exon structure of the human Raftlin gene. The first methionine is indicated as Met, and the numbered boxes represent exons. (B) Targeting constructs of chicken Raftlin. Hygro, hisD, and Neo denote the hygromycin-, histidinol- and neomycin-resistance genes, respectively. The small arrows indicate the primers for screening of homologous recombinants. (C) Genomic PCR of wild-type (WT) and Raftlin-deficient DT40 cells (Raftlin–). Wild-type allele (WT) and mutated alleles containing hygromycin-, histidinol- and neomycin-resistance genes (Hygro, HisD and Neo, respectively) were amplified by PCR. (D) Raftlin expression in wild-type and mutated cells. RT–PCR (upper panel) and western blotting (lower panel) of chicken Raftlin were performed using wild-type (+/+/+), single-recombinated (+/+/–), double-recombinated (+/–/–) and knock out (–/–/–) cells. The primers for RT–PCR were designed on the bases of the sequences of the first and third exons. (E) Western blotting of wild-type (WT), Raftlin-deficient cells (Raftlin–), and transformants of chicken Raftlin cDNA (Raftlin–/cRaftlin) and human Raftlin cDNA (Raftlin–/hRaftlin) into Raftlin-deficient cells using anti-chicken Raftlin Ab and anti-human Raftlin Ab.
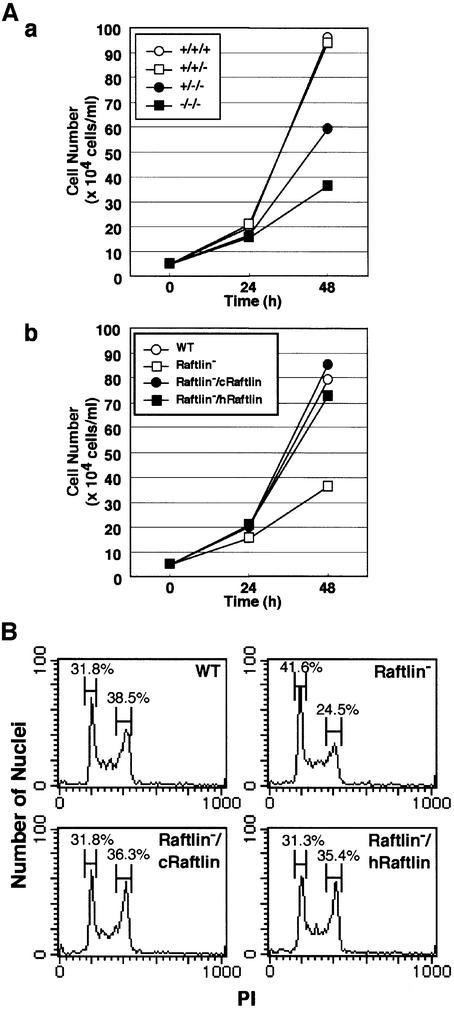
Raftlin expression levels affected proliferation of DT40 cells (Figure 6). The growth rate of DT40 cells was reduced in proportion to the number of knockout alleles (Figure 6A, a). Reintroduction of cRaftlin or hRaftlin in Raftlin– cells resulted in the recovery of a high growth rate (Figure 6A, b). The doubling times at the log phase of wild-type, Raftlin–/cRaftlin and Raftlin–/hRaftlin cells were 12.0, 11.7 and 12.4 h, respectively, while that of Raftlin– cells was 16.7 h. Propidium iodide (PI) staining (Figure 6B) revealed a significant reduction of the population in the G2/M phase and an increase in the G1/G0 phase in Raftlin– cells compared with the wild-type DT40 cells. The proportion of the G2/M and G1/G0 phase recovered to normal levels by the reintroduction of either cRaftlin or hRaftlin. No increase was detected in apoptosis in Raftlin– cells under normal growth conditions when using annexin V staining (data not shown). These data suggest that Raftlin is required for a normal growth rate in DT40 cells.
Fig. 6. Proliferation assay of Raftlin-deficient DT40 cells. (A) Growth curves of DT40 cells. Cells were suspended at 5 × 104 cells per ml in the medium. They were cultured for the indicated time and the cell numbers were counted. (B) Cell cycle analysis. Various mutant DT40 cells were treated in hypotonic solution containing PI and subjected to DNA content analysis by FACScan.
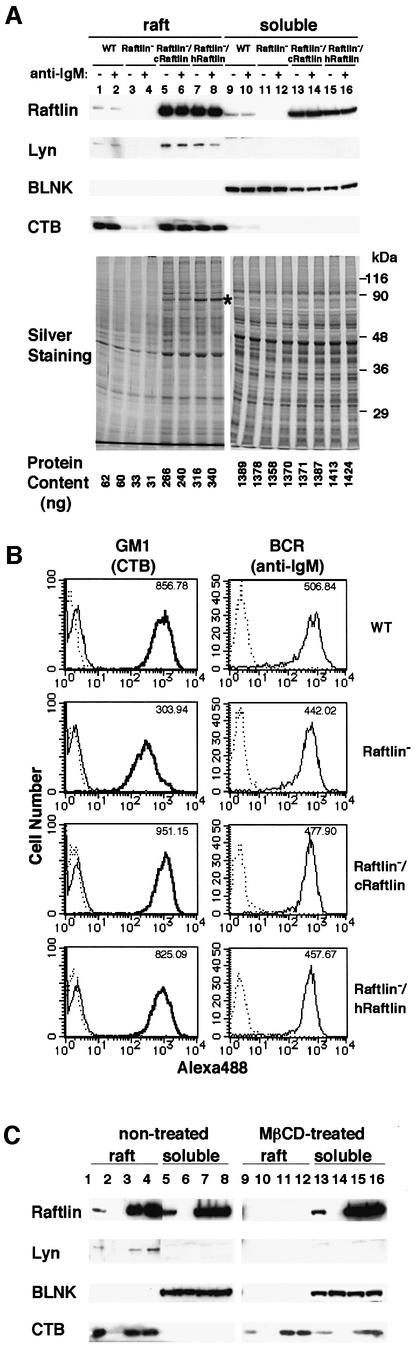
Raftlin is necessary for the formation or maintenance of lipid rafts
The investigation next turned to examining whether Raftlin influences the composition of lipid rafts. Combined raft fractions (fractions 1–4) and soluble fractions (fractions 8–13) from the same number of various DT40 cells were analyzed by western blotting and silver staining (Figure 7A). BLNK, an adapter protein of B cells, was not detected in raft fractions (Figure 7A, BLNK), while Lyn and GM1 detected by CTB were exclusively detected in the raft fractions regardless of BCR stimulation (Figure 7A, Lyn and CTB, lanes 1, 2, 9 and 10). BLNK was shown to localize to membrane fractions after BCR stimulation (Fu et al., 1998; Ishiai et al., 1999), but it may bind to lipid rafts loosely. About 50% of endogenous cRaftlin in DT40 cells was present in raft fractions (Figure 7A, Raftlin, lanes 1, 2, 9 and 10). Surprisingly, the amount of Lyn and GM1 was greatly reduced in the lipid raft fraction of Raftlin– cells compared with the wild-type DT40 cells (lanes 3 and 4) when the same number of cells was compared. Reduction of GM1 in Raftlin– cells was confirmed by FACS analysis, while the level of cell surface expression of BCR on mutant cells was comparable to that of parental cells (Figure 7B). The protein contents and composition were measured by silver staining of SDS polyacrylamide gels, as well as by a protein concentration assay in the lipid raft fraction (Figure 7A, silver staining). Although the composition of the raft proteins in Raftlin– cells seemed to be unaltered (Supplementary figure 7), the amount of proteins in the lipid raft fraction was reduced to ∼50% of that of the wild-type cells (Figure 7A, compare lanes 1 and 2 with 3 and 4). Interestingly, overexpression of cRaftlin and hRaftlin in Raftlin– cells resulted in a marked increase in the amount of Lyn, as well as that of total proteins in the lipid raft fractions (Figure 7A, lanes 5–8). Exogenous cRaftlin and hRaftlin resided more in the raft fractions than in the non-raft fractions (Figure 7A, Raftlin, compare lanes 5–8 with lanes 13–16), which is consistent with the increase in lipid rafts. These data indicate that the amount/content of lipid rafts was reduced by the absence of Raftlin and increased by Raftlin overexpression. Thus, Raftlin is suggested to play an important role in the formation and/or maintenance of lipid rafts.
Fig. 7. Raftlin is necessary for the integrity of lipid rafts. (A) Western blot analysis of proteins in raft and soluble fractions. DT40 cells unstimulated (–) or stimulated with 5 µg/ml M4 for 3 min (+) were lysed and fractionated in a sucrose gradient. Combined raft fractions (fractions 1–4) from 1.5 × 106 cells and combined soluble fractions (fractions 8–13) from 1.5 × 105 cells were loaded onto SDS–PAGE (10%) and visualized by silver staining (Silver Staining). The asterisk indicates chicken and human Raftlin, and the amount of protein in each fraction is shown at the bottom of the panel. For western blotting, raft and soluble fractions from 1 × 106 cells were resolved by SDS–PAGE and stained with anti-Raftlin Ab, anti-Lyn Ab, anti-BLNK Ab and CTB. (B) BCR and GM1 expression on the DT40 cell surface. (Left column) Various type DT40 cells were stained using Alexa488- conjugated CTB with (thin continuous lines) or without (thick continuous lines) 10-fold excess of non-labeled CTB. Unstained cells were used as a negative control (dotted lines). (Right column) To detect surface IgM levels, various genotypes of DT40 cells were stained with M4 antibody followed by Alexa488-conjugated anti-mouse IgM (continuous lines). The cells stained with only Alexa488-conjugated anti-mouse IgM were used as a negative control (dotted lines). The numbers indicate the average of Alexa488 intensity. (C) The effect of cholesterol depletion on the raft proteins and GM1. Wild-type (lanes 1, 5, 9, 13), Raftlin– (lanes 2, 6, 10, 14), Raftlin–/cRaftlin, (lanes 3, 7, 11, 15) and Raftlin–/hRaftlin cells (lanes 4, 8, 12, 16) were treated with or without MβCD, lysed, and fractionated in a sucrose gradient. Combined raft (fractions 1–4; lanes 1–4, 9–12) and combined soluble fractions (fractions 8–13; lanes 5–8, 13–16) from 1 × 106 cells were resolved by SDS–PAGE and blotted with anti-Raftlin, anti-Lyn and anti-BLNK Abs, and CTB–HRP.
Next, we disrupted lipid rafts by treating cells with methyl-β-cyclodextrin (MβCD), which depletes cholesterol, a major component of rafts (Chen and Resh, 2002). The amounts of Raftlin, Lyn and GM1in the raft fractions were greatly decreased (Figure 7C, compare lanes 1, 3 and 4 with lanes 9, 11 and 12), and Raftlin and GM1 levels were increased in the soluble fractions (lanes 13, 15 and 16). Reduction of Lyn protein levels by MβCD treatment resembles that by Raftlin gene deletion. These observations support our hypothesis that Raftlin is important for the integrity of lipid rafts. Our observations also raise the possibility that Lyn is unstable outside lipid rafts.
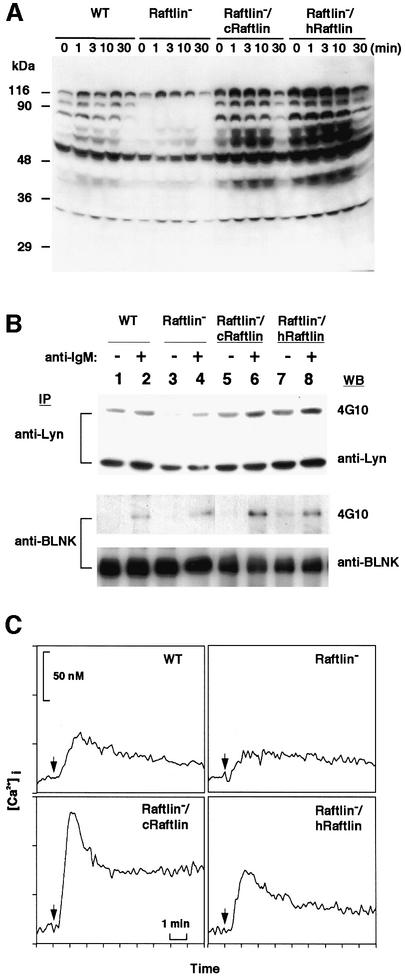
Suppression of BCR signaling in the absence of Raftlin
Reduction of lipid rafts in Raftlin-deficient cells may affect the efficiency of BCR signal transduction. To pursue this possibility, we measured anti-BCR antibody-induced tyrosine phosphorylation of cellular proteins (Figure 8A and B) and intercellular calcium mobilization (Figure 8C). BCR-induced overall tyrosine phosphorylation of Raftlin– cells was markedly reduced compared with that of wild-type DT40 cells. In contrast, BCR-mediated tyrosine phosphorylation of cellular proteins was enhanced by Raftlin overexpression in Raftlin–/cRaftlin and Raftlin–/hRaftlin cells (Figure 8A). Reduction of phosphorylation of Lyn and BLNK was confirmed by immunoprecipitation with specific antibodies and anti-phosphotyrosine antibody (4G10) blotting (Figure 8B). Reduction of phosphorylation of overall cellular proteins in Raftlin-deficient cells might be due to a reduction in the amount and activity of Lyn.
Fig. 8. Loss of Raftlin expression reduced BCR-signal transduction. (A) BCR-induced tyrosine phosphorylation of cellular proteins. Various mutant DT40 cells were stimulated with anti-IgM antibody (M4) (5 µg/ml) for the indicated time. The whole-cell lysates prepared from 106 cells were loaded onto SDS–PAGE (10%) and analyzed by western blotting with anti-phosphotyrosine antibody (4G10). (B) BCR-induced tyrosine-phosphorylation of Lyn and BLNK. Indicated DT40 variants (8 × 106 cells for Lyn and 5 × 106 cells for BLNK) were stimulated with (+) or without (–) M4 (5 µg/ml) for 2 min. The cells were lysed with NP40 lysis buffer and immunoprecipitated using anti-Lyn or anti-BLNK Abs. The precipitants were divided into two parts and blotted with the indicated antibodies. (C) BCR-induced calcium mobilization. Various mutant DT40 cells were stimulated with rabbit anti-mouse IgM followed by M4. Arrows indicate the time points of M4 addition.
BLNK is an essential adaptor molecule for the activation of PLC-γ2 by BCR (Fu et al., 1998). Thus, BCR-mediated Ca2+ mobilization might be affected by Raftlin protein levels. As shown in Figure 8C, BCR-induced intracellular Ca2+ increase was reduced in Raftlin– cells but actually enhanced in Raftlin–/cRaftlin and Raftlin–/hRaftlin cells compared with that of wild-type cells. These data suggest that BCR-mediated signals are significantly reduced in the absence of Raftlin. Since Raftlin does not interact directly with BCR and downstream molecules of BCR signaling (Figure 4B), we assumed that Raftlin is a positive regulator of BCR signaling by increasing the amount of lipid rafts itself or that of signaling molecules, including Lyn, in lipid rafts.
Discussion
In this study, proteins in lipid rafts of B cells are identified. Several proteomic studies have identified raft-resident proteins, including novel proteins, and a few have characterized the physical and functional roles of these proteins in lipid rafts. We confirmed that cytoskeletal proteins and proteins involved in signal transduction are enriched in raft fractions in B cells. We further characterized a major raft-resident protein, Raftlin, using Raftlin-deficient cells and Raftlin-overexpressing cells in DT40 B-cells.
Importantly, the content of the raft fraction was reduced in Raftlin-deficient cells. As a result, growth and BCR signaling were impaired in Raftlin-deficient cells. These phenotypes were recovered by re-expression of human or chicken Raftlin in Raftlin-deficient cells. Therefore, changes in lipid rafts may affect not only BCR signaling but also basal proliferation. Signals from serum growth factors may also be affected by the absence of Raftlin. The reduction of tyrosine phosphorylation of Raftlin-deficient cells before BCR ligation could also be due to the reduction in the signaling by serum growth factors. Raftlin may promote raft assembly indirectly, being a consequence of signaling events.
BCR signaling in mature B cells has been shown to be initiated by the translocation of the BCR into lipid rafts that include the Src family kinase Lyn and exclude the phosphatase CD45. Numerous reports suggest that intact lipid rafts are required for efficient BCR activation and calcium mobilization. For example, Aman and Ravichandran (2000) and Guo et al. (2000) reported that BCR-mediated calcium flux was severely diminished in the presence of raft-disrupting agents such as MβCD. However, Petrie et al. (2000) observed an increase in BCR-induced intracellular calcium mobilization upon disruption of lipid rafts by MβCD in Ramos cells and tonsil B cells. Furthermore, Fc gamma RIIB1 and Cbp/PAG, which transmit negative signals by SHIP and CSK, respectively, have been shown to localize in the rafts (Brdicka et al., 2000; Kawabuchi et al., 2000; Aman et al., 2001). Therefore, lipid rafts also play a role in negative signaling via the BCR. The lack of Raftlin decreases the amount of protein and lipid components, including Lyn and GM1 in the raft fractions. BCR- induced signalings, such as tyrosine phosphorylation and calcium mobilization, were attenuated in Raftlin-deficient cells. These data are consistent with the idea that lipid rafts are necessary for BCR signal initiation. However, to verify the relationship between rafts and BCR activation more clearly, the mechanism of the reduction of raft content in Raftlin-deficient cells and the increase in Raftlin-overexpressing cells must be clarified. Furthermore, it is also important to clarify the negative signals of BCR in our Raftlin-deficient and -overexpressing cells.
At present, it is not clear how Raftlin modulates the content and/or the quantity of lipid rafts. One possibility is that Raftlin has some enzymatic activity linked to the lipid metabolism, thereby influencing the amount of rafts. No Raftlin-related molecules were found in Drosophila or Caenorhabditis elegans; however, counterparts were found in Zebrafish and Fugu. No significant differences were observed in the phospholipid, glycolipid and cholesterol content at the whole-cell level between Raftlin-deficient and wild-type cells. It is therefore unlikely that Raftlin is involved in the basic metabolism of lipid synthesis and transport, although the possibility that Raftlin is involved in the trafficking or synthesis of a vertebrate-specific lipid cannot be ruled out. Raftlin may bind to lipids for a stable lipid microdomain such as caveolin. Caveolin binds to cholesterol tightly and is involved in cholesterol trafficking (Fielding and Fielding, 1996; Smart et al., 1996). Furthermore, the cholesterol content in caveolae may affect signaling (Ikonen and Parton, 2000). Since most lymphocytes do not express caveolin, Raftlin may have caveolin-like functions in B cells. Another possibility is that Raftlin may anchor cytoskeletal proteins into rafts. We are currently trying to identify molecules (proteins and lipids) that associate with Raftlin.
The association of the BCR with lipid rafts has been shown to change during development. In mature resting B cells, the BCR is excluded from lipid rafts, and antigen binding results in association of the BCR with rafts. By contrast, in immature B cells, antigen binding to the BCR does not induce stable association of the BCR with rafts, and the BCR signals for apoptosis outside rafts (Sproul et al., 2000). However, it has not been clarified how the size, amount and composition of lipid rafts change during the development of B cells. This study raises the possibility that raft-resident molecules such as Raftlin may influence the nature of lipid rafts and modulate the intensity and duration of BCR signals. This possibility is currently being explored by the creation of Raftlin-gene-disrupted mice.
The BCR might either be internalized directly from rafts or move laterally from rafts and then be internalized for the purpose of antigen processing or downregulation. At present, it is difficult to determine whether the BCR is internalized raft-dependently or -independently. Our preliminary data suggest that the internalization rate of BCR in DT40 cells is not affected by the absence of Raftlin (K.Saeki and A.Yoshimura, unpublished data). Thus, internalization of BCR may be independent of rafts in DT40 cells.
Materials and methods
Antibodies and cells
Anti-human Raftlin and anti-chicken Raftlin Abs were obtained by immunizing rabbits with bacterially expressed GST fusion proteins containing full-length human and chicken Raftlins, respectively. Anti-chicken Lyn and anti-chicken BLNK Abs have been described previously (Takata et al., 1994; Ishiai et al., 1999). We obtained anti-Lyn monoclonal antibody (MAb) from Transduction Laboratories, anti-STAT5b and anti-GFP (green fluorescent protein) Abs from Santa Cruz Biotechnology, Alexa-conjugated immunoglobulin Gs (IgGs) and Alexa488-conjugated CTB from Molecular Probes, Inc., anti-human IgM and anti-mouse IgM from MBL Medical and Biological Laboratories Co. Ltd, M4, anti-chicken IgM MAb, from Southern Biotechnology Associates, Inc., 4G10, anti-phosphotyrosine MAb, from Upstate Biotechnology, Inc., and horseradish peroxidase (HRP)-conjugated and non-labeled CTB from Sigma. Raji cells and Daudi cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin and streptomycin. Wild-type and its derivative mutant DT40 cells were cultured in RPMI 1640 supplemented with 10% FCS, 1% chicken serum, 50 µM 2-mercaptoethanol, penicillin and streptomycin. HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS and antibiotics.
Subcellular fractionation of Raji cells
Raji cells (6 × 107 cells) were lysed in 2.5 ml of Triton X-100 lysis buffer (50 mM Tris–HCl pH 8.0, 10 mM MgCl2, 150 mM NaCl, 20 mM NaF, 1 mM Na3VO4, 1% Triton X-100, 5 mM 2-mercaptoethanol, 5% glycerol, and protease inhibitor cocktail; Roche-Boehringer), incubated on ice for 1 h, and mixed with equal volume of 80% sucrose in buffer A (50 mM Tris–HCl pH 7.4, 50 mM NaCl, 10 mM MgCl2, 1 mM Na3VO4 and protease inhibitor cocktail). The mixture was transferred to a centrifuge tube and sequentially overlaid with 5 ml of 35% sucrose in buffer A and 1.2 ml of 5% sucrose in buffer A. After centrifugation at 100 000 g at 4°C for 16 h, 0.5 ml fractions were collected from the top of the tube.
Identification of the proteins co-purified with lipid rafts
For the sequence analysis of the proteins in the raft fraction, Raji cells (1.8 × 108 cells) were lysed and centrifuged as described above. The raft fraction at the interface between 35 and 5% sucrose was collected, diluted 3-fold with buffer A, and centrifuged with 100 000 g at 4°C for 1 h. The pellet was washed with Triton X-100 washing buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% Triton X-100) five times and lysed with SDS–PAGE loading buffer containing 280 mM 2-mercaptoethanol. The sample was subjected to SDS–PAGE using 10% gel and stained with Coomassie Brilliant Blue. The major bands shown in Figure 1 were excised and subjected to in-gel digestion with trypsin, and the eluted peptides were loaded on LC-ESI-MS/MS (FINNIGAN LC Q DECA; Finnigan). The data were searched using Sequest software.
Northern blot analysis
Poly(A)+ RNAs of mouse tissues were prepared using TRIZOL Reagent (Invitrogen Life Technologies) followed by Oligotex-dT30<Super> (Takara Bio, Inc.). For northern hybridization, poly(A)+ RNAs (0.5 µg each) were separated on a 1% agarose gel and transferred to a nylon membrane. Hybridization riboprobes were prepared by in vitro transcription with digoxigenin RNA Labeling Mix (Roche-Boehringer) using appropriate cDNA fragments: a 720 bp fragment as a probe for mouse Raftlin and a 500 bp fragment as a probe for G3PDH. Hybridization and detection were performed as described previously (Nakamura et al., 1999).
Mutant analysis of human Raftlin
Wild-type and mutant full-length human Raftlin cDNAs (G2A, C3S, G2A, C3S) were prepared using PCR with wild-type or mutated 5′ primers and a wild-type 3′ primer, and cloned into pEGFP-N1 (Clontech Laboratories, Inc.). These plasmids were transfected to HEK293T cells using calcium phosphate precipitation (Chen and Okayama, 1987), and Raftlin–GFP fusion proteins were observed by fluorescence microscopy using IX70 (Olympus). The transfected cells (5 × 106 cells) were lysed in 0.3 ml of Triton X-100 lysis buffer, mixed with equal volume of 80% sucrose in buffer A, and fractionated on a sucrose gradient similar to the one described above [600 µl (40% sucrose): 550 µl (35% sucrose):250 µl (5% sucrose)]. After centrifugation for 16 h at 100 000 g at 4°C, 100 µl fractions were collected from the top of the tube.
For the inhibition of fatty acylation, the culture medium of HEK293T cells was changed to DMEM containing 2.5% FCS, 0.25% defatted BSA (Sigma) with or without 100 µM 2-hydroxymyristic acid or 100 µM 2-bromopalmitic acid, as described previously (Webb et al., 2000). After 1 h, the cells were transfected with wild-type human Raftlin–GFP fusion protein and incubated overnight. Raftlin–GFP fusion proteins were observed by fluorescence microscopy and fractionated by sucrose gradient centrifugation as described above.
Immunofluorescence staining and confocal microscopy
For the double staining of Raftlin and BCR, non-stimulated Daudi cells were fixed with formaldehyde, stained with goat anti-human IgM followed by Alexa546-conjugated donkey anti-goat IgG, fixed with formaldehyde, permeabilized with 0.1% Triton X-100, and stained with rabbit anti-human Raftlin Ab followed by Alexa488-conjugated goat anti-rabbit IgG. Other Daudi cells were stimulated with goat anti-human IgM and Alexa546-conjugated donkey anti-goat IgG at 37°C in the culture medium, fixed with formaldehyde, permeabilized with 0.1% Triton X-100, and stained with rabbit anti-human Raftlin Ab followed by Alexa488-conjugated goat anti-rabbit IgG. For the double staining of GM1 and BCR, non-stimulated Daudi cells were fixed with formaldehyde, and stained with goat anti-human IgM followed by Alexa546-conjugated donkey anti-goat IgG and Alexa488-conjugated CTB. Cells stimulated with goat anti-human IgM and Alexa546-conjugated donkey anti-goat IgG for indicated periods were stained with Alexa488-conjugated CTB. For the double staining of GM1 and Raftlin, non-stimulated Daudi cells were fixed and stained with Alexa488-conjugated CTB, then rabbit anti-human Raftlin Ab and Alexa546-conjugated goat anti-rabbit IgG following permeabilization with 0.1% Triton X-100. Stimulated cells were fixed and stained with Alexa488-conjugated CTB, then with rabbit anti-human Raftlin Ab and Alexa546-conjugated goat anti-rabbit IgG after permeabilization. The specimens were observed by confocal microscopy using an LSM 5 Pascal (Carl Zeiss).
Isolation of cRaftlin cDNA
To determine a full-length chicken Raftlin cDNA sequence, 5′ and 3′ RACE was performed using the Marathon cDNA amplification kit (Clontech Laboratories, Inc.) and poly(A)+ RNA from DT40 cells. To design the primers for the RACE, two fragments of cRaftlin cDNA (a 5′ fragment and a 3′ fragment) were obtained from DT40 cells by RT–PCR using oligonucleotide primers corresponding to the amino acid sequences (5′ fragment: -LNKLEKR- for the sense primer of the first PCR, -KRPQVET- for the sense primer of the second PCR, -HPFVQPT- for the antisense primer of the first PCR, and -YQQGFSL- for the antisense primer of the second PCR; 3′ fragment: -HMSDHFR- for the sense primer of the first and second PCR, -KFQWRFS- for the antisense primer of the first PCR, and -VEQWTVL- for the antisense primer of the second PCR) conserved between hRaftlin and mouse Raftlin, and the nucleotide sequences of the two fragments were determined.
Generation of Raftlin-deficient DT40 cells
Chicken genomic fragments containing the second exon of Raftlin were obtained from a λFIXII chicken genomic library by PCR using primers on the bases of the sequences second exon and λ phage. The targeting vectors, pRaftlin-hygro, pRaftlin-hisD and pRaftlin-Neo, were constructed by replacing the genomic fragment containing the second exon that corresponds to Raftlin amino acid residues 1–49, with hygro, hisD and neo cassettes. These cassettes were flanked by 3.3 and 2.7 kb of genomic sequences on the 5′ and 3′ sides, respectively. The targeting vectors were linearized and transfected into DT40 cells by electroporation (550 V, 25 µF). After the isolation of several clones in the presence of various drugs (2 mg/ml hygromycin, 1 mg/ml histidinol and 2 mg/ml G418), genomic DNAs were prepared and analyzed by PCR.
Transformants overexpressing chicken and human Raftlin in Raftlin-deficient cells were prepared by transfection of full-length chicken cDNA and full-length human cDNA inserted into pcDNA4/TO (Invitrogen), and selection using 1 mg/ml Zeocin.
Subcellular fractionation of DT40 cells
Wild-type cells and mutants (5 × 107 cells) were lysed in 0.3 ml of Triton X-100 lysis buffer, mixed with equal volume of 80% sucrose in buffer A, and fractionated on a sucrose gradient similar to the one described above [600 µl (40% sucrose):500 µl (35% sucrose):250 µl (5% sucrose)]. After centrifugation, 100 µl fractions were collected from the top of the tube. For cholesterol depletion analysis, cells (5 × 107) were washed with RPMI and incubated in RPMI or RPMI containing 2% MβCD for 1 h. After washing with PBS, cells were lysed and fractionated by the sucrose gradient centrifugation as described above.
Calcium analysis
Cells (5 × 106) were washed once and loaded with 3 µM fura-2/AM in PBS containing 20 mM HEPES pH 7.2, 5 mM glucose, 0.025% BSA and 1 mM CaCl2. After 30 min of incubation at 37°C, cells were washed twice and diluted to 106 cells/ml with the same buffer. Co-ligation of BCR was carried out by adding rabbit anti-mouse IgM (10 µg/ml) followed by anti-chicken IgM MAb, M4 (2 µg/ml). Continuous monitoring of fluorescence from the cell suspension was performed using a Hitachi F-4010 fluorescence spectrophotometer at excitation wavelengths of 340 and 380 nm, and an emission wavelength of 510 nm. The fluorescence intensities were normalized by subtracting the background levels measured in the presence of EGTA and Triton X-100.
Flow cytometric analysis
BCR and GM1 expression analysis and a cell stage assay were performed using FACScan (FACSCalibur; Becton Dickinson). For the cell stage assay, 4 × 105 cells were incubated in hypotonic fluorochrome solution (50 mg/ml PI, 0.1% sodium citrate, 0.1% Triton X-100), and the PI-stained nuclei were analyzed. For the cell surface expression analysis of BCR and GM1, cells were washed with Hanks’ solution containing 0.1% NaN3 and 2% FCS, and stained with M4 (5 µg/ml) followed by Alexa488-conjugated anti-mouse IgM (10 µg/ml) or Alexa488-conjugated CTB (2 µg/ml) with or without preincubation of non-labeled CTB (20 µg/ml), respectively.
DDBJ/EMBL/GenBank accession No.
The chicken Raftlin cDNA sequence data have been submitted to the DDBJ/EMBL/GenBank database under accession No. AB092511.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank the Kazusa DNA Research Institute for the KIAA0084 cDNA clone, Dr Goizuka (Science University of Tokyo) for the λFIXII chicken genomic library, E.Fujimoto for technical assistance, and Drs M.Kurosaki, M.Matsumoto, H.Yoshida and A.K.Oromilas for helpful suggestions. Part of this work was supported by grants from the Ministry of Education, Science, Technology, Sports and Culture of Japan, the Japan Health Science Foundation, the Human Frontier Science Program, the Japan Research Foundation for Clinical Pharmacology, and the Uehara Memorial Foundation.
References
- Aman M.J. and Ravichandran,K.S. (2000) A requirement for lipid rafts in B cell receptor induced Ca(2+) flux. Curr. Biol., 10, 393–396. [DOI] [PubMed] [Google Scholar]
- Aman M.J., Tosello-Trampont,A.C. and Ravichandran,K. (2001) FcγRIIB1/SHIP-mediated inhibitory signaling in B cells involves lipid rafts. J. Biol. Chem., 276, 46371–46378. [DOI] [PubMed] [Google Scholar]
- Brdicka T. et al. (2000) Phosphoprotein associated with glycosphingo lipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med., 191, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A. and London,E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111–136. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and London,E. (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem., 275, 17221–17224. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and Rose,J.K. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533–544. [DOI] [PubMed] [Google Scholar]
- Burkhardt A.L., Brunswick,M., Bolen,J.B. and Mond,J.J. (1991) Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc. Natl Acad. Sci. USA, 88, 7410–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. and Okayama,H. (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol., 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. and Resh,M.D. (2002) Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem., 277, 49631–49637. [DOI] [PubMed] [Google Scholar]
- Cheng P.C., Dykstra,M.L., Mitchell,R.N. and Pierce,S.K. (1999) A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med., 190, 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P.E. and Fielding,C.J. (1996) Intracellular transport of low density lipoprotein derived free cholesterol begins at clathrin-coated pits and terminates at cell surface caveolae. Biochemistry, 35, 14932–14938. [DOI] [PubMed] [Google Scholar]
- Fridriksson E.K., Shipkova,P.A., Sheets,E.D., Holowka,D., Baird,B. and McLafferty,F.W. (1999) Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry, 38, 8056–8063. [DOI] [PubMed] [Google Scholar]
- Fu C., Turck,C.W., Kurosaki,T. and Chan,A.C. (1998) BLNK: a central linker protein in B cell activation. Immunity, 9, 93–103. [DOI] [PubMed] [Google Scholar]
- Garin J., Diez,R., Kieffer,S., Dermine,J.F., Duclos,S., Gagnon,E., Sadoul,R., Rondeau,C. and Desjardins,M. (2001) The phagosome proteome: insight into phagosome functions. J. Cell Biol., 152, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Kato,R.M., Garcia-Lloret,M., Wahl,M.I. and Rawlings,D.J. (2000) Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity, 13, 243–253. [DOI] [PubMed] [Google Scholar]
- Hooper N.M. (1999) Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol. Membr. Biol., 16, 145–156. [DOI] [PubMed] [Google Scholar]
- Ikonen E. and Parton,R.G. (2000) Caveolins and cellular cholesterol balance. Traffic, 1, 212–217. [DOI] [PubMed] [Google Scholar]
- Ishiai M., Kurosaki,M., Pappu,R., Okawa,K., Ronko,I., Fu,C., Shibata,M., Iwamatsu,A., Chan,A.C. and Kurosaki,T. (1999) BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity, 10, 117–125. [DOI] [PubMed] [Google Scholar]
- Kawabuchi M., Satomi,Y., Takao,T., Shimonishi,Y., Nada,S., Nagai,K., Tarakhovsky,A. and Okada,M. (2000) Transmembrane phospho protein Cbp regulates the activities of Src-family tyrosine kinases. Nature, 404, 999–1003. [DOI] [PubMed] [Google Scholar]
- Kosugi A., Hayashi,F., Liddicoat,D.R., Yasuda,K., Saitoh,S. and Hamaoka,T. (2001) A pivotal role of cysteine 3 of Lck tyrosine kinase for localization to glycolipid-enriched microdomains and T cell activation. Immunol. Lett., 76, 133–138. [DOI] [PubMed] [Google Scholar]
- Mairhofer M., Steiner,M., Mosgoeller,W., Prohaska,R. and Salzer,U. (2002) Stomatin is a major lipid-raft component of platelet alpha granules. Blood, 100, 897–904. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Shirai,T., Morishita,S., Uchida,S., Saeki-Miura,K. and Makishima,F. (1999) p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp. Cell Res., 250, 351–363. [DOI] [PubMed] [Google Scholar]
- Nebl T., Pestonjamasp,K.N., Leszyk,J.D., Crowley,J.L., Oh,S.W. and Luna,E.J. (2002) Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem., 277, 43399–43409. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature, 334, 661–665. [DOI] [PubMed] [Google Scholar]
- Petrie R.J., Schnetkamp,P.P., Patel,K.D., Awasthi-Kalia,M. and Deans,J.P. (2000) Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol., 165, 1220–1227. [DOI] [PubMed] [Google Scholar]
- Pierce S.K. (2002) Lipid rafts and B-cell activation. Nat. Rev. Immunol., 2, 96–105. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. (1996) Clonal selection and learning in the antibody system. Nature, 381, 751–758. [DOI] [PubMed] [Google Scholar]
- Resh M.D. (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys Acta, 1451, 1–16. [DOI] [PubMed] [Google Scholar]
- Rigley K.P., Harnett,M.M., Phillips,R.J. and Klaus,G.G. (1989) Analysis of signaling via surface immunoglobulin receptors on B cells from CBA/N mice. Eur. J. Immunol., 19, 2081–2086. [DOI] [PubMed] [Google Scholar]
- Smart E.J., Ying,Y., Donzell,W.C. and Anderson,R.G. (1996) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J. Biol. Chem., 271, 29427–29435. [DOI] [PubMed] [Google Scholar]
- Sproul T.W., Malapati,S., Kim,J. and Pierce,S.K. (2000) Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J. Immunol., 165, 6020–6023. [DOI] [PubMed] [Google Scholar]
- Takata M. and Kurosaki,T. (1996) A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med., 184, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sabe,H., Hata,A., Inazu,T., Homma,Y., Nukada,T., Yamamura,H. and Kurosaki,T. (1994) Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J., 13, 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haller P.D., Donohoe,S., Goodlett,D.R., Aebersold,R. and Watts,J.D. (2001) Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics, 1, 1010–1021. [DOI] [PubMed] [Google Scholar]
- Webb Y., Hermida-Matsumoto,L. and Resh,M.D. (2000) Inhibition of protein palmitoylation, raft localization and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem., 275, 261–270. [DOI] [PubMed] [Google Scholar]
- Weintraub B.C., Jun,J.E., Bishop,A.C., Shokat,K.M., Thomas,M.L. and Goodnow,C.C. (2000) Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J. Exp. Med., 191, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]