Abstract
The identification of osmo/mechanosensory proteins in mammalian sensory neurons is still elusive. We have used an expression cloning approach to screen a human dorsal root ganglion cDNA library to look for proteins that respond to hypotonicity by raising the intracellular Ca2+ concentration ([Ca2+]i). We report the unexpected identification of GAP43 (also known as neuromodulin or B50), a membrane-anchored neuronal protein implicated in axonal growth and synaptic plasticity, as an osmosensory protein that augments [Ca2+]i in response to hypotonicity. Palmitoylation of GAP43 plays an important role in the protein osmosensitivity. Depletion of intracellular stores or inhibition of phospholipase C (PLC) activity abrogates hypotonicity-evoked, GAP43-mediated [Ca2+]i elevations. Notably, hypotonicity promoted the selective association of GAP43 with the PLC-δ1 isoform, and a concomitant increase in inositol-1,4,5-trisphosphate (IP3) formation. Collectively, these findings indicate that hypo-osmotic activation of GAP43 induces Ca2+ release from IP3-sensitive intracellular stores. The osmosensitivity of GAP43 furnishes a mechanistic framework that links axon elongation with phospho inositide metabolism, spontaneous triggering of cytosolic Ca2+ transients and the regulation of actin dynamics and motility at the growth cone in response to temporal and local mechanical forces.
Keywords: actin cytoskeleton/dorsal root ganglion/mechanotransduction/phosphoinositides/PLC
Introduction
Mechanotransduction enables living organisms to detect touch, vibration, movement and mechanical pain (Hamill and Martinac, 2001). Detection of mechanical and osmotic stimuli is essential for a variety of cellular properties such as regulation of cell volume, shape and motility (Bourque and Oliet, 1997; García-Añoveros and Corey, 1997; Hamill and Martinac, 2001). Molecularly, transduction of mechanical forces into electrochemical signals that underlie the cellular response is carried out by a widely expressed, specialized class of membrane proteins known as mechanosensitive ion channels (MSCs) (Bourque and Oliet, 1997; Gillespie and Walker, 2001). Several putative MSCs have been cloned recently. Members of the epithelial sodium channel/degenerin family and of the TRP channel family, in particular the TRPV subfamily of Ca2+-permeable channels, have been associated with responses to mechanical and osmotic stimuli in neuronal and non-neuronal cells in Drosophila melanogaster, Caenorhabditis elegans and mammals (Colbert et al., 1997; García-Añoveros and Corey, 1997; Suzuki et al., 1999; Liedtke et al., 2000; Strotmann et al., 2000; Walker et al., 2000). Mutations in these channels may produce important dysfunctions in touch, hearing and olfaction, as well as impaired proprioception and, possibly, mechanical pain (Walker et al., 2000; Hamill and Martinac, 2001).
Gating of MSCs in specialized mechanosensory cells appears to be regulated by extracellular and intracellular attachments to proteins that pull the channel open (García-Añoveros and Corey, 1997). In C.elegans, several genes have been cloned and found to encode proteins that are assembled to form with MSCs a ‘mechanosensory apparatus’ for the detection of mechanical forces. This type of arrangement has also been postulated for mechanotransduction in specialized hair cells of vertebrates (Hamill and Martinac, 2001). Mechanosensitivity is present in the peripheral nerve endings of various classes of mammalian primary sensory neurons. These exhibit a wide spectrum of sensitivities to stretch, ranging from displacement by a few microns to injurious mechanical forces. Variability in threshold is associated with different subpopulations of neurons endowed with characteristic electrophysiological properties, that respond to hypotonicity-induced membrane stretch with Ca2+ responses differing markedly in kinetics and amplitude (Viana et al., 2001). However, it is not established whether differences in mechanical threshold among mechanosensitive neurons are due to the presence of distinct molecular entities in their mechanosensory apparatus (Hart et al., 1999). Thus, the identification of osmo/mechanosensory proteins in mammalian sensory neurons remains elusive.
Here, we have used an expression cloning strategy to screen a human dorsal root ganglion (DRG)-derived cDNA library to look for proteins that respond to hypo-osmotic stimuli by raising the intracellular calcium concentration ([Ca2+]i). Unexpectedly, the screen identified GAP43, a hydrophilic, membrane-anchored neuronal protein implicated in axonal growth and synaptic plasticity (Aigner and Caroni, 1993, 1995; Dent and Meiri, 1998; Mueller, 1999), as an osmoreceptive protein that augments the [Ca2+]i in response to hypotonicity. Membrane anchoring of GAP43 was critical for the osmosensitive activity. Analysis of the mechanism of action indicates that [Ca2+]i augmentation was due to activation of the inositol-1,4,5-trisphospate (IP3) signaling pathway that leads to Ca2+ release from IP3-sensitive intracellular stores. It is noteworthy that hypotonicity stimulated the formation of IP3 and favored the association of GAP43 with phospholipase C-δ1 (PLC-δ1). Our findings imply that this previously unrecognized osmosensitive activity of GAP43 may underlie its biological function in axonal growth and synaptic plasticity.
Results
Identification of GAP43 as an osmosensory protein
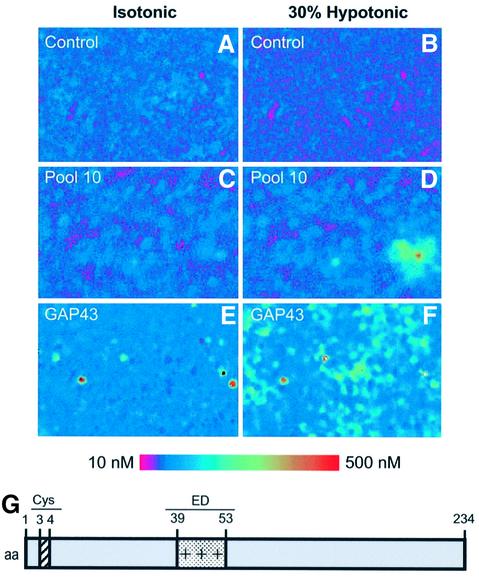
To identify osmosensory proteins, we screened a DRG cDNA library arranged in 50 pools each containing ∼3000 clones. Each pool was transiently transfected into human embryonic kidney-derived (HEK293) cells for functional screening. The functional osmosensitive assay consisted of measuring intracellular Ca2+ elevations in transfected cells upon exposure to a 30% hypo-osmotic solution (217 mOsm/l). For this task, transfected cells were loaded with the calcium-sensitive dye Fura-2 AM and microscopically examined for hypotonicity-evoked changes in fluorescence. As illustrated in Figure 1A and B, untransfected HEK293 cells (control) did not elicit [Ca2+]i changes under either isotonic or hypotonic conditions. Similar results were obtained with cells transfected with the empty plasmid (data not shown). In contrast, cells transfected with cDNA from pool 10 of the library exhibited a significant hypotonicity-evoked increment in cytosolic Ca2+ (Figure 1D). Iterative subdivision and re-assay of the subsequent pools led to the identification of an individual clone containing an 800 bp cDNA insert that conferred osmosensitivity to HEK293 cells (Figure 1F). The open reading frame of this cDNA encoded a protein of 238 amino acids with a predicted mol. wt of 24.8 kDa. A homology BLAST search in non-redundant protein databases identified this protein as GAP43 (also known as neuromodulin or B-50), a neuronal protein implicated in axonal growth and synaptic plasticity (Aigner and Caroni, 1993, 1995; Dent and Meiri, 1998; Mueller, 1999; Bomze et al., 2001; Hulo et al., 2002). GAP43 is a hydrophilic protein anchored to the cell membrane through palmitoyl ation of two key cysteines located at the N-terminus (Figure 1G). In addition, the protein has a basic domain [known as the effector domain (ED), Figure 1G] that is involved in its interaction with phosphoinositides and calmodulin, and contains a phosphorylation site for protein kinase C (PKC; Caroni, 2001).
Fig. 1. Expression cloning of GAP43 protein using Ca2+ imaging. HEK293 cells transiently transfected with clones from a human DRG library were subjected to microscopic fluorescent calcium imaging in isotonic (left) and 30% hypotonic conditions (right). Non-transfected cells exhibited no response to the osmotic–mechanical stimulus (A and B). Cells transfected with pool 10 show a marked increase in cytoplasmic calcium (C and D). This pool was subdivided and re-assayed iteratively until a single positive clone (GAP43) was isolated (E and F). Elevated relative Ca2+ concentrations are indicated by an increased ratio of Fura-2 emission at 340 versus 380 nm excitation wavelenght (see calibrated colored bar). (G) Modular organization of GAP43. Palmitoylation occurs at Cys3 and Cys4. The protein displays a positively charged segment (amino acids 39–53) known as the ED domain.
GAP43-dependent, hypo-osmotic activation of calcium release
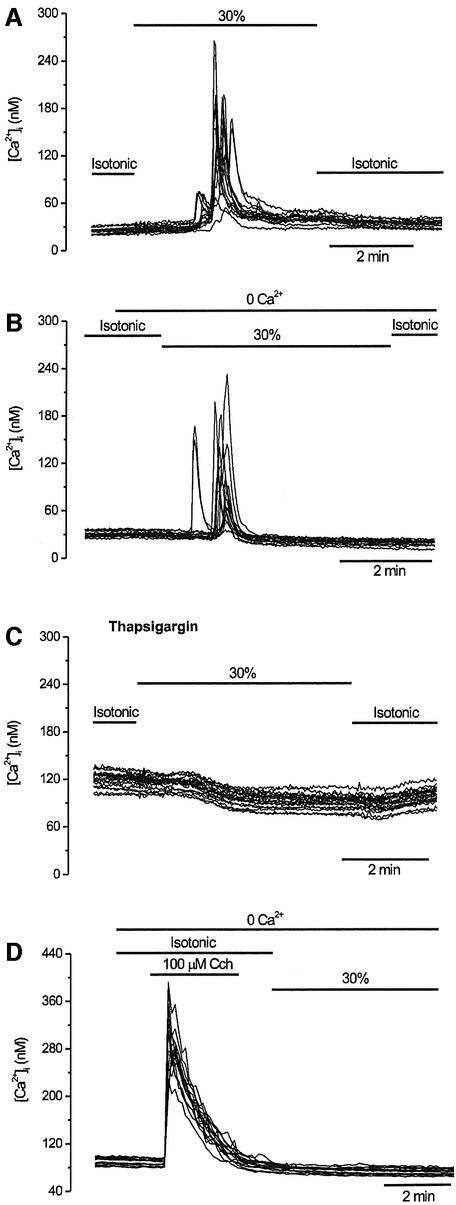
Because GAP43 is not an integral membrane protein, we next investigated whether the hypotonicity-induced, GAP43-mediated [Ca2+]i increase is due to an influx of extracellular Ca2+ or its release from intracellular stores. In the presence of extracellular Ca2+, hypo-osmotically triggered [Ca2+]i changes appeared ∼2 min after external medium change. At variance with exposure to hypotonicity, application of a 30% hyper-osmotic stimulus did not elicit Ca2+ responses in GAP43-expressing cells (GAP43+ cells) (data not shown). Hypo-osmotic responses typically had a rapid rise and decay, with a modest plateau phase in some cells (Figure 2A). Virtually all cells responded once to the hypotonic stimulus, although the latency time of the [Ca2+]i increase was variable from cell to cell. Removal of the external Ca2+ (0Ca2+) did not prevent these osmotically sensitive transient increases in cytosolic Ca2+ (Figure 2B), suggesting that hypotonicity releases Ca2+ from intracellular stores. To substantiate this tenet further, we evaluated whether depletion of the endoplasmic reticulum (ER) with thapsigargin affected hypotonicity-induced [Ca2+]i increases in GAP43+ cells. As illustrated in Figure 2C, treatment with 2 µM thapsigargin for 1 h sub stantially reduced the osmotically evoked [Ca]i increase in GAP43+ cells in the presence of extracellular Ca2+ ([Ca2+]I = 3.8 ± 1.0 nM, n = 150). Similar results were obtained in thapsigargin-treated GAP43+ cells that were hypo-osmotically stimulated in 0Ca2+ (data not shown). Furthermore, carbachol (Cch)-induced Ca2+ release, prior to the hypo-osmotic stimulus, completely abolished hypotonic Ca2+ responses in GAP43+ cells (Figure 2D). Taken together, these results demonstrate that the hypotonicity-induced, GAP43-mediated [Ca2+]i increment is due to release from the ER.
Fig. 2. Swelling-activated Ca2+ signals involve release from intracellular stores. GAP43+ cells were loaded with Fura2-AM to record intracellular calcium signals and activated as indicated. Each panel shows superimposed traces of 15 individual cells recorded in the same experiment. (A) Extracellular Ca2+ (2.4 mM) was present throughout the experiment. After 1 min in standard isotonic solution, the tonicity of the solution was reduced to 70% for 5 min. (B) The external Ca2+ was removed from the medium (and 0.1 mM EGTA was added) 1 min before the start of the 30% hypotonic solution. (C) Responses evoked in GAP43+ cells incubated for 60 min in 2 µM thapsigargin to empty intracellular Ca2+ stores. Identical conditions to those in (A) were used. (D) Pre-treatment of GAP43+ cells with 100 µM carbachol in Ca2+-free solution abolished the subsequent response to 30% hypotonicity.
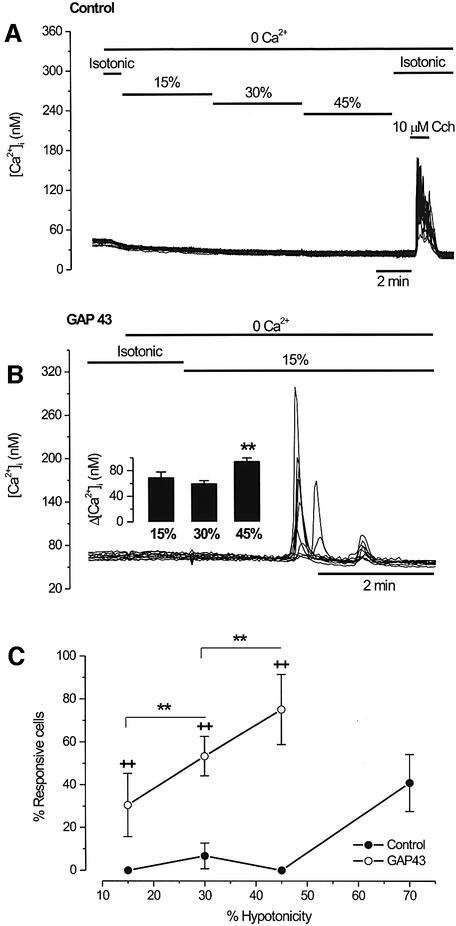
To characterize the osmosensitive release of Ca2+ in GAP43+ cells further, we examined the effects of different levels of hypo-osmolarity to obtain a dose–response relationship. As depicted in Figure 3A and C, the percentage of untransfected HEK293 cells (control) that responded to low-to-moderate levels of hypotonicity (15–45%) increasing the [Ca2+]i was marginal. This lack of response was not due to emptiness of the ER nor to deficiencies in the signaling pathways, since Ca2+ release could be readily evoked by the muscarinic agonist carbachol (Figure 3A). Furthermore, untransfected cells produced significant Ca2+ responses when intense hypo-osmotic stimuli such as 70% were used (Figure 3C). In contrast, [Ca2+]i increases in GAP43+ cells were evoked by hypo-osmotic solutions as low as 15% (Figure 3B). Both the mean amplitude of the Ca2+ transients and the number of responsive GAP43+ cells increased as a function of the hypotonicity of the extracellular solution (Figure 3B, inset and Figure 3C). Note that the magnitude of the [Ca2+]i elevation was significantly higher at strong hypotonicity (Figure 3B, inset). Collectively, these results indicate that GAP43 endows the cells with osmosensitivity, presumably by decreasing their threshold for signaling downstream of osmosensation.
Fig. 3. Swelling-activated intracellular Ca2+ release is dose dependent. HEK293 cells were loaded with Fura2-AM to record intracellular calcium signals, and activated as indicated. (A) Untransfected HEK293 cells (control) were placed in a Ca2+-free (0.1 mM EGTA) isotonic solution and stimulated with solutions of increasing hypotonicity (15, 30 and 45%) for 5 min. Note the absence of response to the osmotic stimulus and the robust response to muscarinic stimulation with 10 µM carbachol. (B) In GAP43+ cells, a mild hypotonic stimulus (15%) in a Ca2+-free solution evokes robust [Ca2+]i signals. The inset shows the mean amplitude of [Ca2+]i elevations for different degrees of hypotonicity. Cells were tested with a single level of hypotonicity. The mean amplitude of the [Ca2+]i signals was larger for the strongest hypotonic stimulus. **P < 0.05 Mann–Whitney rank sum test with N (number of cells) ≥150, and n (number of experiments) ≥3. (C) The percentage of swelling-responsive cells as a function of extracellular hypotonicity. For each experiment, 50 cells in the field were marked at random and scored for a response if the [Ca2+]i was >15 nM. Data are given as mean ± SEM with N ≥ 150 cells, and n ≥ 3. (++) and (**) denote the P < 0.001 significance (Z-test) for the hypotonic responses of GAP43-transfected versus untransfected cells, and for the different hypo- osmolarities tested in GAP43+ cells, respectively.
The palmitoylation sites of GAP43 play an important role in hypotonicity-activated Ca2+ release
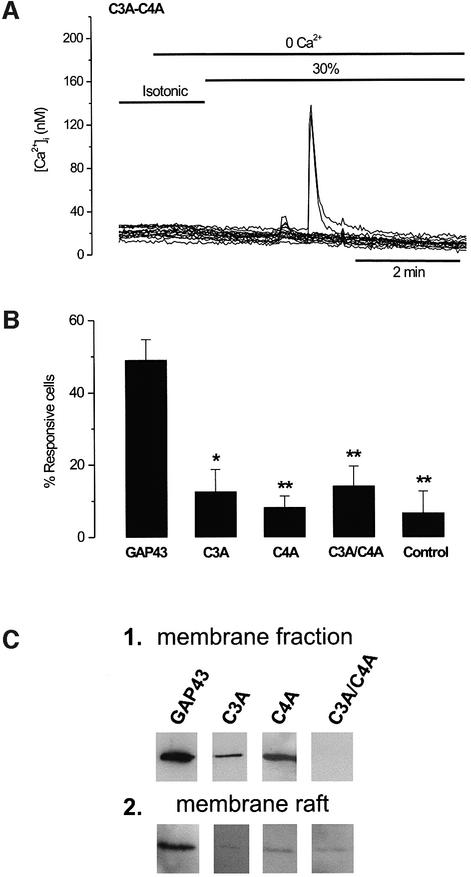
GAP43 is a protein anchored to the membrane through palmitoylation of cysteine residues at the N-terminus (Arni et al., 1998). In addition, this neuronal protein strongly segregates into lipid rafts (Laux et al., 2000). Thus, we questioned whether anchoring to the cellular membrane and interaction with membrane rafts modulated the osmosensory activity of GAP43. To address this issue, both palmitoylation sites were mutated, giving rise to the single mutants C3A and C4A, and the double mutant C3A/C4A. Mutant proteins were expressed in HEK293 cells to evaluate hypotonicity-induced Ca2+ release, membrane location and lipid raft interaction. Functionally, replacement of either C3 or C4 or both by alanine drastically reduced the number of transfected cells responding to hypotonicity, as illustrated for the C3A/C4A double mutant (Figure 4A). Quantitative analysis showed that the number of mutant-transfected cells responding to 30% hypotonicity for C3A (13 ± 6%, mean ± SD, n (number of cells) = 150), C4A (8 ± 3%, n = 230) and C3A/C4A (14 ± 5%, n = 150) was not statistically different from that of untransfected HEK293 cells (7 ± 6%, n = 250) (Figure 4B). Biochemical analysis of the mutant proteins revealed that whereas incorporation of alanine residues at positions C3 or C4 did not abolish partitioning into membranes of mutated GAP43, simultaneous mutation of both cysteine residues (C3A/C4A) fully prevented membrane insertion of the protein, as evidenced by its absence in the low speed cellular pellets (Figure 4C, panel 1). However, mutation of either cysteine or both residues notably impaired the segregation of the protein into membrane rafts, as suggested by the virtual absence of the three mutant species in the low density fractions of Triton X-100 flotation gradients (Figure 4C, panel 2). Taken together, these findings suggest that GAP43 osmosensory activity required the interaction of the protein with plasma membranes, primarily its association with membrane rafts.
Fig. 4. The palmitoylation sites of GAP43 are important for the protein osmosensitivity. (A) [Ca2+]i response in GAP43 C3A/C4A mutant-transfected cells during a 30% hypotonic stimulation in a Ca2+-free solution. (B) Bar histogram showing the percentage of cells hypotonic-responsive to a stimulus protocol similar to that in (A) in wild-type GAP43+ cells and after single and double mutations of the palmitoyl ation sites. Control denotes untransfected HEK293 cells. For each experiment, 50 cells in the field were marked at random and scored for a response if the [Ca2+]i was >15 nM. Data are given as mean ± SEM with N ≥ 150 cells, and n ≥ 3. **P < 0.001 and *P < 0.005 significance (Z-test) of GAP43 mutants versus wild type. (C) Membrane fractions (1) and membrane rafts (2) from a cell expressing the wild-type GAP43 protein and mutant species. Membrane fractions denote crude plasma membranes. Lipid rafts were obtained from detergent-insoluble complexes in Triton X-100 flotation gradients. The figure depicts the top layer of the Optiprep discontinuous gradient. Crude membranes and rafts were separated by SDS–PAGE and analyzed by western immunoblot using an anti-GAP43 monoclonal antibody.
Integrity of the cortical cytoskeleton is not necessary for GAP43-dependent, hypotonicity-activated Ca2+ release
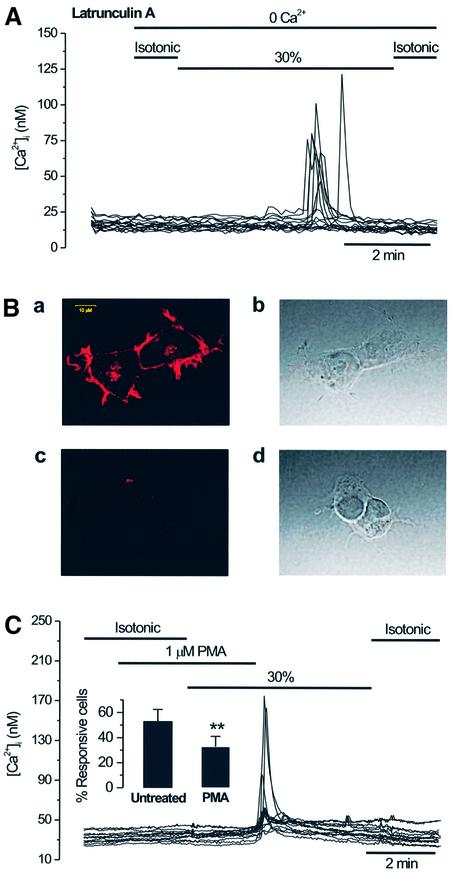
We next investigated the mechanism involved in GAP43-dependent, hypotonicity-induced release of Ca2+. Because GAP43 can bind actin filaments and promote actin cytoskeleton assembly (Qin et al, 1997; Caroni, 2001), we evaluated whether modulation of the actin cytoskeleton was involved in the hypotonicity-evoked Ca2+ release. As illustrated in Figure 5A, incubation of GAP43+ cells with 1 µM of the actin-severing toxin latrunculin A for 35 min did not alter the percentage of cells that release Ca2+ in response to hypotonicity: 52 ± 12% (n = 150) for toxin-treated versus 53 ± 9 (n = 450) for untreated cells. A 10-fold increment in the toxin concentration resulted in a modest decrease in the number of cells responding (39 ± 8%, n = 300), but not in the magnitude of the intracellular Ca2+ increase. Under these experimental conditions, latrunculin A disrupted the cortical actin cytoskeleton. As illustrated in Figure 5B (panel a), the phalloidin–rhodamine staining pattern in HEK293 cells shows an F-actin network primarily concentrated at the cell cortex. Note the uniform fluorescent labeling of a narrow region beneath the plasma membrane. In marked contrast, latrunculin A-treated HEK293 cells were round and exhibited a more diffuse phalloidin labeling, with significant discontinuities in the periphery (Figure 5B, panels c and d). Therefore, the finding that disruption of the F-actin network does not markedly affect hypotonicity-induced Ca2+ release in GAP43+ cells suggests that an intact cortical actin cytoskeleton plays a marginal role in the osmosensory activity of GAP43. Nonetheless, we cannot rule out a contribution of the actin cytoskeleton downstream of Ca2+ release.
Fig. 5. GAP43-dependent, hypotonicity-activated Ca2+ release is not mediated by alterations in the actin cytoskeleton but is inhibited by PKC activation. (A) [Ca2+]i response in GAP43+ cells during a 30% hypotonic stimulation in a Ca2+-free solution, after incubating the cells with 1 µM latrunculin A for 35 min. (B) Latrunculin A disrupts the cortical cytoskeleton. Panels a and c display confocal images of phalloidin–rhodamine staining of untreated and latrunculin A-treated (1 µM, 35 min) HEK293 cells. Panels b and c depict light-transmitted images of the cells exhibited in panels a and c. The calibration bar for all images is shown in panel a. (C) Pre-incubation with 1 µM PMA for 2 min reduces the number of GAP43+ cells responding to hypotonicity in Ca2+-free external medium. Inset: the percentage of untreated and PMA-treated GAP43+ cells that responded to hypotonicity. For each experiment, 50 cells in the field were marked at random and scored for a response if the [Ca2+]i was >15 nM. Data are given as mean ± SEM with N ≥ 150 cells, and n ≥3. **P < 0.001 significance (Z-test).
Activation of PKC signaling modulates GAP43-mediated hypotonic Ca2+ increases
Because GAP43 is a well known substrate of PKC (Widmer and Caroni, 1993; Qin et al., 1997), we next investigated whether activation of this protein kinase modulated the osmosensitivity of GAP43. Figure 5C shows that incubation of GAP43+ cells with 1 µM phorbol-12-myristate-13-acetate (PMA) reduced the number of cells that responded to hypotonicity-evoked [Ca2+]i to 33 ± 8% (n = 250) without altering the amplitude nor the shape of the response. This result indicates a 40% reduction with respect to non-treated GAP43+ cells (53 ± 9%, n = 450) (Figure 5C, inset), and suggests that activation of PKC signaling modulates the cellular osmosensitivity, presumably by direct phosphorylation of GAP43.
GAP43-dependent, hypotonicity-evoked Ca2+ release requires activation of PLC
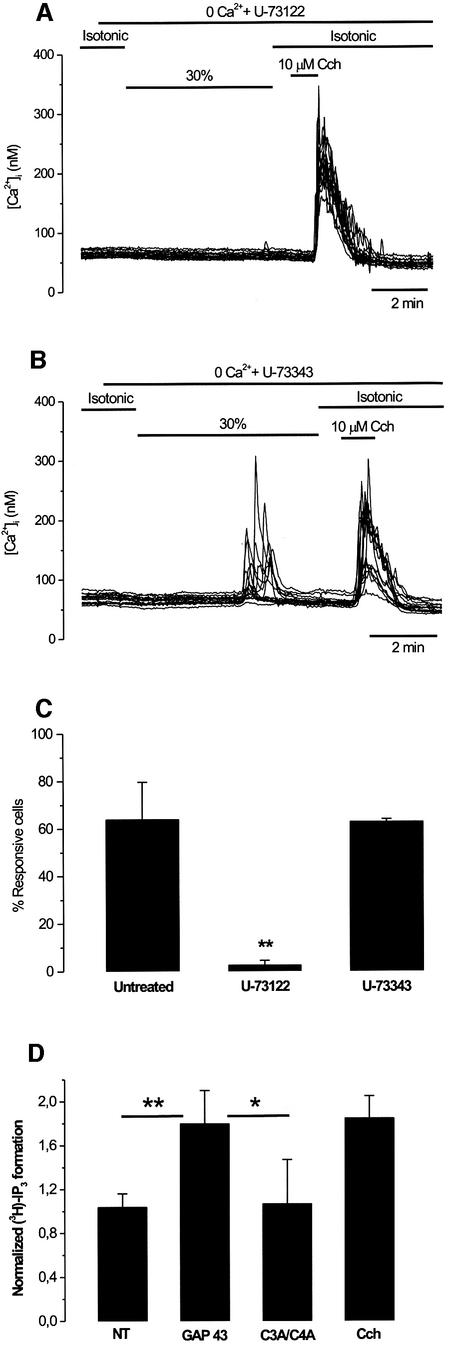
Cumulative evidence shows that GAP43 co-distributes with phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] in membrane rafts (Laux et al., 2000). PI(4,5)P2 can be hydrolyzed by PLC to IP3 which, in turn, can release Ca2+ from the ER. Thus, to determine whether formation of IP3 mediates the hypotonicity-induced Ca2+ release in GAP43+ cells, we inhibited PLC activity with the aminosteroid U-73122 (1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl) amino)hexyl]-1H-pyrrole-2,5-dione). As depicted in Figure 6A, treatment of GAP43+ cells with 3 µM U-73122 resulted in complete abrogation of [Ca2+]i responses triggered by the hypo-osmotic challenge (Figure 6B). Surprisingly, in these cells, Ca2+ release evoked by carbachol was not fully blocked by 3 µM U-73122 treatment (Figure 6A). Quantitative analysis indicates that 3 µM U-73122 inhibited only 40% of 10 µM carbachol-evoked [Ca2+]i responses in HEK293 cells (data not shown). This result contrasts with the strong U-73122 blockade efficacy of muscarinic Ca2+ release in HEK293 cells reported by Short et al. (2000), suggesting the existence of molecular differences between individual HEK293 cells. Nonetheless, significant variability of the muscarinic response to U-73122 inhibition has also been noticed by other groups in different cellular systems (Muto et al., 1997; Cruzblanca et al. 1998; Suh and Hille, 2002).
Fig. 6. GAP43-dependent, hypotonicity-activated Ca2+ release involves IP3 signaling. (A) Pre-incubation for 60 min with the PLC inhibitor U-73122 (3 µM) suppressed the hypotonicity-activated [Ca2+]i response. Note that the response to 10 µM carbacol was unaffected. (B) Pre-incubation for 60 min with the inactive control compound U-73343 (3 µM) did not prevent the hypotonicity-activated [Ca2+]i response. (C) Analysis of the effect of aminosteroid on the percentage of hypotonic-responsive (30% hypotonic stimulus) GAP43+ cells. For each experiment, 50 cells in the field were marked at random and scored for a response if the [Ca2+]i was >15 nM. Data are given as mean ± SEM with N ≥ 150 cells, and n ≥ 3. **P < 0.001 significance (Z-test) for non-treated versus U-73122-treated cells. (D) Hypotonicity induces the synthesis of IP3 in GAP43+ cells. The amount of [3H]IP3 was measured in cells labeled with [2-3H]myo-inositol in isotonic and hypotonic external conditions. Normalized values refer to the ratio of [3H]IP3 after the stimuli (hypotonicity or carbachol) with respect to that before (isotonic). NT denotes non-transfected. Values are given as mean ± SD, with n ≥ 3. **P < 0.01 and *P < 0.05 significance (Student’s t-test) for GAP43 versus NT and GAP43 versus C3A/C4A double mutant, respectively.
At variance with U-73122, incubation of GAP43+ cells with 3 µM U-73343 (1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-2,5-pyrrolidinedione), an analog of U-73122 that does not inhibit PLC activity, did not affect the hypotonicity-activated [Ca2+]i increases in GAP43+ cells (Figure 6B). A quantitative summary of the results is shown in Figure 6C. Taken together, these data suggest that hypotonicity-evoked [Ca2+]i elevations of GAP43+ cells require the synthesis of IP3 which, in turn, releases Ca2+ from IP3-sensitive stores.
To substantiate this tenet further, we determined the levels of IP3 in response to hypotonicity. As depicted in Figure 6D, the 30% hypotonic stimulus provoked an ∼2-fold increase in the formation of IP3 in cells heterologously expressing GAP43. This increase was similar to that elicited by carbachol. No increment in the IP3 level was evoked in cells expressing the C3A/C4A GAP43 double mutant. Therefore, GAP43 appears to transduce hypo-osmotic stimuli into IP3 formation.
Hypo-osmotic stress favors the association of GAP43 with PLC-δ1
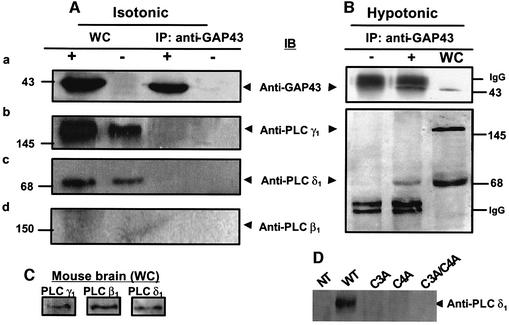
How does hypo-osmotic activation of GAP43 stimulate IP3 production? We hypothesized that GAP43 may regulate PLC activity by interaction with a member of this enzyme family (Rhee, 2001). To test this hypothesis, we used an immunological approach aimed at determining whether complexes containing GAP43 and PLC can be immunopurified with a specific anti-GAP43 monoclonal antibody (mAb). The co-immunopurification of PLC isoforms was then evaluated by western immunoblotting using specific anti-PLC mAbs against isoforms β1, γ1 and δ1. Figure 7A, panels b–d shows that the PLC-γ1 and PLC-δ1, but not the PLC-β1 isoform were endogenously expressed in HEK293 cells. As shown, whole-cell extracts (WC lanes) exhibit an immunoreactive band of 140 kDa that matches that of PLC-γ1 (Figure 7A, panel b), and a band of 68 kDa that correspond to a known proteolytic fragment of PLC-δ1 (Feng et al., 1996) (Figure 7A, panel c). The absence of PLC-β1 immunoreactivity (Figure 7A, panel d) could not be attributed to failure of the antibody since this enzyme isoform was readily detected in mouse brain extracts (Figure 7C). Under isotonic conditions, neither PLC isoform γ1 nor δ1 co-immunopurified with GAP43 (Figure 7A, panels a–c, IP lanes). This lack of GAP43–PLC association may be due to the very low affinity of both proteins. Thus, to address this issue, we attempted to cross-link both proteins using the cell-permeable, thiol-cleavable homobifunctional cross-linking reagent dithiobis(succinimidyl) propionate (DSP). Incubation of GAP43+ cells with 1 mM DSP did not result in co-immunopurification of any PLC isoform using the anti-GAP43 antibody (data not shown), thus supporting the tenet that in isotonic conditions, GAP43 does not interact with these PLC isoforms. As a control for cross-linking activity, the well-known GAP43–calmodulin complex was covalently linked, as evidenced by the co-immunoprecipitation of calmodulin using the anti-GAP43 antibody in GAP43+ cells (data not shown).
Fig. 7. Hypotonicity induces the selective association between GAP43 and PLC-δ1. Whole-cell (WC) extracts from GAP43+ cells (+) and untransfected cells (–) in isotonic (A) or 30% hypotonic media (B) were immunoprobed with specific antibodies against GAP43 (panel a), PLC-γ1 (panel b), PLC-δ1 (panel c) and PLC-β1 (panel d), or immunoprecipitated with anti-GAP43 (IP) and thereafter immunoprobed with the displayed antibodies (IB). (C) Mouse brain extracts immunoblotted with anti-PLC isoform-specific antibodies. (D) Co-immunoprecipitation of PLC-δ1 with GAP43 mutant species. Proteins were separated by 10 or 12% SDS–PAGE and electrotransferred onto cellulose membranes. Immunobands were revealed with ECL plus. Numbers indicate molecular weight markers in kDa.
We next questioned if the interaction/association between PLCs and GAP43 may be favored by exposing the cells to a hypo-osmotic stimulus (Figure 7B). For this purpose, untransfected and GAP43-transfected cells were challenged with a 30% hypotonic stimulus for 5 min, followed by immunoprecipitation with anti-GAP43 mAb. Figure 7B (bottom) shows that PLC-γ1 did not co-immunoprecipitate with GAP43. Similar results were obtained for this PLC isoform in the presence of the cross-linker DSP, further supporting the conclusion that PLC-γ1 does not associate with GAP43 under hypo-osmotic conditions (data not shown). Moreover, since tyrosine phosphorylation is a necessary condition for PLC-γ recruitment and activation, we evaluated whether inhibition of the src-family of tyrosine kinases abrogated GAP43-mediated osmosensitivity. Our findings show that treatment with 1 µM PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), a potent and specific inhibitor of the src family of tyrosine kinases, for 35 min did not alter the GAP43-stimulated release of Ca2+ in response to hypotonicity (see Supplementary data available at The EMBO Journal Online).
In marked contrast, hypotonicity clearly induced the association of GAP43 with PLC-δ1, as demonstrated by the co-immunopurification of this enzyme isoform with GAP43 when an anti-GAP43 antibody was used (Figure 7B, bottom), thus indicating that both proteins associate when cells are under hypo-osmotic stress. Mutation of either one or both palmitoylation sites in GAP43 fully abolished the interaction between GAP43 and PLC-δ1 (Figure 7D). Taken together, these findings suggest that the hypotonicity-induced association of GAP43 and PLC-δ1 is a molecular event that underlies hypotonic Ca2+ release in GAP43+ cells.
Discussion
Transduction of mechanical and osmotic stimuli is essential for a diversity of cellular processes (Hamill and Martinac, 2001). Thus a significant effort is being devoted to defining the molecular components implicated in mechano- and osmosensation, particularly those located in the plasma membrane that transduce external stimuli. We have screened a cDNA library from DRG to search for osmoreceptors. The mechanically insensitive HEK293 cell line was used to express recombinantly the cDNA clones contained in the library. The salient contribution of this study is the identification of GAP43, a 238 amino acid, hydrophilic, neuronal protein that plays a central role in axonal growth and synaptic plasticity (Aigner and Caroni, 1993, 1995; Dent and Meiri, 1998; Mueller, 1999; Bomze et al., 2001; Hulo et al., 2002), as an osmosensory protein that releases Ca2+ in response to hypotonicity, but not to hypertonicity. GAP43 appears to lower the threshold for signaling downstream of osmosensation, as suggested by the response of GAP43+ cells to low-to-moderate hypotonic stimuli in comparison with the drastic hypotonicity required by untransfected HEK293 cells.
Structurally, GAP43 is a cytosolic protein anchored to the plasmalemma through palmitoylation of two cysteine residues at the N-terminus (Widmer and Caroni, 1993; Gamby et al., 1996; Qin et at, 1997; Laux et al. 2000). We found that the protein osmosensory activity was fully compromised by mutation of either palmitoylation site. This functional impairment was not correlated with the ability of the protein to integrate in the plasmalemma, since both single mutants still partitioned into the cell membrane. In contrast, however, we observed that removal of a single or both palmitoylation sites markedly prevented the segregation of the protein into membrane rafts. Therefore, palmitoylation of GAP43 is required for its osmosensitivity, primarily because of its importance for the segregation of the protein into membrane rafts. This conclusion is supported further by the finding that treatment with PMA, a phorbol ester that activates PKC, reduces GAP43 osmosensitivity, presumably as a result of GAP43 phosphorylation. PKC-mediated phosphorylation of GAP43 at Ser42 in the basic ED domain, or incorporation of negatively charged residues at this position, has been shown to weaken notably the binding of GAP43 to phosphoinositides (Laux et al. 2000). As a result, GAP43 interaction with lipid rafts is perturbed, affecting axonal growth and guidance (Laux et al., 2000; Caroni, 2001).
Mechanistically, hypotonicity-induced augmentation in [Ca2+]i is due to release from the ER, as evidenced by its independence from external Ca2+ and abrogation after treatment with thapsigargin. A signaling pathway for this activity may involve alteration of the cortical actin cytoskeleton by GAP43 that could couple hypotonicity to Ca2+ release in GAP43+ cells. This is plausible since GAP43 is known to interact with cytoskeletal proteins including actin (Qin et al., 1997; Caroni, 2001). However, the low sensitivity of GAP43 osmosensory activity to actin cytoskeleton disruption by the toxin latrunculin A argues against a major role for this transduction pathway.
Because GAP43 interacts with phosphoinositides (Laux et al. 2000), an alternative mechanism is the transduction of hypo-osmolarity to Ca2+ release through IP3 signaling. The finding that hypotonicity induced an ∼2-fold increment in the level of IP3, along with the observation that inhibition of PLC activity completely abolished hypo-osmolarity-induced Ca2+ release in GAP43+ cells, substantiates this notion. It is noteworthy that hypotonicity promoted the selective assembly of GAP43 with the PLC-δ1 isoform, an event that could modulate the activity of the enzyme. Since GAP43 sequesters PI(4,5)P2 into lipid rafts, hypotonic stimulation could enhance the local recruitment of PLC-δ1 to these plasmalemma microdomains of PI(4,5)P2, thus facilitating its hydrolysis and formation of IP3. This tenet is consistent with the finding that segregation of GAP43 into membrane rafts modulates its osmosensory activity.
A wealth of data demonstrate that GAP43 is implicated in morphogenic activity and anatomical plasticity in the nervous system, specially during development and axonal nerve regeneration. For instance, transgenic animals that overexpress the protein exhibit spontaneous and ectopic nerve sprouting, while GAP43 knockout mice display abnormalities in axon guidance and topography in the barrel cortex (Maier et al., 1999). All these findings suggest that GAP43 participates in signal transduction pathways in the growth cone that modulate the rate, extent and track of axons (Dent and Meiri 1998; Shen et al., 2002). In support of this notion, GAP43 interacts in a mutually exclusive way with several second messengers in nerve terminals, including calmodulin, PKC, the α-subunit of the GTP-binding protein Go or Gi proteins, rabaptin-5 and PI(4,5)P2 (Widmer and Caroni, 1993; Chao et al., 1996; Gamby et al., 1996; Qin et al., 1997; Nakamura et al., 1998; Neve et al., 1998; Laux et al., 2000; Tejero-Diaz et al., 2000). The relevance of these interactions is still under intense investigation, although there is a strong argument for a critical role in modulating actin dynamics and motility (Caroni, 2001).
A central question arises as to what could be the physiological relevance of the osmosensory activity of GAP43. Recently, a unifying model for the regulation of the actin cytoskeleton through modulation of PI(4,5)P2 rafts by GAP43-like proteins has emerged (Laux et al., 2000; Caroni, 2001). In this model, GAP43-mediated local sequestration of PI(4,5)P2 into lipid rafts releases the inhibitory activity that the phosphoinositide exerts on actin-regulating proteins such as profilin, cofilin and gelsolin (Janmey, 1998; Laux et al., 2000). As a result, actin dynamics are augmented, cytoskeleton stability is decreased and growth cones are elongated. Our finding that GAP43 modulates PI(4,5)P2 metabolism in response to osmotic stress is compatible with this model, and provides further insights into the mechanism by which this neuronal protein may modulate actin-based structures, as well as axon elongation and guidance. Hence, in isotonic conditions, the masking of PI(4,5)P2 by GAP43 would promote actin cortex dynamics and, therefore, growth cone extension (Laux et al. 2000; Caroni 2001). Osmo/mechanical stimuli arising from growth or track changes would favor the association of PLC-δ1 and GAP43 in membrane rafts, facilitating the formation of IP3 which, in turn, would release Ca2+ from IP3-sensitive stores. An increment in [Ca2+]i would inhibit actin dynamics, thus promoting actin polymerization. Phosphorylation of GAP43 by PKC may also contribute by modulating its interaction with PI(4,5)P2 (Caroni, 2001), as well as the protein osmosensitivity. Consequently, the actin cytoskeleton would be stabilized and axonal growth slowed or, even, collapsed. In support of this notion, growth cones generate Ca2+ transients with a frequency that is inversely proportional to the rate of axon outgrowth (Goldberg and Grabham, 1999; Gomez and Spitzer, 1999). Suppression of these cytosolic Ca2+ waves accelerates axonal extension, whereas its stimulation notably slows growth cones (Gomez and Spitzer, 1999). In this context, the osmotically induced, GAP43-mediated transient elevation of [Ca2+]i may be used by growing axons to modulate temporally and locally the rate and trajectories of neuritic extension. Hence, the question that emerges is: do changes in osmotic/mechanical tension play a role in regulating axonal development? Cumulative evidence is making a case for regulatory effects of mechanical tension in axonal development (Zheng et al., 1991; Heidemann and Buxbaum, 1994; Chada et al., 1997). For instance, axonal retraction and guidance may involve active force generation by the neurite shaft (Chada et al., 1997). Recently, it has been reported that endoneural tubes provide strictly mechanical guidance which, together with chemical cues, may contribute to axonal growth during peripheral regeneration (Fournier and Strittmatter, 2002; Nguyen et al., 2002). These findings, taken together with ours, support the tenet that both mechanical and molecular stimuli are involved in growth cone and axonal guidance. The osmosensitivity of GAP43 furnishes a mechanistic framework that links axon elongation to the spontaneous triggering of cytosolic Ca2+ transients in the growth cone, the metabolism of phosphoinositides, regulation of cytoskeleton stability and the generation of regulatory osmo/mechanical forces.
In conclusion, we have reported that GAP43 is an osmosensory protein that triggers transient elevations of [Ca2+]i in response to mild to moderate hypotonicity. The protein lowers the threshold for signaling downstream of osmosensation. The mechanism involves the activation of the IP3-sensitive Ca2+ release channel, which appears to result from the specific association of GAP43 and PLC-δ1 that may facilitate IP3 formation. The use of an expression cloning approach and a recombinant system has unveiled an unsuspected function of GAP43 that may play an important role in modulating the rate, extent and trajectory of growing axons. Further experimental work, however, is necessary to understand the neuronal mechanism of GAP43 osmosensitivity and its relationship to axonal generation and synaptic plasticity.
Materials and methods
Expression cloning and Ca2+ imaging
A human DRG cDNA library (Invitrogen) was used. Independent bacterial clones (4.4 × 106), with an average insert size of 1.3 kb, were divided into 50 pools each containing ∼3000 c.f.u. HEK293 cells were transiently transfected with 2 µg of plasmid DNA from individual pools using Lipofectamine 2000 (Invitrogen). The day prior to the transfection, HEK293 cells were trypsinized and replated in a 24-well plate, on coverslips treated with poly-d-lysine or laminin for 30 min, at a density of 2.5–3.0 × 105 cells per dish and maintained in supplemented Dulbecco’s modified Eag;le’s medium (DMEM). At 48 h post-transfection, cells were incubated with 5–10 µM Fura-2 AM with 0.02% pluronic acid (Molecular probes) for 1 h at 37°C for ratiometric calcium imaging (Viana et al., 2001). Briefly, cells were perfused continuously (2–4 ml/min) in the recording chamber with isotonic solution warmed at 27 ± 1°C (66 mM NaCl, 3 mM KCl, 1.3 mM MgCl2, 2.4 mM CaCl2, 10 mM glucose, 10 mM HEPES pH 7.4, 141 mM d-mannitol; 310 mOsm/kg). Solutions of different osmolarities were employed. The osmolarity of each solution was measured using a cryoscopic osmometer (Gonotec). To maintain a constant ionic composition, only the concentration of d-mannitol was reduced in all hypo-osmotic solutions. In Ca2+-free solutions, 100 µM EGTA was added to chelate residual Ca2+. The perfusion could be switched to one of several test solutions via a multibarrelled stopcock. Exchange of solutions was completed in ∼20–30 s. For each library pool, 10–15 microscopic fields were analyzed.
Fluorescence measurements were carried out with a Zeiss Axioskop FS upright microscope fitted with a Sensys CCD camera (Roper Scientific) through an Olympus ×20 water immersion objective. Fura-2 was excited at 340 and 380 nm with a Lambda 10-2 filter wheel (Sutter Instruments), and emitted fluorescence filtered with a 510 nm long-pass filter. Calibrated ratios were displayed on-line with AIW software (Axon Instruments). Images were acquired and stored at 0.5 Hz. Data are reported as mean ± SEM. Statistical significance (*P < 0.05 and **P < 0.001) was assessed by Z-test as indicated.
Phalloidin–rhodamine staining and confocal microscopy
HEK293 cells seeded on glass coverslips were rinsed briefly in 0.1 M phosphate-buffered saline (PBS) and fixed for 15 min in 4% paraformaldehyde in PBS. Latrunculin A-treated (1 µM, 35 min) and untreated HEK293 cells were permeabilized for 10 min with 0.2% Triton X-100 in 0.1 M PBS and washed twice for 20 min with PBS. Subsequently, cells were incubated for 30 min in 1 µM phalloidin– rhodamine (Sigma) and washed for 30 min in PBS. Staining was visualized with an upright confocal laser scanning microscope (Olympus Fluoview 300) using a 543 nm helium/neon laser.
Isolation of plasma membrane fractions from transfected cells and immunoblotting
Crude plasma membranes were prepared essentially as described (Cabedo et al., 2002). Briefly, transfected cells were washed with ice-cold PBS and lysed with buffer A [2 mM MgCl2, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 mM HEPES pH 7.4]. Cell lysates were centrifuged at 6000 g at 4°C for 10 min to prepare low speed pellets, which were washed with buffer B (buffer A + 1 M NaCl), shaken for 15 min at 4°C, centrifuged, and washed with buffer A for desalting. Final pellets, prepared from equivalent amount of cells, were mixed with β-mercaptoethanol-containing SDS sample buffer, boiled and separated by SDS–PAGE. Proteins were electrotransferred onto nitrocellulose membranes, blocked with 10% fat-free skim milk in Tris-buffered saline (TBS) and incubated with an anti-GAP-43 mAb (Chemicon). Membranes were washed with TBS–Tween (0.3%), incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG and developed with the enhanced chemiluminescence system (ECL; Amersham).
Preparation of membrane rafts
Analysis of detergent-insoluble complexes in flotation gradients was performed essentially as described (Cabedo et al., 2002). Briefly, ∼2.5 × 106 transiently transfected cells were washed with ice-cold PBS and scraped off in buffer C (20 mM HEPES pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF, 0.5% Triton X-100), passed 10 times through a 29 gauge needle, extracted at 4°C for 30 min, and brought to 35% Optiprep. A 1 ml aliquot of the extract was overlayered sequentially with 8 ml of 30% Optiprep in 0.5× buffer C and 400 µl of buffer C in an SW41 tube. After centrifugation (178 000 g at 4°C for 4 h), 10 fractions were collected from the top to the bottom of the gradient; 200 µl of each fraction were precipitated with trichloroacetic acid, neutralized and analyzed by immunoblotting using the anti-GAP43 antibody.
Protein immunoprecipitation and immunoblotting
Protein immunoprecipitation was carried out as described (Caprini et al., 2001). HEK293 cells were washed twice in PBS, scraped off from the wells into 1 ml of RIPA buffer (150 mM NaCl, 50 mM Tris–HCl pH 8.0, 5 mM EDTA, 0.1 mM PMS, 0.01 mM iodacetamine, 0.5% sodium deoxycholate, 1% IGEPAL, 0.1% SDS), sonicated (four times for 10 s) and centrifuged at 14 000 r.p.m. for 30 min at 4°C. The supernatant was used as a whole-cell extract or immunoprecipitated with the anti-GAP43 mAb (3 µg of mAb/mg of protein) at 4°C overnight. Immunocomplexes were captured with agarose-conjugated protein G (75 µl; Pierce), washed six times with 500 µl of RIPA at 4°C, dissociated with 75 µl of SDS–PAGE sample buffer, boiled for 5 min, and analyzed by SDS–PAGE and immunoblotting. Nitrocellulose membranes were blocked with 3% bovine serum albumin (Sigma) and probed with anti-GAP43, anti-PLC-β1, anti-PLC-δ1 or anti-calmodulin (Upstate Biotechnology, USA) and anti-PLC-γ1 (Chemicon, USA) mAbs.
Intact cell chemical cross-linking experiments
HEK293 cells were washed twice in PBS and incubated with 1 mM DSP (Pierce) at 22°C for 30 min following the manufacturer’s instructions. The cross-linking reaction was stopped with 20 mM Tris–HCl pH 7.4. After incubating the reaction mixture for an additional 15 min, cells were washed twice with ice-cold PBS, scraped off the dish, and cellular pellets were resuspended in RIPA buffer. Cross-linked complexes were immunopurified with the anti-GAP43 antibody. The material bound via the cross-linking agent to the GAP43 immune complex was released by incubating the washed beads for 30 min at 37°C with elution buffer (10 mM Tris, pH 7.5, 5 mM EDTA, 0.2 M DTT, 1.2% β-mercaptoethanol), followed by a brief centrifugation. Immunoprecipi tates were analyzed by SDS–PAGE followed by immunoblotting with specific antibodies as indicated.
GAP43 mutation
The MLCC motif of the GAP43 protein was mutated to MLAC (C3A), MLCA (C4A) and MLAA (C3A/C4A) using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). All mutations were verified by sequencing.
IP3 formation
HEK293 cells (2.5 × 106) were transiently transfected with GAP43 or C3A/C4A double mutant cDNA and pre-labeled with [2-3H]myo-inositol (2 µCi/ml) in Medium-199 (low IP3 levels, Sigma) supplemented with 5% fetal calf serum for 48 h. Cells were then challenged with isotonic or hypotonic solution or 100 µM carbachol for 5 min. Formation of IP3 was terminated with 5% ice-cold trichloroacetic acid. After neutralization, samples were passed through a HiTrapQFF column (Amersham Biosciences). [3H]IP3 was eluted with 1.1 M ammonium formate/0.1 M formic acid. Radioactivity was counted in a β-counter.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Eva Quintero and Consuelo Martínez Moratalla for technical assistance, Alvaro Villarroel for anti-calmodulin monoclonal antibody, and Luis M.Gutierrez for latrunculin A. A.G. was a fellow of the CSIC-IP3 program from European Social Funds, and M.C. was a fellow of the Marie Curie Host Fellowship Program. This work was supported by grants from La Fundación La Caixa (01/085-00 to A.F.-M.), the Spanish Ministry of Science and Technology (MCYT) (SAF2000-0142 to A.F.-M., SAF2001-1641 to F.V. and BFI2002-03788 to C.B.), the Instituto de la Salud Carlos III (FIS-01/1162 to C.B.) and The Generalitat Valenciana (GV01-01 to A.F.-M).
References
- Aigner L. and Caroni,P. (1993) Depletion of 43-kDa growth-associated protein in primary sensory neurons leads to diminished formation and spreading of growth cones. J. Cell Biol., 123, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner L. and Caroni,P. (1995) Absence of persistent spreading, branching and adhesion in GAP-43-depleted growth cones. J. Cell Biol., 128, 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arni S., Keilbaugh,S.A., Ostermeyer,A.G. and Brown,D.A. (1998) Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J. Biol. Chem., 273, 28478–28485. [DOI] [PubMed] [Google Scholar]
- Bomze H.M., Bulsara,K.R., Iskandar,B.J., Caroni,P. and Pate Skene,J.H. (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neurosci., 4, 38–43. [DOI] [PubMed] [Google Scholar]
- Bourque C.W. and Oliet,S.H.R. (1997) Osmoreceptors in the central nervous system. Annu. Rev. Physiol., 59, 601–619. [DOI] [PubMed] [Google Scholar]
- Cabedo H., Luna,C., Fernández,A.M., Gallar,J. and Ferrer-Montiel,A. (2002) Molecular determinants of the sensory and motor neuron-derived factor insertion into plasma membrane. J. Biol. Chem., 277, 19905–19912. [DOI] [PubMed] [Google Scholar]
- Caprini M., Ferroni,S., Planells-Cases,R., Rueda,J., Rapisarda,C., Ferrer-Montiel,A.V. and Montal,M. (2001) Structural adaptability between the putative voltage sensor of Kv channels and the prokaryotic KcsA channel. J. Biol. Chem., 276, 21070–21076. [DOI] [PubMed] [Google Scholar]
- Caroni P. (2001) Actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts. EMBO J., 20, 4332–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S., Lamoureux,P., Buxbaum,R.E. and Heidemann,S.R. (1997) Cytomechanics of neurite outgrowth from chick brain neurons. J. Cell Sci., 110, 1179–1186. [DOI] [PubMed] [Google Scholar]
- Chao S., Benowitha,L.I., Krainc,D. and Irwin,N. (1996) Use of a two-hybrid system to investigate molecular interactions of GAP-43. Mol. Brain Res., 40, 195–202. [DOI] [PubMed] [Google Scholar]
- Colbert H.A., Smith,T.L. and Bargmann,C.I. (1997) OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation and olfactory adaptation in Caenorhabditis elegans. J. Neurosci., 17, 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzblanca H., Koh,D.S. and Hille,B. (1998) Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl Acad. Sci. USA, 95, 7151–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E.W. and Meiri,K.F. (1998) Distribution of phosphorylated GAP-43 (neuromodulin) in growth cones directly reflects growth cone behavior. J. Neurobiol., 35, 287–299. [DOI] [PubMed] [Google Scholar]
- Feng J.-F., Rhee,S.G. and Im,M.-J. (1996) Evidence that phospholipase δ1 is the effector in the Gh (transglutaminase II)-mediated signaling. J. Biol. Chem., 271, 16451–16454. [DOI] [PubMed] [Google Scholar]
- Fournier A.E. and Strittmatter,S.M. (2002) Regenerating nerves follow the road more traveled. Nat. Neurosci., 5, 821–822. [DOI] [PubMed] [Google Scholar]
- Gamby C., Waage,M.C., Allen,R.G. and Baizer,L. (1996) Analysis of the role of calmodulin binding and sequestration in neuromodulin (GAP-43) function. J. Biol. Chem., 271, 26698–26705. [DOI] [PubMed] [Google Scholar]
- García-Añoveros J. and Corey,D.P. (1997) The molecules of mechanosensation. Annu. Rev. Neurosci., 20, 567–594. [DOI] [PubMed] [Google Scholar]
- Gillespie P.G. and Walker,R.G. (2001) Molecular basis of mechanosensory transduction. Nature, 413, 194–202. [DOI] [PubMed] [Google Scholar]
- Goldberg D.J. and Grabham,P.W. (1999) Braking news: calcium at the growth cone. Neuron, 22, 423–425. [DOI] [PubMed] [Google Scholar]
- Gomez T.M. and Spitzer,N.C. (1999) In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature, 397, 350–355. [DOI] [PubMed] [Google Scholar]
- Hamill O.P. and Martinac,B. (2001) Molecular basis of mechanotransduction in living cells. Physiol. Rev., 81, 685–740. [DOI] [PubMed] [Google Scholar]
- Hart A.C., Kass,J., Shapiro,J.E. and Kaplan,J.M. (1999) Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J. Neurosci., 19, 1952–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S.R. and Buxbaum,R.E. (1994) Mechanical tension as a regulator of axonal development. Neurotoxicology, 15, 95–107. [PubMed] [Google Scholar]
- Hulo S., Alberi,S., Laux,T., Mueller,D. and Caroni,P. (2002) A point mutant of GAP-43 induces enhanced short-term and long term hippocampal plasticity. Eur. J. Neurosci., 15, 1976–1982. [DOI] [PubMed] [Google Scholar]
- Janmey P.A. (1998) The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev., 78, 763–781. [DOI] [PubMed] [Google Scholar]
- Laux T., Fukami,K., Thelen,M., Golub,T., Frey,D. and Caroni,P. (2000) GAP43, MARCKS and CAP23 modulate PI(4,5)P2 at plasmalemmal rafts and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol., 149, 1455–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W., Choe, Y, Martí-Renom,M.A., Bell,A.M., Denis,C.S., Sali,A., Hudspeth,A.J., Friedman,J.M. and Heller,S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell, 103, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier D.L., Shyamala,M., Donovan,S.L., Soppet,D., Tessarollo,L., McCasland,J.S. and Meiri,K.F. (1999) Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl Acad. Sci. USA, 96, 9397–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B.K. (1999) Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci., 22, 351–388. [DOI] [PubMed] [Google Scholar]
- Muto Y., Nagao,T. and Urushidani,T. (1997) The putative phospholipase C inhibitor U73122 and its negative control, U73343, elicit unexpected effects on the rabbit parietal cell. J. Pharmacol. Exp. Ther., 282, 1379–1388. [PubMed] [Google Scholar]
- Nakamura F., Strittmatter,P. and Strittmatter,S.M. (1998) GAP-43 augmentation of G-protein mediated signal transduction is regulated by both phosphorylation and palmitoylation. J. Neurochem., 70, 983–992. [DOI] [PubMed] [Google Scholar]
- Neve R.L., Coopersmith,R., Mcphie,D.L., Santeufemio,C., Pratt,K.G., Murphy,C.J. and Lynn,S.D. (1998). The neuronal growth-associated protein GAP-43 interacts with rabaptin-5 and participates in endocytosis. J. Neurosci., 18, 7757–7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q.T., Sanes J.R. and Lichtman,J.W. (2002) Pre-existing pathways promote precise projection patterns. Nat. Neurosci., 5, 861–867. [DOI] [PubMed] [Google Scholar]
- Qin H., Dent,E.W. and Meiri,K.F. (1997) Modulation of acting filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. J. Neurosci., 17, 3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.G. (2001) Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem., 70, 281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Mani,S., Donovan,S.L., Schwob,J.E. and Meiri,K.F. (2002) Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J. Neurosci., 22, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short A.D., Winston,G.P. and Taylor,C.W. (2000) Different receptors use inositol trisphosphate to mobilize Ca2+ from different intracellular pools. Biochem. J., 351, 683–686. [PMC free article] [PubMed] [Google Scholar]
- Strotmann R., Harteneck,C., Nunnenmacker,K., Schultz,G. and Plant,T.D. (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol., 2, 695–702. [DOI] [PubMed] [Google Scholar]
- Suh B.-C. and Hille,B. (2002) Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron, 35, 507–520. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sato,J., Kutsuwada,K., Ooki,G. and Imai M. (1999) Cloning of a stretch-inhibitable nonselective cation channel. J. Biol. Chem., 274, 6330–6336. [DOI] [PubMed] [Google Scholar]
- Tejero-Diez P., Rodríguez-Sanchez,P., Martin-Cofreces,N.B. and Diez-Guerra,F.J. (2000) bFGF stimulates GAP-43 phosphorylation at ser41 and modifies its intracellular localization in cultured hippocampal neurons. Mol. Cell. Neurosci., 16, 766–780. [DOI] [PubMed] [Google Scholar]
- Viana F., de la Peña,E., Pecson,B., Schmidt,R.F. and Belmonte,C. (2001) Swelling-activated calcium signaling in cultured mouse primary sensory neurons. Eur. J. Neurosci., 13, 722–734. [DOI] [PubMed] [Google Scholar]
- Walker R.G., Willingham,A.T. and Zucker,C.S. (2000) A Drosophila mechanosensory transduction channel. Science, 287, 2229–2234. [DOI] [PubMed] [Google Scholar]
- Widmer F. and Caroni,P. (1993) Phosphorylation-site mutagenesis of the growth-associated protein GAP-43 modulates its effects on cells spreading and morphology. J. Cell Biol., 120, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Lamoureux,P. Dennerll,S.V., Buxbaum R.E. and Heidemann,S.R. (1991) Tensile regulation of axonal elongation and initiation. J. Neurosci., 11, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]