Abstract
Yeast Mot1p, an abundant conserved member of the Snf2p-ATPase family of proteins, both dissociates TBP from DNA in vitro using the energy of ATP and represses gene transcription in vivo, yet paradoxically, loss of Mot1p function also leads to decreased transcription of certain genes. We conducted experiments utilizing fluorescently labeled DNA, TBP, fluorescence anisotropy spectroscopy and native gel electrophoresis to study Mot1p action. We have made a number of observations, the most intriguing being that a stable Mot1p–TBP complex has the ability to bind TATA DNA with high affinity, albeit with dramatically altered specificity. We propose that this altered TBP–DNA recognition is integral to Mot1p’s ability to regulate transcription, and further postulate that the Mot1p–TBP complex delivers TBP to TAF-independent mRNA encoding genes.
Keywords: ATPase/DNA binding/Mot1p/TBP/transcription regulation
Introduction
A large number of proteins have the ability to bind TBP, a protein universally required for eukaryotic transcription (Pugh, 2000): these proteins are known collectively as TBP associated factors (TAFs). Polymerase-specific TBP–TAF complexes subserve critical functions during preinitiation complex (PIC) formation by the nuclear DNA-dependent RNA polymerases, Pol I, II and III. TAFs play two key roles in transcription. First, they act positively by dedicating a fraction of TBP to Pol-specific initiation factors, forming the distinct SL1, TFIID and TFIIIB TBP–TAF complexes (Albright and Tjian, 2000). Secondly, TAFs also act negatively; for example, the two TBP-binding proteins Mot1p and NC2 (Pugh, 2000) antagonize the ability of TBP to generate PICs in vitro by forming Mot1p–TBP and NC2–TBP complexes. The formation of these alternate TBP–TAF complexes are thought to modulate transcription on a broad scale, in part by competition between the formation of positive-acting (i.e. SL1, TFIID, TFIIIB) and negative-acting (Mot1p, NC2) complexes. Formation of such complexes will be dictated by thermodynamic considerations; while measurements of cellular TAF and TBP content have been made (see below), the corresponding binding affinities for most of these interactions have yet to be reported. Intranuclear concentration, compartmentalization and interaction affinities of these molecules will factor heavily into TBP–TAF dynamics and hence global transcription control.
MOT1 (modifier of transcription 1) was defined through a screen to identify genes encoding potential global transcriptional regulators (Davis et al., 1992). Yeast cells carrying the recessive mot1-1 allele express a reporter gene in the absence of the requisite Ste12p transactivator, which suggests that Mot1p functions as a repressor. This idea is supported by several additional observations. First, Auble and Hahn (1993) identified an activity, ADI (ATP-dependent inhibitor), capable of dissociating TBP from TATA DNA in an ATP-dependent fashion; they subsequently showed that ADI was Mot1p (Auble et al., 1994). Secondly, we discovered that Mot1p formed a unique, readily isolated TBP–TAF complex, distinct from TFIID and TFIIIB (Poon and Weil, 1993; Poon et al., 1994, 1995). Thirdly, a mutant form of MOT1, bur3-1 (mot1-301) (Prelich, 1997), was again identified in a screen similar to the original Mot screen (Prelich and Winston, 1993). Fourthly, overexpression of mutated Mot1p dominantly inhibits yeast growth (Auble et al., 1994, 1997; Adamkewicz et al., 2001) and this effect can be reversed by co-overexpression of TBP. Fifthly, Mot1p is associated with the Leu3p transcription repressor (Wade and Jaehning, 1996). Lastly, chromatin immunoprecipitation (ChIP) experiments showed greater TBP occupancy on select genes when Mot1p was inactivated (Li et al., 1999). The accumulated data are consistent with the idea that Mot1p acts as a general repressor of transcription targeting TBP. However, in certain contexts Mot1p acts positively. Transcription of several genes decreases in cells carrying particular mot1 alleles (Collart, 1996; Madison and Winston, 1997; Prelich, 1997). Secondly, both yeast and human (h) Mot1p (B-TFIID or BTAF1; Tora, 2002) positively affect basal transcription in vitro (Timmers and Sharp, 1991; Muldrow et al., 1999). Finally, recent gene array and ChIP assays document association of Mot1p with actively transcribed genes (Andrau et al., 2002; Dasgupta et al., 2002; Geisberg et al., 2002). Therefore, the ultimate functional outcome of Mot1p’s action, repression or activation, probably depends upon the exact promoter and/or enhancer context of a gene.
Saccharomyces cerevisiae Mot1p, encoded by a single copy essential gene, is a member of the evolutionarily conserved Snf2p/Swi2p ATPase family of proteins (Davis et al., 1992). Mot1p orthologs share significant sequence identity with the yeast prototype, particularly the N-terminal TBP-binding and C-terminal ATPase domains (van der Knaap et al., 1997, 2000; Chicca et al., 1998; Adamkewicz et al., 2001; Pereira et al., 2001). Mot1p contains multiple HEAT-related protein interaction motifs (Andrade et al., 2001) that have been proposed to mediate Mot1p–TBP inter- and/or regulatory intramolecular interactions (Pereira et al., 2001). Overexpressed Mot1p is monomeric in solution (Adamkewicz et al., 2000) but two forms of Mot1p are present in yeast cell extracts: a TBP-associated form and a form devoid of TBP (Poon et al., 1994). hMot1p behaves similarly (Timmers et al., 1992; van der Knaap et al., 1997; Chicca et al., 1998). Mot1p is abundant: ∼5000–10 000 molecules/yeast cell (Bai, 1997; Lee and Young 1998; Campbell 1999) and ∼150 000 molecules/HeLa cell (Chicca et al., 1998); TBP content is 20 000–30 000 in yeasts and 200 000 in human cells. A significant fraction of TBP is bound by Mot1p, consistent with the latter’s role as an important regulator of transcription.
ATPase activity is integral to Mot1p function (Auble and Hahn, 1993; Auble et al., 1994; Adamkewicz et al., 2001). Monomeric Mot1p has a low intrinsic ATPase activity that is enhanced 5- to 10-fold by TBP (Auble et al., 1997; Adamkewicz et al., 2001). Exactly how Mot1p uses ATP energy to dissociate Mot1p–TBP–TATA DNA complexes is not yet clear, nor are the products of ATP-driven Mot1p-catalyzed ternary complex dissociation known. Thus, after Mot1p-catalyzed ternary complex disruption, the products could either be free components (TBP, Mot1p, DNA) or Mot1p–TBP and TATA DNA. Current models of Mot1p action ascribe a critical role to the 20 bp of DNA 5′ of TATA. Such DNA has been proposed to serve as a handle or fulcrum from which Mot1p exerts its action(s) to dissociate TBP from TATA. It is hypothesized that Mot1p undergoes a DNA-anchored structural transition upon ATP hydrolysis, which results in the active dissociation of TBP from DNA by inducing conformational changes in the TBP–DNA complex (Auble and Hahn, 1993; Auble et al., 1994, 1997; Adamkewicz et al., 2000, 2001; Darst et al., 2001). These changes could involve directed TBP displacement via some sort of Mot1p wedge, induced conformational changes in TBP causing a reduction in its affinity for DNA, or induced changes in the DNA moiety of the TBP–DNA complex. An alternative model (Pereira et al., 2001) invokes the action of the conserved hMot1p N-terminal TBP-interacting domain behaving like the TAF N-terminal domain (TAND; Kotani et al., 1998) of yeast Taf1p. Taf1p TAND competes with TATA DNA by binding to the concave surface of TBP, preventing DNA binding. In this model the hMot1p TAND-like domain toggles on and off the concave surface of TBP, cyclically inhibiting TBP–TATA interactions. It has been reported that Mot1p–TBP complexes are unable to bind DNA and that the Mot1p–TBP complex is disrupted by ATP (Darst et al., 2001). Definitive data clearly distinguishing and characterizing all these possible Mot1p activities and mechanisms are lacking.
In this study we used complementary biophysical and biochemical tools to analyze the mechanism of action of Mot1p. Using these approaches we have made a number of novel observations regarding the behavior of this TAF that give us new insight into how Mot1p may positively and negatively modulate transcription.
Results
We previously used fluorescence to monitor the effects of yeast Taf1p on the DNA binding properties of TBP (Bai et al., 1997; Banik et al., 2001). Taf1p does not form a stable Taf1p–TBP–TATA ternary complex, even though Taf1p addition to TBP–TATA binary complex results in TBP–DNA complex dissociation at equilibrium. In contrast, Mot1p, which also induces the disruption of TBP–DNA complexes (in the presence of ATP), readily forms both Mot1p–TBP (Poon et al., 1994; Adamkewicz et al., 2001) and Mot1p–TBP–TATA complexes (Auble and Hahn, 1993; Auble et al., 1994; Adamkewicz et al., 2000). Mot1p ternary complexes are fairly stable and have been studied extensively using electrophoretic mobility shift assays (EMSA) with 32P-labeled TATA probes. In previous studies we demonstrated the power of parallel spectroscopy and EMSA with fluorescent probes for studying TBP–TAF interaction dynamics. We used these tools both to address unanswered questions regarding Mot1p and to re-examine tenets of current models of Mot1p action.
Fluorescence anisotropy efficiently monitors Mot1p–TBP–TATA formation
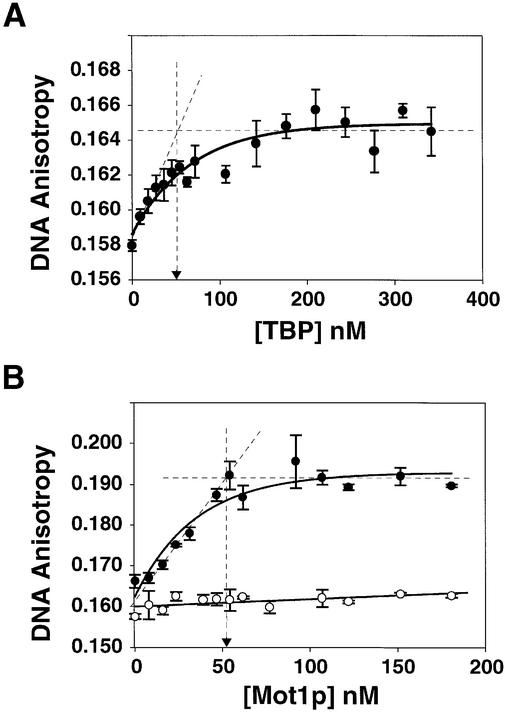
To test the efficacy of spectroscopy-based methods for studying Mot1p, we generated a 43 bp duplex containing a consensus TATA box, TATAAAA. This was labeled with a rhodamine derivative (ROX) and the labeled DNA, termed 43Rox, was used for fluorescence anisotropy. Published studies have shown Mot1p is able to bind a TBP–TATA complex to form an electrophoretically stable Mot1p–TBP–[32P]DNA species. To determine whether we could detect this ternary complex formation via spectroscopy, we first formed the binary TBP–TATA substrate needed by incubating 50 nM 43Rox DNA with increasing amounts of TBP (0–340 nM). When TBP bound to 43Rox, anisotropy (r) increased in a dose-dependent manner with saturation (Figure 1A). We previously determined that TBP bound a shorter TATA probe with high affinity (Kd 4 nM; Bai et al., 1997; Banik et al., 2001). Consistent with this result the TBP–43Rox binding isotherm exhibited the properties expected for a reaction conducted in the stoichiometric binding regime (i.e. concentrations ≥10× the Kd; see dashed lines Figure 1; ∼Kd for TBP–43Rox interaction ≤1 nM); TBP forms a 1:1 complex with 43Rox at saturation (vertical dashed line and filled arrowhead, x-axis at ∼50 nM TBP). These results show that TBP efficiently bound 43Rox with high affinity, and further indicated that the molecules used for our studies were ∼100% active.
Fig. 1. Measurement of Mot1p–TBP–TATA ternary complex formation using fluorescence anisotropy spectroscopy. (A) TBP (9–342 nM) was added to 50 nM 43Rox DNA, equilibrium established (5–10 min at room temperature, 20–23°) and anisotropy monitored. Average anisotropy (n = 3) and standard error (bars) was plotted as a function of TBP concentration. The dashed lines represent the linear portions of the binding isotherm. Extrapolation from the point of intersection to the x-axis (dashed vertical line ending in the filled arrowhead) shows that saturation occurred at ∼1:1 TBP:TATA ratio. (B) Mot1p (8–181 nM) was added to 50 nM binary TBP–TATA complex (r = ∼0.165), and fluorescence measured and plotted (n = 3; filled circles, error bars) as a function of Mot1p concentration. As a control, an equivalent amount of 43Rox DNA was mixed with Mot1p alone (open circles). Dashed lines and filled arrowhead as in (A).
To test whether purified recombinant Mot1p was capable of forming a ternary complex detectable by spectroscopy, we generated 50 nM binary TBP–43Rox TATA binary complexes, and to this added increasing amounts of Mot1p (Figure 1B, closed circles; 0–180 nM Mot1p). As a control, the same amounts of Mot1p alone were added to 43Rox DNA (Figure 1B, open circles); no direct Mot1p–DNA binding was observed. Mot1p efficiently formed a complex with anisotropy greater than TBP–43Rox (binary r = ∼0.165; ternary r = ∼0.195). The putative Mot1p–TBP–DNA complex was formed in a Mot1p dose-dependent fashion, was readily saturable, appeared to occur in the stoichiometric binding regime (see dashed lines in Figure 1B) and saturated at a 1:1 ratio of Mot1p to TBP–DNA complex (see filled arrowhead on x-axis in Figure 1B), again indicating that the reacting species were nearly 100% active. These data demonstrated that Mot1p interactions could be productively studied using fluorescence anisotropy.
Mot1p forms ternary complexes with equal efficiency on DNAs with long and short extensions upstream of TATA
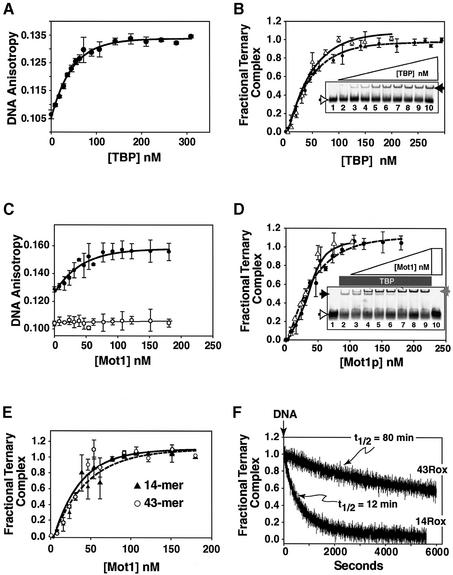
It has been reported that Mot1p displays a strict dependence upon TATA 5′ sequences, both for ternary complex formation and TBP dissociation (Darst et al., 2001). This observation was interpreted to mean that Mot1p makes critical, albeit non-specific, contacts with the 17+ nucleotides immediately 5′ of TATA. These residues were termed a ‘handle’, and Mot1p-upstream DNA interactions have factored heavily into the development of models of Mot1p mechanism (Auble and Hahn, 1993; Auble et al., 1994, 1997; Auble and Steggera, 1999; Adamkewicz et al., 2000; Darst et al., 2001). We tested this idea by using a very short TATA probe for ternary complex formation. The DNA used, termed 14Rox, contains only 3 bp up and 4 bp down from TATA. We first documented efficient TBP–TATA complex formation with 14Rox by anisotropy and EMSA (binding isotherms are shown in Figure 2A and B, respectively). TBP readily bound to 14Rox. Note that while saturation occurred at about the same TBP concentration in both assays (i.e. ∼60 nM TBP; compare curves in Figure 2B), there was significant free DNA in the EMSA due to the dissociation of the TBP–TATA complex during electrophoresis (cf. Hoopes et al., 1992), despite the fact that we took pains to minimize dissociation (see Materials and methods). We have measured the t1/2 of the TBP–14Rox TATA complex to be ≤6 min, thus even our short 10–15 min electrophoretic run represents almost three half-lives of the complex.
Fig. 2. DNA sequences upstream of TATA are not required for efficient Mot1p–TBP–TATA complex formation. (A) TBP–TATA binary complexes (r = ∼0.133) were generated, monitored and the binding results displayed as detailed in Figure 1A (n = 3; 50 nM 14Rox DNA, TBP 9–342 nM). (B) EMSA gels were scanned to detect Rox DNA and TBP–Rox DNA complex, fluorescence quantitated and TBP–TATA complex formation determined. Graphed is the average fractional complex formed (EMSA assay, open triangles; n = 3) compared with fluorescence anisotropy [filled circles; data from (A)]. Shown in the inset is a typical EMSA; free 14Rox DNA (open arrowhead, left) and TBP–TATA complex (filled arrowhead, right); 14Rox DNA at 50 nM (DNA alone lane 1, gel); and TBP 10–200 nM (lanes 2–10). (C) An increasing amount of Mot1p (8–181 nM) was added to 50 nM of TBP–DNA complex (r = ∼0.13). Ternary complex formation was monitored and presented as in Figure 1B (filled circles); in the control Mot1p alone was added to free 14Rox (r = ∼0.105) (open circles). (D) Equivalent assays (lanes 3–9) and controls (lanes 1, 2 and 10) with 50 nM TBP–TATA DNA complex and Mot1p (10, 20, 30, 40, 50, 75 or 100 nM) were analyzed by EMSA. Presence and relative concentrations of TBP and Mot1p are schematically indicated above the gel. Gels were scanned as in (B); free DNA, binary and ternary complexes, open (left/bottom), black (left/top) and gray (right) arrowheads, respectively. Graphed is the average fractional complex formed as determined by EMSA (open triangles; n = 3 for these gel assays) versus fluorescence anisotropy [filled circles, data from (C)]. (E) Shown is a plot comparing relative fractional ternary complex formation on 43Rox TATA DNA (circles, calculated from Figure 1B) or 14Rox TATA DNA [triangles, calculated from (C)] probes. (F) Fifty nanomolar 1:1:1 Mot1p–TBP–DNA ternary complexes were generated on either 14Rox or 43Rox TATA probes as indicated, then 1500 nM of the respective unlabeled DNAs added (arrow, top) anisotropy monitored and fractional complex calculated and plotted as a function of time.
To determine whether Mot1p formed ternary complexes on 14Rox we performed the complementary binding experiments to those of Figure 2A and B. Increasing amounts of Mot1p were added to preformed TBP–TATA complex and potential ternary complex formation monitored by anisotropy or EMSA (Figure 2C and D). Both methods showed that Mot1p formed ternary complexes on 14Rox DNA. When the relative efficiency of ternary complex formation on the 43Rox and 14Rox probes was directly compared (Figure 2E), no significant differences were observed, which indicates that Mot1p does not require any DNA upstream of TATA (over 3 bp) for efficient ternary complex formation. We next examined the t1/2s of Mot1p–TBP–TATA ternary complexes formed on 43Rox and 14Rox (Figure 2F). Ternary complex formed on the 43Rox DNA was much longer-lived (t1/2 ∼80 min) than the ternary complex formed on 14Rox (t1/2 ∼12 min). Therefore, DNA 5′ of TATA stabilized but was not required for ternary complex formation. Such dramatic half-life differences, coupled with extended periods of electrophoresis during EMSA (i.e. 60–90 min), likely led others to deduce that 5′-DNA was required for ternary complex formation (Darst et al., 2001).
Mot1p dissociates ternary complexes formed on 14Rox using its ATPase activity
We next examined whether Mot1p could efficiently dissociate TBP–DNA complexes formed on the 14Rox probe. Here we used time-based spectroscopy complemented with EMSA; samples were withdrawn directly from the cuvette at appropriate times and the molecular species present determined by EMSA. Addition of TBP to free 14Rox (Figure 3A, blue trace, r = ∼0.11, labeled 1; pre-addition sample, free DNA) resulted in rapid TBP–TATA binary complex formation (red trace, r = ∼0.14, labeled 2). Upon addition of Mot1p to this binary complex a ternary complex was rapidly generated, although at a slightly slower rate (green trace, r = ∼0.16, labeled 6). Mot1p did not bind DNA (blue trace, r = ∼0.11, labeled 4). These results are consistent with our equilibrium analyses (Figures 1 and 2). When saturating levels of ATP were added (1 mM), only the Mot1p–TBP–TATA complex showed any significant change in anisotropy (compare the blue, red and green traces in Figure 3A, right). Upon addition of ATP, anisotropy rapidly dropped in the ternary complex reaction and then quickly rose to about the level of TBP–TATA complex (r = ∼0.14). EMSA indicated the molecular species present in the cuvette at various points in these binding reactions (Figure 3B). Interestingly, following ATP-induced ternary complex dissociation, there was a mixture of free DNA binary and ternary complex in sample 7, indicating that a new equilibrium had been established (Figure 3A, right, green trace) which was populated by a mixture of these two protein–DNA complexes, not just binary TBP–DNA as might have been surmised by the anisotropy value. This result underscores the power of combining spectroscopy and EMSA. If limiting concentrations of ATP were added (0.5–4 µM; Mot1p ATPase Km,ATP ∼100 nM; not shown), upon ATP hydrolysis, ternary Mot1p–TBP–TATA rapidly reformed (Supplementary figure 1 available at The EMBO Journal Online) at rates consistent with measured Mot1p ATPase turnover numbers (Supplementary table 1).
Fig. 3. Mot1p dissociates TBP from Mot1p–TBP–DNA complexes formed on 14mer TATA DNA via its ATPase function. (A) Left: 50 nM TBP was added to 50 nM 14Rox DNA (DNA alone, first 60 s blue trace, r = ∼0.11, sampled and labeled 1). Binding was monitored for 10 min (red trace) and sampled (labeled 2). Fifty nanomolar Mot1p was added to 50 nM TBP–DNA binary complex and anisotropy monitored, red t = 0 trace changing to green trace upon Mot1p addition, this reaction was sampled (labeled 6). Note that when the cuvette was accessed for addition of components a large spike in signal was generated. Blue trace (labeled 4) Mot1p added at ∼60 s measures anisotropy after the addition of Mot1p to 14Rox DNA alone. Right: 1 mM ATP was added to each reaction (see arrow labeled ATP) and anisotropy monitored with time; these reactions also sampled for EMSA (labeled 3, 5 and 7). (B) Shown is the fluorescence scan of EMSA fractionation of 10 µl aliquots of samples 1–7 from (A). (C and D) Reactions formed and analyzed exactly as in (A) and (B), except that 50 nM Mot1p K1301A protein was utilized.
These data showed that Mot1p efficiently catalyzed the ATP-dependent dissociation of TBP–DNA complexes formed on 14Rox. Confirming this idea are the results of a similar experiment that utilized a mutant form of Mot1p, termed K1303A (Auble et al., 1994). Mot1p K1303A, mutated in the ATP binding/hydrolysis domain, exhibits dramatically reduced ATPase activity and little to no ATP-dependent TBP–TATA dissociation activity, and dominantly inhibits yeast cell growth when overexpressed from a GAL enhancer/promoter (Auble et al., 1994, 1997). We found that purified Mot1p K1303A formed a stable ternary complex with TBP–14Rox as efficiently as wild type (Figure 3C, green trace), but failed to dissociate upon ATP addition (Figure 3C); EMSA confirmed the spectro scopy findings (Figure 3D). Addition of non-hydrolyzable ATP analogs to ternary complexes containing wild-type Mot1p behaved like K1303A Mot1p and failed to induce dissociation (not shown). These data support the hypothesis that ATP hydrolysis, via Mot1p’s conserved ATPase, was absolutely required for efficient ternary complex dissociation, and also demonstrated that a DNA handle is not required for Mot1p-mediated complex dissociation.
Fate of Mot1p, TBP and TATA DNA following a cycle of ATP-driven Mot1p-catalyzed ternary complex dissociation
We performed a series of time-based competition assays to determine the fate of the components of the Mot1p– TBP–TATA ternary complex following ATP hydrolysis. These experiments differed from those of Figure 3 in that we utilized both labeled (14Rox) DNA and labeled (Alexa-350) TBP in a ‘two-color’ experiment. These two fluorophores are spectrally distinct (excitation and emission) and therefore the behavior of each can be readily distinguished. TBP–DNA and Mot1p–TBP–DNA complexes were formed, binding monitored by the DNA signal (Figure 4A, left, Rox, black trace) and TBP signal (Alexa 350, Figure 4B, left, black trace). Owing to limitations of our fluorimeter these two reactions were performed back-to-back rather than simultaneously. When the Alexa-labeled TBP bound DNA, anisotropy decreased but subsequently increased upon Mot1p addition (Figure 4B, black trace). This behavior, a drop followed by a rise in r, is probably due either to changes in the local environment or to dynamics of the probe on TBP when bound by DNA. Mot1p–TBP (Figure 4B, pink trace; shown for comparative purposes) and Mot1p–TBP–DNA complexes exhibited similar anisotropy values; the corresponding Rox-DNA signals (Figure 4A) indicated that our assignment of Mot1p–TBP–DNA ternary complex in the Alexa–TBP binding assay was correct. Upon addition of ATP (to 1 mM) anisotropy decreased and then quickly rose (Figure 4A) to a value intermediate between that of the free probes and ternary complex (Figure 4A and B, red traces), results consistant with the data in Figure 3. When cognate cold competitors were added to the reactions (either unlabeled 14mer DNA or unlabeled TBP; Figure 4A and B, blue traces), identical decay curves, consistent with the known t1/2s of the 14Rox TATA ternary complex, were observed, regardless of which competitor was added (i.e. 12 min; cf. Figure 2F). When repeated in the presence of ATP, anisotropy rapidly dropped to the values of the respective probes (r = ∼0.11, 14Rox DNA; Figure 4A, right; r = ∼0.145, Alexa–TBP; Figure 4B, right, green traces). Qualitatively similar results were obtained in EMSA using Rox-labeled DNA (cf. Figure 4A; not shown). As our fluorescence imager is unable to excite in the UV range, the complementary EMSA of the Alexa 350–TBP experiments of Figure 4B could not be performed. Taken together, the experiments in Figure 4 indicated that upon a round of ATP-driven Mot1p-mediated dissociation ternary complex dissociated to free TBP, free Mot1p and free DNA.
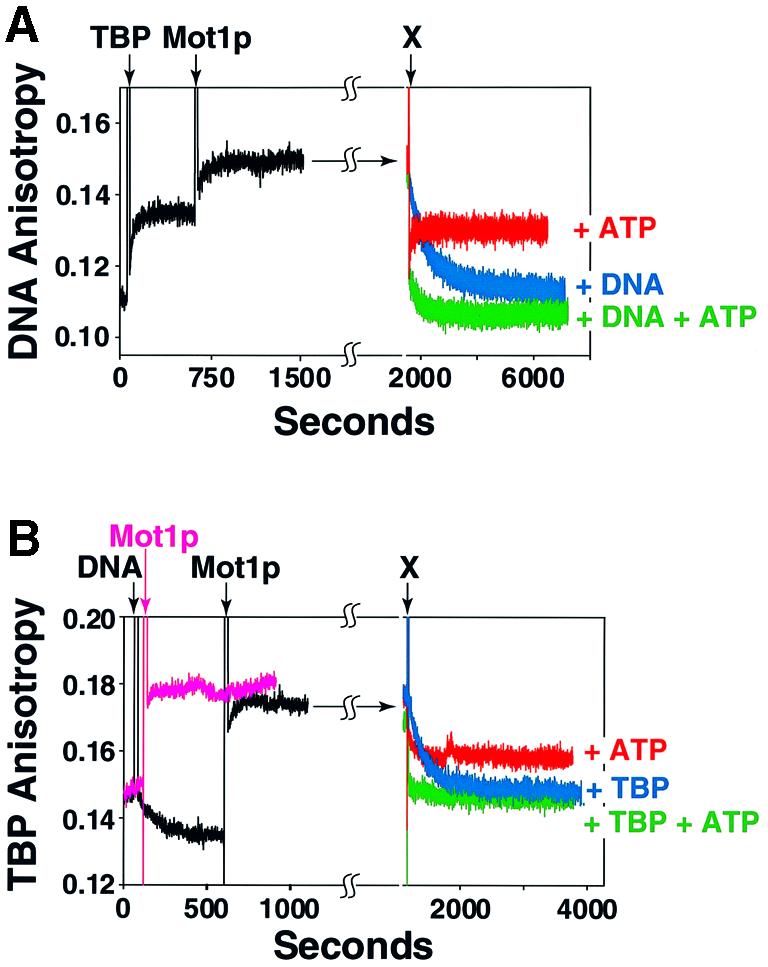
Fig. 4. Mapping the fate of ternary complex components following ATP-driven Mot1p-mediated dissociation. (A) A 1:1:1 ternary complex was formed by adding 50 nM of TBP (arrow, Alexa-labeled TBP added, marked TBP) followed by 50 nM Mot1p (arrow, marked Mot1p) to a 50 nM solution of 14Rox TATA DNA. Anisotropy was monitored and plotted as a function of time. Either 1 mM ATP (red trace, labeled +ATP), 1500 nM cold 14mer Ad2 MLP TATA (blue trace, labeled +DNA) or both 1 mM ATP and 1500 nM 14mer DNA (green trace, labeled +DNA +ATP) added to ternary complex (all additions marked by the arrow marked X, top); anisotropy was monitored and plotted as a function of time. (B) A parallel reaction performed exactly as in (A) except the added 50 nM Alexa 350-labeled TBP was monitored as fluorophore; as a control, Mot1p–TBP complex formation was monitored (pink trace). When present, cold TBP added to 1500 nM.
Mot1p binds TBP directly with high affinity and in an ATP-independent fashion
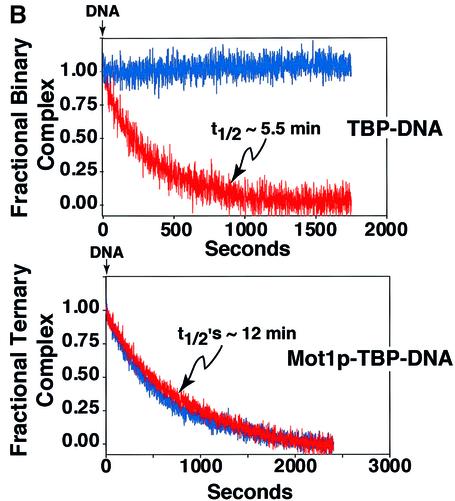
The data shown in Figure 4 suggested that Mot1p bound TBP with relatively high affinity. To investigate Mot1p–TBP association and to further test whether ATP induced dissociation of these two proteins, as reported previously (Darst et al., 2001), we performed studies using TMR-labeled TBP, monitoring binding by anisotropy (Figure 5A) and EMSA (Figure 5B). Mot1p bound TBP, forming a 1:1 binary complex; formation of this complex was unaffected by ATP [compare blue (–ATP) and red (+ATP) curves, Figure 5B]. Curve-fitting indicated that the apparent affinity constant (Kd,app) for Mot1p–TBP interaction was ∼1 nM. Using cold TBP as competitor we determined that the t1/2 of this Mot1p–TBP complex was ∼12 min and was unaffected by ATP [compare blue (–ATP) and red (+ATP) curves, Figure 5C]. These data showed that Mot1p binds TBP directly, with high affinity and in an ATP-independent manner.
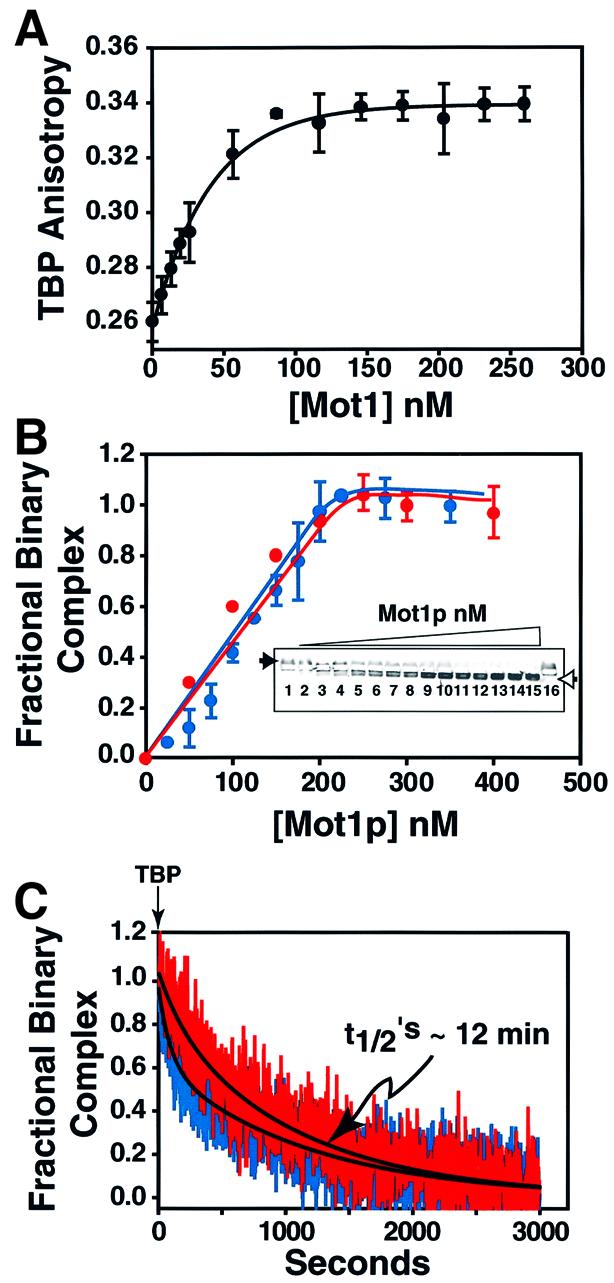
Fig. 5. Direct high affinity ATP-independent interaction of Mot1p with TBP. (A) A 50 nM solution of TMR-labeled TBP was titrated with increasing amounts of Mot1p (7–260 nM) anisotropy measured and plotted as a function of Mot1p concentration (n = 2). (B) Binding of TBP to Mot1p as monitored by horizontal EMSA. Note that free TMR-labeled TBP migrates towards the cathode (filled arrowhead) and TBP–Mot1p binary complex towards the anode (open arrowhead). Assay conducted as above, except that 250 nM TBP was used, and an Mot1p concentration range of 25–400 nM. Shown in the insert is a typical gel. Graphs indicate quantitation of EMSA data (n = 2) blue curve binding –ATP, red curve binding +1 mM ATP. (C) At t = 0, 1500 nM unlabeled TBP was added to 50 nM preformed TMR-labeled TBP–Mot1p binary complex. Anisotropy was monitored with time after addition of competitor TBP; blue, red curves dissociation –ATP, +ATP (1 mM); black lines, calculated exponential decay curve fit.
The Mot1p–TBP complex binds TATA DNA
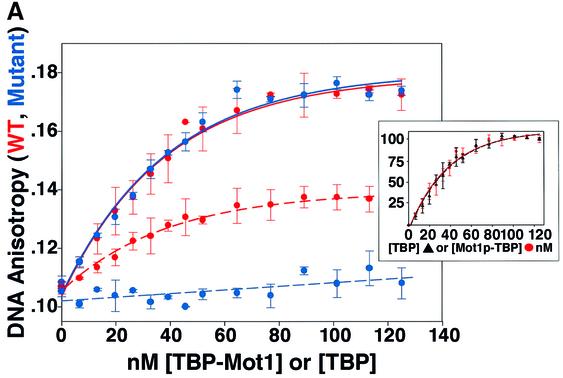
Given the nanomolar binding affinity of Mot1p for TBP, its long-lived ATP-independent nature, and high intranuclear concentrations (i.e. 5000–10 000 molecules in ≤6 × 10–16 l, or 10–20 µM minimum; Bai, 1997; Lee and Young, 1998; Campbell, 1999) it seemed reasonable that, in vivo, Mot1p would readily bind any available (free) TBP, forming Mot1p–TBP complex. We wondered whether this complex had the ability to bind DNA. As shown by the anisotropy data presented in Figure 6, we found that this was the case. The Mot1p–TBP complex bound TATA DNA (Figure 6A, red solid curve) efficiently (Kd,app ∼5 nM); the TBP binding isotherm is included as a control (Figure 6A, red dashed curve; Kd,app ∼5 nM). These data indicated that the stable Mot1p–TBP complex had the ability to bind DNA.
Fig. 6. Mot1p association dramatically alters TBP DNA binding specificity. (A) To 50 nM Rox14mer wild-type TATAAA DNA (red) or 50 nM Rox14mer mutant TAAGAA DNA (blue) was added the indicated nanomolar amounts of TBP (dashed lines) or preformed Mot1p–TBP binary complex (solid lines). Anisotropy was measured and plotted as a function of protein concentration (n = 2, blue dashed and solid lines; n = 3, red dashed line; n = 4, blue solid line; bars indicate standard error). Insert shows relative of binding of TBP and Mot1p–TBP to wild-type TATA DNA; fractional binding versus protein. (B) To 50 nM preformed TBP-14Rox wild-type TATA complex (top), or ternary Mot1p–TBP–14Rox wild-type TATA DNA complex (bottom) was added 1500 nM unlabeled wild-type TATAAAA (red trace) or 1500 nM Mut TAAGAAA competitor DNAs (blue trace). Anisotropy was monitored with time after addition of competitor. Curve fitting (not shown) indicated that the TBP–DNA complex has a t1/2 of ∼5.5 min (wild-type TATA DNA competitor); Mot1p–TBP–TATA DNA complex t1/2 ∼13 min (wild-type TATA DNA competitor) or t1/2 ∼11 min (Mut TATA DNA competitor).
Association with Mot1p dramatically alters the DNA binding selectivity of TBP
We next used labeled and unlabeled wild-type and TATA mutant DNAs to examine the affinity and specificity of DNA binding by TBP when associated with Mot1p. We reasoned that binding would be dependent upon TATA sequence integrity given that TBP was the protein moiety mediating DNA interactions. However, we found that Mot1p-associated TBP had lost the ability to discriminate between wild-type (TATAAAA) and mutant (Mut) (TAAGAAA) TATA DNAs [compare red (WT DNA) and blue (Mut DNA) solid and dashed curves in Figure 6A; dashed curves represent the control TBP-binding isotherms]. These properties were also observed in DNA competition experiments where Mot1p–TBP–TATA ternary complexes were competed with equal efficiency with unlabeled WT (red curve) and Mut (blue curve) TATA DNA (Figure 6B, bottom). In both direct binding (Figure 6A, dashed curves) and DNA competition assays (Figure 6B, top), TBP alone exhibited the predicted binding affinity and specificity (see also Supplementary figure 2). TBP and Mot1p–TBP bound DNA with comparable efficiencies (see Figure 6A inset). Poly(dA–dT)– poly(dA–dT) and poly(dG–dC)–poly(dG–dC) behaved identically to the wild-type and mutant TATA DNAs in similar competition studies (Supplementary figure 3). Taken together, these results indicated that when associated with Mot1p, TBP completely lost the ability to selectivity recognize DNA.
Discussion
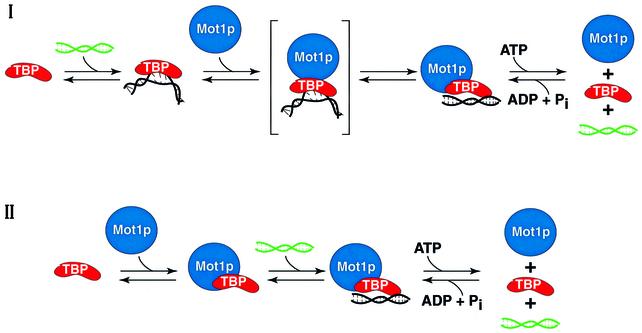
As in summarized in Figure 7, in this report we have described a number of observations relevant to the mechanism of Mot1p. First, we have shown that rapid, high-affinity binding of Mot1p to the TBP–TATA DNA complex does not display a requirement for DNA upstream of TATA. Secondly, ternary Mot1p–TBP– TATA complexes formed on DNA lacking 5′-DNA upstream of TATA are efficiently dissociated by the action of Mot1p’s ATPase to free components upon ATP addition (Figure 7, scheme I). Thirdly, we have shown that Mot1p binds to TBP directly, with nanomolar affinity, in an ATP-independent fashion. Fourthly, binary Mot1p– TBP complexes are capable of binding DNA directly (Figure 7, scheme II), and these complexes display ATP-dependent ternary complex dissociation in a fashion indistinguishable from scheme I in Figure 7. Finally, we demonstrated that when associated with Mot1p, TBP displays dramatically different DNA recognition properties and binds DNA with much reduced selectivity (Figure 7, schemes I and II). We suggest that the repressive functions of Mot1p are mediated through the reactions depicted in scheme I, while activation functions are mediated primarily via the reactions outlined in scheme II (Figure 7).
Fig. 7. Possible mechanisms of Mot1p action. Presented schematically is the information regarding Mot1p detailed in this report. Mot1p can bind either a TBP–TATA DNA binary complex (scheme I) or TBP (scheme II) to form ternary Mot1p–TBP–DNA complexes, wherein TBP interacts with TATA DNA in a dramatically altered fashion (see text). A presumptive intermediate on the path to ternary complex in scheme I is illustrated in brackets. Note that the TBP–TATA DNA complex (and presumptive Mot1p–TBP–TATA complex indicated in parentheses) is highly resistant to poly(dG–dC)–poly(dG–dC), whereas, in contrast, binding of the Mot1p–TBP binary complex to DNA (either wild-type or mutant TATA) is, as expected, sensitive to competition by poly(dG–dC)–poly(dG–dC). Upon ATP addition both types of ternary complex can be dissociated to free components, Mot1p (blue), TBP (red) and B-form TATA DNA (green) as shown. The two different modes of TBP–DNA interaction defined by our work are illustrated by the black, bent and splayed DNA associated TATA DNA in the TBP binary complex shown in scheme I (i.e. Kim et al., 1993) and the black, relatively ‘straight’ unbent form of DNA shown as part of the Mot1p–TBP–DNA ternary complexes (schemes I and II).
Lack of requirement for a DNA ‘handle’ for ternary complex formation and dissociation
It has been argued that Mot1p displays a strict requirement for TATA-proximal 5′-flanking sequences for the formation (and subsequent dissociation) of Mot1p–TBP–DNA complexes (Darst et al., 2001). Our data indicate that this is not the case (Figures 1–4), rather the reason for the conclusion that TATA 5′-DNA plays a role in Mot1p function most likely derives from the fact that ternary complexes formed on long and short DNA display dramatically different half-lives (Figure 2F). Thus, Mot1p functions just as efficiently in the absence of a DNA ‘handle’ as in the presence of one. This fact indicates that Mot1p does not make any essential contacts with DNA upstream of TATA. Consequently, the ability of Mot1p to use ATPase-derived energy (in an ATP concentration-dependent fashion; see Supplementary figure 1) to remove TBP from DNA is not underpinned by Mot1p–upstream DNA interactions, but rather must be an intrinsic property of the Mot1p protein when part of a ternary complex. We feel that the ability of Mot1p to remove TBP from DNA is almost certainly related to the altered DNA binding characteristics exhibited by TBP when it is bound to Mot1p (Figure 6; Supplementary figures 2 and 3). In this regard it is important to note that the ATPase-deficient K1303A mutant form of Mot1p both efficiently forms ternary complex (Figure 3) and alters TBP–DNA interaction specificity like wild type (compare with Supplementary figure 5).
Mot1p–TBP–DNA complexes dissociate to free components upon ATP hydrolysis catalyzed by Mot1p ATPase
In the presence of ATP, through the action of its evolutionarily conserved ATPase, Mot1p induces ternary complex dissociation to free components (i.e. TBP, TATA DNA and Mot1p; Figures 3 and 4) each of which can immediately (re-)participate in binary (TBP–DNA and Mot1p–TBP) and ternary complex formation. This is the first demonstration that yeast Mot1p ternary complexes dissociate to free components after a cycle of ATP hydrolysis. Such dissociation will free up the three reactants for subsequent transcriptional events. This fact is highly relevant given the features of the interactions between Mot1p and TBP (see below).
Mot1p forms a stable ATP-independent Mot1p–TBP complex
Mot1p binds TBP with high affinity to form a relatively long-lived binary complex (Kd ≤1 nM, t1/2 12 min; Figures 4 and 5). In contrast to a recent report (Darst et al., 2001), we found this interaction to be independent of ATP (Figure 5). These data are important considering the fact that both Mot1p and TBP are so abundant (Bai, 1997; Lee and Young, 1998; Campbell, 1999). Given the nanomolar Mot1p–TBP binding affinity and micromolar intranuclear concentrations, it is difficult to imagine that without active, cyclical complex dissociation Mot1p would always be complexed with TBP, effectively ‘freezing’ a large fraction of available free TBP in this form. Our data presented here (Figures 3 and 4; Supplementary figure 4), and previously published (Poon et al., 1994; Campbell, 1999) and unpublished (mass spectrometry-based sequencing of Mot1p-associated proteins), indicates that this is not the case; about half of total Mot1p is not TBP-associated in vivo. How this is accomplished is not known. However, based upon our data, at least after a cycle of Mot1p-catalyzed dissociation, there is a window of opportunity for both ‘liberated’ Mot1p and TBP to interact with other proteins. TBP could then form TFIIIB, TFIID, SL1 or other types of TBP–TAF complexes (Sanders et al., 2002b). We recently reported that yeast TBP dynamically associates with the 14-polypeptide TAF protein assembly that comprises TFIID (Sanders et al., 2002a; t1/2 of association 10–15 min), suggesting a level of TBP and TAF association dynamics heretofore under appreciated. How Mot1p–TBP interaction dynamics might factor into this process is not yet known.
Association of TBP with Mot1p dramatically alters TBP DNA binding selectivity
Our experiments showed that Mot1p–TBP complexes have the ability to directly bind DNA. To examine the specificity of this DNA binding, we performed a series of binding and competition experiments utilizing wild-type and mutant forms of TATA to simulate the range of sequences present in natural promoters (Singer et al., 1990) and poly(dA–dT) and poly(dG–dC) (Figure 6; Supplementary figures 2 and 3). Surprisingly, we observed that when associated with Mot1p, either in Mot1p– TBP–DNA or Mot1p–TBP forms, TBP interacted with DNA with relaxed selectivity, recognizing wild-type TATAAAA and various mutated forms of TATA DNA equally. These characteristics represent a dramatic change in the DNA binding properties of TBP. Such alteration in the mode of TBP–DNA recognition could be caused either by Mot1p-induced changes in the way that TBP contacts DNA, perhaps effected by conformational changes in TBP and/or TATA DNA, or by direct contributions to DNA binding by a (positively charged) domain of Mot1p when it interacts appropriately with TBP, which may follow a TBP-effected Mot1p conformational change. Such altered interactions might involve a Mot1p TAND-like domain (Pereira et al., 2001). Mot1p amino acids 140–250, resident within the N-terminal TBP interaction domain (Adamkewicz et al., 2001; Pereira et al., 2001), are 19% arginine + lysine and display a pI of ∼9.9. K1303A Mot1p behaves the same as wild type in altering TBP binding specificity (Supplementary figure 5), which indicates that this alteration in TBP–DNA recognition is a property of Mot1p likely independent of ATP or ATP-induced conformational changes.
Given these results, one might ask how a ternary complex that displays such relaxed DNA binding specificity can even form (in vitro) in the presence of non-specific DNA [i.e. poly(dG–dC); Auble and Hahn, 1993; data not shown]. Our explanation is that in scheme I, ternary complex only forms through a pre-existing highly specific TBP–TATA complex. Thus, though the resulting complex (scheme I) ultimately is converted to a low specificity TBP–DNA complex, it started out as a classical ‘specific’ TBP–TATA DNA complex. By this reasoning, ternary complexes formed by scheme II should be (and are) non-selective (compare with Supplementary figure 5).
Mechanism of repression by Mot1p
We suggest that the altered TBP–DNA binding properties we document (Figure 6; Supplementary figures 2, 3 and 5) are integral to the ability of Mot1p to modulate transcription. Mot1p likely acts as a repressor by removing promoter-bound TBP from the TATA box of transcribed genes, consequently repressing RNA synthesis. This action of Mot1p may be integral to attenuation of transactivation, and an essential and normal event in gene regulation. Mot1p-mediated TBP dissociation could be accomplished, at least in part, by changing the way that TBP interacts with DNA, as we have detailed. Integral to complete TBP–DNA complex disruption is the ATPase function of Mot1p (Auble and Hahn, 1993; Auble et al., 1994; Figures 3 and 4; Supplementary figures 3 and 5). However, it must be kept in mind that in vivo it is likely that the situation is more complex, potentially involving additional proteins not present in our purified system (Supplementary figure 4). Finally, although the TBP-dissociating functions of Mot1p have been well studied, it remains to be elucidated exactly how the energy of ATP hydrolysis is harnessed by Mot1p to effect TBP–DNA dissociation. Such information will come through additional detailed kinetic and mechanistic analyses.
Mechanism of Mot1p activation of transcription and implications for TAF-independent mRNA gene transcription
It is possible that Mot1p activates transcription only indirectly, perhaps as a consequence of removing TATA-bound TBP after Pol II promoter clearance. However, we show here that a stable, rapidly formed Mot1p–TBP complex has the ability to bind DNA. Furthermore, Mot1p has recently been shown to be associated with actively transcribed genes (Dasgupta et al., 2002; Geisberg et al., 2002), appearing on induced genes with kinetics similar to TBP (Andrau et al., 2002). We postulate that the Mot1p–TBP complex could be involved in transcription activation directly by delivering TBP to select target genes for PIC formation. In this setting, as for all other Pol II transactivation events, the question of selective targeting of TBP/TBP–TAF complexes must be addressed. We hypothesize that the requisite selectivity is achieved by specific interactions between DNA-bound transactivator proteins and the stable Mot1p–TBP complex, in much the same fashion that such specific activator–coactivator interactions (Albright and Tjian, 2000) have been proposed to occur between Gal4p and SAGA (TBP), and Rap1p and TFIID (TAF–TBP) on appropriate target genes (Bhaumik and Green, 2001; Larschan and Winston, 2001; Li et al., 2002; Mencia et al., 2002). In this scenario, Mot1p functions as the coactivator. Thus, Mot1p–TBP complexes may be responsible for escorting TBP to the TAF-independent (Kuras et al., 2000; Li et al., 2000) mRNA encoding genes, perhaps the subset of genes with non-canonical TATA boxes that, based on our data (Figure 6; Supplementary figures 1, 2 and 5) and that of Geisberg et al. (2002), would be predicted to be bound more avidly by the Mot1p–TBP complex than by TBP or TFIID alone. We are currently working to test this and other ideas regarding Mot1p.
Materials and methods
Recombinant proteins
N-terminally His6-tagged yeast wild-type TBP, TBPCys–/+ and yeast wild-type and K1303A forms of Mot1p were purified (>90%) as described previously (Bai et al., 1997; Muldrow et al., 1999). TBPCys–/+ contains amino acid substitutions (Ala61→Cys, Cys78→Ala, Cys164→Ala) that facilitate labeling with cysteine-reactive, maleimide-based fluorophores without altering TBP function (Banik et al., 2001).
DNA probes
Rox (5-carboxy-X-rhodamine; Molecular Probes Inc., Eugene, OR) labeled and unlabeled duplex DNAs from the Ad2 MLP TATA box were from Biosearch Technologies (Novato, CA). The 14 and 43 bp DNAs used varied in the number of bp upstream of TATA. The wild-type sequences (top strand) were: 5′ R-GGCTATAAAAGGGG and 5′ R-ATCCG GAGGACTGTCCTCCGGCGAAGGGGGGCTATAAAAGGGG. R is the site of attachment of the Rox fluorophore if present; TATA box is underlined. Mutant TATA DNAs contained various nucleotide changes (Wobbe and Struhl, 1990).
Protein probes
Alexa 350 or TMR (tetra-methyl rhodamine) labeled TBPs were prepared by mixing 40 nmol of purified TBPCys–/+ in buffer A (20 mM HEPES pH 7.0, 500 mM NaCl, 5% glycerol) for 12 h at 4° in the dark with a 15-fold excess of Alexa 350- or TMR-maleimide (Molecular Probes). Labeled TBP was purified on Sephadex G-25 in buffer A + 5 mM 2-mercaptoethanol and then dialyzed against three changes of 1000 vol. of buffer A over 12 h. Labeling stoichiometry was determined by fluorescence and UV spectrometry and SDS–PAGE. The properties of TBPCys–/+ are detailed in Banik et al. (2001).
EMSA
Protein–DNA complexes were separated by gel electrophoresis on vertical 5% polyacrylamide (30:0.8 acryl:bis) gels (10 × 10 × 0.8 cm) cast in 25 mM Tris–HCl, 250 mM glycine, 5 mM MgCl2, pH 8.4 (ionic strength = 1.5 mmho) and run (350V ×15 min) in the same buffer (plus 5% glycerol) in an Owl Scientific apparatus (Model P8DS-1) with cooling using a 35% ethylene glycol solution chilled to –7°C. Gels were scanned [excitation at 532 nm, detection using 605 nm (Rox) or 555 nm (TMR) band-pass filters] with a Bio-Rad FX imager and quantitated using Bio-Rad Quantity One software. In Figure 5, Mot1p–TBP complexes were separated from free TMR-labeled TBP by horizontal EMSA (Banik et al., 2001).
Binding assays
Complex formation was in 20 mM HEPES pH 7.9, 100 mM NaCl, 5% glycerol, 5 mM MgCl2, 100 µg/ml BSA (ionic strength = 9.4 mmho); EMSA reactions were in 10 µl, anisotropy reactions in 150 µl. Anisotropy was measured using a PTI T-format fluorimeter. All binding data were analyzed as detailed elsewhere (Perez-Howard et al., 1995; Banik et al., 2001).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We would like to thank all laboratory members for helpful comments and suggestions; Drs U.Banik and J.Beechem for their input in the early stages of this work; and Drs Al Beth and Marc Timmers for valuable comments on the manuscript. This work was supported by the NIH (GM52461, GM58588).
References
- Adamkewicz J.I., Mueller,C.G., Hansen,K.E., Prud’homme,W.A. and Thorner,J. (2000) Purification and enzymic properties of Mot1 ATPase, a regulator of basal transcription in the yeast Saccharomyces cerevisiae. J. Biol. Chem., 275, 21158–21168. [DOI] [PubMed] [Google Scholar]
- Adamkewicz J.I., Hansen,K.E., Prud’homme,W.A., Davis,J.L. and Thorner,J. (2001) High affinity interaction of yeast transcriptional regulator, Mot1, with TATA box-binding protein (TBP). J. Biol. Chem., 276, 11883–11894. [DOI] [PubMed] [Google Scholar]
- Albright S.R. and Tjian,R. (2000) TAFs revisited: more data reveal new twists and confirm old ideas. Gene, 242, 1–13. [DOI] [PubMed] [Google Scholar]
- Andrade M.A., Petosa,C., O’Donoghue,S.I., Muller,C.W. and Bork,P. (2001) Comparison of ARM and HEAT protein repeats. J. Mol. Biol., 309, 1–18. [DOI] [PubMed] [Google Scholar]
- Andrau J.-C., Van Oevelen,C.J.C., Van Teeffelen,H.A.A.M., Weil,P.A., Holstege F.C.P. and Timmers,H.T.M. (2002) Mot1 is essential for TBP recruitment to selected promoters during in vivo gene activation. EMBO J., 21, 5173–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble D.T. and Hahn,S. (1993) An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev., 7, 844–856. [DOI] [PubMed] [Google Scholar]
- Auble D.T. and Steggerda,S.M. (1999) Testing for DNA tracking by MOT1, a SNF2/SWI2 protein family member. Mol. Cell. Biol., 19, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble D.T., Hansen,K.E., Mueller,C.G., Lane,W.S., Thorner,J. and Hahn,S. (1994) Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev., 8, 1920–1934. [DOI] [PubMed] [Google Scholar]
- Auble D.T., Wang,D., Post,K.W. and Hahn,S. (1997) Molecular analysis of the SNF2/SWI2 protein family member MOT1, an ATP-driven enzyme that dissociates TATA-binding protein from DNA. Mol. Cell. Biol., 17, 4842–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y. (1997) Molecular identification and characterization if the yeast TBP associated factors. PhD Thesis, Vanderbilt University, Nashville, TN.
- Bai Y., Perez,G.M., Beechem,J.M. and Weil,P.A. (1997) Structure–function analysis of TAF130: identification and characterization of a high-affinity TATA-binding protein interaction domain in the N terminus of yeast TAF(II)130. Mol. Cell. Biol., 17, 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik U., Beechem,J.M., Klebanow,E., Schroeder,S. and Weil,P.A. (2001) Fluorescence-based analyses of the effects of full-length recombinant TAF130p on the interaction of TATA box-binding protein with TATA box DNA. J. Biol. Chem., 276, 49100–49109. [DOI] [PubMed] [Google Scholar]
- Bhaumik S.R. and Green,M.R. (2001) SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev., 15, 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A.M. (1999) Characterization of novel TBP-associated factors in the yeast Saccharomyces cerevisiae. PhD Thesis, Vanderbilt University, Nashville, TN.
- Chicca J.J., Auble,D.T. and Pugh,B.F. (1998) Cloning and biochemical characterization of TAF-172, a human homolog of yeast Mot1. Mol. Cell. Biol., 18, 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M.A. (1996) The NOT, SPT3 and MOT1 genes functionally interact to regulate transcription at core promoters. Mol. Cell. Biol., 16, 6668–6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst R.P., Wang,D. and Auble,D.T. (2001) MOT1-catalyzed TBP–DNA disruption: uncoupling DNA conformational change and role of upstream DNA. EMBO J., 20, 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Darst,R.P., Martin,K.J., Afshari,C.A and Auble,D.T. (2002) Mot1 activates and represses transcription by direct, ATPase-dependent mechanisms. Proc. Natl Acad. Sci. USA, 99, 2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.L., Kunisawa,R. and Thorner,J. (1992) A presumptive helicase (MOT1 gene product) affects gene expression and is required for viability in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol., 12, 1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisberg J.V., Moqtaderi,Z., Kuras,L. and Struhl,K. (2002) Mot1 associates with transcriptionally active promoters and inhibits association of NC2 in Saccharomyces cerevisiae. Mol. Cell. Biol., 22, 8122–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B.C., LeBlanc,J.F. and Hawley,D.K. (1992) Kinetic analysis of yeast TFIID–TATA box complex formation suggests a multi-step pathway. J. Biol. Chem., 267, 11539–11547. [PubMed] [Google Scholar]
- Kim Y., Geiger,J.H., Hahn,S. and Sigler,P.B. (1993) Crystal structure of a yeast TBP/TATA-box complex. Nature, 365, 512–520. [DOI] [PubMed] [Google Scholar]
- Kotani T., Miyake,T., Tsukihashi,Y., Hinnebusch,A.G., Nakatani,Y., Kawaichi,M. and Kokubo,T. (1998) Identification of highly conserved amino-terminal segments of dTAFII230 and yTAFII145 that are functionally interchangeable for inhibiting TBP–DNA interactions in vitro and in promoting yeast cell growth in vivo. J. Biol. Chem., 273, 32254–32264. [DOI] [PubMed] [Google Scholar]
- Kuras L., Kosa,P., Mencia,M. and Struhl,K. (2000) TAF-containing and TAF-independent forms of transcriptionally active TBP in vivo. Science, 288, 1244–1248. [DOI] [PubMed] [Google Scholar]
- Larschan E. and Winston,F. (2001) The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev., 15, 1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.I. and Young,R.A. (1998) Regulation of gene expression by TBP-associated proteins. Genes Dev., 12, 1398–1408. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Bhaumik,S.R. and Green,M.R. (2000) Distinct classes of yeast promoters revealed by differential TAF recruitment. Science, 288, 1242–1244. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Virbasius,A., Zhu,X. and Green,M.R. (1999) Enhancement of TBP binding by activators and general transcription factors. Nature, 399, 605–609. [DOI] [PubMed] [Google Scholar]
- Li X., Bhaumik,S., Zhu,X., Li,L., Shen,W., Dixit,B. and Green,M.R. (2002) Selective recruitment of TAFs by yeast upstream activating sequences. Implications for eukaryotic promoter structure. Curr. Biol., 12, 1240–1244. [DOI] [PubMed] [Google Scholar]
- Madison J.M. and Winston,F. (1997) Evidence that Spt3 functionally interacts with Mot1, TFIIA and TATA-binding protein to confer promoter-specific transcriptional control in Saccharomyces cerevisiae. Mol. Cell. Biol., 17, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencia M., Moqtaderi,Z., Geisberg,J.V., Kuras,L. and Struhl,K. (2002) Activator-specific recruitment of TFIID and regulation of ribosomal protein genes in yeast. Mol. Cell, 9, 823–833. [DOI] [PubMed] [Google Scholar]
- Muldrow T.A., Campbell,A.M., Weil,P.A. and Auble,D.T. (1999) MOT1 can activate basal transcription in vitro by regulating the distribution of TATA binding protein between promoter and nonpromoter sites. Mol. Cell. Biol., 19, 2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G.H., Schroeder,S.C., Bai,Y., Weil,P.A. and Piston,D.W. (1998) Quantitative imaging of TATA-binding protein in living yeast cells. Yeast, 14, 813–825. [DOI] [PubMed] [Google Scholar]
- Pereira L.A., van der Knaap,J.A., van den Boom,V., van den Heuvel,F.A. and Timmers,H.T. (2001) TAF(II)170 interacts with the concave surface of TATA-binding protein to inhibit its DNA binding activity. Mol. Cell. Biol., 21, 7523–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Howard G.M., Weil,P.A. and Beechem,J.M. (1995) Yeast TATA binding protein interaction with DNA: fluorescence determination of oligomeric state, equilibrium binding, on-rate and dissociation kinetics. Biochemistry, 34, 8005–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon D. and Weil,P.A. (1993) Immunopurification of yeast TATA-binding protein and associated factors. Presence of transcription factor IIIB transcriptional activity. J. Biol. Chem., 268, 15325–15328. [PubMed] [Google Scholar]
- Poon D., Campbell,A.M., Bai,Y. and Weil,P.A. (1994) Yeast Taf170 is encoded by MOT1 and exists in a TATA box-binding protein (TBP)–TBP-associated factor complex distinct from transcription factor IID. J. Biol. Chem., 269, 23135–23140. [PubMed] [Google Scholar]
- Poon D., Bai,Y., Campbell,A.M., Bjorklund,S., Kim,Y.J., Zhou,S., Kornberg,R.D. and Weil,P.A. (1995) Identification and characterization of a TFIID-like multiprotein complex from Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 92, 8224–8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G. (1997) Saccharomyces cerevisiae BUR6 encodes a DRAP1/NC2alpha homolog that has both positive and negative roles in transcription in vivo. Mol. Cell. Biol., 17, 2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G. and Winston,F. (1993) Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics, 135, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh B.F. (2000) Control of gene expression through regulation of the TATA-binding protein. Gene, 255, 1–14. [DOI] [PubMed] [Google Scholar]
- Sanders S.L., Garbett,K.A. and Weil,P.A. (2002a) Molecular characterization of Saccharomyces cerevisiae TFIID. Mol. Cell. Biol., 22, 6000–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S.L., Jennings,J., Canutescu,A., Link,A.J. and Weil,P.A. (2002b) Proteomics of the eukaryotic transcription machinery: identification of proteins associated with components of yeast TFIID by multidimensional mass spectrometry. Mol. Cell. Biol., 22, 4723–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer V.L., Wobbe,C.R., Struhl,K. (1990) A wide variety of DNA sequences can functionally replace a yeast TATA element for transcriptional activation. Genes Dev., 4, 636–645. [DOI] [PubMed] [Google Scholar]
- Timmers H.T. and Sharp,P.A. (1991) The mammalian TFIID protein is present in two functionally distinct complexes. Genes Dev., 5, 1946–1956. [DOI] [PubMed] [Google Scholar]
- Timmers H.T., Meyers,R.E. and Sharp,P.A. (1992) Composition of transcription factor B-TFIID. Proc. Natl Acad. Sci. USA, 89, 8140–8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L. (2002) A unified nomenclature for TATA box binding protein (TBP)-associated factors (TAFs) involved in RNA polymerase II transcription. Genes Dev., 16, 673–675. [DOI] [PubMed] [Google Scholar]
- van der Knaap J.A., Borst,J.W., van der Vliet,P.C., Gentz,R. and Timmers,H.T. (1997) Cloning of the cDNA for the TATA-binding protein-associated factorII170 subunit of transcription factor B-TFIID reveals homology to global transcription regulators in yeast and Drosophila. Proc. Natl Acad. Sci. USA, 94, 11827–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap J.A., van den Boom,V., Kuipers,J., van Eijk,M.J., van der Vliet,P.C. and Timmers,H.T. (2000) The gene for human TATA-binding-protein-associated factor (TAFII) 170: structure, promoter and chromosomal localization. Biochem. J., 345, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade P.A. and Jaehning,J.A. (1996) Transcriptional corepression in vitro: a Mot1p-associated form of TATA-binding protein is required for repression by Leu3p. Mol. Cell. Biol., 16, 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobbe C.R. and Struhl,K. (1990) Yeast and human TATA-binding proteins have nearly identical DNA sequence requirements for transcription in vitro. Mol. Cell. Biol., 10, 3859–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]