Abstract
Voltage-dependent anion channels (VDACs) are pore-forming proteins (porins) that form the major pathway for movement of adenine nucleotides through the outer mitochondrial membrane. Electrophysiological studies indicate that VDAC-like channel activity is also prevalent in the cell membranes of many mammalian cells. However, the multitopological localization of porins outside the mitochondrion has remained an extremely controversial issue. Herein, we show that usage of two alternative first exons of the murine VDAC-1 gene leads to expression of two porins differing within their N termini. One porin (plasmalemmal VDAC-1) harboring a hydrophobic leader peptide is primarily targeted through the Golgi apparatus to the cell membrane. In contrast, the second isoform lacking the N-terminal leader (mitochondrial VDAC-1) is translocated more efficiently into the outer mitochondrial membrane. Thus, our data provide unique genetic evidence in favor of a multitopological localization of a mitochondrial porin.
Voltage-dependent anion channels (VDACs) represent a multigene family of evolutionarily conserved and well characterized pore-forming proteins (porins) found in the outer mitochondrial membranes of all eukaryotes. Traditionally, VDACs have been thought to be localized exclusively in the outer mitochondrial membrane (1, 2), where they control homeostasis by transport of ATP and ADP (3). However, several lines of evidence indicate the presence of VDACs in the cell membrane, including electrophysiological studies showing large-conductance anion channels with VDAC-like voltage sensitivity, single-channel conductance, and ionic selectivity in a variety of cell types (2, 4, 5).
Nevertheless, the multitopological nature of VDACs has remained controversial. Although subcellular localization of tagged proteins expressed in cells transiently transfected with human VDAC-1 and VDAC-2 cDNAs indicated exclusive translocation into mitochondria (5), the presence of VDAC-like channels has been detected recently in plasma membrane organelles, the caveolae (6). Thus, the molecular identity of the large-conductance, VDAC-like plasmalemmal anion channel reported in a number of different patch-clamp studies (7, 8) remained unclear. Because cell membrane targeting of proteins is frequently controlled by N-terminal leader sequences, we have investigated in detail the 5′ structures of the murine VDAC-1 gene. Herein, we show that the usage of two alternative first exons of the murine VDAC-1 gene leads to expression of two porins differing in their N termini. Positive evidence for plasmalemmal localization of VDAC was obtained by functional expression of both myc-tagged VDAC-1 isoforms. One porin (plasmalemmal VDAC-1 or pl-VDAC-1) harboring a signal sequence is primarily targeted through the endoplasmic reticulum (ER) to the Golgi apparatus into the cell membrane. In contrast, the second myc-tagged isoform (mitochondrial VDAC-1 or mt-VDAC-1) lacking the N-terminal leader is translocated more effectively into the mitochondrial membrane. An effective plasmalemmal translocation was evidenced further by patch-clamp studies, indicating a significant increase of large-conductance anion channels in the cell membrane of cells stably transfected with pl-VDAC-1.
Materials and Methods
Cloning of the VDAC-1 Gene.
Plaques of a λ FixII murine genomic library (n = 3 × 105; SVJ129, Stratagene) were screened with the entire cDNA probe encoding the bovine VDAC-1 gene (7). Three overlapping phage inserts spanning the entire VDAC-1 locus were isolated and mapped according to standard protocols (9).
Antibodies and Western Blot Analysis.
Western transfer of homogenized cells was done by semidry blotting of SDS/12.5% PAGE gels followed by antibody incubation with the c-myc monoclonal antibody (9E10, Calbiochem) in a dilution of 1:50. Rabbit anti-mouse IgG coupled to horseradish peroxidase was used as secondary antibody (Dako) in a dilution of 1:4,000.
Rapid Amplification of cDNA Ends–PCR (RACE-PCR) and Reverse Transcription–PCR (RT-PCR).
For RACE-PCR, a kit was used, following the manufacturer's instructions precisely (Marathon cDNA Amplification Kit, CLONTECH). The profile was as follows: 1 min at 55°C, 1 min at 72°C, and 1 min at 95°C. RACE products were subcloned into the plasmid pT7 and sequenced entirely on both strands (Applied Biosystems 373 sequencer). Locations of the following primers with respect to the murine VDAC-1 gene are shown in Fig. 1A: mt1, 5′-CCG CAG CCC CCG CCG TAG CTG-3′; mt2, 5′-TCG GAG GCG GTG ACG GCG GGA-3′; mt3, 5′-GCT GCT CCC GCC GTC ACC GCC-3′; pl1, 5′-ACC CAC ATC TGG ATG CCT GAG-3′; pl2, 5′-TGC CAC AAC AAA AGC ACG AGA-3′; pl3, 5′-TGT GTT CAT TCT TTC TCG TGC-3′; V1, 5′-GGT GAA GAC ATC CCT GGC AGA TTT G-3′; and V2, 5′-CCA GTG TTC GGC GAG AAT GAC-3′. Detailed protocols for RT-PCR have been described (9). Briefly, 1 μg of poly(A)+-selected mRNA from murine whole brain was reverse transcribed, and 35 cycles of RT-PCR were performed according to the following profile: 1 min at 65°C, 1 min at 72°C, and 1 min at 95°C.
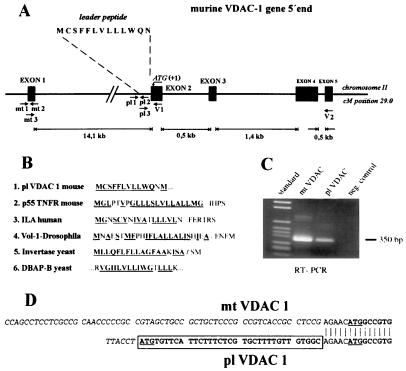
Figure 1.
Expression of alternative mRNAs encoding mt-VDAC-1 and pl-VDAC-1 porins. (A) Schematic representation of the 5′ genomic structure of the murine VDAC-1 gene. pl 1, pl 2, and pl 3 indicate location of primers used for RT-PCR and RACE-PCR. Indicated are the first four exons and their relative lengths. Exon 1 encodes 5′ untranslated RNA of mt-VDAC-1 mRNA. The ORF of mt-VDAC-1 starts 4 bases into the second exon (indicated as residue +1); the ORF of pl-VDAC-1 begins 39 bases 5′ adjacent to this point. kb, kilobase. (B) Comparison of hydrophobic signal leader sequences between pl-VDAC-1 and integral membrane proteins. EMBL data library or GenBank accession numbers are, for p55 tumor necrosis factor, X59238 (27); for activation-dependent T cell mRNA (ILA), L12964 (28); for Drosophila integrin α-subunit (Volado), AF034199 (29); for Saccharomyces cerevisiae SUC2 gene encoding invertase, K03294 (30); and for yeast dipeptidyl aminopeptidase B, X15484 (31). (C) PCR amplification of exons 1–5 of mt-VDAC-1 and of pl-VDAC-1. Primer pairs mt3/V2 and pl3/V2 are indicated in A. Bands of the expected size of 350 bp were amplified and sequenced. Negative control was performed with H2O instead of RNA. (D) Sequence of the longest RACE-PCR products obtained with primer V1. The ATG protein start codon of the previously known mt-VDAC-1 form is underlined; the hydrophobic leader peptide of pl-VDAC-1 is boxed.
Transfection Experiments.
Fully encoding cDNA fragments of pl-VDAC-1 and mt-VDAC-1 were amplified by PCR and ligated into the EcoRI cloning site of pSG5 (ref. 10; Stratagene). The pl-VDAC-1 construct harbored the exon 1 including the hydrophobic leader sequence, and the mt-VDAC-1 construct harbored the 5′ untranslated sequence of exon 1. Both constructs were modified by an 11-aa myc epitope introduced via the 3′ PCR primer. In addition, fully encoding cDNA fragments of pl-VDAC-1 and mt-VDAC-1 were amplified by PCR and were cloned in-frame into the EcoRI/BamHI cloning sites of pEGFP-N3 (CLONTECH). The pl-VDAC-1–GFP construct harbored the exon 1 including the hydrophobic leader sequence, and the mt-VDAC-1–GFP construct harbored the 5′ untranslated sequence of exon 1. Both constructs have a fusion protein, the green fluorescence protein (GFP), at their C termini. Transfections of HeLa and COS7 cells with the myc and GFP constructs were performed by using the Lipofectamine method according to the manufacturer's protocol (GIBCO).
Immunocytochemistry and Cytochemistry.
For immunocytochemistry of the myc transfectants, cells from subconfluent cultures were fixed after 48 h in ice-cold acetone for 20 min and stained by indirect immunofluorescence with a 1:100 dilution of the monoclonal c-myc antibody and a 1:100 dilution of FITC-conjugated sheep anti-mouse IgG (Dako) or a 1:200 dilution of Cy3-conjugated sheep anti-mouse IgG (Sigma). Visualization of mitochondria was performed by incubation of cells in 300 nM MitoTracker Green FM (Molecular Probes) at 37°C for 45 min followed by acetone fixation (11). Visualization of the Golgi was achieved by Bodipy FL C5-ceramide (Molecular Probes; ref. 12). Briefly, cells were incubated in 5 μM dye dissolved in DMEM at 2°C for 30 min, washed in DMEM, incubated at 37°C for 30 min, and finally fixed in ice-cold acetone and further processed for myc immunostaining. Both dyes were visualized in the green FITC channel, and the myc immunostaining was visualized in the red rhodamine channel with confocal laser-scanning microscopy (MRC 600, Bio-Rad).
Immunocytochemical labeling of the trafficking pathways of the GFP transfectants was obtained with the following antibodies. The ER was labeled by an antibody directed to calnexin (13); the Golgi was labeled by the clone 58K-9 (Sigma); and the mitochondria was labeled by a monoclonal antibody toward the subunit I of the complex IV of cytochrome c (Molecular Probes). Immunocytochemistry was performed as recently described (7).
Fluorescence-Activated Cell Sorting (FACS) Analysis.
For FACS, GFP- and pl-VDAC-1- or GFP- and mt-VDAC-1-cotransfected cells were incubated for 30 min in 10 mM EDTA, harvested by centrifugation, and resuspended in PBS. Then, the number of transiently transfected green fluorescent cells was counted at 515 nm by flow cytometry (FACScan, Becton Dickinson). The percentage of GFP-positive cells was quantitated after 48 h.
Northern Blot Analysis.
For Northern hybridization, 10 μg of poly(A)+-selected mRNA from murine whole brain was separated on 1.2% paraformaldehyde/agarose gels, blotted onto Nylon membranes, and hybridized according to standard protocols (14). Hybridization was performed in 50% (vol/vol) formamide at 43°C, and the final wash was in 0.1% SSC/0.01% SDS at 65°C for 30 min.
Electrophysiological Experiments.
A clonal PC12 parental cell line (termed G7) was cotransfected with the vectors containing mt-VDAC-1–myc or pl-VDAC-1–myc and with the vector containing the puromycin-resistance gene by using the Lipofectamine procedure (see above). At 48 h after transfection, the cells were split into 100-mm dishes with 9.0 ml of selection medium containing 2 μg/ml puromycin. After 30 days, individual puromycin-resistant subclones were isolated and grown for immunofluorescence and electrophysiological assays. Analysis of large-conductance anion channels was performed by using the patch-clamp technique with inside-out configuration. Pipettes were pulled from borosilicate glass capillaries (6–9 MΩ) and filled with solution containing 138 mM NaCl, 1.9 mM CaCl2, 1.8 mM glucose, and 5 mM Hepes (pH 7.4). Seal resistances were in the range of 2–8 GΩ. Current data obtained with an Axonpatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA) were low-pass filtered in the patch amplifier at 2 kHz and stored on a personal computer through an interface (Digidata 1200, Axon Instruments). Acquisition and analysis were performed by using pclamp6 software (Axon Instruments). Large-conductance anion channel behavior was characterized from excised patches under symmetrical conditions (NaCl solution in both pipette and bath) from channel recordings in response to depolarizing voltage pulses ranging from +45 to −45 mV. The membrane potential was held at 0 mV, then stepped to test holding potentials for 60 s, and then returned to 0 mV. Unitary conductance and open probabilities were calculated only for transitions between the closed and the open states of the channels (11).
Results and Discussion
Because cell membrane-targeting of proteins frequently is controlled by N-terminal leader sequences (see legend to Fig. 1B and references cited therein), we have investigated in detail the 5′ structure of the murine VDAC-1 gene. Three λ phages of a murine SVJ129 genomic library were isolated containing 30 kb of overlapping DNA fragments spanning the entire VDAC-1 gene. Fluorescence in situ hybridization with one of the phage inserts as a probe revealed specific signals on chromosome 8, indicating that we indeed isolated the genomic locus of VDAC-1 (data not shown). Nine exons were mapped at positions identical to those in a recent report (15). The first untranslated exon is located ≈14 kb 5′ adjacent to the second exon harboring the predicted protein start codon of the known VDAC-1 porin (referred to as nucleic acid residue +1 in Fig. 1A). However, closer inspection revealed a second putative in-frame protein start codon located immediately 5′ upstream from the second exon at position −39. Computer analysis predicted this frame to encode an N-terminal leader peptide harboring a central hydrophobic core characteristic for signal peptides found in many integral membrane proteins of yeast, insects, and mammals (see Fig. 1B and references cited therein). Thus, we speculated that two alternative proteins might be expressed from the VDAC-1 gene: one targeted by means of a N-terminal leader peptide into the cell membrane, hence referred to as pl-VDAC-1, and the other being the previously known mitochondrial form lacking a leader, hence referred to as mt-VDAC-1.
Three sets of experiments were performed to verify that two distinct, alternative mRNAs are transcribed. First, we used specific primers matching the 5′ ends of pl-VDAC-1 and mt-VDAC-1, respectively, and a third primer matching the sequences in the common fifth exon for RT-PCR amplification of the two messages (primer pairs mt3/V2 and pl3/V2 are shown in Fig. 1A). These primer pairs span several exon/intron borders and thus avoid amplification of contaminating genomic DNA. In both cases, the expected 350-bp cDNA fragments were amplified from murine whole-brain mRNA preparations and verified by sequencing (Fig. 1C). Secondly, we amplified the 5′ mRNA ends by RACE-PCR (9) by using the downstream primer V1 matching residues +31 to +54 of exon 2 (shown in Fig. 1A). The RACE-PCR products were subcloned, and the sequences of the longest RACE clones representing 5′ mRNA ends of mt-VDAC-1 and pl-VDAC-1, respectively, are shown in Fig. 1D. These data indicate that the mt-VDAC-1 message is being initiated at least 65 nucleic acid residues upstream from the translation start codon (designated residue +1 in Fig. 1A) and that the pl-VDAC-1 message initiates at least 5 bases upstream from the start codon of the hydrophobic leader. Finally, we hybridized Northern blots prepared from whole-brain mRNA with cDNA probes derived from the specific 5′ gene ends. These probes were amplified by PCR from genomic phage DNA by using primer pairs mt1/mt2 and pl1/pl2 (Fig. 1A). To avoid overlaying of signals similar in size, we probed two different Northern blots and checked RNA integrity and equal poly(A)+-selection efficiency by ethidium bromide staining of the gels and control hybridizations with a β-actin probe (Fig. 2A). Results shown in Fig. 2A reveal two different mRNAs both ≈2 kb in size. Quantitation of signal intensity on a PhosphorImager indicated that the mt-VDAC-1 mRNA is ≈5-fold more highly expressed in whole brain than the pl-VDAC-1 mRNA. Although not performed quantitatively, differences in signal intensities of mt-VDAC-1 and pl-VDAC-1 determined by using RT-PCR (Fig. 1C) and Northern blots (Fig. 2A) were in excellent agreement. Taken together, these data clearly prove that both predicted mRNAs encoding mt-VDAC-1 and pl-VDAC-1 are transcribed in murine brain.
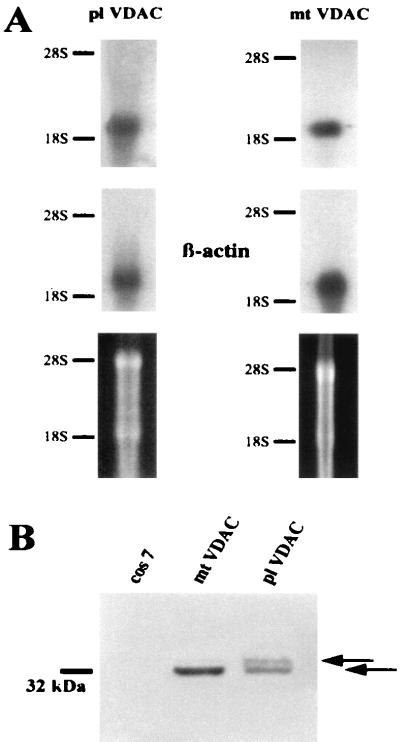
Figure 2.
Expression of mt-VDAC-1 and pl-VDAC-1 mRNA and proteins. (A) Northern blot of murine whole-brain mRNA hybridized with specific 5′ probes of pl-VDAC-1 and mt-VDAC-1 or with a β-actin probe as a control. The lower lane indicates ethidium bromide staining of the agarose gel used for Northern blotting. (B) Western blots of COS7 cells transiently transfected with expression constructs of mt-VDAC-1–myc and pl-VDAC-1–myc. The mt-VDAC-1–myc-transfected cells show a single band with the myc antibody at the expected position of 32 kDa; pl-VDAC-1–myc-transfected cells have two bands, a lower one in the range of the mt-VDAC-1 at 32 kDa and an upper one about 2 kDa above (arrows). The second band is considered to contain the unprocessed pl-VDAC-1–myc protein, because the difference in size matches exactly the length of the leader sequence.
Consistent with the presence of two mRNAs are Western blots from COS7 cells transiently transfected with expression constructs of both porins. Although mt-VDAC-1-transfected cells probed with the myc antibody showed a single band at the expected position of 32 kDa, pl-VDAC-1-transfected cells had two bands, a lower one in the range of the mt-VDAC-1 at 32 kDa and an upper band about 2 kDa higher. A reasonable explanation for this second band is that it contains the uncleaved pl-VDAC-1 protein, because the difference in size matches the length of the leader sequence of ≈2 kDa (13 aa; Fig. 2B).
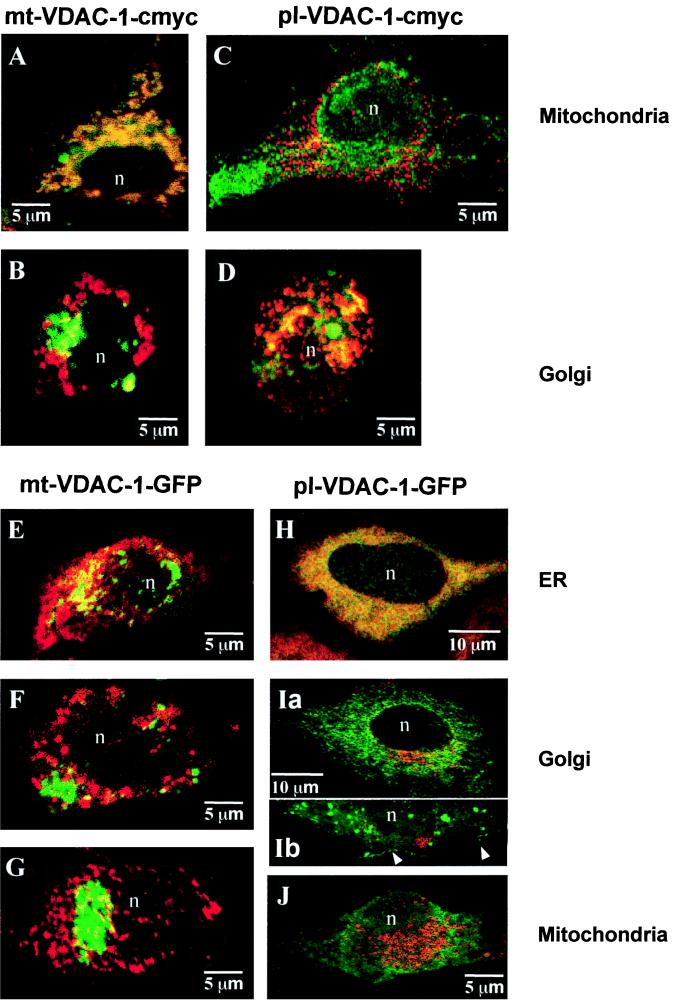
Next, we aimed to express the two predicted VDAC-1 porins and study their subcellular localization. Because the specificity of monoclonal anti-VDAC antibodies, which have been used previously for immunodetection of VDAC-1 on the cell surface (16), has been questioned recently (2), we constructed C-terminally myc-tagged and GFP-tagged expression plasmids of mt-VDAC-1 and pl-VDAC-1. An epitope specifically recognized by the monoclonal c-myc antibody 9E10 that causes little or no nonnuclear background staining was used. The myc-tagged as well as the GFP-tagged cDNAs were amplified by PCR, ligated into simian virus 40 promoter-driven expression plasmids (9), and resequenced in their entirety. In vitro translation of the myc-expression plasmids resulted in the expected peptides of ≈32 kDa for mt-VDAC-1–myc and 34 kDa for pl-VDAC-1–myc, respectively (data not shown). Transient transfections into COS cells and into the teratocarcinoma cell line PA-1 clone 9117 (17, 18) were performed. Myc-tagged mt-VDAC-1 and pl-VDAC-1 proteins were visualized in red with a Cy3-labeled sheep anti-mouse serum, and mitochondria or the Golgi vesicles were counterstained with dyes (12, 19) visible in the green FITC channel. Laser-scanning micrographs clearly revealed that the tagged mt-VDAC-1 porin largely overlapped with mitochondria resulting in orange fluorescence (Fig. 3A). In agreement with the study of Yu et al. (5), red fluorescent myc-tagged mt-VDAC-1 signals did not appreciably colocalize with the green Golgi signals (Fig. 3B). In contrast, pl-VDAC-1–myc staining to a large extent colocalized with the Golgi apparatus mainly in the perinuclear region (Fig. 3D) but did not show a mitochondrial-like staining pattern (Fig. 3C). Presumably because the Golgi-stain is quite specific and does not mark the secretory pathway in its entirety from the ER to the cell membrane, pl-VDAC-1–myc signals did not always completely overlap with the Golgi signal. In addition to the myc tag, we performed labeling of both constructs by using the GFP to identify the secretory pathways of the VDAC-1 proteins. HeLa cells and COS cells were transiently transfected with mt-VDAC-1–GFP and pl-VDAC-1–GFP, respectively. The GFP transfection allowed in vivo preselection of the cells on the level of translational efficiency by estimating the amount of green fluorescence. Only those cell clones that did not show toxic effects by the pl-VDAC-1–GFP construct (see below) were used for immunolabeling. Antibodies to different compartments were used for labeling subcellular structures (see Materials and Methods). The pl-VDAC-1–GFP transfection resulted in a complete green staining of the ER (Fig. 3H) as well as a labeling of the Golgi compartment (Fig. 3Ia). We regularly found green fluorescence in vesicular structures and staining of circumscribed cell membrane domains (Fig. 3Ib) indicative of a secretory targeting of pl-VDAC-1–GFP to the cell membrane. No overlap of green fluorescence was found with the mitochondrial marker (cytochrome c, subunit I, Fig. 3J), which is consistent with the idea of a preferred cell membrane insertion of the pl-VDAC-1–GFP. The mt-VDAC-1–GFP, however, did not show overlap with any of the markers (Fig. 3 E–G). Instead, green fluorescence occurred in the form of aggregates that resembled the recently described aggresomes (20) with respect to their cytoplasmic distribution. Because the myc-tagged mt-VDAC-1 was imported effectively into the mitochondrial membrane (Fig. 3A), a reasonable explanation for the mistargeting of the mt-VDAC-1–GFP is that the signal responsible for mitochondrial import is obscured by the large GFP tag. The entirely different subcellular distribution of both GFP-tagged isoforms can be taken as indirect proof that both isoforms travel along different pathways. From the electrophysiological data (see below), we conclude that at least some mt-VDAC-1–myc escapes into the cell membrane in the transfected cells, but we were unable to detect this escape by immunofluorescence.
Figure 3.
Immunolocalization of transfected mt-VDAC-1–myc and pl-VDAC-1–myc by laser-scanning microscopy. (A) Double fluorescence of mt-VDAC-1–myc staining (red) versus mitochondrial staining with MitoTracker Green FM (green). (B) Golgi staining with Bodipy FL C5-ceramide (green). Significant overlap of mt-VDAC-1–myc (red) immunoreactivity is evident with the mitochondrial stain (green) resulting in orange fluorescence (A), whereas no overlap of mt-VDAC-1–myc labeling (red) occurs with the Golgi marker, which shows a green signal (B). (C and D) pl-VDAC-1–myc immunoreactivity (red) versus mitochondrial staining (green; C) or Golgi staining (green; D). pl-VDAC-1–myc shows little overlap with the mitochondrial marker (C) and a typical perinuclear Golgi-like distribution with significant overlap with the Golgi marker (D). (E–G) mt-VDAC-1–GFP staining in transiently transfected cells and labeling with markers for the ER (E), Golgi (F), and mitochondria (G; all in red). mt-VDAC-1–GFP translation results in an accumulation of aggregates that do not significantly overlap with staining of the compartment markers. (H–J) pl-VDAC-1–GFP staining after transient transfection. The same order of markers was used as indicated for mt-VDAC-1–GFP. Complete overlap of pl-VDAC-1–GFP with the ER resulting in orange fluorescence (H). In Ia, partial staining of the Golgi together with the ER is evident, whereas Ib shows vesicular and cell membrane staining (arrows). In J, no overlap of the mitochondrial staining with the pl-VDAC-1–GFP fluorescence is evident. In all panels, n > 5.
The data above indicate that mt-VDAC-1 mRNA encodes a protein that is translocated primarily into the mitochondria and that the pl-VDAC-1 mRNA encodes a protein that is guided from the ER through the Golgi into the cell membrane.
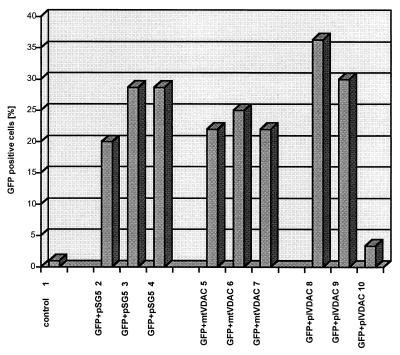
pl-VDAC-1-transfected cells frequently revealed swollen cytoplasm and clusters of clumped Golgi vesicles (data not shown). We therefore investigated whether high levels of pl-VDAC-1 expression exerted deleterious effects on cell viability. A GFP expression plasmid was cotransfected with a series of different amounts of mt-VDAC-1 and pl-VDAC-1 expression plasmids, and the percentage of viable, green-fluorescent cells after 48 h was determined by FACS analysis. Experiments were repeated in triplicate and indicated clearly that the survival of transfected cells decreased in response to increasing amounts of transfected pl-VDAC-1 expression plasmid (Fig. 4). A similar effect was not obtained by the mt-VDAC-1 expression plasmid.
Figure 4.
Cytotoxicity resulting from overexpression of pl-VDAC-1. The percentage of GFP-positive cells after 48 h was quantitated: bar 1, 250 ng of pSG5 expression plasmid without GFP expression cassette; bars 2–4, GFP + pSG5 at ratios of 1:1, 1:3, and 1:5, respectively; bars 5–7, GFP + mt-VDAC-1 at ratios of 1:1, 1:3, and 1:5, respectively; and bars 8–10, GFP + pl-VDAC-1 at ratios of 1:1, 1:3, and 1:5, respectively. A significant dominant-negative effect is evident with a ratio of 1:5 of GFP + pl-VDAC-1 (bar 10), which is not evident after transfection with GFP + mt-VDAC-1 (bar 7).
To obtain direct evidence that transfected pl-VDAC-1 constitutes VDAC-like channels in plasmalemma, we performed patch-clamp analyses of membranes excised from PC12 cells stably transfected with pl-VDAC-1 and mt-VDAC-1 myc-tagged expression plasmids. Untransfected PC12 cells (clone G7) revealed VDAC activity in 4% (2 of 57) of sampled patches. Of the 23 puromycin-resistant subclones transfected with pl-VDAC-1, 15 were immunopositive for myc, and of the 19 clones transfected with mt-VDAC-1, 7 showed immunoreactivity with myc antibodies after immunofluorescence. Electrophysiological recordings were performed on six of the immunopositive clones for pl-VDAC-1 and on six of the immunopositive clones for mt-VDAC-1. Large-conductance anion channel activity was present in 18% (29 of 160) and in 17% (8 of 46) of these membrane patches, respectively. Similar to the behavior of VDAC (11), the conductance of the closed state of the channels recorded in transfectants was about half the conductance of the fully open channels. Because virtually no transitions were recorded between the closed state and the zero current level, analyses were limited to transitions between the closed and fully open states.
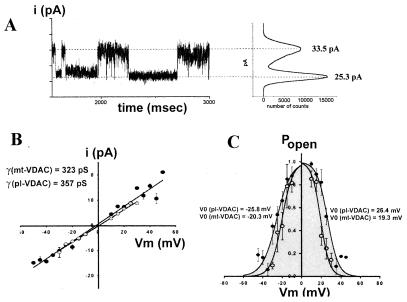
An example of channel activity recorded in such active patches is shown in Fig. 5. Fig. 5A Left shows activity of single large-conductance anion channels in an excised inside-out membrane patch, and Fig. 5A Right shows an all-point histogram indicating conductance of the open state and closed state. In this example of a recording where four channels were present, only one channel opening is shown. Unitary current–voltage (i–V) relationships constructed for both pl-VDAC-1–myc and mt-VDAC-1–myc PC12 transfectants exposed to symmetrical NaCl solution (Fig. 5B) were found to be linear, with slope conductances of 357 pS (pl-VDAC-1) and of 323 pS (mt-VDAC-1). The mean value of single-channel conductance measured from the open to the closed state for all patches at all voltages was 367 ± 16 pS and 321 ± 9 pS for pl-VDAC-1 and mt-VDAC-1, respectively. These values are exactly in the range of the unitary conductance of VDACs described recently (7, 11). The presence of VDAC-like membrane activity after mt-VDAC-1 transfection seems less surprising if one considers the background of expression from which the clones were selected. Because pl-VDAC-1–myc has a dose-dependent negative-dominant effect in transfected cells (see above), selection was biased toward low-level expressers; however, mt-VDAC-1–myc transfection did not show this effect, and selection was more random including overexpressing clones. It is most likely that targeting is not absolute in an overexpressing system, as has been shown for other integral membrane proteins (21), allowing mt-VDAC-1 to be inserted into the cell membrane as well.
Figure 5.
Electrophysiological characteristics of large-conductance anion channels recorded from PC12 cells transfected with two VDAC-1 cDNAs. (A) Current fluctuations in an excised, inside-out membrane patch from PC12 cells transfected with mt-VDAC-1–myc cDNA at a holding potential of −25 mV by using symmetrical (138 mM NaCl) internal and external solution (Left). i, current. The all-points histogram (Right) constructed for the entire recording duration, which is shown, in part, at Left, shows two peaks; the peak at 25.3 pA represents basal current with all the channels closed, and the other, at 33.5 pA, shows the main open state of one single channel. (B) Current versus voltage (i–V) relationship obtained for single channels from excised, inside-out patches of PC12 cells transfected with cDNAs encoding mt-VDAC-1 (open circles) and pl-VDAC-1 (closed circles). Each point in the graph is the mean current amplitude between the open and the closed state at a given potential fitted by a Gaussian distribution with pclamp6 software. (C) Voltage dependence of the probability of the channel being in the open state (Popen) obtained for pl-VDAC-1 (closed circles) and mt-VDAC-1 (open circles) transfectants. Popen was calculated from the time the channel spent in the open state compared with the total time (1 min) of the recording at a given voltage. The curves were fitted as the sum of two Boltzmann equations of the form Popen = 1/{1 + e[(Vm−V0)/A]}, where Popen is the probability of finding the channel in the open state at a giving holding potential (Vm), A is the slope factor corresponding to the voltage-sensitivity of the activation, and V0 is the voltage producing half-maximal open probability. The fitted values of V0 are shown in the graph.
Thus, our transfection experiments provide clear evidence of a translocation of pl-VDAC-1 into the cell membrane, indicative of the existence of a targeting pathway of this porin into the cell membrane of eukaryotic cells. In all likelihood, the hydrophobic leader sequence can be regarded to be responsible for this targeting process. The occurrence of plasmalemmal channels with the typical biophysical profile of mt-VDACs after stable transfection with the pl-VDAC-1 construct supports the idea that the VDAC-like activity found in a number of different cell membranes (7, 22–25) relies on the presence of channels of the VDAC type. The large-conductance anion selective channels obtained by pl-VDAC-1 transfection are strikingly similar to the VDACs of the outer mitochondrial membrane. These similarities include large main-state unitary conductance, a closed state with about half of the fully open channel size, and the rather symmetric voltage sensitivity of open probability. A feasible argument against this interpretation is an artifactual insertion of pl-VDAC-1 into the cell membrane in a system driven by strong promoters, as is the case for the simian virus 40 promoter. This argument is unlikely for the following reasons. Artifactual insertion by overexpression is expected to generate a more or less stochastic translocation of the products into both the cell membrane and the outer mitochondrial membrane regardless of the translated splice form. In our case, however, we consistently found two significantly variant pathways for the plasmalemmal and the mitochondrial isoforms. Although pl-VDAC-1 was clearly trafficking through the Golgi, the mt-VDAC-1 was never found in the Golgi but accumulated in the mitochondria. Such a consistency in trafficking is likely to be the consequence of a directed transport rather than a stochastic process. The toxic effect observed for the pl-VDAC-1 also speaks in favor of a directed process. Although this effect was clearly dose dependent (Fig. 4), indicating that overexpression of pl-VDAC-1 is deleterious for the transfected cells, a similar effect was not observed after mt-VDAC-1 expression. A reasonable explanation for this difference is that the pl-VDAC is inserted much more effectively into the cell membrane, leading to irreversible changes in membrane permeability as is expected for this large-conductance channel. One could speculate that the regulative capacity of the cell, which normally keeps this channel in a closed state (26), is exhausted when excessive amounts of pl-VDAC-1 are incorporated in the cell membrane.
Taken together, our data provide clear molecular and functional evidence for the multitopological nature of VDAC-1 channel porins and thus settle an important issue of the nature and origin of large-conductance anion channels in the cell membrane that has remained controversial for many years.
Acknowledgments
We are grateful to Dr. Y. Gao, who evaluated the stably transfected VDAC clones through immunostaining, and Dr. A. Andrade-Rozental and Dr. S. Suadicani, who participated in the establishment of the stably transfected cell lines. This work was supported by Deutsche Forschungsgemeinschaft Grants Bu672/2-3 to R.B. and SSP Glia to R.D., by National Institutes of Health Grants NS07512 and NS34931 to D.C.S., and by Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo Grant 94/4378-5 to E.S.
Abbreviations
- VDAC
voltage-dependent anion channel
- ER
endoplasmic reticulum
- pl
plasmalemmal
- mt
mitochondrial
- RACE
rapid amplification of cDNA ends
- RT-PCR
reverse transcription–PCR
- kb
kilobase
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060242297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060242297
References
- 1.Hodge T, Colombini M. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 2.Yu W-H, Forte M. J Bioenerg Biomembr. 1996;28:93–100. doi: 10.1007/BF02110638. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtseva T, Colombini M. Biophys J. 1997;72:1954–1960. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayser H, Kratzin H-D, Thinnes F-P, Gotz H, Schmidt W-E, Eckart K, Hilschmann N. Biol Chem Hoppe-Seyler. 1989;370:1253–1264. [PubMed] [Google Scholar]
- 5.Yu W-H, Wolfgang W, Forte M. J Biol Chem. 1995;270:13998–14006. doi: 10.1074/jbc.270.23.13998. [DOI] [PubMed] [Google Scholar]
- 6.Bathori G, Parolini I, Tombola F, Szabo I, Messina A, Oliva M, De Pinto V, Lisanti M, Sargiacomo M, Zoratti M. J Biol Chem. 1999;274:29607–29612. doi: 10.1074/jbc.274.42.29607. [DOI] [PubMed] [Google Scholar]
- 7.Dermietzel R, Hwang T-K, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes F-P, Fishman G-I, Spray D-C. Proc Natl Acad Sci USA. 1994;91:499–503. doi: 10.1073/pnas.91.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thinnes F-P. J Bioenerg Biomembr. 1992;24:71–75. doi: 10.1007/BF00769533. [DOI] [PubMed] [Google Scholar]
- 9.Bosserhoff A-K, Hein R, Bogdahn U, Buettner R. J Biol Chem. 1996;271:490–495. doi: 10.1074/jbc.271.1.490. [DOI] [PubMed] [Google Scholar]
- 10.Umesono K, Murakami K-K, Thompson C-C, Evans R-M. Cell. 1991;28:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombini M. J Memb Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- 12.Pagano R-E, Martin O-C, Kang H-C, Haugland R-P. J Cell Biol. 1991;113:1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doms R-W, Helenius A, White J. J Biol Chem. 1985;260:2973–2981. [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 15.Sampson M-J, Lovell R-S, Craigen W-J. J Biol Chem. 1997;272:18966–18973. doi: 10.1074/jbc.272.30.18966. [DOI] [PubMed] [Google Scholar]
- 16.Reymann S, Florke H, Heiden M, Jakob C, Stadtmuller U, Steinacker P, Lalk V-E, Pardowitz I, Thinnes F-P. Biochem Mol Med. 1995;54:75–87. doi: 10.1006/bmme.1995.1011. [DOI] [PubMed] [Google Scholar]
- 17.Munro S, Pelham H-R-B. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 18.Tainsky M-A, Yim S-O, Krizman D-B, Kannan P, Chiao P-J, Mukhopadhyay T, Buettner R. Oncogene. 1991;6:1575–1582. [PubMed] [Google Scholar]
- 19.Whitaker J-E, Moore P-L, Haugland R-P, Haugland R-P. Biochem Biophys Res Commun. 1991;175:387–393. doi: 10.1016/0006-291x(91)91576-x. [DOI] [PubMed] [Google Scholar]
- 20.Johnston J-A, Ward C-L, Kopito R-R. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N-M, Friend D-S, Gilula N-B. J Cell Sci. 1995;108:3725–3734. doi: 10.1242/jcs.108.12.3725. [DOI] [PubMed] [Google Scholar]
- 22.Blatz A-L, Magleby K-L. Biophys J. 1983;43:237–241. doi: 10.1016/S0006-3495(83)84344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarze W, Kolb H-A. Pflügers Arch. 1984;402:281–291. doi: 10.1007/BF00585511. [DOI] [PubMed] [Google Scholar]
- 24.Orkand P-M, Blanco R, Marrero H, Orkand R-K. Ann NY Acad Sci. 1991;633:586–588. doi: 10.1111/j.1749-6632.1991.tb15670.x. [DOI] [PubMed] [Google Scholar]
- 25.Jalonen T. Glia. 1993;9:227–237. doi: 10.1002/glia.440090308. [DOI] [PubMed] [Google Scholar]
- 26.Mangan P-S, Colombini M. Proc Natl Acad Sci USA. 1987;84:4896–4899. doi: 10.1073/pnas.84.14.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett K, Taylor-Fishwick D-A, Cope A-P, Kissonerghis A-M, Gray P-W, Feldmann M, Foxwell B-M. Eur J Immunol. 1991;21:1649–1656. doi: 10.1002/eji.1830210710. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz H, Tuckwell J, Lotz M. Gene. 1993;134:295–298. doi: 10.1016/0378-1119(93)90110-o. [DOI] [PubMed] [Google Scholar]
- 29.Grotewiel M-S, Beck C-D-O, Wu K-H, Zhu X-R, Davis R-L. Nature (London) 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- 30.Sarokin L, Carlson M. Mol Cell Biol. 1984;4:2750–2757. doi: 10.1128/mcb.4.12.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts C-J, Pohlig G, Rothman J-H, Stevens T-H. J Cell Biol. 1989;108:1363–1373. doi: 10.1083/jcb.108.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]