Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. Kim Kyun-Hwan, Seong Baik L. The EMBO Journal. 2003;22:2104–2116. doi: 10.1093/emboj/cdg210.
In the above paper, there was a printing error in Figure 1C. The correct figure is reproduced below. The publishers would like to apologize for the error and any confusion caused.
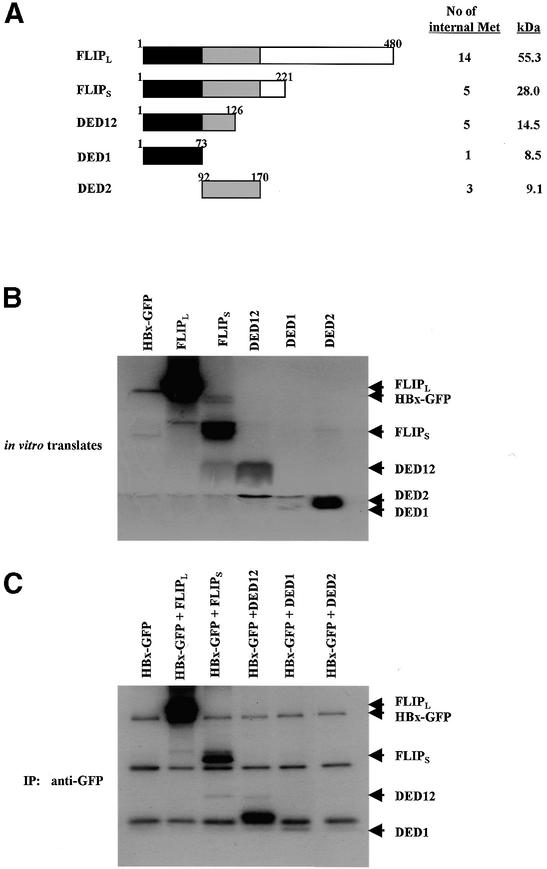
Fig. 7. The N-terminal domain of c-FLIP (DED1) is crucial for association with HBx. (A) Schematic representation of full-length and truncated c-FLIP, along with a number of internal methionines. (B) The same amounts of in vitro translated c-FLIP deletion mutants were analyzed by SDS–PAGE. Note that the expression levels of DED12 and DED1 are very low compared with that of DED2. (C) The 1:1 mixture of in vitro translated HBx–GFP and c-FLIP deletion mutants was incubated for 1 h, and immunoprecipitated with anti-GFP antibody. The immune complex was analyzed by 15% SDS–PAGE, and autoradiographed. These autoradiograms are representative data from two independent binding assays.