Abstract
Cultures of plastic-adherent cells from bone marrow have attracted interest because of their ability to support growth of hematopoietic stem cells, their multipotentiality for differentiation, and their possible use for cell and gene therapy. Here we found that the cells grew most rapidly when they were initially plated at low densities (1.5 or 3.0 cells/cm2) to generate single-cell derived colonies. The cultures displayed a lag phase of about 5 days, a log phase of rapid growth of about 5 days, and then a stationary phase. FACS analysis demonstrated that stationary cultures contained a major population of large and moderately granular cells and a minor population of small and agranular cells here referred to as recycling stem cells or RS-1 cells. During the lag phase, the RS-1 cells gave rise to a new population of small and densely granular cells (RS-2 cells). During the late log phase, the RS-2 cells decreased in number and regenerated the pool of RS-1 cells found in stationary cultures. In repeated passages in which the cells were plated at low density, they were amplified about 109-fold in 6 wk. The cells retained their ability to generate single-cell derived colonies and therefore apparently retained their multipotentiality for differentiation.
Keywords: marrow stromal cells
In addition to stem cells for hematopoietic cells, bone marrow contains stem-like cells that are precursors of nonhematopoietic tissues (1–9). The precursors of nonhematopoietic tissues were initially referred to as plastic-adherent cells or colony-forming-unit fibroblasts (1, 5, 7) and subsequently as either mesenchymal stem cells (8) or marrow stromal cells (MSCs). The cells have attracted interest because of their ability to serve as a feeder layer for the growth of hematopoietic stem cells, their multipotentiality for differentiation, and their possible use for both cell and gene therapy (see refs. 8–11).
Friedenstein et al. (1) initially isolated MSCs by their adherence to tissue culture surfaces, and essentially the same protocol was used by many subsequent investigators (see refs. 2–5,7, 8, and 12–21). The isolated cells were shown to be multipotential in that they differentiated in culture or after implantation in vivo into osteoblasts, chondrocytes, adipocytes, and myotubes. After systemic infusion of MSCs, progeny of the cells appeared in a variety of tissues, including bone (22–25), cartilage (22, 23), lung (22, 23, 26), spleen (26), and thymus (26). Also, engraftment of donor MSCs into repairing muscle was observed after either local injection or systemic injection (27). Engraftment into muscle of a dystrophin-deficient mouse was seen after systemic infusion of rare marrow cells defined as a “side population” (SP) or SP cells that may be precursors of MSCs (28, 29).
In addition, MSCs or related cells were also shown to engraft into the central nervous system. The presence of donor-derived astrocytes was observed after systemic infusion of whole marrow into immunodeficient mice (30). After direct infusion into basal ganglia of rats, either rat or human MSCs integrated and migrated in a manner similar to paraventricular astrocytes that have many of the properties of neural stem cells (31). After mouse MSCs were infused into the paraventricular region of newborn mice, some of the cells differentiated into astrocytes (32). Others appeared in large numbers in neuron-rich regions and probably differentiated into neurons (32). The potential for interconvertibility of cells between bone marrow and the central nervous system was also emphasized by the observation (33) that neural stem cells can reconstitute the hematopoietic system in mice that have undergone marrow ablation.
Despite the great interest in MSCs, there is still no well-defined protocol for isolation and expansion of the cells in culture. Most experiments have been carried out with cultures of MSCs that are isolated primarily by their tight adherence to tissue culture dishes as described by Friedenstein et al. (1, 6). Several groups of investigators developed protocols to prepare more homogeneous populations (34–40), but none of these protocols has gained wide acceptance.
In the present report, we have identified a subpopulation of cells in cultures of MSCs that are small, proliferate rapidly, undergo cyclical renewal when the cells are replated, and are precursors of more mature cells in the same cultures. Therefore, we have referred to the cells as recycling stem (RS) cells. In addition, we have defined conditions under which RS cells can be amplified about 109-fold in 6 wk.
Materials and Methods
Isolation and Culture of Human MSCs (hMSCs).
hMSCs were obtained from 20-ml aspirates from the iliac crest of normal donors ranging in age from 19 to 49 yr (41). With several donors, duplicate samples were obtained on the same occasion from the right and left iliac crest. Each 20 ml of aspirate was diluted 1:1 with Hanks' balanced salt solution (HBSS; GIBCO) and layered over about 10 ml of ficoll (Ficoll-Paque; Pharmacia). After centrifugation at 2,500 × g for 30 min, the mononuclear cell layer was removed from the interface and suspended in HBSS. Cells were centrifuged at 1,500 × g for 15 min and resuspended in complete medium (MEM, α medium without deoxyribonucleotides or ribonucleotides; GIBCO); 20% FCS lot selected for rapid growth of MSCs (Atlanta Biologicals, Norcross, GA); 100 units/ml penicillin (GIBCO), 100 μg/ml streptomycin (GIBCO); and 2 mM l-glutamine (GIBCO). All of the cells were plated in about 25 ml of medium in a 176-cm2 culture dish (Nunc) and incubated at 37°C with 5% humidified CO2. After 24 h, nonadherent cells were discarded, and the adherent cells were thoroughly washed twice with PBS. Fresh complete medium was added and replaced every 3 or 4 days for about 14 days. The cells were harvested with 0.25% trypsin and 1 mM EDTA (GIBCO) for 5 min at 37°C, replated in a 79-cm2 plate, again cultured for about 14 days, and harvested. About 8 ml of complete medium was mixed with the trypsinized cells to inactivate the trypsin, and the cells were counted with an automated instrument (Coulter) or a hemacytometer. The cells were recovered by centrifugation and suspended at a concentration of 1 to 2 × 106 cells per ml in 5% DMSO and 30% FCS. Aliquots of about 1 ml each were slowly frozen and stored in liquid nitrogen. One or two aliquots of each frozen stock was assayed (41) for colony-forming units (cfus). The values for cfus per 100 cells ranged from 12 to 42. To grow cultures at varying cell densities, a frozen stock of MSCs was thawed at 37°C, diluted with complete medium, and recovered by centrifugation to remove the DMSO. The cells were suspended in medium and plated at about 5,000 cells/cm2. After incubation for about 1 day, nonadherent cells were removed, the adherent cells were harvested with EDTA/trypsin, dissociated by passage through a narrowed Pasteur pipette, counted, and replated at the densities indicated in 10 ml medium on 79-cm2 plates.
FACS Analysis.
Cultured MSCs were detached with EDTA/trypsin, suspended in 0.5 ml PBS at concentrations of 20,000 to 100,000 per ml and assayed in a flow cytometer (FACsort; Becton Dickinson). To identify surface epitopes, large samples were prepared by plating about 18,000 MSCs at a density of three cells/cm2 in 1 liter of medium in an interconnecting system of tissue culture flasks (6,000 cm2; Cell Factory, Nunc). The cells were cultured for 6 days and then harvested with EDTA/trypsin. The cells were washed by centrifugation in PBS and resuspended in PBS at a concentration of about 100,000 cells/ml. The cells were fixed in 1% methanol or acetone at 4°C for 10 min and washed with PBS. Nonspecific antigens were blocked by incubating the cells at room temperature for 1 h in 1% BSA, 0.1% FCS, and 0.1% goat serum. The cells were washed by centrifugation in three volumes of PBS, and the cell pellet was suspended in 0.5 ml of a primary antibody solution containing 20 μg/ml of antibody, 1% BSA, and 0.1% goat serum. After incubation for 40 min at 4°C, the cells were washed in PBS. The primary antibodies were mouse anti-human, obtained from the following sources: CD90 (Chemicon); CD117 (Chemicon); CD11b (FITC-labeled; Chemicon); CD38 (Chemicon); Stro-1 (IgM Hybridoma Bank at the University of Iowa); CD45 (PharMingen); CD31 (Biomeda); CD34 and CD43 (Santa Cruz Biotechnology). For an isotype control, nonspecific mouse Ig (DAKO) was substituted for the primary antibody. For antibodies that required a second antibody for detection, the cell pellet was incubated under the same conditions for 20 min with antimouse IgG labeled with FITC (Santa Cruz). The cells were then washed in PBS and suspended in 1 ml of PBS for FACS analysis.
Results
Effect of Plating Density on Expansion of MSCs.
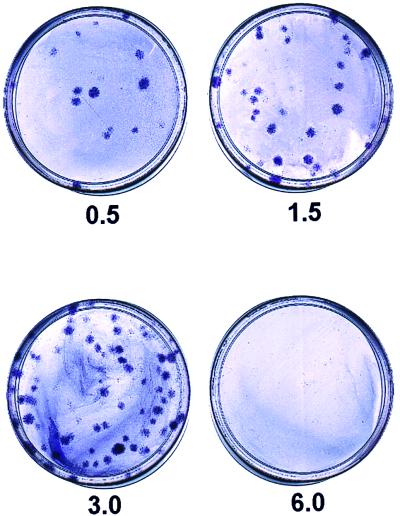
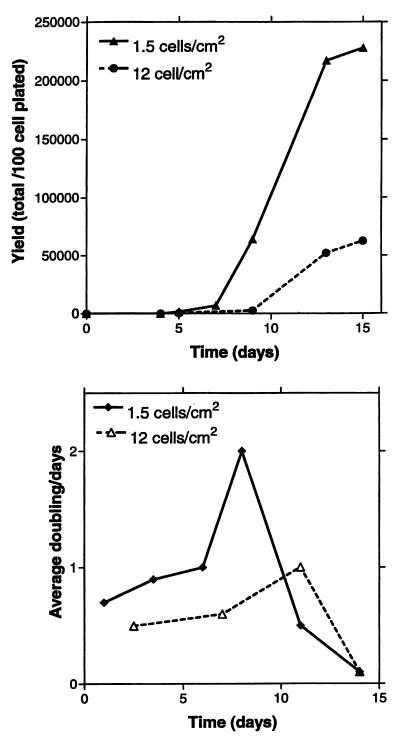
As noted in previous reports (17), human MSCs form single-cell derived colonies when plated at low density so as to form single-cell-derived colonies. Here, we found that the number of colonies formed per 100 cells plated remained constant when we varied the density of plating from 0.5 to 12 cells/cm2. However, we noticed that the size of the colonies decreased markedly when the cells were plated at the higher densities. Colonies of maximal size were obtained when cells were plated at 1.5 or 3.0 cells/cm2 with MSCs from most stock samples of bone marrow aspirates (Fig. 1). The colonies were smaller when plated at 6 or 12 cells/cm2. The colony size was not affected by exchanging the medium every other day or after 10 days (not shown). However, the yield of cells decreased as the size of the colonies decreased. When cells were plated at a low density of 1.5 or 3.0 cells/cm2, the cells expanded up to 2,000-fold over about 10 days (Fig. 2A). In contrast, cells plated at 12 cells/cm2 expanded only about 60-fold. When plated at low density, the cells typically exhibited three phases of growth: a lag phase, a log phase of rapid growth, and then a stationary phase (Fig. 2A). During the log phase, the cells plated at low density doubled an average of about twice per 24 h, i.e., doubling times of about 12 h (Fig. 2B). When cells were plated at 6 cells/cm2 instead of 1.5 cm2, the most rapid growth was at day 6 instead of day 8 (not shown). If cells were plated at 12 cells/cm2, the doubling time was 24 h or greater throughout the culture period (Fig. 2B).
Figure 1.
Size of colonies observed after plating of MSCs at 0.5, 1.5, 3.0, or 6.0 cells/cm2. After incubation for 14 days in 79-cm2 plates, the colonies were stained with 0.5% Crystal violet in methanol for 5–10 min at room temperature. Colonies from cells plated at 6 cells/cm2 are too small to be seen in the photograph.
Figure 2.
Effect of plating density on yield of cells, and average doubling times. The cells were plated onto 79-cm2 plates, and yields of cells on days indicated were assayed with a hemocytometer. (A) Yield of cells per 100 cells plated with initial plating densities of 1.5 and 12.0 cells/cm2. (B) Average cell doublings per day when MSCs were plated at 1.5 and 12.0 cells/cm2. Values are 3-day averages from data presented in A.
Identification of a Subpopulation of Progenitors.
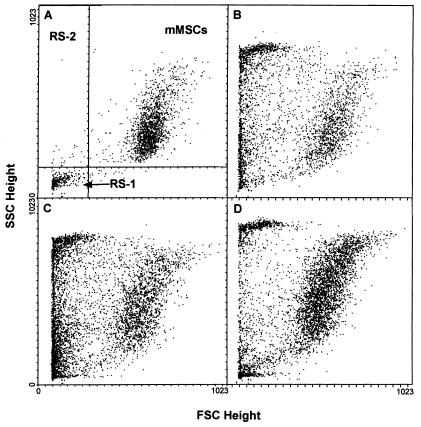
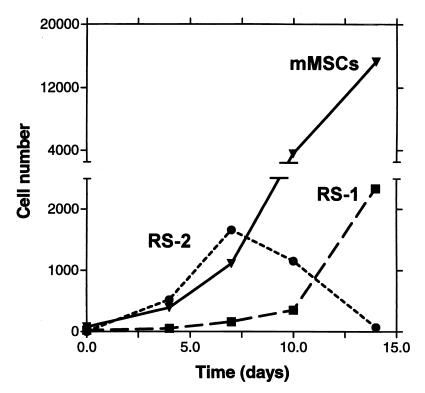
Cells from stationary cultures of MSCs were assayed for size and granularity by forward light and side light scattering by FACS. Two populations of cells were seen in stationary cultures (Fig. 3A). Most of the cells were large and had a medium content of granules. However, there was a minor population of small and agranular cells. Staining with propidium iodide demonstrated that about 98% of the cells in both populations were viable. Staining with the cell-cycle specific antigen Ki-67 indicated that the small, agranular cells were not in cell cycle, whereas the larger cells were in cell cycle (not shown). A similar pattern of large and small cells was observed with cultures plated at 12 cells/cm2 and examined after 5 days (not shown). However, a different pattern was seen with cultures that were plated at 1.5 or 3.0 cells/cm2 and examined at 5 days. About 13% of the cells were small and agranular cells, but a new subpopulation of small and granular cells accounted for about 30% of the total population (Fig. 3B). Staining with Ki-67 demonstrated that the small and granular cells were in cell cycle (not shown). For convenience, we refer here to the small and agranular cells as RS-1, the small and granular cells as RS-2, and the large and moderately granular cells as mature MSCs (mMSCs). Graphing of the data from the experiment shown in Fig. 3 indicated that RS-2 cells first appeared and expanded during the lag period (Fig. 4). During the log phase of growth, the RS-2 cells decreased in number and the mMSCs rapidly expanded. During the late log phase, the RS-2 cells disappeared, and the RS-1 cells expanded.
Figure 3.
FACS analysis of cells for size and granularity after different periods of incubation. Preliminary experiments indicated that the precursor–product relationships among the cell populations were more apparent in cultures that grew slowly (not shown). Therefore, the stock sample of MSCs selected for the experiment was previously shown to expand slowly in culture and have an unusually low cfu value of about 12% (see ref. 41). The cells were plated at a suboptimal density of 3 cells/cm2 that was equivalent to 1.0 cell/cm2 or less for samples that expanded more rapidly and had higher cfu values (see Fig. 1). (A) Analysis of cells in stationary culture at day 14. Lines indicate gating used to define RS-1 cells, RS-2 cells, and mMSCs. (B) Analysis on day 5 after initial plating. (C) Analysis on day 7 after initial plating. (D) Analysis on day 10 after initial plating. FSC, forward scattering of light that assays cell size; SSC, side scattering of light that assays cell granularity. Values in arbitrary units.
Figure 4.
Time course of number of cells observed after initial plating of cells. Values are taken from the data presented in Fig. 3, adjusted for the total number of cells in the cultures.
Correlation Between RS Cells and cfus.
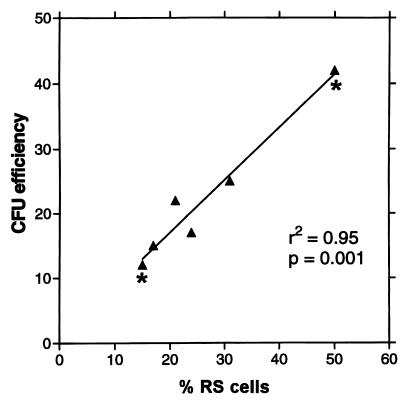
The data in Figs. 3 and 4 suggest that the earliest progenitors in the cultures are RS-1 and RS-2 cells. Therefore, the number of RS-1 and RS-2 cells in any sample of MSCs should reflect the number of cells that generate single-cell derived colonies in cfu assays. As indicated in Fig. 5, a linear relationship was observed between the number of RS cells and cfu values obtained for a series of samples (r2 = 0.95; P value = 0.001). Moreover, the slope of the plot was 0.82 ± 0.96 SD. Therefore, the results indicated that each RS cell formed a colony with an average efficiency of about 82%. As also indicated in Fig. 5, the same relationship between cfus and total RS cells (RS-1 plus RS-2) was found with samples from the same culture at different times after plating. For example, one culture was assayed on day 5 and day 12. On day 5, about 48 of every 100 cells was an RS cell and about 42 colonies were formed. On day 12, about 12 cells per 100 were RS cells and about 12 colonies were formed. Because most of the RS cells on day 5 were RS-2 cells and most of the RS cells on day 12 were RS-1 cells, the results indicated that both populations generated single-cell derived colonies.
Figure 5.
Proportionality between percent of RS cells and percent of cfus in samples of MSCs. ▴, samples from cultures that were plated at varying density and incubated for 14 days; *, cultures from the same initial sample of MSCs incubated for 5 days (higher value) or for 14 days (lower value) after plating at 3 cells/cm2. % RS, total number of RS-1 and RS-2 cells in the samples; cfu efficiency, colonies per 100 cells plated.
Epitope Profile of the Cell Cultures.
All of the cells in the cultures of MSCs were consistently negative for CD34, a marker for early hematopoietic stem cells (Table 1). They were also negative for other markers for hematopoietic cells (CD11B, CD43, CD45). A small number (less than 10%) of all three cell types were dimly positive for the endothelial cell marker CD31. Also, a small number were dimly positive for CD38, a marker for B lymphocytes, and some thymocytes, T lymphocytes, NK cells, and macrophages. The mMSCs were dimly positive for the hematopoietic stem cell marker CD117 (c-Kit), but the RS-1 and RS-2 cells were negative. As reported previously (41), the mMSCs were moderately positive for Stro-1, an epitope for osteogenic MSCs (35). However, RS-1 and RS-2 cells were negative for Stro-1. One marked difference in the epitopes among the three subpopulations of cells was that the mMSCs were positive for CD90 (Thy-1), a marker for thymocytes and peripheral T lymphocytes. RS-1 cells were dimly positive, but the RS-2 cells were negative.
Table 1.
Epitope profile of the cells
| Epitope | RS-1 cells | RS-2 cells | mMSCs cells |
|---|---|---|---|
| CD34 | Negative | Negative | Negative |
| CD11B (Mac-1) | Negative | Negative | Negative |
| CD43 | Negative | Negative | Negative |
| CD45 | Negative | Negative | Negative |
| CD31 | Dim | Dim | Dim |
| CD38 | Dim | Dim | Dim |
| CD117 (c-Kit) | Negative | Negative | Dim |
| STRO-1 | Negative | Negative | Dim |
| CD90 (Thy-1) | Dim | Negative | Positive |
Extensive Expansion of the MSCs by Plating at Low Density.
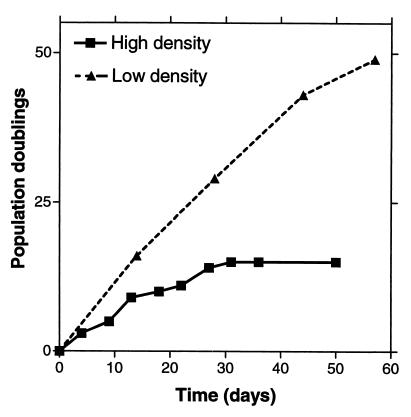
Because plating of MSCs at low density promoted rapid growth of the cultures (Figs. 1 and 2), we examined the extent to which the cells could be expanded in culture by repeated passage at low density. In one experiment, the cells were plated at low density, a large colony was isolated from the plate with a cloning ring, and the cells in the colony replated at low density. The procedure was repeated so that only the cells from the largest colonies were passed. The cells continued to grow through about 50 population doublings (Fig. 6). In contrast, when a similar sample of MSCs was passed at high density, the cells stopped growing after about 15 doublings.
Figure 6.
Population doublings obtained when cells were plated at low density and high density. ▴, cells that were plated at 1.5 cells/cm2, incubated for 10 to 14 days; a large colony was then isolated by ring cloning for each subsequent passage under the same conditions; ■, MSCs from a sample with a comparable value for cfus that was passed by plating at a high density of 5,000 cells/cm2 (see ref. 41).
In a second series of experiments, several samples of MSCs were passed at low density with a more conventional protocol in which all of the cells from a culture were harvested with EDTA/trypsin and an aliquot was replated at 3.0 cells/cm2 for the next passage. The procedure was repeated for a total of three passages. The fold expansion of the cells varied from about 150-fold to over 2,000-fold during single passages of 14 days (Table 2). With the four separate samples from two donors, the average fold expansion per passage was 600-fold and therefore about 2 × 109-fold over three passages in 6 wk. If all of the cells from each passage had been plated, over 1013 MSCs would have been obtained from each 20 ml aspirate. The average percent cfus at the end of the third passage was about the same as the initial value for the samples (28% vs. 33%). Therefore, calculated cumulated yield of cfu cells was also about 1013.
Table 2.
Expansion of human MSCs in culture*
| Sample/Passage‡ | Observed values for MSCs per 530 cells plated | Calculated cumulative totals†
|
|
|---|---|---|---|
| MSCs | CFUs | ||
| Donor 58R | |||
| First | 8.0 × 104 | 7.0 × 107 | 2.2 × 107 |
| Second | 1.1 × 106 | 1.5 × 1010 | 0.95 × 1010 |
| Third | 2.3 × 105 | 6.5 × 1013 | 1.40 × 1013 |
| Donor 58L | |||
| First | 8.6 × 104 | 1.1 × 108 | 0.25 × 108 |
| Second | 4.2 × 105 | 1.1 × 1010 | 4.1 × 1010 |
| Third | 3.1 × 105 | 5.0 × 1013 | 1.5 × 1013 |
| Donor 59R | |||
| First | 1.9 × 105 | 2.8 × 108 | 1.1 × 108 |
| Second | 2.4 × 105 | 1.3 × 1011 | 5.5 × 1010 |
| Third | 7.5 × 105 | 1.8 × 1014 | 5.2 × 1013 |
| Donor 59L | |||
| First | 1.4 × 105 | 2.4 × 108 | 0.91 × 108 |
| Second | 1.7 × 105 | 7.8 × 1010 | 3.7 × 1010 |
| Third | 5.0 × 105 | 7.4 × 1013 | 2.2 × 1013 |
For each passage, 530 MSCs were plated in 176-cm2 plates for an average plating density of 3.0 cells/cm2. The cultures were incubated for 14 days, harvested with EDTA/trypsin, and an aliquot of 530 cells replated at a density of 3.0 cells/cm2.
† Calculated cumulative total of MSCs and CFUs if all the MSCs from the frozen stock and each passage had been replated at 3.0 cells/cm2.
‡ The samples were from bone marrow aspirates obtained at the same time from two normal volunteers (58 and 59), one from the right iliac crest (58R and 59R) and one from the left iliac crest (58L and 59L). Each of the aspirates was about 20 ml, from which 1.2 to 2.7 × 107 nucleated cells were obtained. The nucleated cells were plated at high density to obtain frozen stocks of early passage MSCs (see text).
Discussion
Numerous previous reports demonstrated that MSCs are relatively easy to expand in culture by capitalizing on their tendency to adhere and proliferate on tissue culture surfaces (see refs. 1, 6, 15–18). Some species and strain-dependent variations are seen. In particular, murine MSCs from several strains are found to be difficult to propagate and are usually contaminated by hematopoietic precursors (see refs. 15 and 42). In contrast, human MSCs are relatively easy to propagate and are largely free of hematopoietic precursors after two or three passages. In previous reports, however, human MSCs were passed by plating at relatively high densities of 5,000 cells/cm2 and expanded 3- to 5-fold as the cells grew to confluency over about 2 wk (see ref. 18). As demonstrated here, the cells propagate in a much more dramatic manner if they are plated at extremely low densities of 1.5 or 3.0 cells/cm2. At lower densities, the cultures expanded an average of 600-fold in 2 wk. In some passages, up to 2,000-fold expansion in 12 days was observed. We are currently exploring the possibility that the slow growth of the cells after high density plating is explained either by cell-to-cell contact or factors that the cells secrete into the medium.
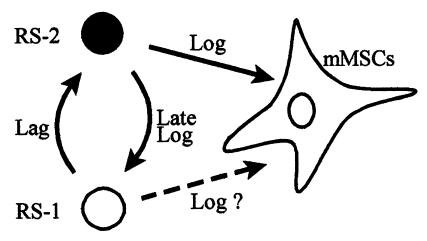
The rapid expansion of the MSCs in culture was found to depend on the presence of a minor population of small cells. Previous reports made conflicting claims as to whether cultures of human MSCs are homogeneous. Some indicated that the cells are homogeneous by morphology and several other criteria such as surface epitopes (see ref. 21). Many, however, emphasized heterogeneity. In particular, Mets and Verdonk (3) emphasized the presence of large and flat cells that they referred to as type II cells and smaller spindle-shaped cells they referred to as type I cells. The type II cells propagated very slowly and the type I more rapidly. The FACS analyses carried out here demonstrated that stationary cultures of MSCs contained a major population of large cells here referred to as mMSCs and a minor population of small and agranular cells (RS-1 cells). After replating the cultures at low density, a new population of small and granular RS-2 cells appeared. During the log phase growth, the population of mMSCs rapidly expanded, the RS-2 cells declined in number, and the RS-1 cells increased. As noted by Mets and Verdonk (3) and other investigators (see ref. 9), mMSCs are relatively mature cells that divide slowly and become the predominant cell as the cultures approach senescence (see ref. 41). Therefore, the simplest explanation for these observations is that the cells referred to here as RS-1 cells generate RS-2 cells during the lag phase, the RS-2 cells give rise to mMSCs during the log phase, and then the RS-2 cells regenerate RS-1 cells during the late log phase (Fig. 7). This sequence is consistent with the time course in which the cells appear and disappear in the cultures and the linear relationship between the number of RS cells and the number of cells in the same samples that can generate single-cell derived colonies.
Figure 7.
Scheme for the precursor–product relationships of cells in cultures of MSCs. As discussed in the text, the large mMSCs replicate poorly (2, 41). Therefore, the RS-2 cells that appear during the lag phase must arise from RS-1 cells (Fig. 4). During the early log phase of growth, the RS-2 cell decline in number as the mMSCs appear in large numbers. Therefore, the RS-2 cells are probably precursors of the mMSCs. However, the data do not completely exclude the possibility that RS-2 cells rapidly generate RS-1 cells, and the RS-1 cells then give rise to mMSCs (dashed arrow). Also, the earliest mMSCs probably continue to replicate. During the late log phase, the RS-2 cells decline in number, and the subpopulation of RS-1 expands. Therefore, the RS-2 cells probably recycle into RS-1 cells.
The ease with which stem-like cells in cultures of human MSCs can be expanded presents a marked contrast to the difficulties that have been encountered in expanding hematopoietic stem cells (see ref. 43) or the relatively slow rate at which neural progenitor cells expand in culture (44). The ability to rapidly expand human MSCs in culture will be of obvious importance in using the cells for cell and gene therapy (see refs. 8 and 9). Under the conditions developed here, one bone marrow aspirate obtained under local anesthesia can generate about 1013 cells, a number that approaches the total number of cells in the adult body. Of special importance was that after extensive expansion of the cells, the number of cfus remained unchanged. cfu values for cultures of hMSCs were previously shown to be closely correlated to the ability of the cells to differentiate into both osteoblasts or adipocytes (41). Therefore, the results suggest that the expanded cells maintain their multipotentiality.
Abbreviations
- MSCs
marrow stromal cells
- mMSCs
mature MSCs
- RS cells
recycling stem cells
- cfu
colony-forming unit
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070034097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070034097
References
- 1.Friedenstein A J, Gorskaja U, Kalugina N N. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 2.Castro-Malaspina H, Gay R E, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenz S, Broxmeyer H E, Moore M A S. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 3.Mets T, Verdonk G. Mech Ageing Dev. 1981;16:81–89. doi: 10.1016/0047-6374(81)90035-x. [DOI] [PubMed] [Google Scholar]
- 4.Piersma A H, Brockbank K G M, Ploemacher R E, van Vilet E, Brakel-van Peer K M J, Visser P J. Exp Hematol. 1985;13:237–243. [PubMed] [Google Scholar]
- 5.Howlett C R, Cave J, Williamson M, Farmer J, Ali S Y, Bab I, Owen M E. Clin Orthoped Rel Res. 1986;213:251–263. [PubMed] [Google Scholar]
- 6.Friedenstein A J, Chailakhyan R K, Gerasimov U V. Cell Tissue Kinetics. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 7.Owen M E, Friedenstein A J. Cell and Molecular Biology of Vertebrate Hard Tissues. Chichester, U.K.: Ciba Foundation Symposium; 1988. pp. 42–60. [PubMed] [Google Scholar]
- 8.Caplan A I. J Orthoped Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 9.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 10.Chiang G G, Rubin H L, Cherington V, Wang T, Sobolewski J, McGrath C A, Gaffney A, Emani S, Sarver P H, Levine P H, et al. Hum Gene Ther. 1999;10:61–76. doi: 10.1089/10430349950019192. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz E M, Prockop D J, Fitzpatrick L A, Winston W K K, Gordon P L, Neel M, Sussman P, Orchard P, Marx J C, Pyeritz R E, et al. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 12.Anklesaria P, Klassen V, Sakakeeny M A, FitzGerald T J, Harrison D, Rybak M E, Greenberger J S. Exp Hematol. 1987;15:636–644. [PubMed] [Google Scholar]
- 13.Beresford J N, Bennett J H, Devlin C, Leboy P S, Owen M E. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S L, Yang J W, Rifas L, Zhang S-F, Avioli L V. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 15.Clark B R, Keating A. Ann NY Acad Sci. 1995;770:70–78. doi: 10.1111/j.1749-6632.1995.tb31044.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsov S A, Friedenstein A J, Robey P G. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuznetsov S A, Krebsbach P H, Satomura K, Kerr J, Riminucci D, Benayahu D, Robey P G. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 18.Bruder S P, Jaiswal N, Haynesworth S E. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Wakitani S, Saito T, Caplan A I. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 20.Lecka-Czernik B, Gubrij I, Moerman E J, Kajkevona O, Lipschitz D A, Manolagas S C, Jilka R L. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 21.Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J E, Moorman M A, Simonetti D W, Craig S, Marshak D R. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Pereira R F, Halford K W, O'Hara M D, Leeper D B, Sokolov B P, Pollard M D, Bagasra O, Prockop D J. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira R F, O'Hara M D, Laptev A V, Halford K W, Pollard M D, Class R, Simon D, Livezey K, Prockop D J. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Z, Nguyen Q, Frenkel B, Nillson S K, Milne M, van Wijnen A J, Stein J L, Quesenberry P, Lian J B, Stein G S. Proc Natl Acad Sci USA. 1999;96:7294–7299. doi: 10.1073/pnas.96.13.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson S K, Dooner M S, Weier H U, Frenkel B J, Lian J B, Stein G S, Quesenberry P S. J Exp Med. 1999;189:729–734. doi: 10.1084/jem.189.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating A, Guinn B, Larava P, Wang X-H. Exp Hematol. 1996;24:1056. [Google Scholar]
- 27.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 28.Goodell M A, Rosenzweit M, Kim H, Marks D F, DeMaria M, Paradis G, Grupp S A, Sieff C A, Mulligan R C, Johnson R P. Nat Med. 1997;12:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 29.Gussoni E, Soneoka Y, Stickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–393. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 30.Eglitis M A, Mezey E. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azizi S A, Stokes D G, Augelli B J, DiGirolamo C M, Prockop D J. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopen G C, Prockop D J, Phinney D G. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjornson C R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 34.Long M W, Robinson J A, Ashcraft E A, Mann K G. J Clin Invest. 1995;95:881–887. doi: 10.1172/JCI117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons P J, Torok-Storb B. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 36.Waller E K, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo G R, Terstappen L. Blood. 1995;85:2422–2435. [PubMed] [Google Scholar]
- 37.Rickard D J, Kassem M, Hefferan T E, Sarkar G, Spelsberg T C, Riggs B L. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 38.Gronthos S, Simmons P J. J Hematother. 1996;5:15–23. doi: 10.1089/scd.1.1996.5.15. [DOI] [PubMed] [Google Scholar]
- 39.Joyner C J, Bennett A, Triffitt J T. Bone. 1997;21:1–6. doi: 10.1016/s8756-3282(97)00074-4. [DOI] [PubMed] [Google Scholar]
- 40.Stewart K, Walsh S, Screen J, Jefferiss C M, Chainey J, Jordan G R, Beresford J N. J Bone Miner Res. 1999;14:1345–1256. doi: 10.1359/jbmr.1999.14.8.1345. [DOI] [PubMed] [Google Scholar]
- 41.DiGirolamo C M, Stokes D, Colter D, Phinney D G, Class R, Prockop D J. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 42.Phinney D G, Kopen G, Isaacson R L, Prockop D J. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 43.Glimm H, Eaves C J. Blood. 1999;97:2161–2168. [PubMed] [Google Scholar]
- 44.Carpenter M K, Cui X, Hu Z Y, Jackson J, Sherman S, Seiter A, Wahlberg L U. Exp Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]