Abstract
Stimulation of the Ras/extracellular signal-regulated kinase (ERK) pathway can modulate cell growth, proliferation, survival, and motility. The p90 ribosomal S6 kinases (RSKs) comprise a family of serine/threonine kinases that lie at the terminus of the ERK pathway. Efficient RSK activation by ERK requires its interaction through a docking site located near the C terminus of RSK, but the regulation of this interaction remains unknown. In this report we show that RSK1 and ERK1/2 form a complex in quiescent HEK293 cells that transiently dissociates upon mitogen stimulation. Complex dissociation requires phosphorylation of RSK1 serine 749, which is a mitogen-regulated phosphorylation site located near the ERK docking site. Using recombinant RSK1 proteins, we find that serine 749 is phosphorylated by the N-terminal kinase domain of RSK1 in vitro, suggesting that ERK1/2 dissociation is mediated through RSK1 autophosphorylation of this residue. Consistent with this hypothesis, we find that inactivating mutations in the RSK1 kinase domains disrupted the mitogen-regulated dissociation of ERK1/2 in vivo. Analysis of different RSK isoforms revealed that RSK1 and RSK2 readily dissociate from ERK1/2 following mitogen stimulation but that RSK3 remains associated with active ERK1/2. RSK activity assays revealed that RSK3 also remains active longer than RSK1 and RSK2, suggesting that prolonged ERK association increased the duration of RSK3 activation. These results provide new evidence for the regulated nature of ERK docking interactions and reveal important differences among the closely related RSK family members.
The Ras/extracellular signal-regulated kinase (ERK) pathway regulates a variety of cellular processes that include cell proliferation, survival, growth, and motility (for reviews, see references 21 and 26). Among the substrates of ERK are the members of the p90 ribosomal S6 kinase (RSK) family of serine/threonine kinases (10). In humans, the RSK family consists of four isoforms (RSK1 to -4) and two structurally related cousins, called RSK-like protein kinase (RLPK/MSK1) and RSK-B (MSK2). RSK family members are unusual among serine/threonine kinases in that they contain two distinct kinase domains, both of which are catalytically functional (9). The C-terminal kinase domain is believed to be involved in autophosphorylation (2, 9, 31), a critical step in RSK activation, whereas the N-terminal kinase domain, which is homologous to members of the AGC superfamily of kinases, is responsible for the phosphorylation of all known exogenous substrates of RSK.
Activated RSK has both cytoplasmic and nuclear substrates. RSK plays an active role in nuclear signaling by phosphorylating the cyclic AMP response element binding protein (CREB) (33), c-Fos (5), and IκB (27). Phosphorylation of Bad (3, 29) and C/EBPβ (4) by RSK can protect cells from apoptosis. RSK has also been implicated in cell cycle regulation. In Xenopus extracts, RSK phosphorylates and inhibits Myt1, a p34cdc2 inhibitory kinase (20). Moreover, RSK phosphorylates histone H3 (25), suggesting that RSK may regulate chromatin remodeling.
The precise mechanism of RSK activation remains elusive. The current model suggests that following mitogen stimulation, ERK phosphorylates RSK1 Thr590 (according to avian RSK1 numbering; human 573), located in the activation loop of the C-terminal kinase domain, and possibly Ser381 (turn motif; human 363) and Thr377 (human 359) in the linker region between the two kinase domains. Activation of RSK1 is absolutely dependent on ERK docking near the C terminus of RSK1 (12, 30). Activation of the C-terminal kinase domain leads to autophosphorylation of Ser398 (hydrophobic motif; human 380), also located in the linker region (32). This creates a docking site for PDK1 (11), which then phosphorylates Ser239 (human 221) in the activation loop of the N-terminal kinase domain (15, 24), allowing RSK to phosphorylate all its targets. Interestingly, Ser749 (human 732) is located near the ERK docking site and represents another mitogen-regulated phosphorylation site in RSK1. This residue is thought to be regulated by autophosphorylation from the N-terminal kinase domain, as it fits a RSK consensus phosphorylation sequence (K/RXXS/T) (1, 17), but its role in RSK activation and function remains unknown.
RSK and ERK have previously been shown to form a complex in various cell types (14, 28, 34). Recent reports have demonstrated that the ERK binding region is localized within the last 15 amino acids of RSK1 and RSK2 (LAQRRVRKLPSTTL), with the boldface amino acids conserved among all RSK isoforms (12, 30). The functional relationship of ERK and RSK has been studied in Xenopus laevis, and RSK has been shown to associate with inactive ERK and to dissociate upon oocyte maturation and ERK activation (13). However, the regulation of the RSK-ERK complex in mammalian cells is less clear. In COS7 cells, complexes between endogenous ERK1/2 and ectopically expressed RSK were found not to be affected by the activation of ERK (34). Clearly, understanding the mechanisms that regulate the interaction between RSK and ERK will be important in resolving their functional interactions.
Here, we report that RSK1 is constitutively bound to ERK1/2 in quiescent HEK293 cells but that mitogen stimulation leads to a transient and complete dissociation of the complex. Through mutational analysis of the RSK1 C-terminal region, we found that ERK1/2 binding minimally requires residues 739LAQRR743 and that Ser749 phosphorylation regulates interaction between ERK1/2 and RSK1. We confirmed that Ser749 can be phosphorylated by the N-terminal kinase domain of RSK1 in vitro, and consistent with these results, mutations that affect RSK1 kinase activity prevent ERK1/2 dissociation from RSK1 following mitogen stimulation in vivo. Analysis of different RSK isoforms revealed that RSK1 and RSK2 dissociate from ERK1/2 following mitogen stimulation but that RSK3 remains associated with active ERK1/2. ERK dissociation from RSK1 and RSK2 correlated with shorter duration of activity compared to RSK3, indicating that ERK dissociation may shorten RSK activation.
MATERIALS AND METHODS
Reagents.
Fetal bovine serum (FBS) was purchased from HyClone Laboratories, Inc. (Logan, Utah), RSK phosphospecific antibodies were supplied by R&D Systems (Minneapolis, Minn.), ERK1/2 antibodies were described previously (7), epidermal growth factor (EGF) was purchased from Invitrogen (Carlsbad, Calif.), and U0126 was purchased from Biomol (Plymouth Meeting, Pa.).
Plasmid constructions.
Avian RSK1 was cloned into pKH3 with a triple hemagglutinin (HA) tag at the N terminus (23). RSK-CTT is a C-terminally truncated RSK1 lacking the last 11 amino acids (RRVKKLPSTTL), which encode an ERK docking site (12, 30). Point mutations in RSK1 were introduced using the QuickChange methodology from Stratagene (La Jolla, Calif.). All avian RSK1 constructs contain a sequence specific to the avian isoform, recognized by antibodies described previously (23). Human RSK1, murine RSK2, and human RSK3 sequences were cloned into pKH3, resulting in HA-tagged constructs.
Cell culture and transfection.
HEK293 cells were maintained in 5% CO2 at 37°C in Dulbecco's modified Eagle medium supplemented with 10% FBS and 100 μg of penicillin-streptomycin/ml. The cells were transfected using calcium phosphate 16 to 18 h after being plated and were incubated for 5 h with the DNA precipitates and starved in Dulbecco's modified Eagle medium containing 0.5% bovine serum albumin for 16 to 18 h prior to stimulation. Following stimulation, the cells were washed twice in cold phosphate-buffered saline (PBS) and lysed in CLB (10 mM KPO4, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 50 mM β-glycerophosphate, 0.5% NP-40, 0.1% Brij 35, 0.1% deoxycholic acid, 1 mM sodium orthovanadate [Na3VO4], 1 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin/ml, 10 μg of pepstatin/ml). The lysates were centrifuged for 5 min at 4°C and used for immunoprecipitations, immunoblotting, or protein kinase assays.
Immunoprecipitations.
Lysates were incubated with anti-HA antibodies for 2 h and with protein A-Sepharose for an additional hour at 4°C. Beads were washed twice with PBS- 1% NP-40 and once with TNE (10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, pH 7.4) prior to the addition of 45 μl of 2× Laemmli sample buffer.
Protein kinase assays.
The beads from immunoprecipitations were washed twice with PBS- 1% NP-40 and twice with kinase buffer (25 mM Tris, pH 7.4, 2 mM dithiothreitol, 10 mM MgCl2, 5 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride). Kinase assays were performed with glutathione-S-transferase (GST)-S6 as a substrate (1 μg per assay) or with GST-RSK1385-752 containing point mutations as previously described (6, 24) and were completed in the linear range of substrate phosphorylation. The reaction products were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and 32P incorporation was quantified using a Bio-Rad phosphorimager.
Immunoblotting.
Cell lysates were subjected to SDS-PAGE on 8 to 12% acrylamide gels and electroblotted to nitrocellulose. Blocking and primary and secondary antibody incubations of immunoblots were performed in TBST (10 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween 20) supplemented with 5% (wt/vol) dry skim milk powder. Phosphospecific RSK antibodies (R&D Systems) were used according to the instructions from the manufacturer. Horseradish peroxidase-conjugated donkey anti-rabbit and anti-mouse immunoglobulin Gs were used at a dilution of 1:5,000, and immunoreactive bands were detected using enhanced chemiluminescence.
RESULTS
The interaction between RSK1 and ERK1/2 is regulated by mitogen stimulation.
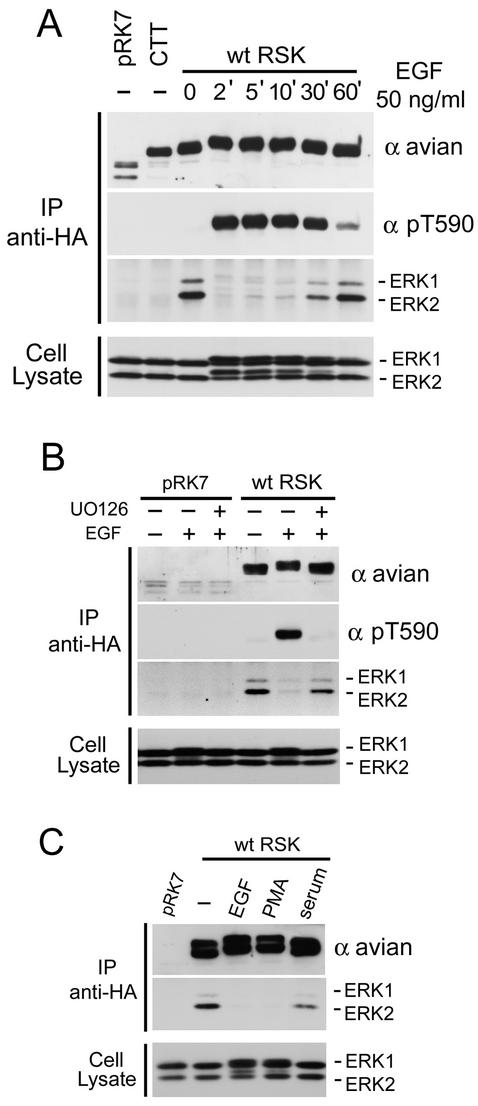
The ERKs have been shown to interact with RSK in Xenopus oocytes (13, 14) and in mammalian cells (30, 34), but how this interaction is regulated remains uncharacterized. To investigate the RSK-ERK interaction, HEK293 cells were transfected with triple-HA-tagged avian RSK1 constructs, serum starved for 16 to 18 h before stimulation with EGF, and RSK1 immunoprecipitated to analyze ERK1/2 association by immunoblotting. RSK1 was used in this study because it has been shown that this isoform can interact with ERK (23), and all amino acid numbering refers to avian RSK1. We have found that in quiescent HEK293 cells, endogenous ERK1/2 associates with immunoprecipitated wild-type (wt) RSK1 (Fig. 1A). This interaction requires the ERK docking site, because RSK-CTT, missing the last 11 amino acids, did not associate with ERK1/2 (Fig. 1A). Interestingly, RSK1 associates preferentially with ERK2 rather than ERK1, despite their equal expression levels as shown in the total cell lysate. To determine the effect of mitogen stimulation on ERK1/2 binding to RSK1, ERK1/2 association was analyzed during a time course following EGF stimulation (50 ng/ml) of HEK293 cells. ERK1/2 completely dissociated from RSK1 within 2 min of EGF stimulation, and only a very faint and transient activated (shifted) form of ERK1/2 was visible in RSK1 immunoprecipitates at the 2- and 5-min time points (Fig. 1A). ERK1/2 dissociation was transient, and the complex reappeared, reaching unstimulated levels within 30 to 60 min of EGF stimulation, correlating perfectly with the loss of ERK1/2 activity (Fig. 1A). This transient effect also correlated with the activation state of RSK1, as shown by the transient RSK1 gel retardation indicative of phosphorylation, and RSK1 phosphorylated Thr590, as determined using phosphospecific antibodies (Fig. 1A).
FIG. 1.
Regulated association between RSK1 and ERK1/2. (A) HEK293 cells were transfected with wt avian RSK1, RSK-CTT (CTT), or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for the indicated times. Anti-HA RSK1 immunoprecipitates (IP) were immunoblotted for ERK1/2 association, for RSK1 using anti-avian epitope antibodies, and for phosphorylated RSK1 Thr590 (pT590) using phosphospecific antibodies. The cell lysates were probed for ERK1/2 to measure MAPK-ERK pathway activation. (B) HEK293 cells were transfected as for panel A. Where indicated, the cells were pretreated (+) with 5 μM U0126 for 30 min prior to stimulation with EGF (50 ng/ml) for an additional 10 min. Anti-HA RSK1 immunoprecipitates and whole-cell lysates were blotted as for panel A. (C) HEK293 cells were stimulated for 10 min with EGF (50 ng/ml), phorbol myristate acetate (PMA) (100 ng/ml), or FBS (10%) (serum), and analyzed as for panel A.
ERK activation was necessary for the EGF-mediated effects, because pretreatment of the cells with the MEK inhibitor U0126 prevented the RSK-ERK complex from dissociation following EGF stimulation (Fig. 1B, lower middle blot). Furthermore, the effects on ERK1/2 docking to RSK1 were not limited to EGF, as phorbol myristate acetate and, to a lesser extent, serum also promoted ERK1/2 dissociation from RSK1, again correlating with the levels of RSK and ERK activation (Fig. 1C). Therefore, RSK1 and ERK1/2 interact in quiescent cells, but the complex dissociates following mitogen stimulation, suggesting that the phosphorylation state of either RSK1 or ERK1/2 regulates their association and dissociation.
Mutational analysis of the ERK docking region in the C-terminal region of RSK1.
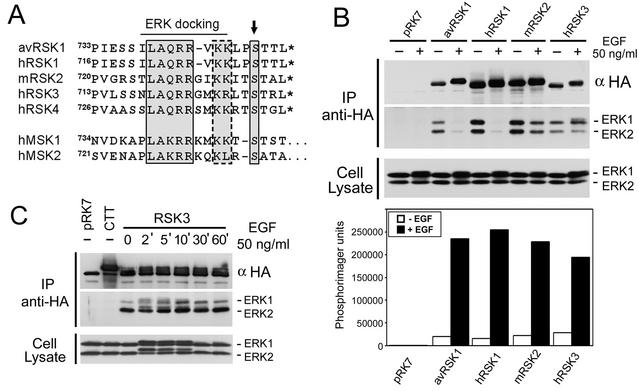
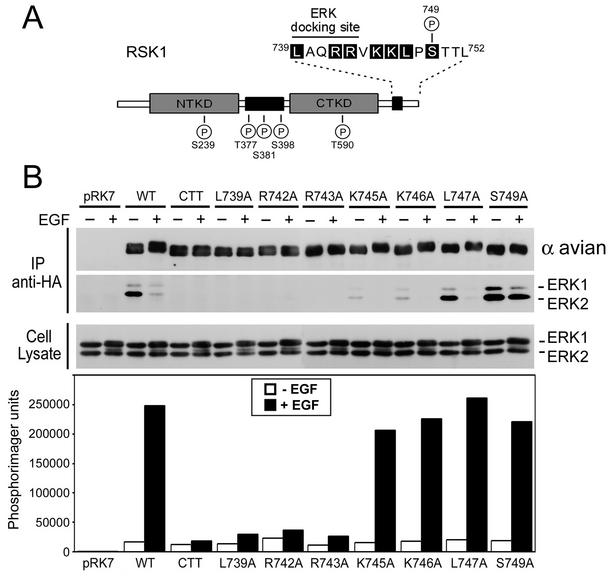
The minimal region necessary for ERK docking has been localized to the last 15 amino acids of human RSK1, 739LAQRRVKKLPSTTL747 (12, 30), with the boldface amino acids homologous among all RSK isoforms (see Fig. 6A). We performed a mutational analysis around this region to identify key residues involved in ERK docking (Fig. 2). HEK293 cells were transfected with RSK1 point mutants, and ERK1/2 association was determined by immunoblotting following RSK immunoprecipitation. We found that the conserved Leu739, Arg742, and Arg743 were important for ERK1/2 interaction, because the RSK1 L739A, R742A, and R743A mutants were unable to bind ERK1/2 with or without EGF stimulation (Fig. 2B). These mutations also prevented EGF-stimulated RSK1 activation, as shown by kinase assays using GST-S6 as a substrate for the N-terminal kinase activity of RSK1 (Fig. 2B). We also generated A740G and Q741A mutants of RSK1, but we found that mutation of these two residues did not reduce ERK1/2 binding or decrease RSK activation following mitogen stimulation (data not shown). RSK1 to -4 have two conserved basic residues located C terminal to the ERK docking site (Lys745 and Lys746 in avian RSK1), and mutations of these residues reduced the strength of interaction with ERK1/2 but only marginally reduced mitogen-stimulated RSK1 activity (Fig. 2B). Mutation of the conserved hydrophobic residue Leu747, however, did not affect ERK docking, nor did it decrease RSK activation. Therefore, the minimal region necessary for ERK docking appears to require residues 739LAQRR743, where the boldface amino acids are essential for ERK1/2 interaction, with a minimal involvement of other conserved residues located C-terminal to the docking motif.
FIG. 6.
Differential regulation of ERK docking to RSK isoforms. (A) Alignments of the C termini of avian (av) and human (h) RSK1, murine (m) RSK2, human RSK3 and RSK4, and human MSK1 and MSK2. The boxed residues indicate the ERK and/or p38 docking sites, the dashed box indicates residues that modulate ERK interaction with RSK1, the arrow indicates the conserved serine that lies within an RSK/MSK consensus phosphorylation sequence, and the asterisks indicate the C termini of the Rsk proteins. (B) HEK293 cells were transfected with wt avian and human RSK1, murine RSK2, human RSK3, or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for 10 min where indicated (+). Anti-HA RSK immunoprecipitates (IP) were blotted for ERK1/2 association and for RSK1 to -3 using anti-HA antibodies. The cell lysates were probed for ERK1/2 to measure MAPK-ERK pathway activation. The phosphotransferase activities of all RSK isoforms were determined by kinase assays using GST-S6 as a substrate. Phosphorimager analysis of GST-S6 was performed, and typical results are shown as a histogram (n = 3). (C) HEK293 cells were transfected with hRSK3, RSK-CTT (CTT), or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for the indicated times. Anti-HA RSK3 immunoprecipitates and lysates were immunoblotted as for panel B.
FIG. 2.
Mutational analysis of the ERK docking region. HEK293 cells were transfected with control vector (pRK7), wt RSK1 (WT), RSK-CTT (CTT), and full-length RSK1 with point mutations in the C-terminal region (L739A, R742A, R743A, K745A, K746A, L747A, and S749A) as shown in the schematic (A). (B) Cells were serum starved for 16 to 18 h and stimulated with EGF (50 ng/ml) for 10 min where indicated (+). Anti-HA RSK1 immunoprecipitates (IP) were immunoblotted for ERK1/2 association and for RSK1 using anti-avian epitope antibodies. The cell lysates were probed for ERK1/2 to measure MAPK-ERK pathway activation. The phosphotransferase activities of wt RSK1 and RSK1 mutants were determined by kinase assays using GST-S6 as a substrate. Phosphorimager analysis of GST-S6 was performed, and typical results are shown as a histogram (n = 3). NTKD, N-terminal kinase domain; CTKD, C-terminal kinase domain; circled Ps, regulated phosphorylation sites.
Serine 749 is a mitogen-regulated RSK1 residue (8), and because it is located near the ERK docking site, phosphorylation of Ser749 may regulate ERK binding. We found that mutation of Ser749 to alanine resulted in a RSK1 mutant that constitutively interacts with ERK1/2, with or without mitogenic stimulation (Fig. 2B, last two lanes on right). In the absence of stimulation, the interaction of ERK1/2 with the S749A mutant was even stronger than with wt RSK1, suggesting that Ser749 is phosphorylated basally to a low level, a finding previously reported by Dalby et al. (8). Although the interaction of ERK1/2 was no longer regulated, the kinase activity of the S749A mutant was almost identical to that of wt RSK1, suggesting that ERK1/2 dissociation is not essential for RSK1 kinase activation. Together, these results indicate that phosphorylation of Ser749 is a crucial event in the regulation of ERK1/2 docking before and after RSK1 activation.
Mutational analysis of RSK1 serine 749.
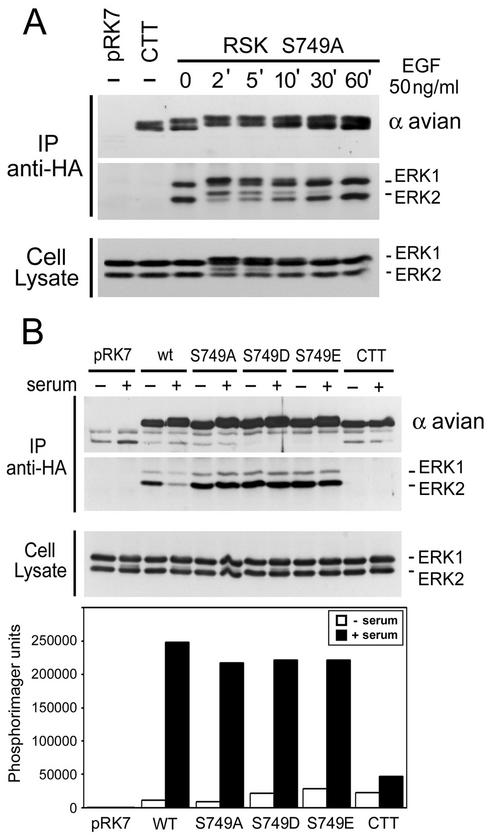
To determine the involvement of Ser749 in ERK docking, HEK293 cells were transfected with the RSK1 S749A mutant and analyzed for coimmunoprecipitated ERK1/2. At all times following EGF stimulation (2 to 60 min), identical amounts of ERK1/2 were found associated with RSK1 S749A, indicating that this residue is essential for ERK1/2 dissociation (Fig. 3A). Moreover, both inactive and activated forms of ERK1/2 associated with RSK1 S749A following mitogen stimulation. Mutation of Ser749 to phosphomimetic residues (aspartic acid or glutamic acid) did not reduce the ability of RSK1 to bind ERK1/2, nor did it rescue its ability to dissociate following stimulation (Fig. 3B). Similar results were also found with a double phosphomimetic mutant of RSK1 with aspartic acid substitutions at Ser749 and Thr750 (data not shown). Interestingly, RSK1 kinase assays revealed that all three RSK1 Ser749 mutants were nearly as active as wt RSK1 following EGF stimulation (Fig. 3B), suggesting again that ERK1/2 dissociation is not necessary for maximal RSK1 activation. These results suggest that Ser749 might play a role in regulating RSK function following activation (see Fig. 7).
FIG. 3.
Mutational analysis of RSK1 serine 749. (A) HEK293 cells were transfected with RSK1 containing an alanine mutation on Ser749, RSK-CTT (CTT), or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for the indicated times. Anti-HA RSK1 immunoprecipitates (IP) were blotted for ERK1/2 association and for RSK1 using anti-avian epitope antibodies. The cell lysates were probed for ERK1/2 to measure MAPK-ERK pathway activation. (B) HEK293 cells were transfected with RSK1 containing an alanine (S749A), aspartic acid (S749D), or glutamic acid (S749E) mutation, serum starved for 16 to 18 h, and stimulated with FBS (10%) (serum) for 10 min where indicated (+). Anti-HA RSK1 immunoprecipitates and whole-cell lysates were blotted as for panel A. The phosphotransferase activities of all RSK1 mutants were determined by kinase assays using GST-S6 as a substrate. Phosphorimager analysis of GST-S6 was performed, and typical results are shown as a histogram (n = 4).
FIG. 7.
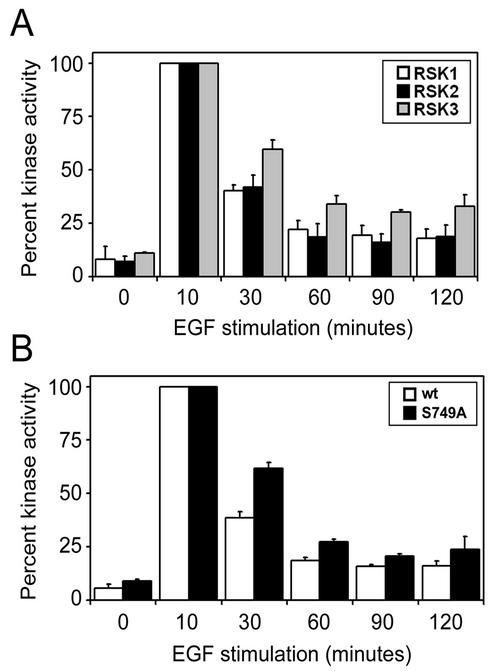
Activation kinetics of RSK isoforms. HEK293 cells were serum starved and stimulated for 0, 10, 30, 60, 90, or 120 min with EGF (50 ng/ml). The phosphotransferase activities of all immunoprecipitated RSK isoforms were determined by kinase assays using GST-S6 as a substrate. Phosphorimager analysis of GST-S6 was performed, and typical results are shown as a histogram (n = 3). Activation levels are shown as percentages of maximum activation at 10 min of EGF stimulation for each RSK construct. Analyses were made among human RSK1, murine RSK2, and human RSK3 (A) and between wt avian RSK1 and RSK1 S749A (B). Each histogram represents the average plus standard error of three independent experiments.
The RSK1 N-terminal kinase domain phosphorylates serine 749 in vitro.
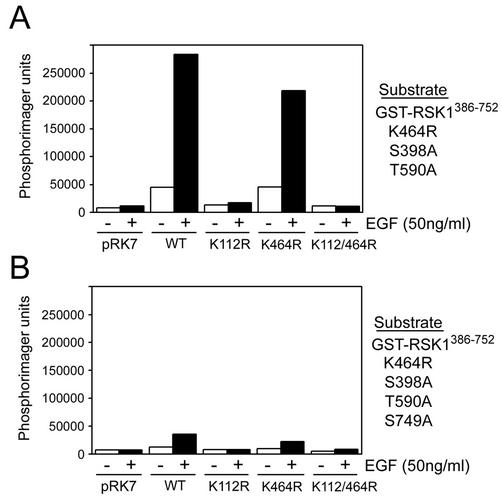
The RSK N-terminal kinase domain consensus phosphorylation sequence suggests that Ser749 may be an RSK autophosphorylation site. To test this, different RSK1 kinase domain mutants (K112R, K464R, and K112/464R) were immunoprecipitated from cells and assayed for the ability to transphosphorylate in vitro bacterially expressed kinase-inactive GST-RSK1. GST fusion proteins containing amino acids 386 to 752 of avian RSK1 (GST-RSK1386-752) with various phosphorylation site mutations were used to monitor RSK1 phosphotransferase activity. Using kinase-inactive GST-RSK1386-752 containing S398A and T590A mutations (GST-RSK1AA386-752), we found that immunoprecipitated wt RSK1 led to a 10-fold increase in 32P incorporation following mitogen stimulation (Fig. 4A). A similar increase was also seen with the C-terminal kinase domain-inactive (K464R) RSK1 mutant but not with the N-terminal (K112R) and double (K112/464R) kinase-inactive RSK1 mutants, indicating that a functional N-terminal kinase domain is required for efficient phosphotransferase activity toward GST-RSK1AA386-752. To address whether Ser749 was the site of phosphorylation, we assayed phosphotransferase activity toward another GST fusion protein containing S398A, T590A, and S749A mutations (GST-RSK1AAA386-752) and found that incubation of GST-RSK1AAA386-752 with wt RSK1 or any RSK1 mutants did not lead to 32P incorporation (Fig. 4B). Together, our results indicate that the RSK1 N-terminal kinase domain phosphorylates Ser749 in vitro and suggest that this residue may be an autophosphorylation site in vivo.
FIG. 4.
The RSK1 N-terminal kinase domain phosphorylates serine 749. HEK293 cells were transfected with wt RSK1 (WT), kinase-inactive alleles (K112R or K464R), double-kinase-inactive RSK1 (K112/464R), or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for 10 min where indicated (+). Anti-HA RSK1 immunoprecipitates were assayed for phosphotransferase activity toward recombinant kinase-inactive (K464R) GST-RSK1386-752 with alanine substitutions for Ser398 and Thr590 (A) or Ser398, Thr590, and Ser749 (B). Kinase reaction products were subjected to SDS-PAGE, and 32P incorporation was assessed by autoradiography and analyzed using a Phosphorimager. Typical results are shown as a histogram (n = 2).
RSK1 phosphotransferase activity regulates ERK1/2 docking.
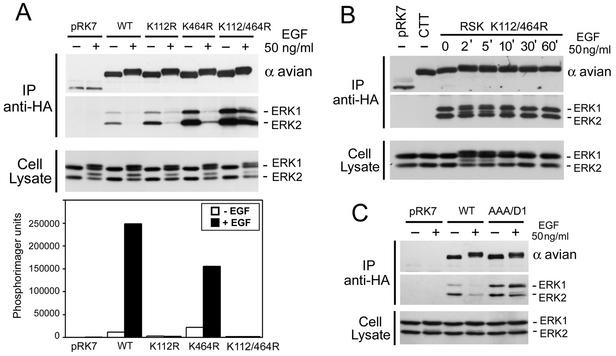
To determine if RSK1 kinase activity regulates ERK1/2 docking, we analyzed the different RSK1 kinase-inactive mutants for the ability to associate with ERK1/2. While wt RSK1 readily dissociated from ERK1/2 upon EGF stimulation, RSK1 with mutations in both kinase domains (K112/464R) did not, indicating that RSK1 kinase activity is necessary for ERK1/2 dissociation (Fig. 5A). Moreover, RSK1 K112/464R remained associated with ERK1/2 at all times (2 to 60 min) following mitogen stimulation (Fig. 5B). The levels of basally associated ERK1/2 were also higher with the RSK1 K112/464R mutant than wt RSK1, suggesting that basal levels of RSK1 activity also promote ERK1/2 dissociation in quiescent cells. RSK1 K464R dissociated from ERK1/2 following stimulation, most likely because this mutant maintained some N-terminal kinase domain phosphotransferase activity, as shown by the kinase assay using GST-S6 as a substrate (Fig. 5A). However, despite its complete lack of kinase activity toward GST-S6, RSK K112R also dissociated from ERK1/2 following mitogen stimulation, indicating that Ser749 phosphorylation may still be regulated in this mutant. To address this, we generated a completely inactivated RSK1 mutant by replacing the APE motif in the activation segment (subdomain VIII) of the N-terminal kinase domain with alanines (RSK1 AAA/D1). We found that this mutant completely lost its ability to dissociate from ERK1/2 following stimulation (Fig. 5C). Taken together, these results indicate that RSK1 N-terminal kinase domain activity is required for ERK1/2 dissociation following mitogen stimulation.
FIG. 5.
ERK interaction is regulated by RSK1 autophosphorylation. (A and C) HEK293 cells were transfected with wt RSK1 (WT), kinase-inactive alleles (K112R, K464R, or AAA/D1), double-kinase-inactive RSK1 (K112/464R), or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for 10 min where indicated (+). Anti-HA-RSK1 immunoprecipitates (IP) were blotted for ERK1/2 association and for RSK1 using anti-avian epitope antibodies. The cell lysates were probed for ERK1/2 to measure MAPK-ERK pathway activation. The phosphotransferase activities of all RSK1 mutants were determined by kinase assays using GST-S6 as a substrate. Phosphorimager analysis of GST-S6 was performed, and typical results are shown as a histogram (n = 2). (B) HEK293 cells were transfected with double-kinase-inactive RSK1 (K112/464R), RSK-CTT (CTT), or control vector (pRK7); serum starved for 16 to 18 h; and stimulated with EGF for the indicated times. Anti-HA RSK1 immunoprecipitates and lysates were immunoblotted as for panel A.
Differential regulation of ERK1/2 association with RSK isozymes.
RSK1 Ser749 is conserved within all RSK isozymes, suggesting that ERK binding might be regulated similarly (Fig. 6A). To determine whether ERK1/2 docking to RSK2 and RSK3 is also regulated following mitogen stimulation, HEK293 cells were transfected with avian and human RSK1, murine RSK2, or human RSK3, and RSK was immunoprecipitated to analyze associated ERK1/2. Consistent with our findings with avian RSK1, human RSK1 dissociated completely from ERK1/2 following mitogen stimulation (Fig. 6B). Unlike avian RSK1, however, human RSK1 did not preferentially associate with ERK2. Following EGF stimulation, RSK2 only partially dissociated from ERK1/2 and human RSK3 remained bound to active and inactive ERK1/2, indicating that ERK docking is regulated differently among RSK isozymes. At all times following EGF stimulation (2 to 60 min), relatively equal amounts of ERK1/2 were found associated with RSK3, and similarly to the RSK1 S749A mutant, both inactive and activated forms of ERK1/2 associated with RSK3 (Fig. 6C). As opposed to previous results (34), the kinase activities of all RSKs were similar following 10 min of EGF stimulation (Fig. 6B), indicating that the differential regulation of ERK1/2 docking to the RSK isozymes does not affect their full activation. Since prolonged ERK docking may affect the duration of RSK activation, we determined whether regulated ERK docking might correlate with different activation kinetics.
ERK association promotes sustained RSK activation.
Unlike RSK1 and RSK2, RSK3 does not dissociate from ERK1/2 following mitogen stimulation (Fig. 6B), which suggest that this isoform may be more efficiently activated by ERK1/2. Although all three RSKs have similar activation levels following a 10-min EGF treatment (Fig. 6B), we determined whether their activation and inactivation were temporally distinct following mitogen stimulation (Fig. 7A). For all three RSK isoforms, maximal activity was reached within 10 min of EGF stimulation in HEK293 cells and was set to 100% activity. Compared to RSK1 and RSK2, we found that RSK3 remained activated to higher levels following 30, 60, 90, and 120 min of EGF stimulation. After 30 min of EGF stimulation, RSK1 and RSK2 activation decreased to ∼40% of their maximum activities, while RSK3 activity decreased to only 60% for the same time point. To determine whether ERK association was indeed responsible for the longer RSK3 activation duration, we tested the activation kinetics of RSK1 S749A in comparison with that of wt RSK1 (Fig. 7B) and found that RSK1 S749A remained activated longer than wt RSK1 (60 versus 35% at 30 min). Taken together, our results indicate that constitutive ERK association promotes prolonged RSK activation and that the differences in ERK association to the RSK isozymes correlate with their differential regulation.
DISCUSSION
Stimulation of the Ras pathway results in the activation of ERK and RSK, but the precise mechanisms involved in RSK activation and inactivation remain elusive. In this study, we characterized the ERK binding domain of RSK1 and found that a serine residue located near the docking site (position 749 in avian RSK1) was involved in the regulation of ERK1/2 binding (Fig. 1 to 3). We also found that Ser749 was efficiently phosphorylated by the RSK1 N-terminal kinase domain in vitro (Fig. 4) and that mutations in the RSK1 kinase domains disrupted ERK dissociation (Fig. 5). Analysis of different RSK isoforms revealed that RSK1 and RSK2 activation induced ERK1/2 release but that mitogen stimulation did not affect the interaction of ERK1/2 with RSK3 (Fig. 6). We also found that RSK3 remains activated longer than RSK1 and RSK2 (Fig. 7), indicating that ERK association promotes RSK activation or antagonizes RSK inactivation.
Our results indicate that the minimal region in RSK1 necessary for ERK1/2 docking consists of residues 739LAQRR743, where the boldface amino acids are essential for ERK1/2 interaction. Mutation of residues Lys745 and Lys746 did not affect RSK1 activation but reduced the overall strength of interaction between RSK1 and ERK1/2. It is possible that ERK1/2 docking to the RSK1 K745A and K746A mutants is not altered in vivo but that the interaction is rapidly disrupted under normal cell lysis conditions. Since an arginine or lysine is always present at both positions in all RSK isoforms, it is possible that these residues play roles in the binding of specific ERK isoforms. We have found that RSK1, RSK2, and RSK3 interact with ERK1/2 to similar levels in serum-starved cells but that only RSK1 and RSK2 dissociate from ERK1/2 following mitogen stimulation of HEK293 and NIH 3T3 cells (data not shown). These results are in disagreement with a report by Zhao et al. (34), who demonstrated that complexes between endogenous ERK1/2 and ectopically expressed RSK1, RSK2, and RSK3 were not affected by activation of ERK1/2 in COS7 cells. Moreover, they found that ERK1/2 associates preferentially with RSK3 and less with RSK2 but could not bind to RSK1 (34). Our results indicate that each RSK isoform interacts similarly with ERK1/2 in cells that were serum starved prior to stimulation. It is possible, however, that ERK1/2 may appear to preferentially associate with RSK3 in cells maintained in the presence of serum, because RSK3 is the only isoform that does not dissociate from ERK1/2 upon activation. Therefore, we suggest that the state of Ras/ERK pathway activation will determine the ratio of RSK1, RSK2, and RSK3 that interacts with ERK1/2.
RSK activation leads to the phosphorylation of four essential residues (Ser239, Ser381, Ser398, and Thr590) and two additional sites (Thr377 and Ser749) with unknown functions (8). We have found that Ser749 regulates ERK1/2 association with RSK1 and that phosphorylation of this residue requires an active N-terminal kinase domain, suggesting that Ser749 is an RSK1 autophosphorylation site that releases ERK1/2 in vivo. While ERK1/2 dissociation was inhibited when both RSK1 kinase domains were mutated (K112/464R), ERK dissociation from RSK1 mutants with only an N-terminal (K112R) or C-terminal (K464R) kinase domain inactivation still occurred, suggesting that Ser749 becomes phosphorylated in these mutants. The K464R mutant displays partial N-terminal kinase domain activity, which can account for the ERK1/2 dissociation, but the K112R mutant was found to be completely inactive toward exogenous substrates (Fig. 5A). This raised the possibility that the RSK K112R mutant retained its ability to phosphorylate Ser749 in cis, despite its inactivity toward exogenous substrates. To address this, we mutated the APE motif in the activation segment (subdomain VIII) of the N-terminal kinase domain of RSK1 and found that this mutant (RSK1 AAA/D1) completely lost its ability to dissociate from ERK1/2 upon stimulation. These results indicated that, indeed, RSK1 K112R retained the ability to phosphorylate Ser749 in cis and that the activity of the N-terminal kinase domain of RSK1 is essential to promote the release of ERK1/2 following mitogen stimulation.
ERK is thought to play at least two roles in RSK1 activation. First, activated ERK phosphorylates RSK1 on Thr590, and possibly on Thr377 and Ser381, and second, ERK brings RSK1 into close proximity to membrane-associated kinases that may phosphorylate RSK1 on Ser381 and Ser398 (23). While ERK interaction is required for both of these functions, the role for a regulated ERK dissociation mechanism remains unknown. On one hand, it has been suggested that ERK substrates need to dissociate from its docking site in order to be phosphorylated (22), but our results indicate that even RSK1 mutants that interact constitutively with ERK1/2 become activated to the same levels as wt RSK1. On the other hand, we have found that RSK proteins that interact constitutively with ERK1/2 remained activated longer than other RSK isoforms with regulated ERK docking mechanisms, suggesting that ERK association regulates the kinetics of RSK activation. The significance of signal duration in intracellular signaling has recently been underscored by a report showing that cells in the G1 phase of the cell cycle can sense ERK signal duration and respond to it by a specific biological function (18). In that study, signal duration was sensed through the timely binding of ERK to specific docking sites, called DEF domains, located in the immediate-early c-Fos protein. Therefore, our results would suggest that the duration of activation of the RSK isozymes may similarly regulate specific cellular targets in order to produce precise biological outcomes.
Although ERK may potentiate RSK3 activation through its constitutive interaction throughout the time course, it is also possible that ERK binding to RSK3 reduces its rate of inactivation. ERK and RSK are generally inactivated in a coordinated fashion subsequent to their activation by growth factors, but the phosphatases involved are still unknown. The nuclear phosphatases MKP1 and MKP2 are good candidates for ERK inactivation and possess ERK docking sites (16, 19). Since these phosphatases bind to the same region on ERK that is recognized by RSK, it is possible that the stable interaction between RSK3 and ERK may repress ERK inactivation by competing for the same docking sites. While this remains a possibility, we cannot exclude the likelihood that ERK binding to RSK suppresses the ability of RSK phosphatases to specifically inactivate RSK.
In conclusion, we have revealed the mechanisms that regulate the interaction between RSK and ERK and found that regulated ERK docking plays important roles in RSK activation kinetics. These results will help in understanding ERK function and the functional differences among the different RSK isozymes, and they suggest that similar mechanisms may also exist in RSK-related kinases, such as the MSKs.
Acknowledgments
We thank Rana Anjum, Leon Murphy, and Celeste Richardson for helpful comments on the manuscript.
This work was supported by the Human Frontier Science Program (P.P.R.), the American Cancer Society (S.A.R.), and the National Institutes of Health grant RO1 CA46595 (J.B.).
REFERENCES
- 1.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 2.Bjorbaek, C., Y. Zhao, and D. E. Moller. 1995. Divergent functional roles for p90rsk kinase domains. J. Biol. Chem. 270:18848-18852. [DOI] [PubMed] [Google Scholar]
- 3.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358-1362. [DOI] [PubMed] [Google Scholar]
- 4.Buck, M., V. Poli, T. Hunter, and M. Chojkier. 2001. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807-816. [DOI] [PubMed] [Google Scholar]
- 5.Chen, R.-H., C. Abate, and J. Blenis. 1993. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA 90:10952-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, R.-H., and J. Blenis. 1990. Identification of Xenopus S6 protein kinase homologs (pp90rsk) in somatic cells: phosphorylation and activation during initiation of cell proliferation. Mol. Cell. Biol. 10:3204-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, R.-H., C. Sarnecki, and J. Blenis. 1992. Nuclear localization and regulation of the erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 12:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalby, K. N., N. Morrice, F. B. Caudwell, J. Avruch, and P. Cohen. 1998. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 273:1496-1505. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, T. L., and J. Blenis. 1996. Evidence for two catalytically active kinase domains in pp90rsk. Mol. Cell. Biol. 16:1212-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frodin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 151:65-77. [DOI] [PubMed] [Google Scholar]
- 11.Frodin, M., C. J. Jensen, K. Merienne, and S. Gammeltoft. 2000. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 19:2924-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavin, A. C., and A. R. Nebreda. 1999. A MAP kinase docking site is required for phosphorylation and activation of p90(rsk)/MAPKAP kinase-1. Curr. Biol. 9:281-284. [DOI] [PubMed] [Google Scholar]
- 13.Gavin, A. C., A. Ni Ainle, E. Chierici, M. Jones, and A. R. Nebreda. 1999. A p90(rsk) mutant constitutively interacting with MAP kinase uncouples MAP kinase from p34(cdc2)/cyclin B activation in Xenopus oocytes. Mol. Biol. Cell 10:2971-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao, K.-M., S.-Y. Chou, S.-J. Shih, and J. E. Ferrell, Jr. 1994. Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc. Natl. Acad. Sci. USA 91:5480-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen, C. J., M. B. Buch, T. O. Krag, B. A. Hemmings, S. Gammeltoft, and M. Frodin. 1999. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 274:27168-27176. [DOI] [PubMed] [Google Scholar]
- 16.Keyse, S. M. 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 17.Leighton, I. A., K. N. Dalby, F. B. Caudwell, P. T. Cohen, and P. Cohen. 1995. Comparison of the specificities of p70 S6 kinase and MAPKAP kinase-1 identifies a relatively specific substrate for p70 S6 kinase: the N-terminal kinase domain of MAPKAP kinase-1 is essential for peptide phosphorylation. FEBS Lett. 375:289-293. [DOI] [PubMed] [Google Scholar]
- 18.Murphy, L. O., S. Smith, R. H. Chen, D. C. Fingar, and J. Blenis. 2002. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4:556-564. [DOI] [PubMed] [Google Scholar]
- 19.Nichols, A., M. Camps, C. Gillieron, C. Chabert, A. Brunet, J. Wilsbacher, M. Cobb, J. Pouyssegur, J. P. Shaw, and S. Arkinstall. 2000. Substrate recognition domains within extracellular signal-regulated kinase mediate binding and catalytic activation of mitogen-activated protein kinase phosphatase-3. J. Biol. Chem. 275:24613-24621. [DOI] [PubMed] [Google Scholar]
- 20.Palmer, A., A. C. Gavin, and A. R. Nebreda. 1998. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17:5037-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 22.Pouyssegur, J., V. Volmat, and P. Lenormand. 2002. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharmacol. 64:755-763. [DOI] [PubMed] [Google Scholar]
- 23.Richards, S. A., V. C. Dreisbach, L. O. Murphy, and J. Blenis. 2001. Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol. Cell. Biol. 21:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards, S. A., J. Fu, A. Romanelli, A. Shimamura, and J. Blenis. 1999. Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr. Biol. 9:810-820. [DOI] [PubMed] [Google Scholar]
- 25.Sassone-Corsi, P., C. A. Mizzen, P. Cheung, C. Crosio, L. Monaco, S. Jacquot, A. Hanauer, and C. D. Allis. 1999. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science 285:886-891. [DOI] [PubMed] [Google Scholar]
- 26.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schouten, G. J., A. C. O. Vertegaal, S. T. Whiteside, A. Israel, M. Toebes, J. C. Dorsman, A. J. van der Eb, and A. Zantema. 1997. IκBα is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 16:3133-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scimeca, J. C., T. T. Nguyen, C. Filloux, and E. Van Obbergen. 1992. Nerve growth factor-induced phosphorylation cascade in PC12 pheochromocytoma cells. Association of S6 kinase II with the mictrotubule-associated protein kinase, ERK1. J. Biol. Chem. 267:17369-17374. [PubMed] [Google Scholar]
- 29.Shimamura, A., B. A. Ballif, S. A. Richards, and J. Blenis. 2000. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr. Biol. 10:127-135. [DOI] [PubMed] [Google Scholar]
- 30.Smith, J. A., C. E. Poteet-Smith, K. Malarkey, and T. W. Sturgill. 1999. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 274:2893-2898. [DOI] [PubMed] [Google Scholar]
- 31.Vik, T. A., and J. W. Ryder. 1997. Identification of serine 380 as the major site of autophosphorylation of Xenopus pp90rsk. Biochem. Biophys. Res. Commun. 235:398-402. [DOI] [PubMed] [Google Scholar]
- 32.Vik, T. A., L. J. Sweet, and R. L. Erikson. 1990. Coinfection of insect cells with recombinant baculovirus expressing pp60v-src results in the activation of a serine-specific protein kinase pp90rsk. Proc. Natl. Acad. Sci. USA 87:2685-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing, J., D. D. Ginty, and M. E. Greenberg. 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959-963. [DOI] [PubMed] [Google Scholar]
- 34.Zhao, Y., C. Bjorbaek, and D. E. Moller. 1996. Regulation and interaction of pp90(rsk) isoforms with mitogen-activated protein kinases. J. Biol. Chem. 271:29773-29779. [DOI] [PubMed] [Google Scholar]