Abstract
MEK is a dual-specificity kinase that activates the extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase upon agonist binding to receptors. The ERK/MAP kinase cascade is involved in cell fate determination in many organisms. In mammals, this pathway is proposed to regulate cell growth and differentiation. Genetic studies have shown that although a single Mek gene is present in Caenorhabditis elegans, Drosophila melanogaster, and Xenopus laevis, two Mek homologs, Mek1 and Mek2, are present in the mammalian cascade. The inactivation of the Mek1 gene leads to embryonic lethality and has revealed the unique role played by Mek1 during embryogenesis. To investigate the biological function of the second homolog, we have generated mice deficient in Mek2 function. Mek2 mutant mice are viable and fertile, and they do not present flagrant morphological alteration. Although several components of the ERK/MAP kinase cascade have been implicated in thymocyte development, no such involvement was observed for MEK2, which appears to be nonessential for thymocyte differentiation and T-cell-receptor-induced proliferation and apoptosis. Altogether, our findings demonstrate that MEK2 is not necessary for the normal development of the embryo and T-cell lineages, suggesting that the loss of MEK2 can be compensated for by MEK1.
The mitogen-activated protein (MAP) kinase signaling pathways consist of protein kinase cascades linking extracellular stimuli to various targets scattered in the cytoplasm, the cytoskeleton, the membrane, and the nucleus (38). There are at least three distinct MAP kinase signaling pathways in mammals, including the extracellular signal-regulated kinases (ERKs), the c-Jun N-terminal kinases, and the p38 MAP kinase (12). These kinases are activated in cascades by phosphorylation on both threonine and tyrosine residues in the regulatory TXY loop present in all MAP kinases. This phosphorylation is carried out via distinct upstream dual-specificity MAP kinase kinases (MAPKKs). The classical pathway, which appears to be the major one in growth factor signaling, uses MAP kinase- or ERK-activating kinases (MEK and MAPKK) and ERK isoforms (MAP kinase) and is named the ERK/MAP kinase-signaling pathway. In mammals, MAPKK constitutes a small family of related proteins, but only MEK1 and MEK2 are known participants in the ERK/MAP kinase cascade (38). Directly downstream of MEK1 and MEK2, ERK1 and ERK2 phosphorylate a large number of substrates located in the cytoplasm and the nucleus (13, 25, 27, 38, 45).
The ERK/MAP kinase pathway is also involved in cell fate determination in Caenorhabditis elegans, Drosophila melanogaster, and Xenopus laevis (21, 28, 41, 44). While two different MEK proteins are present in the ERK/MAP kinase cascade in mammals, a single Mek gene fulfills this role in these species. Sequence analysis revealed that the murine MEK1 protein is more related to the Xenopus MEK than to the mouse MEK2. Indeed, MEK2 protein is only 80% identical and 90% similar to MEK1, whereas Xenopus MEK is 91% identical and 96% similar to murine MEK1 (7, 13, 37). Two regions of MEK2 show reduced homology with MEK1: (i) the amino terminus (33% identical, 66% similar), which includes the ERK docking site and the nuclear exclusion sequence, and (ii) the MEK-specific sequence (21% identical, 36% similar), which is common to MEK proteins from different species (17, 34). The MEK-specific sequence domain of MEK1 contains p21-activated kinase 1 (PAK) phosphorylation sites important for MEK1 function. It is also involved in the interaction with the Raf family members (8, 15, 16, 31, 34). The protein sequence differences observed between MEK1 and MEK2 suggest that MEK2 has diverged from MEK1, most likely to achieve unique functions in mammals. Moreover, many observations indicate functional differences between MEK1 and MEK2. For instance, only MEK1 is activated in Swiss 3T3 or macrophage cells in response to bombesin or tumor necrosis factor alpha, respectively (40, 42). In addition, ERK/MAP kinase cannot be activated in response to bombesin in Mek1−/− mouse embryonic fibroblasts (MEFs) (M. Tremblay and J. Charron, unpublished data). In contrast, MEK2 is specifically activated by lactosylceramide in human aortic smooth muscle cells or by estradiol in mouse cerebral cortex (5, 39). Differential activation of MEK1 and MEK2 by Raf family members in epidermal growth factor (EGF)-stimulated HeLa cells has also been described previously (43). In addition, only MEK1 can form a signaling complex with Ras and c-Raf in serum-stimulated NIH 3T3 cells, suggesting that in these cells the c-Raf signaling pathway preferentially activates MAP kinases via MEK1 (24). Finally, the Rac-PAK pathway has been shown to be involved in the activation of the ERK/MAP kinase cascade by regulating the formation of a specific MEK1-ERK signaling complex, which is dependent on PAK phosphorylation sites present exclusively in MEK1 (16). All of these results support a model in which the transduction of specific signals transits via distinct protein kinase isoforms through the ERK/MAP kinase cascade (1). The presence of these various isoforms at different levels of the pathway may reflect the complexity of the controls required for regulation of the multiple mammalian cellular processes.
To determine the specific role of both MEK1 and MEK2, we generated a mutant mouse line for the Mek1 function. Inactivation of the Mek1 gene causes embryonic death at around 10.5 days of gestation (embryonic day 10.5 [E10.5]) (18). Characterization of the mutant phenotype revealed no major anomaly in the embryo proper, but a remarkably underdeveloped placenta indicated that MEK1 is essential for normal placental development. In addition, in vitro studies revealed the absence of redundant functions between MEK1 and MEK2 in the regulation of haptotaxis cell migration induced by specific extracellular matrixes, demonstrating the essential role of MEK1 in the ERK/MAP kinase pathway during embryonic development.
The Mek1 mutant embryos exhibit a specific phenotype that implies that MEK2 cannot fully complement the lack of Mek1 function. To explore the biological role of Mek2 in vivo, we generated a mutant mouse line deficient in Mek2 gene function by genetic elimination. Mek2−/− mice are viable and fertile and do not present evident growth defects. Moreover, no gross anatomical alteration has been observed. Our results also demonstrate that Mek2 is not required for proliferation and for the reentry of the cells in the cell cycle. Finally, while the ERK/MAP kinase cascade is known to be involved in T-cell development and activation, Mek2 appears dispensable for these functions (2, 3, 14, 33). All together, our findings suggest that Mek1 can compensate for the lack of Mek2 function.
MATERIALS AND METHODS
Mek2 targeting vector and chimeric mouse production.
Mek2 genomic sequences were obtained from a λ phage isolated from a 129/Sv mouse strain-derived genomic library. The targeting vector was made by using a 6.6-kb genomic fragment encompassing exons 3 to 8 of the Mek2 gene, which was fused to the herpes simplex virus-thymidine kinase cassette for selection against random integration (30). To interrupt the Mek2 sequences, a neomycin resistance cassette was inserted in the opposite orientation between nucleotides 630 and 736, according to the Mek2 cDNA sequence (GenBank accession no. NM 023138), thereby resulting in a 1-kb deletion of genomic sequences (Fig. 1A). The targeting vector comprised 3.6 and 2 kb of Mek2-homologous sequences at its 5′ and 3′ extremities, respectively (Fig. 1A). WW6 embryonic stem (ES) cells were electrophoresed with 25 μg of linearized targeting vector DNA as previously described and plated on neor feeder layers (10, 22). After 24 h, selection for G418 and ganciclovir was applied (G418, 400 μg/ml; Invitrogen, Burlington, Ontario, Canada; ganciclovir, 2 μM; Syntex, Palo Alto, Calif.). ES cell clones recovered from drug selection were screened by Southern blot analysis. Positive clones were injected into MF1 blastocysts and transferred into pseudopregnant foster mothers to generate chimeras that were tested for germ line transmission as previously described (9, 18).
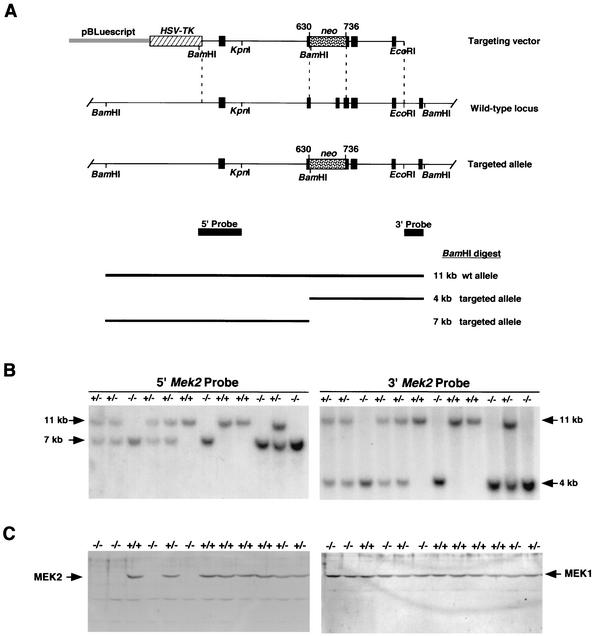
FIG. 1.
Targeted disruption of the Mek2 gene. (A) Mek2 gene-targeting strategy. Translated Mek2 exons are represented by black boxes. A neo selection marker (stippled box) was inserted between the fourth and sixth Mek2 exons, deleting a 1-kb DNA fragment. The targeting vector contains 3.6 and 2 kb of Mek2 homologous genomic sequences on the 5′ and 3′ sides of the neo insertion, respectively. The herpes simplex virus-thymidine kinase (HSV-TK) selection cassette (hatched box) was added at the 5′ end of the homology. wt, wild type. (B) Southern blot analysis. DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Mek2 mutant mice digested with BamHI was hybridized with a 5′ probe (BamHI-KpnI targeting vector fragment) and a 3′ probe (EcoRI-BamHI fragment). The 11-kb band represents the wild-type allele, and the 4- and 7-kb bands correspond to the mutant allele hybridized with the 3′ and the 5′ probes, respectively. (C) Western blot analysis of Mek2−/− mice. Proteins extracted from E11.5 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Mek2 mutant embryos were analyzed by SDS-PAGE. Duplicate blots were probed with antibodies directed against MEK1 or MEK2.
Genotyping of targeted ES cells, mice, and embryos.
Genomic DNA from cultured ES cells, mouse tail biopsies, or embryonic yolk sacs was extracted as previously described (9, 10). Purified DNA was digested with the restriction enzyme indicated in Fig. 1, fractionated by electrophoresis through 0.8% agarose gels, blotted onto a Hybond-NX membrane (Amersham Biosciences, Baie d'Urfé, Quebec, Canada), and hybridized by following procedures recommended by the supplier for either the 5′ Mek2 probe (BamHI-SacI fragment from the targeting vector) or the 3′ Mek2 probe (EcoRI-BamHI genomic fragment), as shown in Fig. 1.
Routine genotyping of DNA isolated from tail biopsies or embryo yolk sacs was performed by PCR. The primers were identified as Mek2 ko/629 (5′-TTGGGGAAGGTCAGCATTGC-3′), Mek2 ko/754 (5′-GCCGCTCACCCCGAAGTCAC-3′), and Mek2 ko/neo (5′-GCACGAGGAAGCGGTCAGCCC-3′). The Mek2 ko/629 and Mek2 ko/754 primers were specific for the wild-type Mek2 allele (annealing to nucleotides 629 to 648 and 754 to 772 of the published Mek2 cDNA sequence [7]), and they amplified a fragment of 1.1 kb. The primer Mek2 ko/neo was specific to the neo sequences (nucleotides 857 to 877; GenBank accession no. V00618). In conjunction with the primer Mek2 ko/754, a fragment of 1.3 kb was amplified from the Mek2-targeted allele. The three primers were used in a 25-μl reaction mixture. Cycling conditions were 93°C for 3 min, followed by 40 cycles of 93°C for 30 s, 55°C for 30 s, and 68°C for 1 min, with a final elongation cycle of 5 min at 68°C. The amplified products were analyzed by electrophoresis on 1.5% agarose gels.
Western blot analysis and MAP kinase mobility shift assay.
Protein extracts were obtained from embryonic tissues or primary mouse embryonic fibroblasts. Embryonic tissues were homogenized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 2.5% β-mercaptoethanol, 10% glycerol, and 0.005% bromophenol blue), and MEFs were extracted in MGM HL buffer (20 mM MOPS [morpholinepropanesulfonic acid, pH 7.0], 10% glycerol, 80 mM β-glycerophosphate, 5 mM EGTA, 0.5 mM EDTA, 1 mM Na3VO4, 5 mM NaPPi, 50 mM NaF, 1% Triton X-100, 1 mM benzamindine, 1 mM dithiothreitol, and 1 mM phenylmethlysulfonyl fluoride). Total protein lysates (20 μg) were resolved by denaturing SDS-10% PAGE, transferred to nitrocellulose by electroblotting, and probed with specific polyclonal or monoclonal antibodies by following procedures recommended by the suppliers. Antibodies used included polyclonal anti-MEK1 antibodies (13, 32), monoclonal anti-MEK2 M24529 antibodies (Transduction Laboratory, Lexington, Ky.), polyclonal anti-ERK2 antibodies (36), polyclonal anti-phospho-p38 MAP kinase antibodies, and monoclonal anti-phospho-c-Jun N-terminal kinase/stress-activated protein kinase G9 antibodies (Cell Signaling Technology, Mississauga, Ontario, Canada). Antibody binding was revealed with the ECL Plus Western blotting detection system (Amersham Biosciences). Quantification was performed by using a Molecular Dynamics PhosphorImager.
The MAP kinase mobility shift assay was performed as described by Chen et al. (11), with the exception that total protein lysates were electrophoresed on SDS-10% polyacrylamide gels with a 150:1 acrylamide/bis-acrylamide ratio. The ERK2 polyclonal antibody was used to probe the Western blot (36).
Cell cycle analysis.
Primary mouse embryonic fibroblasts were prepared from wild-type and Mek2−/− embryos as previously described (18). For cell cycle analysis, they were plated in 60-mm-diameter tissue culture dishes at 5 × 105 cells per dish in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin, 100 μg of streptomycin, and 2 mM glutamine (Invitrogen). A few hours later, after attachment and spreading of the cells, the serum concentration was reduced to 0.1% for 24 h. Reentry of the cells into the cell cycle was induced by addition of 20% FBS and measured after various periods of time. Cells were collected by trypsinization and stained with propidium iodide as previously described (4). Cell cycle analysis was performed on a Beckman Coulter Epics Elite model ESP by using the Multicycle program.
Lymphocyte preparations and flow cytometry.
Single-cell suspensions were prepared by gently pressing lymphoid organs (spleen, thymus, and lymph nodes) from 3- to 6-week-old wild-type and Mek2−/− mice between frosted-glass slides. Total lymphocytes were purified by filtration through Nitex nylon mesh (pore size, 53 μm). Cells (106) were stained with anti-CD4, -CD8, -CD25, -CD44, or -CD69 antibodies by standard procedures (29). Flow cytometry was performed on a Beckman Coulter Epics Elite model ESP and analyzed by using Expo version 2 software.
T-lymphocyte proliferation assays.
T lymphocytes were obtained from the spleens and thymuses of 4- to 8-week-old wild-type and Mek2−/− mice (19). They were plated in Pro-bind flat-bottomed, 96-well microtiter plates (Falcon, Franklin Lakes, N.J.) at 2 × 105 cells/well in RPMI medium supplemented with 10% FBS, 100 U of penicillin, 100 μg of streptomycin, 2 mM glutamine (Invitrogen), and 50 μM β-mercaptoethanol. The plates were coated with 50 μl of antibody dilutions for 2 h and washed twice with phosphate-buffered saline just before the addition of the cells. The plates were incubated at 37°C in a 5% CO2 atmosphere, and cells in each well were pulsed with 1 μCi of tritiated thymidine (Amersham Biosciences) for the last 18 h of a 72-h culture period. The cells were harvested with a Tomtec cell harvester and counted with a Wallac model 1450 microbeta scintillation counter.
Statistical analysis.
Analysis of variance with two criteria of classification (genotype and sex) was used to compare the growth curves of wild-type, Mek2+/−, and Mek2−/− mice. Mixed-model analysis was performed by using PROC MIXED from SAS Systems to assess differences in the proliferation rates of thymocytes and T cells between genotypes at all induction levels where the day of main effect is regarded as random while the genotype is considered fixed. Student's t tests were performed to compare T-cell subpopulations in wild-type and Mek2−/− lymphoid organs.
RESULTS AND DISCUSSION
Disruption of the Mek2 gene in mice by homologous recombination.
To define the specific role of Mek2 in vivo, we generated a null allele of the Mek2 gene by homologous recombination into ES cells. A targeting vector, in which the neo selection cassette was inserted into the Mek2 gene, deleting sequences from the fourth, fifth, and sixth exons, was designed (Fig. 1A). The neo insertion interrupts the Mek2 coding sequences after the 196th amino acid, located between the kinase subdomains VI and VII (20). Therefore, homologous recombination results in the loss of Mek2 sequences that encode the subdomains VII and VIII of the MEK2 kinase domain. The neo cassette also interrupts translation of the MEK2 protein.
After electrophoresis of the linearized targeting vector, WW6 ES cells were selected with G418 and ganciclovir. Out of 818 neomycin-resistant ES cell clones analyzed, three clones carried the disrupted Mek2 allele, as determined by Southern blot analysis with a 3′ external probe (Fig. 1B). The presence of a new BamHI site, introduced by the insertion of the neo selection marker into the Mek2 gene, was revealed by the detection of a 4-kb band in addition to the wild-type 11-kb band. Moreover, the 5′ probe allowed the detection of the expected 7-kb band for the targeted allele, showing that the integration of the targeting vector occurred by homologous recombination at both ends (Fig. 1B). The three correctly targeted ES clones were injected into MF1 blastocysts, and two of them (clones 7.6.c and 9.12.f) generated chimeras that transmitted the disrupted Mek2 allele through the germ line. Germ line chimeras were mated with 129/SvEv mice to establish mouse lines. The Mek2+/− mice of both sexes were normal and fertile compared to their wild-type littermates. To assess the consequences of the lack of Mek2 function in the mouse, Mek2+/− mice were intercrossed and the progeny were analyzed. Animals derived from both ES cell clones displayed the same phenotype, and the results presented were generated from the mouse line derived from the 7.6.c ES cell clone.
Mek2-deficient mice are viable.
Mek2−/− animals were born, and they appeared phenotypically normal compared to their heterozygous and wild-type littermates. Among the 313 viable offspring from heterozygous matings, expected Mendelian ratios were obtained: 81 (26%) were wild type, 164 (52%) were heterozygous, and 68 (22%) were homozygous, indicating that the Mek2 mutation does not cause embryonic lethality. Moreover, the Mek2−/− mice were fertile. Thus, these results demonstrated that Mek2 is not required for mouse development. They also suggested that Mek1 might compensate for Mek2 gene function.
To confirm that the targeted allele does not produce any form of MEK2 protein, Western blot analysis of protein extracts from E11.5 embryos or primary MEFs was performed by using two different antibodies directed against the amino terminus of MEK2. MEK2 protein was observed in extracts from wild-type and Mek2+/− embryos and MEFs. In Mek2−/− specimens, no full-length or truncated forms of MEK2 protein were detected (Fig. 1C and data not shown). These results established that the Mek2 mutation generated a null allele.
Immunoblotting with an MEK1 antibody showed that MEK1 protein was present in all embryonic and MEF samples regardless of their genotype. No obvious variation in the level of MEK1 between wild-type and Mek2−/− samples was observed, suggesting that there is no compensation by overexpression of Mek1 (Fig. 1C and data not shown).
Normal growth of Mek2−/− mice and MEFs.
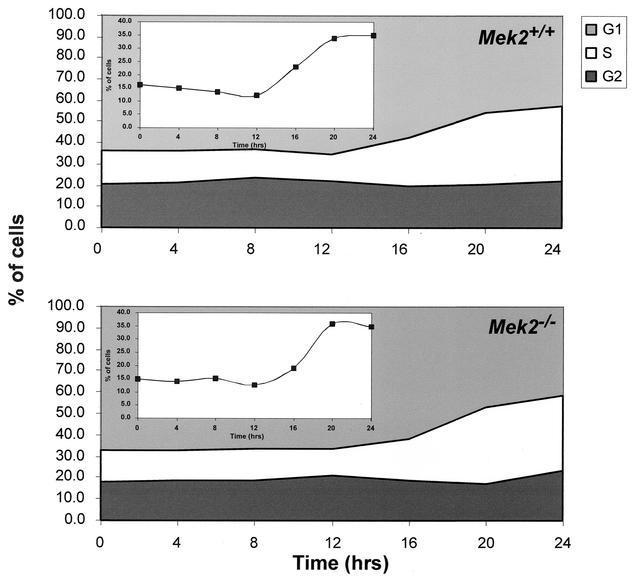
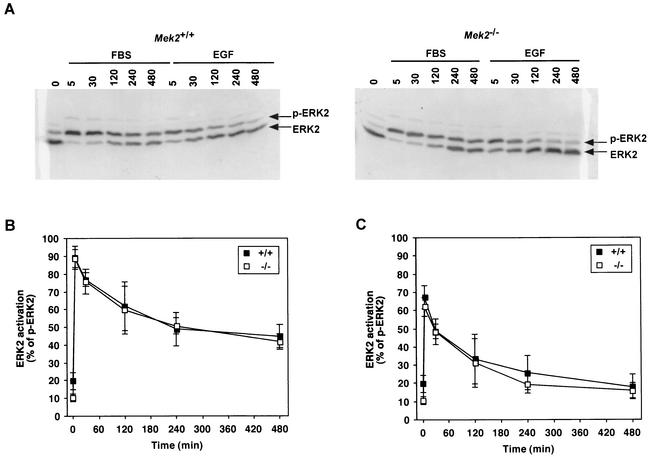
To investigate the specific role of Mek2 in the mitogen response, we first examined the proliferation rate of primary MEFs derived from wild-type and Mek2−/− embryos. No significant difference in the doubling times was observed between the wild-type and the mutant cultures (30 ± 4 h for the wild-type MEFs [n = 5]; 35 ± 6.5 h for the Mek2−/− MEFs [n = 4]; P = 0.62). We also investigated the ability of the Mek2−/− MEFs to reinitiate DNA synthesis. Primary wild-type and Mek2−/− MEFs were serum deprived (in 0.1% FBS) for 24 h before reinitiation of DNA synthesis by treatment with 20% FBS. Both MEF cultures showed identical kinetics of DNA synthesis (Fig. 2). In agreement with this observation, no difference in temporal activation of ERK1 and ERK2 during reentry into the cell cycle was observed between primary wild-type and Mek2−/− MEFs in response to FBS and EGF (Fig. 3). Moreover, no statistically significant difference in growth rate was noted among wild-type, Mek2+/−, and Mek2−/− male and female mice (Fig. 4). All together, these results indicated that MEK2 is not essential for cell proliferation and ERK1/ERK2 activation in response to mitogens.
FIG. 2.
Cell cycle analysis of wild-type and Mek2−/− MEFs. Confluent cultures of wild-type and Mek2−/− primary MEFs were serum deprived in 0.1% FBS for 24 h. Reentry into the cell cycle was induced with 20% FBS. Cell samples were collected at 4-h intervals for 24 h by trypsinization, stained with propidium iodide, and analyzed for DNA content by flow cytometry. The percentages of cells in the G1, S, and G2/M phases at each time point are represented. The insets show the percentages of cells in S phase. Representative results from five wild-type and four Mek2−/− MEF cultures are shown.
FIG. 3.
Time course of serum-stimulated ERK2 phosphorylation in wild-type and Mek2−/− MEFs. Primary MEFs were serum deprived for 24 h and subsequently stimulated with 20% FBS or 1 ng of EGF/ml for the indicated times (0, 5, 30, 120, 240, and 480 min). (A) Total cell extracts from wild-type and Mek2−/− MEFs were separated by SDS-PAGE to resolve ERK2 and phospho-ERK2 (p-ERK2), which were revealed by immunoblotting by using an antibody directed against ERK2. (B and C) Quantification with a PhosphorImager of the active form of ERK2 (percentage of phospho-ERK2) in wild-type and Mek2−/− MEFs when they were stimulated with 20% FBS (B) or 1 ng of EGF/ml (C). The means and standard deviations of the results from five wild-type and four Mek2−/− MEF cultures are represented.
FIG. 4.
Normal growth of Mek2−/− mice. Growth was assessed for wild-type (circles), Mek2+/− (triangles), and Mek2−/− (squares) female (open symbols) and male (filled symbols) mutant mice. Body weight was determined daily for the first 28 days and then weekly up until the eighth week. Standard deviations of the mean body weights are represented. No statistically significant difference was observed among the various genotypes (wild-type males, n = 30; wild-type females, n = 20; Mek2+/− males, n = 10; Mek2+/− females, n = 13; Mek2−/− males, n = 10; Mek2−/− females, n = 15).
Disruption of Mek2 does not alter normal T-cell development.
Experiments using transgenic mice producing the dominant negative form of members of the ERK/MAP kinase cascade or mice deficient in specific components of the cascade indicated that the ERK/MAP cascade plays a significant role in thymocyte growth and differentiation (2, 3, 14, 33). Precursor T cells mature in the thymus, and different populations corresponding to sequential development stages can be monitored by measuring the surface expression of CD4 and CD8 molecules (26, 35). The most immature thymocytes do not express CD4 and CD8 and are therefore named double negative (DN). Among the DN subset, four populations can be identified by their expression of CD44 and CD25 molecules, with the most immature CD44+ CD25− cells becoming CD44+ CD25+ and then CD44− CD25+ and finally CD44− CD25−. The last subset gives rise to CD4+ CD8+ double-positive (DP) thymocytes, which represent around 80% of all thymocytes. At this stage, DP thymocytes undergo either negative selection, i.e., apoptosis induction, if their T-cell receptor (TCR) is engaged with a too-high affinity by endogenous ligands, or positive selection, i.e., differentiation, if their TCR is engaged with a moderate affinity. DP thymocytes undergoing positive selection are characterized by the up-regulation of CD69 and TCR molecules at the cell surface. These cells finally mature to the single-positive (SP) stage by losing either CD4 or CD8 markers and exit the thymus to seed peripheral lymphoid organs such as lymph nodes and the spleen. During the DN stage, an alternative pathway of differentiation, less well characterized, leads to the generation of CD4− CD8− T cells expressing a γδTCR, instead of the αβTCR in the classical pathway. Transgenic mice expressing a dominant inactive form of MEK1, which is able to block MAP kinase activation in the T-cell lineage, present a defect in thymocyte differentiation from DP status to SP status, characterized by a decrease in the number of DP cells, which express high surface levels of CD69 (CD69high) (3). In parallel, Erk1−/− mice show defects in thymocyte differentiation leading to a reduction of the SP T-cell population as well as a decreased number of αβTCRhigh- and CD69high-expressing cells (33).
To determine whether Mek2 is involved in lymphocyte development and function, we examined thymus, spleen, and lymph node T-cell differentiation from wild-type and Mek2−/− mice by flow cytometry analysis. Mature T cells were measured as SP CD4+ or CD8+ cells. All Mek2−/− lymphoid organs examined had mature T cells in the same proportion as wild-type specimens, indicating that there was no major effect on thymocyte maturation (Fig. 5 and Table 1). The early stages of DN thymocyte differentiation were assessed by the determination of the CD25+ CD44− and CD25− CD44− subpopulations in the CD4− CD8− DN subset. Again, no difference was observed between wild-type and Mek2−/− samples (Table 1). In addition, no significant difference was observed in the numbers of βTCRhigh- and CD69high-expressing thymocytes in CD4 and CD8 DP or SP thymocytes (Table 1). We have also verified that the subpopulation of DN thymocytes that expresses the γδTCR was affected (23). The proportion of the γδTCR population was unchanged in Mek2−/− mice compared to that of wild-type specimens.
FIG. 5.
Flow cytometric analysis of CD4 and CD8 expression on T lymphocytes from wild-type and Mek2−/− lymphoid organs. Thymus, spleen, and lymph node cells from 4- to 6-week-old wild-type and Mek2−/− mice were stained with the designated antibodies and analyzed by flow cytometry. The percentage of cells in each quadrant is indicated. The results of a representative staining experiment are shown. A summary of all flow cytometry analyses performed on thymocytes is presented in Table 1. No statistically significant differences were observed between wild-type and Mek2−/− specimens.
TABLE 1.
Thymocyte populations in Mek2-deficient mice
| Subpopulation | Proportion (%) (mean ± SE)
|
P value | |
|---|---|---|---|
| Mek2+/+ | Mek2−/− | ||
| CD4− CD8− DN thymocytesa | 6.6 ± 1.2 | 7.5 ± 1.5 | 0.64 |
| CD4+ CD8+ DP thymocytesa | 79.4 ± 1.9 | 79.7 ± 2.1 | 0.92 |
| CD4+ SP thymocytesa | 10.5 ± 1.0 | 9.7 ± 1.8 | 0.69 |
| CD8+ SP thymocytesa | 3.4 ± 0.5 | 3.1 ± 0.6 | 0.69 |
| CD25+ CD44− subset in DN thymocytesb | 47.8 ± 3.3 | 48.8 ± 1.0 | 0.87 |
| CD25− CD44− subset in DN thymocytesb | 45.1 ± 3.3 | 47.6 ± 1.0 | 0.68 |
| γδTCR+b | 9.1 ± 1.4 | 8.0 ± 0.9 | 0.57 |
| βTCRhigha | 16.8 ± 8.2 | 17.3 ± 4.9 | 0.86 |
| CD69high subsetb | |||
| DP thymocytes | 2.7 ± 0.1 | 2.5 ± 0.1 | 0.48 |
| CD4+ SP thymocytes | 62.1 ± 1.2 | 60.2 ± 1.8 | 0.86 |
| CD8+ SP thymocytes | 32.1 ± 3.1 | 31.4 ± 0.4 | 0.57 |
Data for these populations were from 10 experiments.
Data for these populations were from two experiments.
Finally, the sizes of the lymphoid organs were comparable between wild-type and Mek2−/− specimens, and a normal number of cells, compared to that from wild-type specimens (data not shown), was isolated from the different Mek2−/− lymphoid tissues.
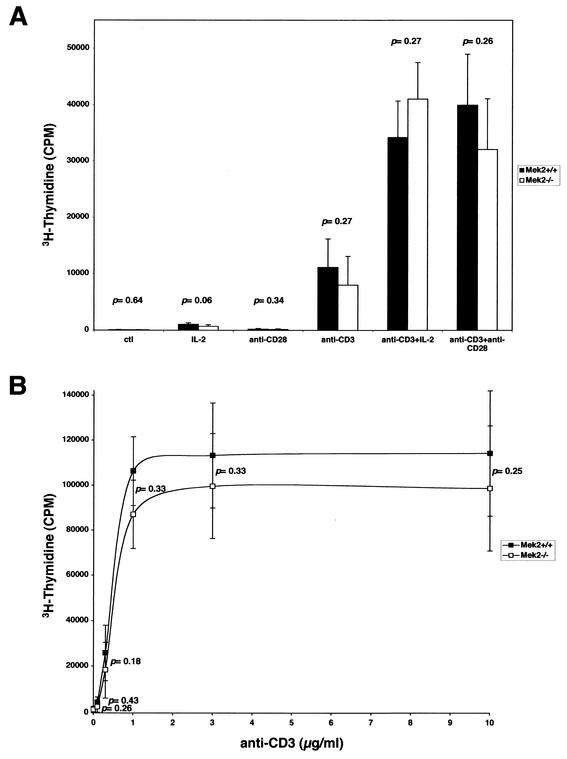
To determine whether T lymphocytes from the Mek2−/− mice were functional, we tested the ability of thymocytes and mature T cells to proliferate in response to signals that are mediated through the TCR. Thymocytes and T cells isolated from thymus and spleen were stimulated with anti-CD3ɛ, interleukin-2 (IL-2), and anti-CD28 alone or in combination. Proliferation was assessed by incorporation of tritiated thymidine. Thymocytes derived from wild-type and Mek2−/− mice responded to a level just above the background level in the presence of anti-CD28 or IL-2 alone. A stronger response was seen in the presence of anti-CD3ɛ. Coinduction with anti-CD3ɛ plus anti-CD28 or IL-2 led to a synergetic induction in both wild-type and Mek2−/− cultures (Fig. 6A). However, no statistically significant difference was observed for any of the conditions tested. In parallel, the proliferation rates of mature T cells derived from either wild-type or Mek2−/− spleens in response to anti-CD3ɛ (Fig. 6B) were found to be comparable.
FIG. 6.
Mitogen responsiveness of T cells derived from Mek2−/− mice. (A) Total thymocytes from wild-type and Mek2−/− mice (2 × 105 cells) were cultured in medium (ctl) or in the presence of IL-2, anti-CD3ɛ, or anti-CD28 alone or in combination in 96-well plates. Cells were stimulated with the indicated concentrations of mitogens, 10 U of IL-2/ml, anti-CD3ɛ (plated at 10 μg/ml), or anti-CD28 (plated at 10 μg/ml) for 72 h. The results shown are the means ± the standard errors of the means of results for nine different experiments done in triplicate. No statistically significant difference was observed. (B) Total T cells from wild-type and Mek2−/− spleens (2 × 105 cells) were cultured in the presence of various concentrations of anti-CD3ɛ (plated at 0.1 to 10 μg/ml). The results shown are the means ± the standard errors of the means of results from six different experiments done in triplicate. No statistically significant difference was observed. CPM, counts per minute.
As another measure of thymocyte function, the response to apoptotic signals was tested. It is known that CD4+ CD8+ DP thymocytes undergo apoptosis when their TCR is engaged by a high-affinity ligand (6). Therefore, Mek2−/− thymocytes were tested for their ability to respond to apoptotic signals induced by anti-CD3ɛ and anti-CD28 antibodies. In CD4 and CD8 DP and SP cells, the level of apoptosis determined by flow cytometry with an anti-annexin V antibody revealed no statistical difference between wild-type and Mek2−/− thymocytes (data not shown). Taken together, these results indicate the nonessential role of MEK2 in the thymocyte response to proliferation or apoptosis signals induced by the activation of the TCR.
Conclusion.
The mutation introduced in the Mek2 gene abolishes the production of MEK2 protein. Nevertheless, Mek2-deficient mice are normal in their overall general behavior, fertility, body weight, and life span. The presence in mammals of MEK1 suggests that functional redundancy can explain the absence of the phenotype observed in Mek2−/− mice. The MEK2 protein does not seem to possess specific functions essential for the animal that cannot be rescued by MEK1. While the Mek1 gene can compensate for the absence of MEK2 protein, the Mek2 gene cannot substitute for the crucial role of MEK1 during placenta development. The differences between the phenotypes can be explained by functional distinctions between the proteins, as shown by several cell culture and biochemical studies (5, 16, 39, 40, 42). Differences between MEK1 and MEK2 amino acid sequences exist, and these are most likely involved in the ability of each MEK isoform to interact with specific components of the ERK/MAP cascade or to be regulated by specific kinases.
MEK functional disparity can also be attributed to differential expression of each Mek gene during embryogenesis. Despite our observations that both Mek genes show a ubiquitous expression profile in the embryo and the placenta, we cannot rule out the possibility of a defined expression pattern for a subset of cells (18). Finally, levels of MEK activity can be a limiting factor. Modulation in levels of activity can be achieved by regulating the amount of protein or the duration of the activation or by varying the contribution of both MEK isoforms to the signaling cascade to mediate specific responses to various signals. Characterization of mutant mice carrying various combinations of Mek mutant alleles should help to determine the specific role of each gene in signal transduction. So far, we have observed that about 85% of Mek1+/−; Mek2+/− double heterozygous mice die before birth, indicating that the absence of one allele of each Mek gene has more deleterious effects on mouse development than the absence of both Mek2 alleles. These observations also suggest that Mek2 makes a functional contribution during embryogenesis.
Acknowledgments
We thank Lucie Jeannotte and Josée Aubin for critical reading of the manuscript, Pedro-O. De Campos-Lima for helpful discussions, Luc Caron and John H. Grose for ERK2 antibody, Nancy Roberge and Marcelle Carter for skilled technical assistance with flow cytometry analyses and blastocyst microinjection, respectively, and François Harel for statistical analyses.
This work was supported by grants from the Canadian Institutes of Health Research (grant MOP-42523) and the Cancer Research Society Inc. to J.C. J.C. is a Senior Scholar of the Fonds de la Recherche en Santé du Québec, and L.-F.B. held a studentship from NSERC.
REFERENCES
- 1.Acharya, U., A. Mallabiabarrena, J. K. Acharya, and V. Malhotra. 1998. Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92:183-192. [DOI] [PubMed] [Google Scholar]
- 2.Alberola-Ila, J., K. A. Hogquist, K. A. Swan, M. J. Bevan, and R. M. Perlmutter. 1996. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 184:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberola-Ila, J., K. A. Forbush, R. Seger, E. G. Krebs, and R. M. Perlmutter. 1995. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature 373:620-623. [DOI] [PubMed] [Google Scholar]
- 4.Aubry, S., and J. Charron. 2000. N-Myc shares cellular functions with c-Myc. DNA Cell Biol. 19:353-364. [DOI] [PubMed] [Google Scholar]
- 5.Bhunia, A. K., H. Han, A. Snowden, and S. Chatterjee. 1996. Lactosylceramide stimulates Ras-GTP loading, kinases (MEK, Raf), p44 mitogen-activated protein kinase, and c-fos expression in human aortic smooth muscle cells. J. Biol. Chem. 271:10660-10666. [DOI] [PubMed] [Google Scholar]
- 6.Bommhardt, U., Y. Scheuring, C. Bickel, R. Zamoyska, and T. Hünig. 2000. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J. Immunol. 164:2326-2337. [DOI] [PubMed] [Google Scholar]
- 7.Brott, B. K., A. Alessandrini, D. A. Largaespada, N. G. Copeland, N. A. Jenkins, C. M. Crews, and R. L. Erikson. 1993. MEK2 is a kinase related to MEK1 and is differentially expressed in murine tissues. Cell Growth Differ. 4:921-929. [PubMed] [Google Scholar]
- 8.Catling, A. D., H.-J. Schaeffer, C. W. M. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for Raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charron, J., B. A. Malynn, P. Fisher, V. Stewart, L. Jeannotte, S. P. Goff, E. J. Robertson, and F. W. Alt. 1992. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 6:2248-2257. [DOI] [PubMed] [Google Scholar]
- 10.Charron, J., B. A. Malynn, E. J. Robertson, S. P. Goff, and F. W. Alt. 1990. High-frequency disruption of the N-myc gene in embryonic stem and pre-B cell lines by homologous recombination. Mol. Cell. Biol. 10:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Q., M. S. Kinch, T. H. Lin, K. Burridge, and R. L. Juliano. 1994. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 269:26602-26605. [PubMed] [Google Scholar]
- 12.Cobb, M. H. 1999. MAP kinase pathways. Prog. Biophys. Mol. Biol. 71:479-500. [DOI] [PubMed] [Google Scholar]
- 13.Crews, C. M., A. Alessandrini, and R. L. Erikson. 1992. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 258:478-480. [DOI] [PubMed] [Google Scholar]
- 14.Crompton, T., K. C. Gilmour, and M. J. Owen. 1996. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86:243-251. [DOI] [PubMed] [Google Scholar]
- 15.Dang, A., J. A. Frost, and M. H. Cobb. 1998. The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J. Biol. Chem. 273:19909-19913. [DOI] [PubMed] [Google Scholar]
- 16.Eblen, S. T., J. K. Slack, M. J. Weber, and A. D. Catling. 2002. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22:6023-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda, M., Y. Gotoh, and E. Nishida. 1997. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giroux, S., M. Tremblay, D. Bernard, J. F. Cardin-Girard, S. Aubry, L. Larouche, S. Rousseau, J. Huot, J. Landry, L. Jeannotte, and J. Charron. 1999. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9:369-372. [DOI] [PubMed] [Google Scholar]
- 19.Guo, Z., G. Clydesdale, J. Cheng, K. Kim, L. Gan, D. J. McConkey, S. E. Ullrich, Y. Zhuang, and B. Su. 2002. Disruption of Mekk2 in mice reveals an unexpected role for MEKK2 in modulating T-cell receptor signal transduction. Mol. Cell. Biol 22:5761-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, J. C., and N. Perrimon. 1994. A temperature-sensitive MEK mutation demonstrates the conservation of the signaling pathways activated by receptor tyrosine kinases. Genes Dev. 8:2176-2187. [DOI] [PubMed] [Google Scholar]
- 22.Ioffe, E., Y. Liu, M. Bhaumik, F. Poirier, S. M. Factor, and P. Stanley. 1995. WW6: an embryonic stem cell line with an inert genetic marker that can be traced in chimeras. Proc. Natl. Acad. Sci. USA 92:7357-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway, C. A., A. V. Chervonsky, and D. Sant'Angelo. 1997. T-cell receptors: is the repertoire inherently MHC-specific? Curr. Biol. 7: R299-R300. [DOI] [PubMed] [Google Scholar]
- 24.Jelinek, T., A. D. Catling, C. W. M. Reuter, S. A. Moodie, A. Wolfman, and M. J. Weber. 1994. RAS and RAF-1 form a signalling complex with MEK-1 but not MEK-2. Mol. Cell. Biol. 14:8212-8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, G. L., and R. R. Vaillancourt. 1994. Sequential protein kinase reactions controlling cell growth and differentiation. Curr. Opin. Cell Biol. 6:230-238. [DOI] [PubMed] [Google Scholar]
- 26.Killeen, N., B. A. Irving, S. Pippig, and K. Zingler. 1998. Signaling checkpoints during the development of T lymphocytes. Curr. Opin. Immunol. 10:360-367. [DOI] [PubMed] [Google Scholar]
- 27.Klemke, R. L., S. Cai, A. L. Giannini, P. J. Gallagher, P. de Lanerolle, and D. A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornfeld, K., K. L. Guan, and H. R. Horvitz. 1995. The Caenorhabditis elegans gene mek-2 is required for vulval induction and encodes a protein similar to the protein kinase MEK. Genes Dev. 9:756-768. [DOI] [PubMed] [Google Scholar]
- 29.Malynn, B. A., J. Demengeot, V. Stewart, J. Charron, and F. W. Alt. 1995. Generation of normal lymphocytes derived from N-myc-deficient embryonic stem cells. Int. Immunol. 7:1637-1647. [DOI] [PubMed] [Google Scholar]
- 30.Mansour, S. L., K. R. Thomas, and M. R. Capecchi. 1988. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336:348-352. [DOI] [PubMed] [Google Scholar]
- 31.Nantel, A., K. Mohammad-Ali, J. Sherk, B. I. Posner, and D. Y. Thomas. 1998. Interaction of the Grb10 adapter protein with the Raf1 and MEK1 kinases. J. Biol. Chem. 273:10475-10484. [DOI] [PubMed] [Google Scholar]
- 32.Pages, G., A. Brunet, G. L'Allemain, and J. Pouyssegur. 1994. Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1). EMBO J. 13:3003-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pages, G., S. Guerin, D. Grall, F. Bonino, A. Smith, F. Anjuere, P. Auberger, and J. Pouyssegur. 1999. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286:1374-1377. [DOI] [PubMed] [Google Scholar]
- 34.Papin, C., A. Denouel, G. Calothy, and A. Eychene. 1996. Identification of signalling proteins interacting with B-Raf in the yeast two-hybrid system. Oncogene 12:2213-2221. [PubMed] [Google Scholar]
- 35.Rodewald, H. R., and H. J. Fehling. 1998. Molecular and cellular events in early thymocyte development. Adv. Immunol. 69:1-112. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau, S., F. Houle, J. Landry, and J. Huot. 1997. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15:2169-2177. [DOI] [PubMed] [Google Scholar]
- 37.Russell, M., C. A. Lange-Carter, and G. L. Johnson. 1995. Regulation of recombinant MEK1 and MEK2b expressed in Escherichia coli. Biochemistry 34:6611-6615. [DOI] [PubMed] [Google Scholar]
- 38.Seger, R., and E. G. Krebs. 1995. The MAPK signaling cascade. FASEB J. 9:726-735. [PubMed] [Google Scholar]
- 39.Setalo, G., Jr., M. Singh, X. Guan, and C. D. Toran-Allerand. 2002. Estradiol-induced phosphorylation of ERK1/2 in explants of the mouse cerebral cortex: the roles of heat shock protein 90 (Hsp90) and MEK2. J. Neurobiol. 50:1-12. [DOI] [PubMed] [Google Scholar]
- 40.Seufferlein, T., D. J. Withers, and E. Rozengurt. 1996. Reduced requirement of mitogen-activated protein kinase (MAPK) activity for entry into the S phase of the cell cycle in Swiss 3T3 fibroblasts stimulated by bombesin and insulin. J. Biol. Chem. 271:21471-21477. [DOI] [PubMed] [Google Scholar]
- 41.Umbhauer, M., C. J. Marshall, C. S. Mason, R. W. Old, and J. C. Smith. 1995. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature 376:58-62. [DOI] [PubMed] [Google Scholar]
- 42.Winston, B. W., L. K. Remigio, and D. W. Riches. 1995. Preferential involvement of MEK1 in the tumor necrosis factor-α-induced activation of p42mapk/erk2 in mouse macrophages. J. Biol. Chem. 270:27391-27394. [DOI] [PubMed] [Google Scholar]
- 43.Wu, X., S. J. Noh, G. Zhou, J. E. Dixon, and K. L. Guan. 1996. Selective activation of MEK1 but not MEK2 by A-Raf from epidermal growth factor-stimulated Hela cells. J. Biol. Chem. 271:3265-3271. [DOI] [PubMed] [Google Scholar]
- 44.Wu, Y., M. Han, and K. L. Guan. 1995. MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes Dev. 9:742-755. [DOI] [PubMed] [Google Scholar]
- 45.Zheng, C. F., and K. L. Guan. 1993. Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J. Biol. Chem. 268:23933-23939. [PubMed] [Google Scholar]