Abstract
Nonsense-mediated mRNA decay (NMD) is an RNA surveillance pathway that detects and destroys aberrant mRNAs containing nonsense or premature termination codons (PTCs) in a translation-dependent manner in eukaryotes. In yeast, the NMD pathway bypasses the deadenylation step and directly targets PTC-containing messages for decapping, followed by 5′-to-3′ exonuclease digestion of the RNA body. In mammals, most PTC-containing mRNAs are subject to active nucleus-associated NMD. Here, using two distinct transcription-pulsing approaches to monitor mRNA deadenylation and decay kinetics, we demonstrate the existence of an active cytoplasmic NMD pathway in mammalian cells. In this pathway, a nonsense codon triggers accelerated deadenylation that precedes decay of the PTC-containing mRNA body. Transcript is stabilized when accelerated deadenylation is impeded by blocking translation initiation; by ectopically expressing two RNA-binding proteins, UNR and NSAP1; or by ectopically expressing a UPF1 dominant-negative mutant. These results are consistent with the notion that the nonsense codon can function in the cytoplasm by promoting rapid removal of the poly(A) tail as a necessary first step in the decay process.
Eukaryotic cells have evolved various mechanisms to recognize and eliminate functionally aberrant RNAs (25). Nonsense-mediated mRNA decay (NMD) exemplifies one such type of quality control mechanism that selectively degrades mRNAs with premature termination codons (PTCs) (13, 19, 24), thus preventing the synthesis of potentially deleterious truncated proteins and rendering recessive a large fraction of human pathological mutations (10).
In mammalian cells, most PTC-containing mRNAs are specifically eliminated while still associated with the nucleus (nucleus-associated NMD) (17, 48). It has been reported that the steady-state pool of PTC-containing β-globin mRNA (BBBPTC) in the cytoplasm, presumably escaping nucleus-associated NMD, is immune to NMD (1, 8). Although nonsense globin mRNAs are degraded in the nuclear fraction of transfected mammalian cell lines, earlier studies using transgenic animals indicated that they are degraded in the cytoplasm of erythrocytes (20, 21). In yeast, the NMD pathway is deadenylation independent and is distinct from the deadenylation-dependent decay pathway for normal mRNAs (14, 31). PTCs are recognized during translation termination, resulting in direct decapping and subsequent 5′-to-3′ exonucleolytic digestion of the RNA body (26). It has been assumed that a similar deadenylation-independent mechanism also operates in mammalian cells to eliminate PTC-containing mRNAs (33, 49, 51). A preliminary observation (34) that degradation of PTC-containing β-globin mRNA (BBBPTC) seems to occur parallel to its deadenylation has been used to support this view. However, in this earlier study, the observation was based on a single experiment, and consequently several critical issues were not addressed. For example, it was not clear from the decay plot whether the nonsense codon significantly accelerated deadenylation. Moreover, if mammalian NMD is deadenylation independent, one would expect that interfering with deadenylation would not lead to any significant stabilization of PTC-containing β-globin mRNA (BBBPTC). This is a critical issue that has not actually been tested. As current models of mammalian NMD were proposed with the assumption that it is deadenylation independent and nucleus associated, elucidation of the relationship between deadenylation and NMD could provide important new insights into the NMD process and a better understanding of this intriguing surveillance mechanism.
Recent advances in our understanding of decay machinery and the process of mammalian RNA turnover point to some major differences between yeast and mammalian systems. For example, poly(A) shortening followed by 3′-to-5′ exonucleolytic digestion of the RNA body via the exosome is the major decay pathway in mammalian in vitro-reconstituted systems (5, 27, 46), whereas the major pathway in yeast involves deadenylation-dependent decapping followed by 5′-to-3′ exonuclease digestion of the RNA body (2). Increasing evidence indicates that the 3′ poly(A) tail of mRNAs plays a critical role in regulating the fate of mRNA by communicating with the 5′ cap, with the internal ribosomal entry site, and with various RNA stability determinants (11, 38, 43, 45, 47). Recent studies also indicate that the 3′ poly(A) tail interacts with the translation termination codon. Hoshino et al. (15) showed that mammalian eRF3, a translation termination-releasing factor, interacts strongly with poly(A)-binding protein (PABP), suggesting that the poly(A) tail associated with PABP participates in the recognition of a stop codon and thus in mammalian NMD. In view of the distinction between NMD in yeast and in mammalian cells and the interaction of the poly(A) tail with the translation termination complex, and the unresolved issues described above, we examined the relationship between deadenylation and NMD in the cytoplasm of mammalian cells.
MATERIALS AND METHODS
Plasmid construction.
The construction of plasmids pSVα1/GAPDH (6), pBBB, pBBBPTC (34), pTet-BBB (50), pBBB+hp (hp, hairpin) (7), pTet-Myc-Ovep, pTet-Myc-HuR (32), pSG-UNRFlag, and pSV-mNSAP1 (11) has been described previously. To generate pBBBPTC+hp and pTet-BBBPTC, an XbaI linker (New England Biolabs) containing stop codons in all three reading frames was inserted into the unique AccI site (blunt ended with T4 DNA polymerase) located within the second exon of the rabbit β-globin gene in pBBB+hp and pTet-BBB, respectively. Plasmids pCI-neo-FLAG-UPF1-WT and pCI-neo-FLAG-UPF1-R844C were kindly provided by Lynne Maquat (37).
RNA blot analysis and preparation of NIH 3T3 cytoplasmic extracts.
Cell culture, DNA transfection, isolation of total cytoplasmic RNA, Northern blot analysis, and lysate preparation were conducted as described previously (35). Briefly, NIH 3T3 cells (for the serum-inducible c-fos promoter system) or its subline B2A2 cells stably harboring tTA (for the Tet-regulated promoter system) (50) were transfected for ∼16 h with a total of 20 μg of DNA. When transcription was driven by the serum-inducible c-fos promoter, the cells were first serum starved for 25 h, followed by stimulation with 20% bovine serum (Gibco BRL). When the Tet-regulated promoter system was used, the cells were cultured in medium containing 25 ng of Tet/ml for another 24 h. Transient expression from the Tet-regulated gene was induced for 150 min by changing to Tet-free medium. Tet (500 ng/ml) was then added to block transcription, and total cytoplasmic RNA was extracted at various times. Gene-specific DNA probes for Northern blot analysis were prepared by the method of random oligonucleotide priming; 32P label was introduced by inclusion of [α-32P]dCTP (>6,000 Ci/mmol; DuPont). The quantitation of data was obtained by scanning the radioactive blots with an imager (Packard). Deadenylation and decay curves were plotted as described previously (34, 36).
For lysate preparations, one 100-mm-diameter culture dish of transfected cells from a parallel time course experiment was harvested for preparation of cytoplasmic extract (32). The protein concentrations of samples used in Western blot analysis were analyzed with the bicinchoninic acid protein assay reagent (Pierce).
Western blot analysis.
Proteins in cytoplasmic lysates were analyzed on a sodium dodecyl sulfate-8% polyacrylamide gel and transferred to nitrocellulose membranes, and immunoreactive bands were visualized using an ECL Western blotting kit (Amersham, Arlington Heights, Ill.). The antibodies used are described below (see the legend to Fig. 4). M2 antibody against the Flag tag in human Upf1 (hUpf1) protein was from Sigma and was used at 1:1,000 dilution. Purified monoclonal antibody against α-tubulin (DM1A) was from Sigma and was used at 1:40,000 dilution as a positive control for cytoplasmic protein preparations.
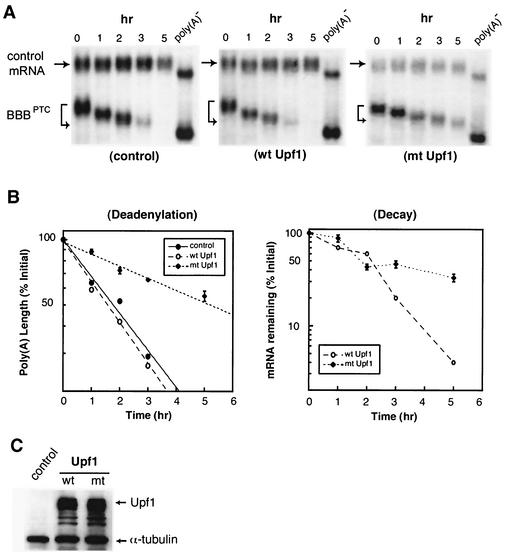
FIG. 4.
Ectopic expression of a dominant-negative mutant of human UPF1 protein slows down deadenylation of PTC-containing β-globin mRNA, leading to message stabilization. (A) Northern blots showing deadenylation and decay of BBBPTC mRNA in proliferating NIH 3T3 cells in the absence (control) or presence of ectopically expressed wild-type (wt) or R844C mutant (mt) hUpf1 protein. (B) Comparison of deadenylation and decay rates of BBBPTC mRNA in the absence (control) or presence of ectopically expressed wt Upf1 or mt Upf1 protein. Transient transfection, time course experiments, RNA isolation, poly(A)− RNA preparation, poly(A) length comparisons, and data quantitation and plotting were as described in the legend to Fig. 1. (C) Western blot analysis showing that wt and mt Upf1 proteins were expressed at similar levels. Cytoplasmic lysates prepared from NIH 3T3 B2A2 cells transfected with BBBPTC in the absence (control) or presence of either wt Upf1 or mt Upf1 protein were resolved on a sodium dodecyl sulfate-8% polyacrylamide gel. The blot was probed with antibody against the Flag tag for detection of Upf1 and its mutant proteins and a control monoclonal antibody against α-tubulin for equal loading of protein samples.
RESULTS
Nonsense codon triggers accelerated deadenylation of β-globin mRNA, preceding decay of the RNA body in the cytoplasm.
To assess the relationship between poly(A) tail removal and NMD in the cytoplasm of mammalian cells, we examined the deadenylation kinetics of a PTC-containing β-globin mRNA in serum-stimulated NIH 3T3 cells. The PTC was introduced into the rabbit β-globin gene at codon 36 in the second exon, 130 nucleotides from the downstream exon-intron junction (Fig. 1A, BBBPTC). Nonsense codons at and around this position in human β-globin mRNAs are sufficient to trigger NMD (1, 40, 51). Transcription of the β-globin gene was driven by the serum-inducible c-fos promoter, which allows rapid and transient induction of transcription upon serum stimulation of quiescent NIH 3T3 cells (34). This transcriptional pulsing produces a pool of newly transcribed mRNAs that are homogeneous in regard to the size of the poly(A) tail once they arrive in the cytoplasm. Cytoplasmic poly(A) shortening can thus be monitored by extracting cytoplasmic mRNA from transfected cells at various time points, making an unequivocal determination of in vivo deadenylation kinetics possible (22, 50).
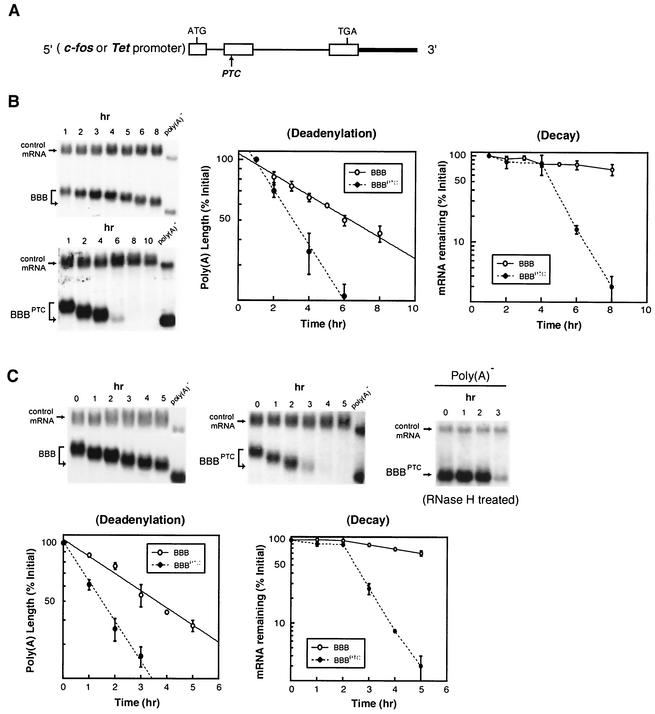
FIG. 1.
Nonsense codon triggers accelerated deadenylation of β-globin mRNA in the cytoplasm, preceding the decay of the mRNA body. (A) Physical map of β-globin gene whose transcription is driven by the c-fos or Tet-regulated promoter. The open rectangles, thin lines, and thick lines depict exons, introns, and the 3′ flanking region of the rabbit β-globin gene, respectively. The sequences encoding the translation initiation codon (ATG) and the termination codon (TGA) are indicated. The arrow marks the point where the PTC was introduced. (B) Left, Northern blots showing deadenylation and decay of wild-type (BBB) and PTC-containing (BBBPTC) β-globin mRNAs transcribed from the c-fos promoter in serum-induced NIH 3T3 cells. Middle, comparison of deadenylation rates of BBB and BBBPTC mRNAs. Right, comparison of decay rates of BBB and BBBPTC mRNAs. (C) Top, Northern blots showing deadenylation and decay of BBB and BBBPTC mRNAs transiently transcribed from the Tet-regulated promoter following a transcription pulse in proliferating NIH 3T3 B2A2 cells. Bottom left, comparison of deadenylation rates of BBB and BBBPTC mRNAs. Bottom right, comparison of decay rates of BBB and BBBPTC mRNAs. (B and C) NIH 3T3 cells (B) or subline cells stably harboring tTA for the Tet-regulated promoter system (C) were transiently cotransfected with a control plasmid (pSVα/GAPDH) and one of the test plasmids (pBBB or pBBBPTC). Total cytoplasmic mRNA was isolated at various time intervals after serum stimulation of quiescent cells (B) or after the addition of Tet (C) to proliferating cells and analyzed by Northern blot analysis (see Materials and Methods). α-globin/GAPDH control mRNAs were expressed constitutively and served as an internal standard. The times given above the blots correspond to hours after serum stimulation (B) or after Tet addition (C). Poly(A)− RNA was prepared in vitro by treating RNA samples with oligo(dT) and RNase H. Poly(A) lengths were compared by calculating from the difference in electrophoretic mobility between each message and cognate poly(A)− RNA. The quantitation of data was obtained by scanning the radioactive blots with an imager (Packard). Deadenylation and decay curves were plotted as described previously (34, 36). All curves shown in the plots are based on the average results of multiple experiments with standard deviations.
The BBBPTC mRNA displayed accelerated deadenylation before decay of the RNA body in the cytoplasm (Fig. 1B, BBBPTC), whereas wild-type β-globin mRNA (BBB) underwent much slower deadenylation and little decay of the message (Fig. 1B, BBB). The deadenylation process, quantitated by plotting the fraction of surviving poly(A) as a function of time, showed that in vivo deadenylation of both wild-type and PTC-containing β-globin mRNAs followed first-order kinetics. The process was significantly faster for the BBBPTC mRNA than for wild-type mRNA (Fig. 1B). The decay plot in Fig. 1B clearly showed that BBBPTC mRNA underwent accelerated deadenylation before decay of the RNA body.
The experiments described above were carried out in serum-induced NIH 3T3 cells undergoing a G0-to-G1 transition. To be sure that bona fide NMD was being examined, and to rule out the possibility that cytoplasmic deadenylation before degradation for PTC-containing β-globin mRNA was peculiar to serum-induced conditions, we performed additional time course experiments in proliferating NIH 3T3 cells. In this case, transcription pulsing from the BBBPTC gene was accomplished by the tetracycline-regulated promoter system (50). Following a short period of mRNA synthesis in the absence of tetracycline, transcription was abruptly shut off by adding the drug, and the deadenylation and decay of cytoplasmic mRNA were monitored. As shown in Fig. 1C, BBBPTC mRNA underwent accelerated deadenylation before decay of the RNA body in proliferating NIH 3T3 cells, just as in serum-induced NIH 3T3 cells (Fig. 1B). RNase H-directed removal of the poly(A) tail using RNA samples from various time points confirmed that shortening occurred from the 3′ poly(A) tail (Fig. 1C, top right).
The observed rapid decay of PTC-containing β-globin mRNA using transcriptional pulsing approaches is in contrast to previous studies showing that, once it has escaped the nucleus-associated NMD, the steady-state pool of PTC-containing β-globin mRNA transcribed from a constitutive promoter is not subject to NMD in the cytoplasm (1, 8). To better address this issue, we proceeded to perform the time course experiments monitoring mRNA decay under conditions mimicking those of the two earlier investigations. The tetracycline-regulated promoter-driven BBB and BBBPTC constructs were transiently transfected into NIH 3T3 B2A2 cells cultured in the absence of tetracycline to allow a constitutive expression of BBB and BBBPTC transcripts following transfection. Thirty-six hours later, RNA stability was determined by blocking ongoing transcription with tetracycline. Consistent with previous studies, the results (Fig. 2A) also show that the steady-state pool of cytoplasmic BBBPTC transcript decays at a rate identical to that of the normal BBB mRNA under such circumstances. The steady-state levels of BBBPTC transcript synthesized in the cytoplasm are only 6% of those of normal β-globin mRNA, indicative of an efficient nucleus-associated NMD (Fig. 2B, right). In contrast, the levels of BBBPTC transcript that arrived in the cytoplasm following transcription pulsing (Fig. 2B, left) were ∼60% of normal β-globin mRNA. Taken together, our results obtained using transcriptional-pulsing approaches revealed that an active NMD pathway exists in the cytoplasm of mammalian cells that is distinct from the nucleus-associated NMD. In this cytoplasmic pathway, deadenylation precedes the decay of the RNA body. Since the comparison was done using the same tetracycline-regulated promoter to drive transcription, our results also rule out the possibility that the difference is due to the use of different promoters.
FIG. 2.
Mode of transcription of PTC-containing β-globin gene affects nonsense-mediated decay for the transcript. (A) Steady-state pool of cytoplasmic BBBPTC transcript decays at a rate identical to that of the normal BBB mRNA after being constitutively expressed. The Tet-regulated promoter-driven BBB and BBBPTC constructs were transiently transfected into NIH 3T3 B2A2 cells cultured in the absence of tetracycline to allow constitutive expression of BBB and BBBPTC transcripts following transfection. Thirty-six hours later, mRNA stability was determined by blocking ongoing transcription with tetracycline. RNA isolation, data quantitation, and plotting were as described in the legend to Fig. 1. Left, Northern blots showing decay of the cytoplasmic steady-state pool of BBB (wild-type) or BBBPTC (PTC) mRNA constitutively transcribed from the Tet-regulated promoter in proliferating NIH 3T3 B2A2 cells. Right, comparison of decay rates of BBB and BBBPTC mRNAs. (B) Northern blots showing the direct side-by-side comparison of the levels of wild-type (wt) BBB and BBBPTC transcripts transcribed from the Tet-regulated promoter in a transient (left) or constitutive (right) mode. Transfection, RNA isolation, and data quantitation were as described in Materials and Methods and in the legend to Fig. 1.
Blocking translation of PTC-containing β-globin mRNA impedes the deadenylation step.
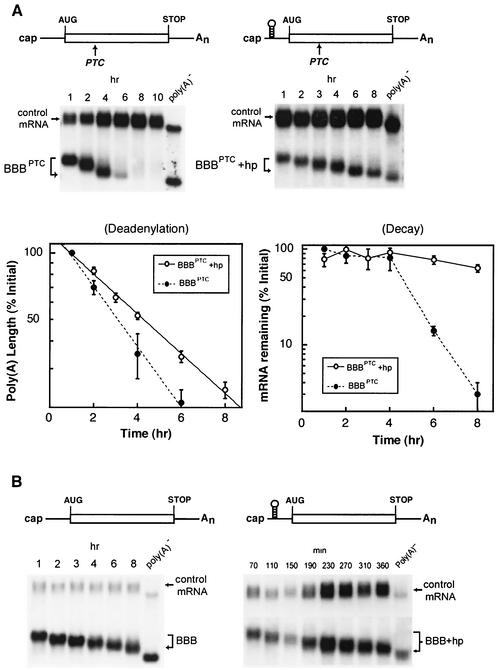
NMD is known to be tightly coupled to translation and to involve ribosome transit and possible formation of a posttermination surveillance complex (24, 49). Decay of PTC-containing β-globin mRNA was significantly slowed when translation was inhibited by the introduction of an iron-responsive stem-loop structure in the 5′ untranslated region (29). To determine whether increased stability of BBBPTC mRNA by translation blockade is related to impeded deadenylation, a stem-loop structure known to efficiently block translation initiation of normal β-globin mRNA or β-globin mRNA containing an AU-rich element (ARE), as demonstrated by a polysome profile study (7), was inserted into the 5′ untranslated regions of the BBBPTC mRNAs. As shown in Fig. 3A, this manipulation significantly slowed deadenylation and decay of BBBPTC+hp mRNA. Completion of deadenylation was postponed until after the 8-h time point, during which time there was little decay of the transcript. This effect was specific, as insertion of the stem-loop into wild-type β-globin mRNA had little effect on its deadenylation and decay kinetics (Fig. 3B). These data show that the accelerated deadenylation of the PTC-containing mRNA depends on active translation of the message, consistent with operation of a bona fide NMD pathway in the cytoplasm.
FIG. 3.
Targeted blockage of translation of PTC-containing β-globin mRNA impedes the deadenylation step and stabilizes the message. (A) Top, Northern blots showing deadenylation and decay of BBBPTC and BBBPTC+hp mRNAs. Bottom, comparison of deadenylation and decay rates of BBBPTC and BBBPTC+hp mRNAs. (B) Northern blots showing deadenylation and decay of BBB and BBB+hp mRNAs. Transient transfection, time course experiments, RNA isolation, poly(A)− RNA preparation, poly(A) length comparisons, and data quantitation and plotting were as described in the legend to Fig. 1. (Note: deadenylation and decay curves for BBBPTC mRNA are based on the results of multiple experiments, including the ones shown in this figure and in Fig. 1.)
Ectopic expression of a dominant-negative mutant of hUPF1 protein slows down deadenylation of PTC-containing β-globin mRNA.
NMD pathways described in yeast, Caenorhabditis elegans, and mammalian cells all involve Upf or its homologue proteins (12, 23, 30, 49). To further corroborate our finding that deadenylation-dependent NMD in the cytoplasm is a bona fide NMD, we monitored the kinetics of BBBPTC mRNA decay in the presence of a dominant-negative mutant of hUpf1 known to impede NMD in proliferating mammalian cells (37). The mutant hUpf1 protein has an arginine-to-cysteine mutation at residue 844 (R844C) within the RNA helicase domain and was Flag epitope tagged. As shown in Fig. 4, ectopic expression of Upf1(R844C) significantly slowed deadenylation of BBBPTC mRNA in proliferating NIH 3T3 cells and led to partial stabilization of the BBBPTC mRNA, whereas wild-type Upf1 or empty vector had little effect. Although a portion of BBBPTC mRNA still decayed after the 2-h time point, a significant portion of BBBPTC mRNA persisted without much degradation at the 3- and 5-h time points. Western blot analysis (Fig. 4C) showed that the wild-type and mutant Upf1 proteins were expressed at similar levels. These data further suggest that Upf1 plays a role in the PTC-triggered rapid deadenylation in the cytoplasm.
Ectopic expression of UNR and NSAP1 impedes deadenylation and decay of PTC-containing β-globin mRNA.
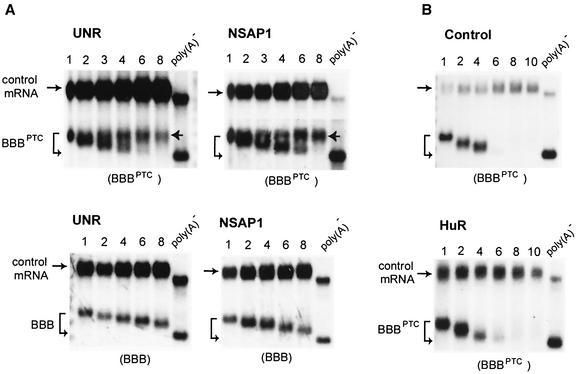
Previously, two RNA-binding proteins, UNR and NSAP1, that block deadenylation of β-globin mRNA bearing instability determinants when ectopically overexpressed in NIH 3T3 cells were identified (11). The results suggested that the two proteins interfere with the normal function of a poly(A) nuclease in cytoplasmic mRNA deadenylation. To further address the relationship between the deadenylation process and the NMD pathway, we examined whether ectopic expression of UNR and NSAP1 could block deadenylation of BBBPTC mRNA and stabilize the message in serum-stimulated NIH 3T3 cells. The results (Fig. 5A, top) showed that deadenylation of a subpopulation of BBBPTC transcript was completely stalled and the corresponding pool of mRNA was stable over the 8-h time course, whereas the remaining message underwent accelerated deadenylation and rapid decay. Importantly, in control experiments using transfection with an empty vector or ectopic expression of HuR, an ARE-binding protein, there were no appreciable changes in deadenylation or decay of BBBPTC mRNA (Fig. 5B). These results substantiate the conclusion that deadenylation precedes mRNA degradation in cytoplasmic NMD of BBBPTC mRNA and support the notion that deadenylation is a necessary step in this pathway.
FIG. 5.
Ectopic expression of UNR and NSAP1 blocks deadenylation of PTC-containing β-globin mRNA, leading to message stabilization. Shown are Northern blots demonstrating deadenylation and decay of BBBPTC mRNAs in serum-stimulated NIH 3T3 cells in the absence (B, Control) or presence of ectopically expressed UNR (A, top left), NSAP1 (A, top right), or HuR (B, bottom) and deadenylation and decay of BBB mRNA in the presence of ectopically expressed UNR (A, bottom left) or NSAP1 (A, bottom right). Transient transfection, time course experiments, RNA isolation, and poly(A)− RNA preparation were as described in the legend to Fig. 1.
To further assess the specificity of the observed partial blockage of deadenylation of BBBPTC mRNA by UNR and NSAP1, we also showed that neither UNR nor NSAP1 had any significant effect on the deadenylation kinetics of wild-type BBB mRNA (Fig. 5A, bottom). Thus, it was striking that deadenylation blocked by UNR and NSAP1 was related to the presence of a nonsense codon. This observation could argue for a fundamental difference between the mRNP structures of normal β-globin mRNA and nonsense-containing β-globin mRNA.
DISCUSSION
In this study, we provided several lines of evidence that demonstrate the presence of an active mammalian cytoplasmic NMD pathway in which removal of the poly(A) tail precedes the decay of the body of PTC-containing β-globin mRNA (Fig. 1 to 5). We show that introduction of a PTC codon in the β-globin transcript leads to accelerated deadenylation both at the G0-to-G1 transition and in the proliferating state of mouse fibroblasts. Moreover, the accelerated deadenylation induced by PTC is dependent upon on-going translation and appears to involve UPF1. Similar observations have been made recently in yeast (3a, 25a). It appears that when yeast decapping activity is inactivated, an alternative, decapping-independent NMD pathway remains able to eliminate PTC-containing transcripts in a process which involves accelerated deadenylation and subsequent 3′-to-5′ exonucleolytic digestion of the RNA body. Importantly, UPF1 is involved in this deadenylation-dependent pathway in yeast. It will be interesting to see how UPF1 functions to modulate deadenylation both in yeast and in mammalian NMD pathways (see further discussion below).
Current models for mammalian NMD were proposed with the assumption that it is deadenylation independent (e.g., references 49 and 51). If this is true, one would expect that interfering with deadenylation would have little effect on the decay of PTC-containing β-globin mRNA. This is apparently not the case. Here, we took three different approaches to slow down the accelerated deadenylation of nonsense-containing transcript: (i) blocking translation initiation; (ii) ectopically expressing two RNA-binding proteins, UNR and NSAP1; and (iii) ectopically expressing a UPF1 dominant-negative mutant. Our results show that regardless of how deadenylation is impeded, it always leads to significant stabilization of nonsense-containing transcript. These observations, as well as the similarities between the deadenylation and decay kinetics of the cytoplasmic NMD pathway described here and the deadenylation-dependent decay pathways for degrading normal mRNAs in yeast and in mammalian cells, are consistent with the notion that deadenylation is a necessary step in mammalian cytoplasmic NMD, which precedes decay of the RNA body.
It is noteworthy that Neu-Yilik et al. (29) showed that nonpolyadenylated β-globin mRNAs ending with a histone 3′ stem-loop terminus remain NMD competent in mammalian cells. In this case, it is possible that deadenylation is bypassed due to the presence of the 3′ histone stem-loop structure, allowing, for example, the termination-surveillance complex and exon-exon junction complex to communicate directly with the 5′ cap binding complex, the decapping enzymes, or the 3′ exosome complex for degrading the transcript.
One obvious pending issue is to pinpoint the responsible poly(A) nuclease. PARN is a likely candidate, as we showed that both UNR and NSAP1 interact strongly with PARN in glutathione S-transferase pulldown assays (unpublished observation). One possibility is that direct binding of UNR or NSAP1 to PARN inactivates PARN′s deadenylase activity or sequesters it from accessing RNA substrate. However, given that two other potential poly(A) nuclease complexes have been identified in mammals through sequence analysis, i.e., mammalian orthologues of yeast poly(A) nuclease complexes CCR4/POP2 (3, 28), as well as PAN2/PAN3 (18), it is also possible that one of these two poly(A) nucleases is the responsible enzyme. A thorough understanding of the regulation, function, redundancy, and interplay of these three different poly(A) nuclease activities and their roles in cytoplasmic deadenylation in mammalian mRNA turnover during cell growth and differentiation will shed light on this issue.
How does a premature termination codon trigger accelerated deadenylation? One possibility is that this is accomplished through direct interaction between the 3′ poly(A)/PABP and the termination surveillance complex during translation. This is based on the following observations. First, accelerated deadenylation is lost when translation of PTC-containing β-globin mRNA is blocked (Fig. 3). Second, mammalian termination-releasing factor 3, eRF3, interacts strongly with PABP (15, 41), allowing the translation termination signal to be conveyed to the 3′ poly(A) tail. Moreover, this interaction has an effect on cooperative binding of PABP to the poly(A) tail (15, 41). Third, three yeast Upf proteins, which are all essential trans-acting factors for NMD, influence the translation termination efficiency of PTC-containing messages by interacting with eRF1 and eRF3 (9, 44). Fourth, overexpression of the hUpf1(R844C) mutant protein retards deadenylation and stabilizes the PTC-containing β-globin mRNA (Fig. 4), suggesting a role for Upf1 protein in controlling the PTC-induced deadenylation via translation termination.
Our results (Fig. 1 and 2) clearly show that the way in which the PTC-containing β-globin gene is transcribed in the nucleus can greatly affect the turnover of PTC-containing β-globin mRNA in the cytoplasm. Following a transcription pulse from the Tet-regulated promoter, nearly 60% of the PTC-containing β-globin mRNA arrives in the cytoplasm (Fig. 2B), apparently not detected and eliminated by the nucleus-associated NMD. In the absence of further transcription, this cytoplasmic pool of BBBPTC mRNA molecules, fairly homogeneous in size and age, undergoes accelerated deadenylation before rapid decay of the RNA body. In contrast, when PTC-containing β-globin mRNA is transcribed from the same Tet-regulated promoter, but in a constitutive manner, only 6% has reached the cytoplasm when examined 36 h later (Fig. 2B), consistent with previous studies showing that BBBPTC mRNA is efficiently eliminated by nucleus-associated NMD (4, 8, 16, 39, 42). It should be noted that this pool of PTC-containing β-globin mRNA represents a steady-state level of mRNA molecules that are heterogeneous in size and age and are immune to NMD in the cytoplasm (Fig. 2A) (1, 8). Mammalian cells appear to have two NMD pathways operating, one in the cytoplasm and one associated with the nucleus. It will be interesting to investigate their relationship and whether and how they coordinate to fulfill their surveillance role.
Our data show that a nonsense codon triggers accelerated deadenylation before the onset of decay of the β-globin mRNA. It will be interesting to test the generality of this cytoplasmic NMD pathway in other well-characterized PTC-containing transcripts which are also known to be subject to nucleus-associated NMD, e.g., T-cell receptor and triosephosphate isomerase (4, 8). Moreover, what is the decay step following deadenylation? Is decay of the RNA body mediated by the exosome, or does decapping trigger decay of the RNA body following deadenylation? Our findings provide new insights into the general mechanism of NMD and suggest an experimental basis for elucidating the roles of trans-acting factors, e.g., eRF3, Upf factors, the exon-junction complex, PARN [as well as other poly(A) nucleases], and PABP in this intriguing cytoplasmic surveillance process.
Acknowledgments
We thank T.-C. Chang, A. van Hoof, and R. Kulmacz for critical reading of the manuscript and for their valuable comments and L. Maquat for providing us with expression plasmids containing Flag-tagged hUpf1 wild-type cDNA and R844C mutant cDNA. Special thanks go to S. Durdan, N. Xu, and W. Zhu for technical assistance.
This work was supported by grants from the National Institutes of Health to A.-B. S. (GM59211 and GM46465).
REFERENCES
- 1.Baserga, S. J., and E. J. Benz, Jr. 1992. Beta-globin nonsense mutation: deficient accumulation of mRNA occurs despite normal cytoplasmic stability. Proc. Natl. Acad. Sci. USA 89:2935-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beelman, C. A., and R. Parker. 1995. Degradation of mRNA in eukaryotes. Cell 81:179-183. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, J. A., C. Adams-Burton, D. L. Pedicord, D. A. Sukovich, P. A. Benfield, M. H. Corjay, J. K. Stoltenborg, and I. B. Dicker. 1998. Human carbon catabolite repressor protein (CCR4)-associative factor 1: cloning, expression and characterization of its interaction with the B-cell translocation protein BTG1. Biochem. J. 336:471-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Cao, D., and R. Parker. 2003. Computational modeling and experimental analysis of nonsense-mediated decay in yeast. Cell 113:533-545. [DOI] [PubMed] [Google Scholar]
- 4.Carter, M. S., S. Li, and M. F. Wilkinson. 1996. A splicing-dependent regulatory mechanism that detects translation signals. EMBO J. 15:5965-5975. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, C. Y., R. Gherzi, S. E. Ong, E. L. Chan, R. Raijmakers, G. J. Pruijn, G. Stoecklin, C. Moroni, M. Mann, and M. Karin. 2001. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell 107:451-464. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. Y., and A. B. Shyu. 1994. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol. Cell. Biol. 14:8471-8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C. Y., N. Xu, and A. B. Shyu. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 15:5777-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, J., and L. E. Maquat. 1993. Nonsense codons can reduce the abundance of nuclear mRNA without affecting the abundance of pre-mRNA or the half-life of cytoplasmic mRNA. Mol. Cell. Biol. 13:1892-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czaplinski, K., M. J. Ruiz-Echevarria, S. V. Paushkin, X. Han, Y. Weng, H. A. Perlick, H. C. Dietz, M. D. Ter-Avanesyan, and S. W. Peltz. 1998. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 12:1665-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frischmeyer, P., and H. Dietz. 1999. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 8:1893-1900. [DOI] [PubMed] [Google Scholar]
- 11.Grosset, C., C.-Y. A. Chen, N. Xu, N. Sonenberg, H. Jacquemin-Sablon, and A.-B. Shyu. 2000. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell 103:29-40. [DOI] [PubMed] [Google Scholar]
- 12.He, F., A. Brown, and A. Jacobson. 1997. Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol. Cell. Biol. 17:1580-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentze, M. W., and A. E. Kulozik. 1999. A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96:307-310. [DOI] [PubMed] [Google Scholar]
- 14.Hilleren, P., and R. Parker. 1999. mRNA surveillance in eukaryotes: kinetic proofreading of proper translation termination as assessed by mRNP domain organization? RNA 5:711-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino, S., M. Imai, T. Kobayashi, N. Uchida, and T. Katada. 1999. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 274:16677-16680. [DOI] [PubMed] [Google Scholar]
- 16.Humphries, R. K., T. J. Ley, N. P. Anagnou, A. W. Baur, and A. W. Nienhuis. 1984. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood 64:23-32. [PubMed] [Google Scholar]
- 17.Ishigaki, Y., X. Li, G. Serin, and L. Maquat. 2001. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 106:606-617. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa, K., T. Nagase, M. Suyama, N. Miyajima, A. Tanaka, H. Kotani, N. Nomura, and O. Ohara. 1998. Prediction of the coding sequences of unidentified human genes. X. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 5:169-176. [DOI] [PubMed] [Google Scholar]
- 19.Li, S., and M. F. Wilkinson. 1998. Nonsense surveillance in lymphocytes? Immunity 8:135-141. [DOI] [PubMed] [Google Scholar]
- 20.Lim, S., J. Mullins, C. Chen, K. Gross, and L. Maquat. 1989. Novel metabolism of several beta zero-thalassemic beta-globin mRNAs in the erythroid tissues of transgenic mice. EMBO J. 8:2613-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim, S., C. Sigmund, K. Gross, and L. Maquat. 1992. Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products. Mol. Cell. Biol. 12:1149-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loflin, P. T., C.-Y. A. Chen, N. Xu, and A.-B. Shyu. 1999. Transcriptional pulsing approaches for analysis of mRNA turnover in mammalian cells. Methods 17:11-20. [DOI] [PubMed] [Google Scholar]
- 23.Lykke-Andersen, J., M. D. Shu, and J. A. Steitz. 2000. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103:1121-1131. [DOI] [PubMed] [Google Scholar]
- 24.Maquat, L. E. 2000. Nonsense-mediated RNA decay in mammalian cells: a splicing dependent means to down-regulate the levels of mRNAs that prematurely terminate transcription, p. 849-868. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational control of gene expression, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Mendell, J., and H. Dietz. 2001. When the message goes awry: disease-producing mutations that influence mRNA content and performance. Cell 107:411-414. [DOI] [PubMed] [Google Scholar]
- 25a.Mitchell, P., and D. Tollervey. 2003. An NMD pathway in yeast involving accelerated deadenylation and exosome-mediated 3′ to 5′ degradation. Mol. Cell 11:1405-1413. [DOI] [PubMed] [Google Scholar]
- 26.Muhlrad, D., and R. Parker. 1994. Premature translational termination triggers mRNA decapping. Nature 370:578-581. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee, D., M. Gao, J. P. O'Connor, R. Raijmakers, G. Pruijn, C. S. Lutz, and J. Wilusz. 2002. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 21:165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagase, T., K. Ishikawa, R. Kikuno, M. Hirosawa, N. Nomura, and O. Ohara. 1999. Prediction of the coding sequences of unidentified human genes. XV. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 6:337-345. [DOI] [PubMed] [Google Scholar]
- 29.Neu-Yilik, G., N. H. Gehring, R. Thermann, U. Frede, M. W. Hentze, and A. E. Kulozik. 2001. Splicing and 3′ end formation in the definition of nonsense-mediated decay-competent human β-globin mRNPs. EMBO J. 20:532-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page, M. F., B. Carr, K. R. Anders, A. Grimson, and P. Anderson. 1999. SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Mol. Cell. Biol. 19:5943-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltz, S. W., F. He, E. Welch, and A. Jacobson. 1994. Nonsense-mediated mRNA decay in yeast. Prog. Nucleic Acid Res. Mol. Biol. 47:271-298. [DOI] [PubMed] [Google Scholar]
- 32.Peng, S.-P., C.-Y. Chen, N. Xu, and A.-B. Shyu. 1998. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 17:3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachs, A. B. 1993. Messenger RNA degradation in eukaryotes. Cell 74:413-421. [DOI] [PubMed] [Google Scholar]
- 34.Shyu, A.-B., J. G. Belasco, and M. G. Greenberg. 1991. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 5:221-232. [DOI] [PubMed] [Google Scholar]
- 35.Shyu, A.-B., J. A. Garcia-Sanz, and E. Mullner. 1996. Analysis of mRNA decay in mammalian cells, p. 450-462. In I. Lefkovits (ed.), The immunology methods manual. Academic Press, London, United Kingdom.
- 36.Shyu, A.-B., M. E. Greenberg, and J. G. Belasco. 1989. The c-fos mRNA is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 3:60-72. [DOI] [PubMed] [Google Scholar]
- 37.Sun, X., H. A. Perlick, H. C. Dietz, and L. E. Maquat. 1998. A mutated human homologue to yeast Upf1 protein has a dominant-negative effect on the decay of nonsense containing mRNAs in mammalian cells. Proc. Natl. Acad. Sci. USA 95:10009-10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svitkin, Y. V., H. Imataka, K. Khaleghpour, A. Kahvejian, H. D. Liebig, and N. Sonenberg. 2001. Poly(A)-binding protein interaction with elF4G stimulates picornavirus IRES-dependent translation. RNA 7:1743-1752. [PMC free article] [PubMed] [Google Scholar]
- 39.Takeshita, K., B. G. Forget, A. Scarpa, and E. J. Benz. 1984. Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood 64:13-22. [PubMed] [Google Scholar]
- 40.Thermann, R., G. Neu-Yilik, A. Deters, U. Frede, K. Wehr, C. Hagemeier, M. W. Hentze, and A. E. Kulozik. 1998. Binary specification of nonsense codons by splicing and cytoplasmic translation. EMBO J. 17:3484-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida, N., S.-I. Hoshino, H. Imataka, N. Sonenberg, and T. Katada. 2002. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J. Biol. Chem. 277:50286-50292. [DOI] [PubMed] [Google Scholar]
- 42.Urlaub, G., P. J. Mitchell, C. J. Ciudad, and L. A. Chasin. 1989. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol. Cell. Biol. 9:2868-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakiyama, M., H. Imataka, and N. Sonenberg. 2000. Interaction of eIF4G with poly(A)-binding protein stimulates translation and is critical for Xenopus oocyte maturation. Curr. Biol. 10:1147-1150. [DOI] [PubMed] [Google Scholar]
- 44.Wang, W., K. Czaplinski, Y. Rao, and S. W. Peltz. 2001. The role of Upf proteins in modulating the translation read-through of nonsense-containing transcripts. EMBO J. 20:880-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Z., N. Day, P. Trifillis, and M. Kiledjian. 1999. An mRNA stability complex functions with poly(A)-binding protein to stabilize mRNA in vitro. Mol. Cell. Biol. 19:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Z., and M. Kiledjian. 2001. Functional link between the mammalian exosome and mRNA decapping. Cell 107:751-762. [DOI] [PubMed] [Google Scholar]
- 47.Wells, S. E., P. E. Hillner, R. D. Vale, and A. B. Sachs. 1998. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2:135-140. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson, M., and A. Shyu. 2002. RNA surveillance by nuclear scanning? Nat. Cell Biol. 4:E144-E147. [DOI] [PubMed] [Google Scholar]
- 49.Wilusz, C. W., M. Wormington, and S. W. Peltz. 2001. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2:237-246. [DOI] [PubMed] [Google Scholar]
- 50.Xu, N., P. Loflin, C.-Y. A. Chen, and A.-B. Shyu. 1998. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy. Nucleic Acids Res. 26:558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, J., X. Sun, Y. Qian, and L. E. Maquat. 1998. Intron function in the nonsense-mediated decay of beta-globin mRNA: indications that pre-mRNA splicing in the nucleus can influence mRNA translation in the cytoplasm. RNA 4:801-815. [DOI] [PMC free article] [PubMed] [Google Scholar]