Abstract
Insulin stimulates glucose transport by promoting translocation of GLUT4 proteins from the perinuclear compartment to the cell surface. It has been previously suggested that the microtubule-associated motor protein kinesin, which transports cargo toward the plus end of microtubules, plays a role in translocating GLUT4 vesicles to the cell surface. In this study, we investigated the role of Rab4, a small GTPase-binding protein, and the motor protein KIF3 (kinesin II in mice) in insulin-induced GLUT4 exocytosis in 3T3-L1 adipocytes. Photoaffinity labeling of Rab4 with [γ-32P]GTP-azidoanilide showed that insulin stimulated Rab4 GTP loading and that this insulin effect was inhibited by pretreatment with the phosphatidylinositol 3-kinase (PI3-kinase) inhibitor LY294002 or expression of dominant-negative protein kinase C-λ (PKC-λ). Consistent with previous reports, expression of dominant-negative Rab4 (N121I) decreased insulin-induced GLUT4 translocation by 45%. Microinjection of an anti-KIF3 antibody into 3T3-L1 adipocytes decreased insulin-induced GLUT4 exocytosis by 65% but had no effect on endocytosis. Coimmunoprecipitation experiments showed that Rab4, but not Rab5, physically associated with KIF3, and this was confirmed by showing in vitro association using glutathione S-transferase-Rab4. A microtubule capture assay demonstrated that insulin stimulation increased the activity for the binding of KIF3 to microtubules and that this activation was inhibited by pretreatment with the PI3-kinase inhibitor LY294002 or expression of dominant-negative PKC-λ. Taken together, these data indicate that (i) insulin signaling stimulates Rab4 activity, the association of Rab4 with kinesin, and the interaction of KIF3 with microtubules and (ii) this process is mediated by insulin-induced PI3-kinase-dependent PKC-λ activation and participates in GLUT4 exocytosis in 3T3-L1 adipocytes.
Stimulation of glucose transport is a major action of insulin and occurs in the insulin target tissues, muscle and fat, by a process involving translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane (34). GLUT4 proteins are contained in intracellular vesicles which are predominantly localized to a perinuclear compartment in the basal state. After insulin stimulation, the GLUT4-containing vesicles are translocated to the plasma membrane (31). Numerous studies have examined the insulin signaling mechanisms leading to translocation of GLUT4 vesicles to the plasma membrane, and it is understood that this process involves multiple steps (34). These steps include release of vesicles from storage pools, transport to the plasma membrane, proper docking, and fusion with the membrane, and these events are regulated by multiple insulin signaling components (5).
It has been shown that different Rab proteins are present in trafficking vesicles (26, 43) and that GLUT4 vesicles can contain a number of associated molecules, such as Rab4, Rab5, Rab11, insulin-responsive amino peptidase, and transferrin receptors (27). In previous reports (6, 35, 36), including those from our laboratory (42), it has been demonstrated that Rab4 plays an important role in the GLUT4 translocation process. On the other hand, intracellular vesicles are generally transported to and from the cell surface by motor proteins, such as kinesin and dynein (22), and these motor proteins have a function in GLUT4 vesicle translocation (8, 11). However, it is unclear how insulin regulates motor protein activity and how motor proteins recognize GLUT4 vesicles in response to insulin stimulation.
In this study, we have examined the interaction between Rab4 and KIF3 (kinesin II in the mouse) as it relates to the process of insulin-induced GLUT4 vesicle exocytosis. We show that insulin can stimulate both Rab4 and KIF3 activities through a phosphatidylinositol 3-kinase- (PI3-kinase) and protein kinase C-λ (PKC-λ)-dependent signaling mechanism and that activated (GTP-bound) Rab4 can associate with KIF3 to mediate movement of GLUT4 vesicles to the plasma membrane in 3T3-L1 adipocytes.
MATERIALS AND METHODS
Materials.
The wild-type and mutant Rab4 cDNA constructs were kindly provided by Stephen Ferguson (The John P. Robarts Research Institute, London, Ontario, Canada). Adenovirus with PKC-λ constructs was kindly gifted by Wataru Ogawa (Kobe University, Kobe, Japan). The GLUT4-enhanced green fluorescent protein (eGFP) expression vector was kindly provided by Jeffrey E. Pessin (University of Iowa, Iowa City). A rabbit polyclonal anti-GLUT4 antibody (F349) was kindly provided by Michael Mueckler (Washington University, St. Louis, Mo.), and a mouse monoclonal anti-GLUT4 antibody (1F8) was purchased from Biogenesis Inc. (Brentwood, N.H.). Monoclonal anti-Rab4, -Rab5, -KIF1A, -KIF3B, -KAP3A, and -PKC-λ antibodies were from Transduction Laboratories (Lexington, Ky.). Polyclonal anti-Rab5, -Rab7, -Akt1, and -PKC-λ antibodies and horseradish peroxidase-linked anti-mouse and -rabbit antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.). The polyclonal anti-Rab4 antibody was from Calbiochem (San Diego, Calif.). The polyclonal anti-Akt antibody was from Cell Signaling (Beverly, Mass.). Sheep immunoglobulin G (IgG) and rhodamine- and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit, -mouse, and -sheep IgG antibodies were obtained from Jackson Immmunoresearch Laboratories Inc. (West Grove, Pa.). A myristoylated peptide of the PKC-λ pseudosubstrate was from Biosource International (Camarillo, Calif.). The glutathione S-transferase (GST)-protein expression vector and GST-protein purification kit were from Amersham-Pharmacia Biotech (Piscataway, N.J.). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Grand Island, N.Y.). All other reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cell treatment and transient transfection.
3T3-L1 cells were cultured and differentiated as described previously (21). For preparation of whole-cell lysates for immunoprecipitation and immunoblotting experiments, 3T3-L1 adipocytes were starved for 4 to 5 h in DMEM containing 0.1% bovine serum albumin (BSA). The cells were stimulated with 17 nM insulin at 37°C for various periods as indicated in the figures.
Differentiated 3T3-L1 adipocytes were transiently transfected by electroporation, as previously described (18). Wild-type or dominant-negative mutant (N121I) Rab4 expression vectors along with the GLUT4-eGFP expression vector were electroporated together for scoring of GLUT4 translocation. Transfected cells were detected by the expression of the GLUT4-eGFP protein, and GLUT4 translocation (ring assay) was scored by using eGFP fluorescence without staining.
For adenovirus infection, 3T3-L1 adipocytes were transduced at a multiplicity of infection (MOI) of 30 PFU/cell for 16 h with the recombinant adenovirus encoding the wild type, a kinase-inactive dominant-negative K273E mutant (DN-PKC-λ), or a constitutively active mutant (CA-PKC-λ) lacking the pseudosubstrate domain, as described previously (24).
Microinjection and immunofluorescence staining.
Microinjection was performed with a semiautomatic Eppendorf microinjection system. Antibodies for microinjection were concentrated and used at 5 mg/ml in microinjection buffer containing 5 mM sodium phosphate (pH 7.2) and 100 mM KCl and were injected into the cytoplasm as previously described (20). The duplexes of each small interfering RNA (siRNA), targeting KAP3A mRNA (target sequence: 5′-GGCUCUUGAUCGGGACAAUTT-3′; corresponding to the cDNA sequence from 928 to 950) and insulin receptor mRNA (target sequence: 5′-TATACCATGAATTCCAGCAACTT-3′; corresponding to the cDNA sequence from 955 to 977), and a negative control (scrambled sequence) were purchased from Dharmacon Research Inc. (Lafayette, Colo.). These sequences were chosen by a World Wide Web-based search program (www.dharmacon.com), and the absence of homology to any other gene was confirmed by a BLAST search (National Center for Biotechnology Information, National Institutes of Health). siRNAs were dissolved at 5 μM in microinjection buffer including fluorescein-conjugated dextran (Molecular Probes, Eugene, Oreg.) to identify the injected cells. Cells were incubated in complete medium for 72 h after microinjection and then serum starved for 4 h, followed by stimulation with insulin for 20 min.
Immunostaining of GLUT4 was performed as described before (16, 18, 19, 42). Briefly, cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. After permeabilization with 0.1% Triton X-100 for 10 min and blocking with 3% FBS in PBS for 10 min, cells were incubated with the rabbit anti-GLUT4 antibody in PBS with 3% FBS overnight at 4°C. After being washed, cells were incubated for 1 h at room temperature with the rhodamine-conjugated goat anti-rabbit antibody for GLUT4 staining and with the FITC-conjugated goat anti-mouse antibody for staining the injected mouse IgG, as a marker for identifying injected cells. Cell surface GLUT4 staining was identified by immunofluorescence microscopy, as previously described (16, 18, 19, 42). Individual cells were scored as positive or negative for surface GLUT4, and approximately 300 cells per coverslip were counted by an observer blind to the experimental conditions.
Immunoprecipitation and Western blotting.
Coimmunoprecipitation experiments were performed as described previously (18).
For the coimmunoprecipitation between Rab4/Rab5 and KIF3, starved 3T3-L1 adipocytes were incubated for 20 min at 37°C in serum-free DMEM containing 2 mM dithiobis(succinimidylpropionate) cross-linker (Pierce), as described elsewhere (33). After stimulation with or without insulin (17 nM), cells were washed twice with ice-cold PBS and were then lysed in a cold solubilizing buffer containing 40 mM Tris, 1 mM EGTA, 100 mM NaCl, 1 mM MgCl2, 1% Nonidet P-40, 10% glycerol, 1 mM Na3VO4, 1 mM phenymethylsulfonyl fluoride (PMSF), and 20 mM NaF, pH 7.5. The soluble fractions were immunoprecipitated with antibodies at 4°C for 6 h or overnight, followed by a 1-h incubation with anti-mouse IgG antibody-, protein A-, or protein G-conjugated beads (Sigma). For coimmunoprecipitation with the anti-KIF3B antibody, the rabbit anti-mouse IgG antibody was added together with anti-rabbit IgG-conjugated beads. The immunoprecipitates were boiled with Laemmli sample buffer containing 100 mM dithiothreitol and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with the anti-Rab4, -Rab5, or -KIF3 antibody.
In vitro GTPγS/GDPβS loading and in vitro binding assay.
For in vitro GTPγS/GDPβS (a nonhydrolyzable GTP/GDP analog) loading, starved 3T3-L1 cell lysates (40 mM HEPES, 1 mM EDTA, 150 mM NaCl, 10 mM MgCl2, 1% Nonidet P-40, 10% glycerol, 1 mM Na3VO4, 1 mM PMSF, 20 mM NaF [pH 7.5]) were incubated with 100 μM GTPγS or GDPβS for 15 min at 37°C and then coimmunoprecipitation with the anti-Rab4 or -Rab5 antibody was performed as described above. These immunoprecipitates were analyzed by immunoblotting with the anti-KIF3B antibody.
Full-length Rab4 cDNA was subcloned into the EcoRI/XhoI site in the pGEX-4T-1 vector (Amersham-Pharmacia Biotech), and sequenced for verification. GST only or GST-tagged Rab4 proteins were purified with glutathione-Sepharose 4B (GS4B) beads as described previously (32). GST-tagged proteins bound to GS4B beads were incubated with 100 μM GTPγS or GDPβS for 15 min at 37°C in loading buffer containing 40 mM HEPES, 1 mM EGTA, 150 mM NaCl, 1 mM MgCl2, and 10% glycerol, pH 7.5, and then washed three times with loading buffer. GS4B beads with GST-GTPγS or GST-Rab4-GTPγS/GDPβS were incubated with 1 mg of protein from 3T3-L1 cell lysates for 2 h at 4°C and then washed three times with loading buffer and boiled with Laemmli sample buffer containing 100 mM dithiothreitol. Eluted proteins were analyzed by SDS-PAGE and immunoblotting with the anti-KIF3B antibody.
Photoaffinity labeling of Rab4 with [γ-32P]GTP-azidoanilide.
Starved 3T3-L1 adipocytes were stimulated with 17 nM insulin for different time periods and then lysed as described above. For photolabeling, whole-cell lysates (800 μg of protein) were incubated with 10 μM [γ-32P]GTP-azidoanilide (Affinity Labeling Technologies, Inc., Lexington, Ky.) (12.2 mCi/μmol) for 3 min, followed immediately by UV irradiation (254 nm) for 3 min on ice. Photoincorporation was stopped by addition of 10 mM dithiothreitol. Samples were then immunoprecipitated with the anti-Rab4 antibody at 4°C, and the immunoprecipitates were resolved by SDS-PAGE. Gels were dried, and signals were quantitated by PhosphorImager (Molecular Dynamics).
Microtubule capture assay.
Microtubule capture assays were performed as described previously with some modifications (10a, 16). Paclitaxel (Taxol)-stabilized microtubules were prepared by incubating 200 μg of tubulin (Sigma) in 50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 6.9]-1 mM EGTA-0.5 mM MgSO4-0.1 mM GTP with 20 mM paclitaxel at 37°C for 30 min and then with 100 μl of prewashed protein A beads (Upstate Biotechnologies, Inc.)-2 mM dithiobis(succinimidylpropionate) cross-linker (Pierce) at 37°C for 15 min with agitation to prepare the microtubule-coated (MTC) beads. MTC beads were washed four times with washing buffer (40 mM Tris, 1 mM EGTA, 1 mM MgCl2, 0.1 mM paclitaxel [pH 6.9]) stringently, with vortexing at 4°C. 3T3-L1 adipocytes were homogenized by being passed several times through a 27-gauge needle with BRB40 buffer (40 mM PIPES, 1 mM EGTA, 1 mM MgCl2 [pH 6.9]) containing 2 mM dithiothreitol, 1 mM AMP-PNP, and 1 mM PMSF at 4°C. The soluble fractions were incubated with MTC beads and 0.1 mM paclitaxel for 10 min at 4°C. After MTC beads were washed three times with cold BRB40 containing 0.1 mM paclitaxel, the beads were boiled in Laemmli sample buffer, and the amount of captured motor proteins with MTC beads was analyzed by SDS-PAGE and immunoblotting with the anti-KIF3B antibody.
RESULTS
Rab4 and PKC-λ are interacting components of the signaling mechanism for insulin-induced GLUT4 translocation in 3T3-L1 adipocytes.
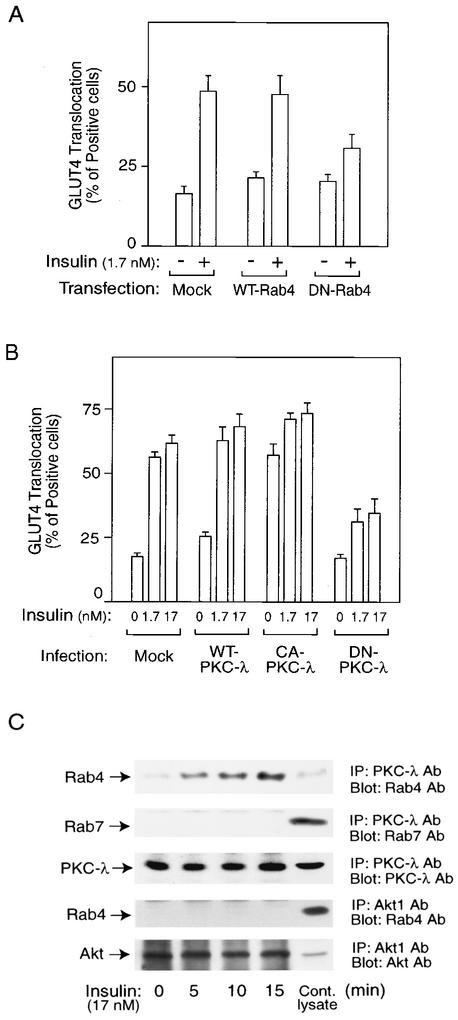
We have previously shown that microinjection of the anti-Rab4 antibody or dominant-negative GTP-binding-defective (N121I) Rab4 protein inhibited insulin-induced GLUT4 translocation in 3T3-L1 adipocytes (42). To support these findings for the present system, we performed transfection experiments with an equal amount of Rab4 and GLUT4-eGFP expression vectors using electroporation. GLUT4 translocation was detected in the GLUT4-eGFP-expressing cells by assessing eGFP localization at the cell surface after insulin treatment. Consistent with our previous data, transfection of the N121I-Rab4 construct into 3T3-L1 adipocytes inhibited insulin stimulation of GLUT4 translocation (Fig. 1A).
FIG. 1.
Molecular complex with Rab4 and PKC-λ plays a role in insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. (A) Mock control, wild-type Rab4 (WT-Rab4), or dominant-negative Rab4 (DN-Rab4) expression vectors together with an eGFP-GLUT4 expression vector were transiently transfected into 3T3-L1 adipocytes by electroporation. Forty-eight hours after incubation, cells were starved and treated with 1.7 nM insulin for 20 min or received no insulin treatment. GLUT4 translocation was detected by assessing cell surface eGFP fluorescence. The percentage of cells positive for GLUT4 translocation was calculated by counting at least 200 cells at each point. The data represent the means ± standard errors (SE) from three independent experiments. (B) Sixty hours after infection with PKC-λ-expressing adenovirus (MOI, 30 PFU/cell), serum-starved 3T3-L1 adipocytes on coverslips were incubated with or without insulin for 20 min. The percentage of cells positive for GLUT4 translocation was calculated as described in Materials and Methods. The data are the means ± SE from three independent experiments. (C) Serum-starved 3T3-L1 adipocytes were stimulated with 17 nM insulin for the indicated time periods, and then cells were lysed and immunoprecipitated (IP) with the anti-PKC-λ or -Akt1 antibody (Ab). Immunoprecipitates were analyzed by Western blotting. These experiments were repeated three times.
It has been shown that PKC-λ is required for insulin-induced glucose transport (20, 24), and, along these lines, we have found that microinjection of an anti-PKC-λ antibody blocks the effects of insulin on GLUT4 translocation (20). In Fig. 1B, we used adenovirus-mediated expression to show that dominant-negative PKC-λ (DN-PKC-λ) inhibited, and constitutively active PKC-λ (CA-PKC-λ) stimulated, insulin-induced GLUT4 translocation. Since PKC-λ can be associated with GLUT4 vesicles (38), we examined the interactions between PKC-λ and Rab4 in coimmunoprecipitations. As seen in Fig. 1C, insulin treatment caused a time-dependent increase in the association of Rab4 with PKC-λ. As a control, no association between Rab7 and PKC-λ or between Akt and Rab4 was observed and insulin had no effect on Rab4 or PKC-λ protein content. Taken together, these results demonstrate that both Rab4 and PKC-λ are necessary for GLUT4 translocation and that these two proteins can associate within the cell in an insulin-dependent manner.
Insulin induces Rab4 activation.
To determine whether the interaction between Rab4 and PKC-λ was functionally significant, we examined the effects of PKC-λ on Rab4 activity. Figure 2A depicts the photoaffinity labeling of Rab4 using [γ-32P]GTP-azidoanilide in 3T3-L1 adipocytes, in the presence or absence of insulin and various inhibitors. As seen, insulin (17 nM, 10 min) stimulated Rab4-GTP binding by 2.8-fold, and this effect was blocked by pretreatment with the PI3-kinase inhibitor LY294002 (50 μM) or with the PKC-λ inhibitor (myristoylated-PKC-λ pseudosubstrate; 50 μM), but not by the MEK inhibitor PD98059 (30 μM). To further explore these observations, we expressed the PKC-λ constructs using adenoviral infection in 3T3-L1 adipocytes. Expression of CA-PKC-λ increased GTP-bound Rab4 by fourfold, and kinase-inactive DN-PKC-λ expression inhibited insulin-induced Rab4 activation (Fig. 2B). Since insulin stimulates PKC-λ activity in a PI3-kinase-dependent manner (39), these data indicate that insulin can stimulate Rab4 by a process involving PI3-kinase and PKC-λ in 3T3-L1 adipocytes.
FIG. 2.
Rab4 is activated via PI3-kinase- and PKC-λ-dependent pathways. After pretreatment with 50 μM LY294002, 30 μM PD98059, 50 μM myristoylated-PKC-λ pseudosubstrate, or dimethyl sulfoxide (DMSO) vehicle for 30 min (A) or 60 h after PKC-λ-expressing adenovirus infection (MOI, 30 PFU/cell) (B), cells were incubated with 17 nM insulin for 10 min. Cells were then lysed and photolabeled with [γ-32P]GTP-azidoanilide. The samples were immunoprecipitated with the anti-Rab4 antibody, and the immunoprecipitates were resolved by SDS-PAGE. Representative results are shown at the top. Data were quantitated by a PhosphorImager as represented in the bar graph. Data represent the means ± standard errors of three independent experiments. NC, negative control (no Rab4 antibody); WT, wild type; CA, constitutively active; DN, dominant negative; Cont., control.
Activated Rab4, but not Rab5, can bind to the kinesin family protein KIF3.
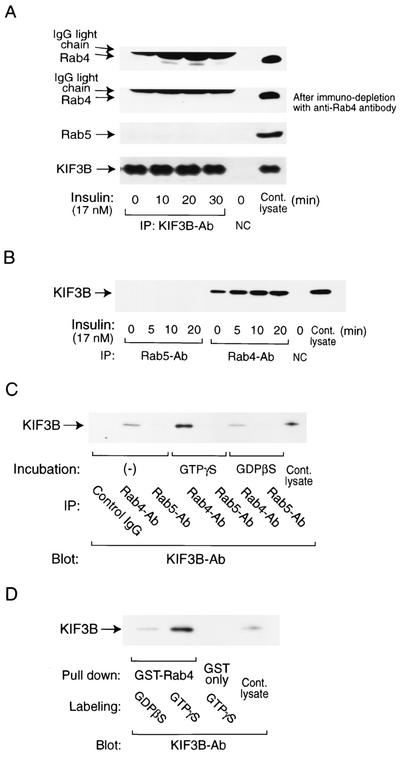
Motor proteins are required to convey vesicular cargo to and from the plasma membrane, and we have previously shown that Rab5 can interact with dynein in the process of GLUT4 internalization (16). The motor protein KIF3 (kinesin II in mice) is ubiquitously distributed in many tissues and facilitates the anterograde transport of intracellular vesicles (15). KIF3 is a heterotrimeric protein, composed of two heavy chains, KIF3A and KIF3B, and one adaptor subunit, KAP3 (kinesin-associated protein 3 or KIFAP3) (25). We determined whether Rab4 was associated with KIF3 by conducting coimmunoprecipitation experiments with anti-KIF3, -Rab4, and -Rab5 antibodies. In response to insulin stimulation, Rab4, but not Rab5, associated with KIF3 in a time-dependent manner, and this was observed in immunoprecipitations with the KIF3 antibody (blot with the Rab4 antibody; Fig. 3A, top) or with the Rab4 antibody (blot with the KIF3B antibody; Fig. 3B). To further demonstrate the specificity of the interaction between Rab4 and KIF3, additional experiments in which the whole-cell lysates were first precleared of Rab4 by immunoprecipitation with the anti-Rab4 antibody were performed (Fig. 3A, second from the top). The resulting supernatants were then subjected to a second immunoprecipitation with the anti-KIF3B antibody, and the immunoprecipitates were immunoblotted for Rab4. The interacting Rab4 band was almost completely eliminated under these conditions, providing further evidence that the protein band coprecipitating with KIF3 from the original lysates is indeed Rab4 and that the interactions are specific. Since insulin can stimulate Rab4 activity (Fig. 2A), these data suggested that activated (GTP-bound) Rab4 binds to KIF3. To assess this, 3T3-L1 cell lysates were saturated with GTPγS in order to activate GTP-binding proteins in vitro and then coimmunoprecipitated with the anti-Rab4 or Rab5 antibody. As seen in Fig. 3C, with GTPγS treatment, KIF3 was specifically precipitated with the anti-Rab4 antibody but not with the anti-Rab5 antibody. Similarly, the GTPγS-labeled recombinant GST-Rab4 protein pulled down the KIF3 protein from unstimulated cell lysates (Fig. 3D), further showing that GTP-bound Rab4 can associate with the motor protein KIF3.
FIG. 3.
Insulin-stimulated or active (GTP-bound) Rab4 associates with KIF3 in vivo and in vitro. (A and B) Cells were treated with insulin (17 nM) for the indicated periods of time. Whole-cell lysates were immunoprecipitated with the anti-KIF3B, -Rab4, or -Rab5 antibody (Ab). For the analysis with prior immunodepletion of Rab4, cell lysates were precleared by immunoprecipitation with the anti-Rab4 antibody first and then immunoprecipitated (IP) with the anti-KIF3B antibody (A, second panel from the top). NC, negative control (no antibody). (C) Whole-cell lysates (1 mg) were incubated with or without GTPγS or GDPβS (100 μM each) for 30 min and then immunoprecipitated with the anti-Rab4 or -Rab5 antibody. (D) Recombinant GST-Rab4 or the control GST protein were purified with GS4B beads and labeled with GTPγS or GDPβS and then incubated in 3T3-L1 cell lysates. All precipitants were analyzed by SDS-PAGE and Western blotting with the anti-Rab4, -Rab5, or -KIF3B antibody. These experiments were repeated three times.
KIF3 is necessary for insulin-induced GLUT4 translocation.
To assess the function of KIF3 in insulin-induced GLUT4 translocation, we microinjected two kinds of anti-KIF3 antibodies; one was directed against the KIF3 adaptor subunit, KAP3A, and the other was directed against the KIF3 heavy chain, KIF3B. In the basal state, cells display GLUT4 staining mostly in a perinuclear localization, with some staining distributed in the cytoplasm (Fig. 4B, upper left); after insulin stimulation, GLUT4 staining is seen at the plasma membrane as a circumferential ring, with a concomitant decrease in intracellular distribution (Fig. 4B, upper right). As shown in Fig. 4A and B, microinjection of these antibodies inhibited insulin-induced GLUT4 translocation. As controls, injection of regular IgG or an antibody against the KIF1A motor protein had no effect.
FIG. 4.
KIF3 is involved in insulin-induced GLUT4 exocytosis in 3T3-L1 adipocytes. (A) Serum-starved 3T3-L1 adipocytes were microinjected with control IgG or the anti-KAP3A, -KIF3B, or -KIF1A antibody (Ab; mouse), followed by stimulation with or without insulin (1.7 nM) for 20 min. Fixed cells were stained with the rabbit anti-GLUT4 antibody and then stained with TRITC (tetramethyl rhodamine isothiocyanate)-conjugated anti-rabbit IgG and FITC-conjugated anti-mouse antibodies. The percentage of cells positive for GLUT4 translocation was calculated as described in Materials and Methods. The data are the means ± standard errors (SE) from four independent experiments. (B) Representative images of cells depicting GLUT4 staining from the experiment in panel A. Arrows, cells scored as positive. Injected cells (open triangles [top]) are shown at the bottom. (C) Seventy-two hours after microinjection of siRNA directed against the insulin receptor (IR), KAP3A, or the control (random sequence), 3T3-L1 adipocytes on coverslips were serum starved for 4 h and stimulated with or without insulin (1.7 nM) for 20 min. Cells were then stained for GLUT4 and scored as described for panel A. No evidence of cell toxicity was observed with any of the siRNA injections. Data represent the means ± SE of three independent experiments. (D) After 3T3-L1 cells were stimulated with 1.7 nM insulin for 20 min, the cells were then microinjected with control IgG (□), anti-KAP3A (⋄), or the anti-dynein heavy-chain antibody (○), followed by incubation in serum-free medium at 37°C for the indicated periods of time. Cell surface GLUT4 was measured by immunofluorescence microscopy. Data represent the means ± SE of three independent experiments.
To further confirm the importance of KIF3 for insulin-stimulated GLUT4 translocation, we utilized siRNA directed against KAP3A mRNA to knock down KAP3A expression, followed by measurement of GLUT4 translocation. The KAP3A siRNA was microinjected into the cytoplasm of 3T3-L1 adipocytes, and 72 h later, GLUT4 translocation was measured. As seen in Fig. 4C, microinjection of KAP3A siRNA led to a 69% decrease in insulin-induced GLUT4 translocation, consistent with the antibody microinjection data (Fig. 4A and B). Given the small number of cells injected by the microinjection technique, we cannot measure the level of the siRNA-targeted mRNA or the level of the protein itself. However, these results are supported by the siRNA injections using positive and negative controls; thus, microinjection of siRNA directed against the insulin receptor completely abolished insulin stimulation, whereas a scrambled negative control siRNA had no effect. Taken together with the antibody injection data, these results are consistent with a role for KIF3 in insulin-induced GLUT4 exocytosis.
The movement of GLUT4 vesicles to the plasma membrane proceeds through a targeted exocytotic pathway, whereas delivery of surface GLUT4 proteins back to the intracellular vesicular compartment involves a distinct regulated endocytotic process. Movement of GLUT4-containing vesicles in either direction entails motor protein-facilitated trafficking along microtubules. We have recently shown that Rab5 interacts with the motor protein dynein to mediate GLUT4 internalization and that insulin can slow this process (16). GLUT4 internalization can be assessed by stimulating cells with insulin, washing them, and then monitoring the disappearance of GLUT4 from the cell surface by immunofluorescence staining. As seen in Fig. 4D, microinjection of the anti-KAP3A antibody had no effect on GLUT4 internalization. As a control, we also injected the antidynein antibody, which retards the rate of internalization, as previously described (16).
Insulin stimulates KIF3 activity through PI3-kinase and PKC-λ in 3T3-L1 adipocytes.
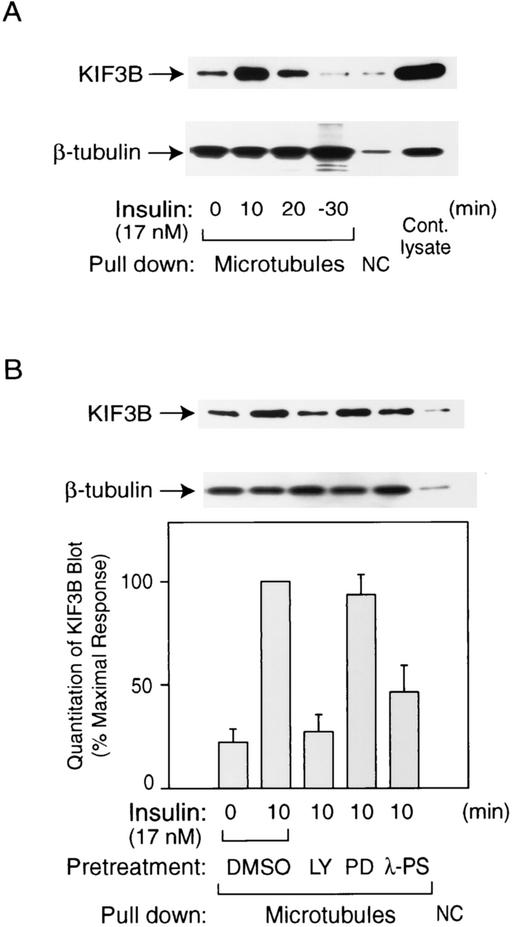
We assessed whether insulin could stimulate the activity of KIF3 to interact with microtubules by using a microtubule capture assay (16). Since activated motor proteins can bind to microtubules (10a), we used MTC beads to precipitate the activated motor protein as a functional measurement of KIF3 activity. As seen in Fig. 5A, KIF3 was activated by insulin stimulation and this effect was reversed 30 min after insulin removal. This activation was inhibited by pretreatment of cells with LY294002 or the PKC-λ pseudosubstrate peptide, but not by PD98059 (Fig. 5B).
FIG. 5.
Microtubule capture activity of KIF3 is stimulated by insulin via PI3-kinase and PKC-λ. 3T3-L1 adipocytes were stimulated with or without insulin (17 nM) for the indicated time periods after pretreatment with 50 μM LY294002 (LY), 30 μM PD98059 (PD), 50 μM myristoylated-PKC-λ pseudosubstrate (λ-PS), or dimethyl sulfoxide (DMSO) vehicle for 30 min (B) or 60 h after PKC-λ-expressing adenovirus infection (MOI, 30) (C). Whole-cell lysates were then incubated with MTC beads for capture assays. Precipitants with MTC beads (microtubules) or protein A beads (NC) were analyzed by SDS-PAGE and immunoblotted with the anti-KIF3B antibody. Equal amounts of microtubules were used in all conditions, as confirmed by immunoblotting with the anti-β-tubulin antibody. The images were quantitated by the National Institutes of Health Image program, and the data represent the means ± standard errors from three or four independent experiments (B and C). NC, negative control; Cont, control virus; WT, wild type; CA, constitutively active; KD, dominant negative.
Figure 5C shows that adenoviral expression of CA-PKC-λ stimulated KIF3 activity by 3.3-fold in the absence of insulin and that DN-PKC-λ expression decreased insulin-stimulated KIF3 activation. Thus, similar to Rab4 activation, KIF3 activity was regulated by PKC-λ in these cells.
DISCUSSION
The Rab family of small GTP binding proteins represents key components of vesicular trafficking, mediating a range of diverse functions such as vesicle formation, vesicle transport, organelle motility, vesicle docking and fusion, and exocytosis and endocytosis (43). Different Rab proteins can participate in these processes depending on the specific functions of the protein involved and its unique subcellular localization. For example, Rab7 and Rab9 are located in late endosomes, participating in late endosome Golgi transport, while Rab11 is observed in recycling endosomes and participates in this process (26). Thus, Rab family proteins can be key determinants of directional movement, regulation, and the identity of intracellular vesicles.
GLUT4 proteins reside in specific vesicular compartments in fat and skeletal muscle tissues, and GLUT4 vesicles contain a number of associated proteins, including Rab4, Rab5, Rab7, and Rab11, and it has been reported that Rab4 and Rab5 can be translocated to the plasma membrane in response to GTPγS treatment in 3T3-L1 adipocytes (27). Several previous studies (6, 35, 36), including some from our own laboratory (42), have already demonstrated that Rab4 plays an important role in insulin-mediated GLUT4 exocytosis, whereas Rab5 is important for the endocytotic process of GLUT4 internalization (16). Consistent with these previous studies, the present results confirm the necessity of Rab4 function for insulin-stimulated GLUT4 translocation by showing that overexpression of dominant-negative Rab4 inhibits the stimulatory effects of insulin on this process.
We also show that insulin stimulates Rab4 activation as well as the association of Rab4 with PKC-λ. Our data also support a role for PKC-λ in GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Thus, microinjection of anti-PKC-λ antibodies inhibited GLUT4 translocation (20), whereas adenovirus-mediated expression of dominant-negative PKC-λ inhibited, and constitutively active PKC-λ stimulated, glucose transport activity. These results are consistent with several other studies in the literature showing that PKC-λ plays an important role in the insulin-induced GLUT4 translocation process (2-4, 9, 24, 41) and that insulin can stimulate the translocation of PKC-λ to GLUT4 vesicles (38). On the other hand, Tsuru et al. presented evidence showing that interference with PKC-λ activity does not influence GLUT4 translocation (40), and it seems that further studies will be necessary to reconcile these different results.
Microtubules provide an intracellular structure along which trafficking vesicles can move under the influence of a number of motor proteins (12). Intracellular vesicle trafficking is dependent on a specific motor protein function which coordinates movement of cargo along the cellular cytoskeletal structure, and different classes of motor proteins demonstrate specific functions with regard to movement, velocity, and direction along the microtubules (13). For example, it has been shown that kinesin II moves toward the plus end of microtubules, whereas dynein traffics toward the minus end (15). A role for motor proteins in facilitating the exocytosis and endocytosis of GLUT4 has been described in previous reports (8, 11, 16). Along these lines, we found that KIF3 (kinesin II in mice) is important for the insulin-stimulated exocytosis of GLUT4 vesicles to the cell surface. It has been shown that the KIF3 motor protein functions only in anterograde transport (14), and this is fully consistent with our findings that microinjection of the anti-KAP3A or -KIF3B antibody inhibits exocytosis but does not affect the endocytosis of GLUT4 proteins.
Insulin stimulation of KIF3's microtubule binding activity proceeds through a process involving PI3-kinase-dependent activation of PKC-λ. Although the precise molecular mechanisms of these events are still unclear, it is interesting that protein phosphatase 2A can affect the dynein motor, suggesting that motor protein activity can be regulated by alterations in serine/threonine phosphorylation (20a). It is possible that PKC-λ-mediated phosphorylation of one or more of the KIF3 subunits is important in these stimulatory events, and this formulation is consistent with the data showing that expression of DN-PKC-λ blocks insulin-stimulated activation of KIF3 as well as insulin-induced GLUT4 translocation.
A number of previous papers have evaluated the role of microtubules in GLUT4 translocation, and, while several of these have provided evidence demonstrating the necessity for microtubule function in GLUT4 exocytosis (10, 29, 30), other studies have come to different conclusions (28, 37). For example, several studies have used nocodazole to depolymerize microtubules, and others have used different methods to disrupt microtubule function, all showing that an intact microtubule cytoskeletal system is necessary for GLUT4 translocation (10, 29, 30). On the other hand, Molero et al. (28) have presented evidence indicating that nocodazole inhibition of GLUT4 translocation is independent of microtubule-depolymerizing effects, whereas Shigematsu et al. (37) found little effect of nocodazole on GLUT4 translocation. Although the present studies do not directly address this issue and, therefore, cannot shed light on these divergent results, given the apparent role of motor proteins in the GLUT4 exocytotic and endocytotic itinerary, it would be logical to propose some role for microtubules in these processes.
Various kinds of linker proteins (binding partners) which couple the motor proteins to their cargo have been described (1, 23), and previous studies of other systems have indicated that various Rab proteins can serve as motor protein binding partners to facilitate motor protein-cargo interactions (7, 17). Furthermore, Rab proteins, particularly Rab4 and Rab5, have been identified in GLUT4 vesicles (27). Previous work from our laboratory has demonstrated the interaction of Rab5 with the motor protein dynein to mediate endocytosis of GLUT4 proteins (16), and the present report presents data indicating that Rab4 interacts with KIF3 to effect exocytosis of GLUT4 vesicles. Since there are many studies showing various roles of Rab4 and kinesin, it is likely that, in addition to the GLUT4 translocation system, both Rab4 and KIF3 interact with other compartments in other systems or cell types to function in membrane cycling events. Based on these data, one can propose a model in which insulin leads to GTP loading and activation of Rab4 within GLUT4 vesicles, thereby enabling recognition of the GLUT4 vesicle by the motor protein kinesin. PI3-kinase-dependent activation of PKC-λ facilitates this process and may promote the activation of kinesin with respect to its microtubule binding and motility functions.
In earlier studies, we showed that Rab5 can interact with the motor protein dynein to facilitate retrograde movement, or endocytosis, of GLUT4 proteins and that insulin can attenuate this process (16). The present study, showing that insulin stimulates the Rab4-KIF3 interaction, which facilitates the exocytotic movement of GLUT4 proteins, provides a balanced picture of exocytosis and endocytosis in which Rab proteins participate in the coupling of motor proteins to their cargo to facilitate GLUT4 translocation to and from the cell surface under the influence of insulin.
Acknowledgments
We thank Stephen Ferguson (The John P. Robarts Research Institute, London, Ontario, Canada) for Rab4 cDNA constructs, Wataru Ogawa (Kobe University, Kobe, Japan) for adenoviral constructs of PKC-λ, Michael Mueckler (Washington University, St. Louis, Mo.) for rabbit polyclonal anti-GLUT4 antibody, and Jeffrey E. Pessin (University of Iowa, Iowa City) for GLUT4-eGFP expression vector.
This work was supported in part by NIH grant DK-33651 and the VA Medical Research Service.
REFERENCES
- 1.Almenar-Queralt, A., and L. S. B. Goldstein. 2001. Linkers, packages and pathways: new concepts in axonal transport. Curr. Opin. Neurobiol. 11:550-557. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay, G., Y. Kanoh, M. P. Sajan, M. L. Standaert, and R. V. Farese. 2000. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology 141:4120-4127. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay, G., M. L. Standaert, U. Kikkawa, Y. Ono, J. Moscat, and R. V. Farese. 1999. Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinases C-zeta and C-lambda. Biochem. J. 337:461-470. [PMC free article] [PubMed] [Google Scholar]
- 4.Braiman, L., A. Alt, T. Kuroki, M. Ohba, A. Bak, T. Tennenbaum, and S. R. Sampson. 2001. Activation of protein kinase Cζ induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol. Cell. Biol. 21:7852-7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter glut4. Nat. Rev. Mol. Cell Biol. 3:267-277. [DOI] [PubMed] [Google Scholar]
- 6.Cormont, M., M. N. Bortoluzzi, N. Gautier, M. Mari, E. Vanobberghen, and Y. Lemarchandbrustel. 1996. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol. Cell. Biol. 16:6879-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echard, A., F. Jollivet, O. Martinez, J. J. Lacapere, A. Rousselet, I. JanoueixLerosey, and B. Goud. 1998. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 279:580-585. [DOI] [PubMed] [Google Scholar]
- 8.Emoto, M., S. E. Langille, and M. P. Czech. 2001. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 adipocytes. J. Biol. Chem. 276:10677-10682. [DOI] [PubMed] [Google Scholar]
- 9.Etgen, G. J., K. M. Valasek, C. L. Broderick, and A. R. Miller. 1999. In vivo adenoviral delivery of recombinant human protein kinase C-zeta stimulates glucose transport activity in rat skeletal muscle. J. Biol. Chem. 274:22139-22142. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher, L. M., G. I. Welsh, P. B. Oatey, and J. M. Tavare. 2000. Role for the microtubule cytoskeleton in GLUT4 vesicle trafficking and in the regulation of insulin-stimulated glucose uptake. Biochem. J. 352:267-276. [PMC free article] [PubMed] [Google Scholar]
- 10a.Gheber, L., S. C. Kuo, and M. A. Hoyt. 1999. Motile properties of the kinesin-related Cin8p spindle motor extracted from Saccharomyces cerevisiae cells. J. Biol. Chem. 274:9564-9572. [DOI] [PubMed] [Google Scholar]
- 11.Guilherme, A., M. Emoto, J. M. Buxton, S. Bose, R. Sabini, W. E. Theurkauf, J. Leszyk, and M. P. Czech. 2000. Perinuclear localization and insulin responsiveness of GLUT4 requires cytoskeletal integrity in 3T3-L1 adipocytes. J. Biol. Chem. 275:38151-38159. [DOI] [PubMed] [Google Scholar]
- 12.HammAlvarez, S. F., and M. P. Sheetz. 1998. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiol. Rev. 78:1109-1129. [DOI] [PubMed] [Google Scholar]
- 13.Hirokawa, N. 1997. The mechanisms of fast and slow transport in neurons: identification and characterization of the new kinesin superfamily motors. Curr. Opin. Neurobiol. 7:605-614. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa, N. 2000. Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1:29-34. [DOI] [PubMed] [Google Scholar]
- 15.Hirokawa, N., Y. Noda, and Y. Okada. 1998. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr. Opin. Cell Biol. 10:60-73. [DOI] [PubMed] [Google Scholar]
- 16.Huang, J., T. Imamura, and J. M. Olefsky. 2001. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl. Acad. Sci. USA 98:13084-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hume, A. N., L. M. Collinson, A. Rapak, A. Q. Gomes, C. R. Hopkins, and M. C. Seabra. 2001. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152:795-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamura, T., J. Huang, S. Dalle, S. Ugi, I. Usui, L. M. Luttrell, W. E. Miller, R. J. Lefkowitz, and J. M. Olefsky. 2001. Beta-arrestin-mediated recruitment of the Src family kinase yes mediates endothelin-1-stimulated glucose transport. J. Biol. Chem. 276:43663-43667. [DOI] [PubMed] [Google Scholar]
- 19.Imamura, T., K. Ishibashi, S. Dalle, S. Ugi, and J. M. Olefsky. 1999. Endothelin-1-induced GLUT4 translocation is mediated via Galpha(q/11) protein and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J. Biol. Chem. 274:33691-33695. [DOI] [PubMed] [Google Scholar]
- 20.Imamura, T., P. Vollenweider, K. Egawa, M. Clodi, K. Ishibashi, N. Nakashima, S. Ugi, J. W. Adams, J. H. Brown, and J. M. Olefsky. 1999. G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol. Cell. Biol. 19:6765-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Inaba, K. 2002. Dephosphorylation of Tctex2-related dynein light chain by type 2A protein phosphatase. Biochem. Biophys. Res. Commun. 297:800-805. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi, K., T. Imamura, P. M. Sharma, J. Huang, S. Ugi, and J. M. Olefsky. 2001. Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J. Clin. Investig. 107:1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal, A., and L. S. B. Goldstein. 2000. Connecting vesicle transport to the cytoskeleton. Curr. Opin. Cell Biol. 12:503-508. [DOI] [PubMed] [Google Scholar]
- 23.Karcher, R. L., S. W. Deacon, and V. I. Gelfand. 2002. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 12:21-27. [DOI] [PubMed] [Google Scholar]
- 24.Kotani, K., W. Ogawa, M. Matsumoto, T. Kitamura, H. Sakaue, Y. Hino, K. Miyake, W. Sano, K. Akimoto, S. Ohno, and M. Kasuga. 1998. Requirement of atypical protein kinase Cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 18:6971-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marszalek, J. R., and L. S. B. Goldstein. 2000. Understanding the functions of kinesin-II. Biochim. Biophys. Acta 1496:142-150. [DOI] [PubMed] [Google Scholar]
- 26.Martinez, O., and B. Goud. 1998. Rab proteins. Biochim. Biophys. Acta 1404:101-112. [DOI] [PubMed] [Google Scholar]
- 27.Millar, C. A., A. Shewan, G. R. X. Hickson, D. E. James, and G. W. Gould. 1999. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes. Mol. Biol. Cell 10:3675-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molero, J. C., J. P. Whitehead, T. Meerloo, and D. E. James. 2001. Nocodazole inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes via a microtubule-independent mechanism. J. Biol. Chem. 276:43829-43835. [DOI] [PubMed] [Google Scholar]
- 29.Olson, A. L., A. R. Trumbly, and G. V. Gibson. 2001. Insulin-mediated GLUT4 translocation is dependent on the microtubule network. J. Biol. Chem. 276:10706-10714. [DOI] [PubMed] [Google Scholar]
- 30.Patki, V., J. Buxton, A. Chawla, L. Lifshitz, K. Fogarty, W. Carrington, R. Tuft, and S. Corvera. 2001. Insulin action on GLUT4 traffic visualized in single 3T3-L1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12:129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pessin, J. E., D. C. Thurmond, J. S. Elmendorf, K. J. Coker, and S. Okada. 1999. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J. Biol. Chem. 274:2593-2596. [DOI] [PubMed] [Google Scholar]
- 32.Ricketts, W. A., J. H. Brown, and J. M. Olefsky. 1999. Pertussis toxin-sensitive and -insensitive thrombin stimulation of Shc phosphorylation and mitogenesis are mediated through distinct pathways. Mol. Endocrinol. 13:1988-2001. [DOI] [PubMed] [Google Scholar]
- 33.Seachrist, J. L., S. A. Laporte, L. B. Dale, A. V. Babwah, M. G. Caron, P. H. Anborgh, and S. S. G. Ferguson. 2002. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J. Biol. Chem. 277:679-685. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd, P. R., and B. B. Kahn. 1999. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 341:248-257. [DOI] [PubMed] [Google Scholar]
- 35.Shibata, H., W. Omata, and I. Kojima. 1997. Insulin stimulates guanine nucleotide exchange on Rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J. Biol. Chem. 272:14542-14546. [DOI] [PubMed] [Google Scholar]
- 36.Shibata, H., W. Omata, Y. Suzuki, S. Tanaka, and I. Kojima. 1996. A synthetic peptide corresponding to the Rab4 hypervariable carboxyl-terminal domain inhibits insulin action on glucose transport in rat adipocytes. J. Biol. Chem. 271:9704-9709. [DOI] [PubMed] [Google Scholar]
- 37.Shigematsu, S., A. H. Khan, M. Kanzaki, and J. E. Pessin. 2002. Intracellular insulin-responsive glucose transporter (GLUT4) distribution but not insulin-stimulated GLUT4 exocytosis and recycling are microtubule dependent. Mol. Endocrinol. 16:1060-1068. [DOI] [PubMed] [Google Scholar]
- 38.Standaert, M. L., G. Bandyopadhyay, L. Perez, D. Price, L. Galloway, A. Poklepovic, M. P. Sajan, V. Cenni, A. Sirri, J. Moscat, A. Toker, and R. V. Farese. 1999. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J. Biol. Chem. 274:25308-25316. [DOI] [PubMed] [Google Scholar]
- 39.Standaert, M. L., L. Galloway, P. Karnam, G. Bandyopadhyay, J. Moscat, and R. V. Farese. 1997. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J. Biol. Chem. 272:30075-30082. [DOI] [PubMed] [Google Scholar]
- 40.Tsuru, M., H. Katagiri, T. Asano, T. Yamada, S. Ohno, T. Ogihara, and Y. Oka. 2002. Role of PKC isoforms in glucose transport in 3T3-L1 adipocytes: insignificance of atypical PKC. Am. J. Physiol. Encocrinol. Metab. 283:E338-E345. [DOI] [PubMed] [Google Scholar]
- 41.Valverde, A. M., R. Lorenzo, P. Navarro, C. Mur, and M. Benito. 2000. Okadaic acid inhibits insulin-induced glucose transport in fetal brown adipocytes in an Akt-independent and protein kinase C zeta-dependent manner. FEBS Lett. 472:153-158. [DOI] [PubMed] [Google Scholar]
- 42.Vollenweider, P., S. S. Martin, T. Haruta, A. J. Morris, J. G. Nelson, M. Cormont, Y. Le Marchand-Brustel, D. W. Rose, and J. M. Olefsky. 1997. The small guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3-L1 cells. Endocrinology 138:4941-4949. [DOI] [PubMed] [Google Scholar]
- 43.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]