Abstract
S100B is a Ca2+-modulated protein of the EF-hand type with both intracellular and extracellular roles. S100B, which is most abundant in the brain, has been shown to exert trophic and toxic effects on neurons depending on the concentration attained in the extracellular space. S100B is also found in normal serum, and its serum concentration increases in several nervous and nonnervous pathological conditions, suggesting that S100B-expressing cells outside the brain might release the protein and S100B might exert effects on nonnervous cells. We show here that at picomolar to nanomolar levels, S100B inhibits myogenic differentiation of rat L6 myoblasts via inactivation of p38 kinase with resulting decrease in the expression of the myogenic differentiation markers, myogenin, muscle creatine kinase, and myosin heavy chain, and reduction of myotube formation. Although myoblasts express the multiligand receptor RAGE, which has been shown to transduce S100B effects on neurons, S100B produces identical effects on myoblasts overexpressing either full-length RAGE or RAGE lacking the transducing domain. This suggests that S100B affects myoblasts by interacting with another receptor and that RAGE is not the only receptor for S100B. Our data suggest that S100B might participate in the regulation of muscle development and regeneration by inhibiting crucial steps of the myogenic program in a RAGE-independent manner.
S100B, a member of a multigenic family of Ca2+-regulated proteins of the EF-hand type, is highly abundant in astrocytes and is expressed in relatively large amounts in a variety of nonneural cell types (for reviews, see references 12, 13, and 36). Besides being implicated in the Ca2+-dependent regulation of several intracellular activities (12, 13, 36), S100B is released by astrocytes into the extracellular space (45) and is also found in serum (13). S100B has been shown to interact with neurons, astrocytes, and microglia and to exert various effects on these cells depending on its concentration. S100B has also been shown to enhance neuronal survival and stimulate neurite outgrowth and astrocyte proliferation at nanomolar concentrations (2, 3, 6, 7, 16, 20, 21, 23, 39, 47) and to cause neuronal and astrocyte apoptosis and stimulate interleukin-6 secretion by neurons and nitric oxide release by astrocytes and microglia at micromolar concentrations (1, 18-20, 25, 27, 31). Therefore, S100B has been hypothesized to play roles in brain development and neuronal protection (2, 3, 6, 7, 16, 20, 21, 23, 39, 47) and in the pathophysiology of neurodegenerative disorders (15, 20, 30, 40, 41), depending on the concentration attained in the extracellular space. Trophic effects of S100B on neurons have been shown to depend on activation of the transcription factor NF-κB (3). The receptor for advanced glycation end products (RAGE), a multiligand receptor of the immunoglobulin superfamily (for reviews, see references 37 and 38), has been shown to bind S100B (17) and to mediate the effects of both low and high levels of S100B on a neuronal cell line (20). Binding of nanomolar amounts of S100B to RAGE on neurons caused nuclear translocation of NF-κB and up-regulation of the antiapoptotic factor Bcl-2, whereas micromolar doses of the protein caused cytochrome c release, activation of the caspase cascade, and neuronal apoptosis (20). Toxic effects of micromolar doses of S100B on RAGE-expressing neurons were eliminated by pretreatment of cells with antioxidants or inhibition of the mitogen-activated protein (MAP) kinase kinase (MEK) signaling pathway, suggesting that RAGE engagement by high doses of S100B determines intracellular accumulation of reactive oxygen species and persistent activation of the MEK-extracellular signal-regulated kinase (ERK1/2) signaling pathway (20). Thus, S100B-RAGE interactions appear to be critical for both trophic and toxic effects of S100B on neurons. However, at present it is not known whether RAGE is the sole S100B receptor, nor is it known whether or by what mechanisms extracellular S100B may affect nonnervous cell types.
In search of novel target cells for S100B, we investigated possible effects of this protein on myoblasts. The rationale for this study was as follows: (i) S100B is expressed in myoblasts and myotubes as well as vertebrate skeletal muscles (5, 42), and myoblasts and/or myotubes might release the protein constitutively or upon appropriate stimuli; (ii) the presence of S100B in normal serum points to S100B release by cell types other than astrocytes and possible effects of S100B on cells outside the nervous system; and (iii) the serum concentration of S100B increases in several nervous and nonnervous pathological conditions (4, 8, 14, 24, 28, 29, 34, 35). We show here that (i) at picomolar to nanomolar doses S100B inhibits rat L6 myogenic differentiation by inactivating p38 MAP kinase and that (ii) while rat L6 myoblasts express RAGE constitutively, S100B causes similar effects on both myoblasts overexpressing full-length RAGE and myoblasts overexpressing a mutant form of RAGE lacking the transducing domain (RAGEΔcyto), which indicates that RAGE is not implicated in the S100B effects on myoblasts described here. These data suggest that extracellular S100B may participate in the regulation of myogenesis by interfering with the activity of p38, a kinase shown to be crucial for myoblast differentiation (33, 48, 49). Also, our data suggest that extracellular effects of S100B are not restricted to the brain and that S100B might be recognized by more than one receptor.
MATERIALS AND METHODS
Purification of S100B and S100A1.
Recombinant S100B and S100A1 were expressed, purified, and characterized as described previously (20). The S100B concentration was calculated using the Mr of the S100B dimer, i.e., 21 kDa.
Cell culture.
L6 myoblasts (clone L6C31) were cultured for 24 h in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Life Technologies), 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a H2O-saturated 5% CO2 atmosphere at 37°C before FBS was decreased to 2% to induce myoblast differentiation and myotube formation. Most experiments with S100B or an anti-S100B antibody (SWant) were done on myoblasts in 2% FBS. Other experiments were performed as described below or in the figure legends. The anti-S100B antibody used here was a whole immunoglobulin G (IgG) fraction isolated from rabbit serum following immunization with pure S100B.
Measurement of S100B.
The S100B content of FBS and myoblast conditioned media was measured by two-site enzyme-linked immunosorbent assay as described previously (2).
Kinase assay.
p38 and Akt kinase assays were performed on myoblast extracts subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting using a polyclonal antibody specific to phosphorylated (Thr180/Tyr182) p38 (1:1,000) or a polyclonal anti-phosphorylated (Ser473) Akt (1:1,000) (both from New England BioLabs). Total p38 and Akt were detected by Western blotting using a polyclonal anti-p38 (1:2,000) and a polyclonal anti-Akt (1:2,000) antibody (both from New England BioLabs), respectively. To produce cell extracts, myoblasts were solubilized with 2.5% sodium dodecyl sulfate, 10 mM Tris-HCl (pH 7.4), 0.1 M dithiothreitol, and 0.1 mM tosylsulfonyl phenylalanyl chloromethyl ketone protease inhibitor (Roche). The immune reaction was developed by enhanced chemiluminescence (ECL) (SuperSignal West Pico; Pierce).
Immunocytochemistry.
To detect myosin heavy chain (MHC), myoblasts cultivated in 2% FBS for 3 days were fixed in cold methanol at −20°C for 7 min and subjected to immunocytochemistry as described previously (42) with a monoclonal anti-developmental MHC antibody (Biogenesis) at a 1:1,000 dilution. The immune reaction product was visualized by using a Vectastain Elite ABC kit (Vector Laboratories Inc.). To detect MHC, myogenin, tubulin, and RAGE by Western blotting, myoblasts were cultivated as detailed in the legends to pertinent figures, washed twice with phosphate-buffered saline, and solubilized as described above. MHC, myogenin, tubulin, and RAGE were detected by using the MHC antibody mentioned above (1:300), a monoclonal anti-myogenin antibody (1:1,000; PharMingen), a monoclonal anti-α-tubulin antibody (1:10,000; Sigma), and a polyclonal anti-RAGE antibody (1:2,000; Chemicon International), respectively. The immune reaction was developed by ECL (SuperSignal West Femto Maximum [Pierce] for RAGE and SuperSignal West Pico for MHC, myogenin, and tubulin).
Transfection assay.
L6 myoblasts were transfected with human RAGE cDNA or RAGEΔcyto cDNA (kindly provided by Henri J. Huttunen, Helsinki, Finland) by using the plasmid vector pcDNA3 and the Lipofectamine technique (Life Technologies). Geneticin (G418; 250 μg/ml; Life Technologies) was added to the medium 48 h after transfection to select the stably transfected clones. Myoblasts overexpressing RAGE or RAGEΔcyto were used in experiments as described above in media containing G418 (125 μg/ml). Transient transfections were carried out using Lipofectamine 2000 (Invitrogen) as recommended by the manufacturer. Briefly, cells cultured in 10% FBS without antibiotics were transfected with expression plasmid MKK6EE (a constitutively active form of MAP kinase kinase 6 [MKK6]) (22) or empty vector and the reporter gene muscle creatine kinase (MCK)-luc. After 24 h, cells were shifted to 2% FBS containing S100B. After another 24 h, cells were harvested to measure luciferase activity.
RT-PCR.
Total cytoplasmic RNA was isolated from L6 myoblasts by using the TRIzol reagent method. Subsequent steps were done as described previously (42), with the following modifications: (i) the polymerase used was AmpliTaq Gold (Perkin Elmer); (ii) the murine RAGE forward and reverse primers were (5′ to 3′) GGAATTGTCGATGAGGGGAC (500 nM) and CAACAGCTGAATGCCCTCTG (500 nM), respectively; and (iii) 1 cycle at 95°C for 10 min was performed for polymerase activation before the performance of 35 cycles for amplification reactions. The expected PCR product for rat RAGE was 453 bp in size. The expression of human RAGE and RAGEΔcyto was verified by reverse transcriptase (RT) PCR using the following RAGE-specific oligonucleotides (5′ to 3′): TGTCGGGATCCAGGATGAGG (forward primer) (250 nM) and ACCACCAATTGGACCTCCTC (reverse primer 1) (250 nM) or ACTACTCTCGCCTGCCTCAG (reverse primer 2) (250 nM). Reverse primer 2 was chosen for its specificity to a region contained in the RAGE C-terminal and transducing domain. The expected PCR products were 462 and 983 bp in size for reverse primers 1 and 2, respectively. In control samples, cDNA mixture from the RT reaction was omitted. After amplification, samples (10 μl) of each PCR mixture were electrophoresed on a 1.3% agarose gel and RT-PCR products were revealed by ethidium bromide staining.
RESULTS
Myoblasts do not release S100B constitutively.
FBS contained ∼80 ng of S100B dimer/ml, i.e., ∼4 nM, as determined by two-site enzyme-linked immunosorbent assay. Serum S100B can be purified to homogeneity by the procedure used to purify S100B from tissues (11) (data not shown). No differences were detected between the S100B content in the culture medium of myoblasts grown in FBS (8.3 ± 0.68 ng/ml [average of three experiments ± standard deviation {SD}]; myoblasts were cultivated in 10% FBS for 24 h) and that in unconditioned FBS (8.2 ± 0.33 ng/ml; 10% FBS in DMEM without myoblasts), and no measurable S100B could be detected in the culture medium of serum-starved myoblasts (cultivated in the absence of FBS for 24 h). Thus, contrary to cultured astrocytes (45), L6 myoblasts do not appear to release S100B normally, although we cannot exclude the possibility that release of minute amounts of S100B might have escaped detection by the method used here.
S100B inhibits myogenic differentiation and myotube formation.
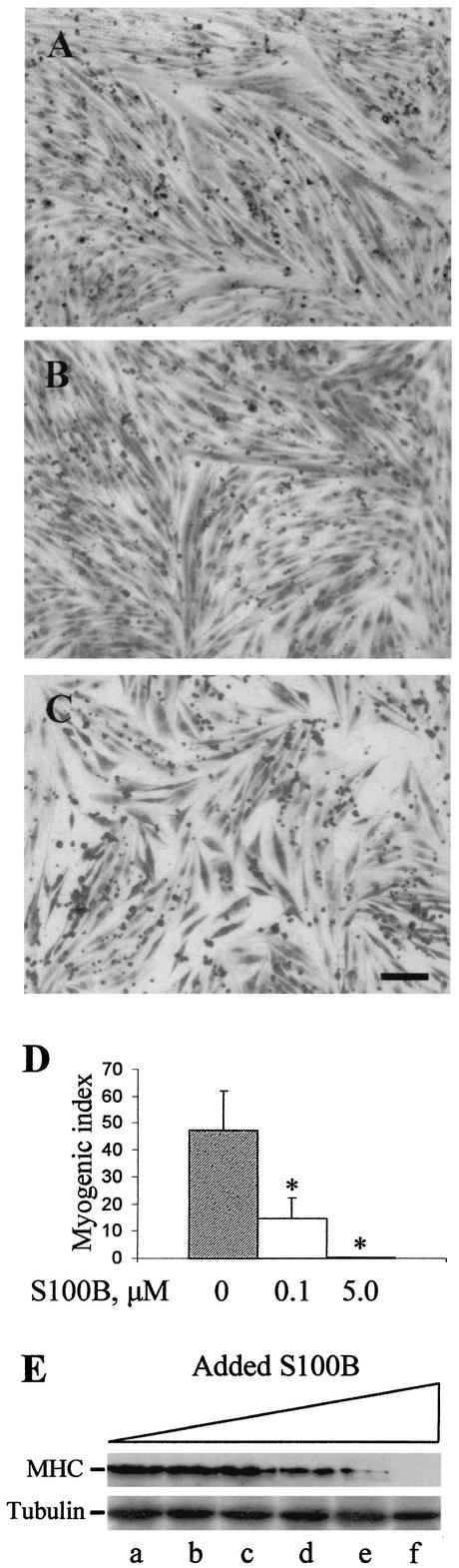
Although our data suggested that L6 myoblasts did not release measurable amounts of S100B, thus questioning the possibility that the protein might act on myoblasts in an autocrine manner, we sought to investigate effects of exposure of myoblasts to S100B given the presence of the protein in normal serum (13). In the first series of experiments, we analyzed the effects of increasing doses of S100B on myoblast fusion and calculated the myogenic index, i.e., the fraction of nuclei residing in cells containing ≥3 nuclei after staining with May-Grünwald Giemsa, as a measure of myoblast fusion. Upon visual inspection, myotube formation was found to be significantly reduced in the presence of 100 nM S100B (i.e., an S100B concentration that induces neurons to differentiate and astrocytes to proliferate [2, 3, 6, 7, 20, 21, 23, 39, 47]) and completely blocked in the presence of 5 μM S100B (Fig. 1A to C). Also, under the latter condition the cell number was distinctly reduced (Fig. 1C). The myogenic index was ∼47% in control cells, ∼15% at 100 nM S100B, and <0.1% at 5 μM S100B (Fig. 1D). At concentrations of <50 nM, no effects of added S100B could be detected by this experimental approach (data not shown). In parallel experiments in which myoblasts were solubilized to detect the differentiation marker, MHC, by Western blotting, we found that added S100B was ineffective up to 5 nM, while it caused a dose-dependent inhibition of MHC expression at ≥50 nM (Fig. 1E), suggesting that S100B interfered with both myoblast differentiation and myotube formation.
FIG. 1.
Effects of S100B on myoblast differentiation and fusion. (A to C) Myoblasts were cultivated for 3 days in 2% FBS in the absence (A) or presence of 100 nM (B) or 5 μM (C) S100B and then fixed and stained with May-Grünwald Giemsa. Note the reduction of fusion at 100 nM S100B and the absence of fusion and strong reduction in cell number and absence of myotubes at 5 μM S100B. Bar = 100 μm. (D) Twenty random fields were analyzed to calculate the myogenic index (i.e., the fraction of nuclei residing in cells containing ≥3 nuclei after staining with May-Grünwald Giemsa) as a measure of myoblast fusion.Results shown are averages of three experiments plus SD. ∗, significantly different from control (P < 0.001). (E) Myoblasts were cultivated for 3 days in 2% FBS in the presence of increasing concentrations of S100B before solubilization and Western blotting with anti-MHC antibody. Lanes: a, no additions; b, 0.5 nM; c, 5 nM; d, 50 nM; e, 0.1 μM; f, 5 μM. About 15 μg of protein was loaded in each lane. The same nitrocellulose filter was treated with Restore Western blot stripping buffer (Pierce) to detach the anti-MHC antibody and was subjected to Western blotting using a monoclonal anti-α-tubulin antibody. Note the dose-dependent decrease in the MHC amount at ≥50 nM S100B, with no MHC detected at 5 μM S100B.
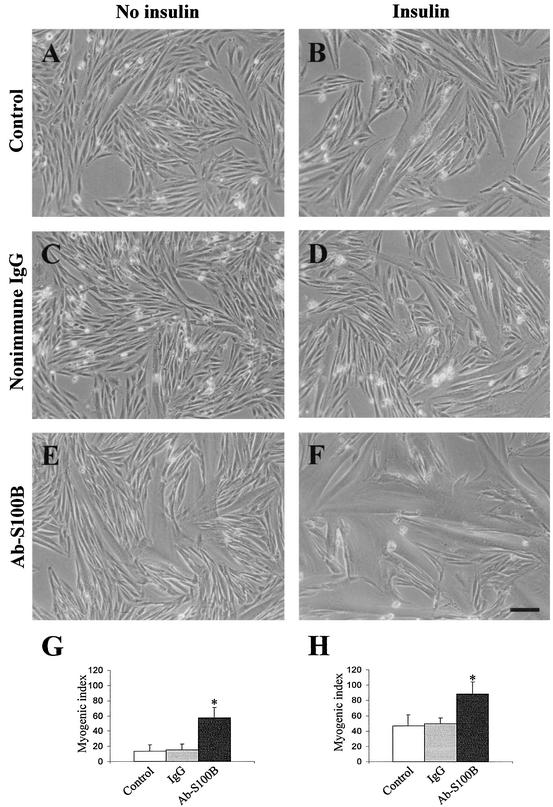
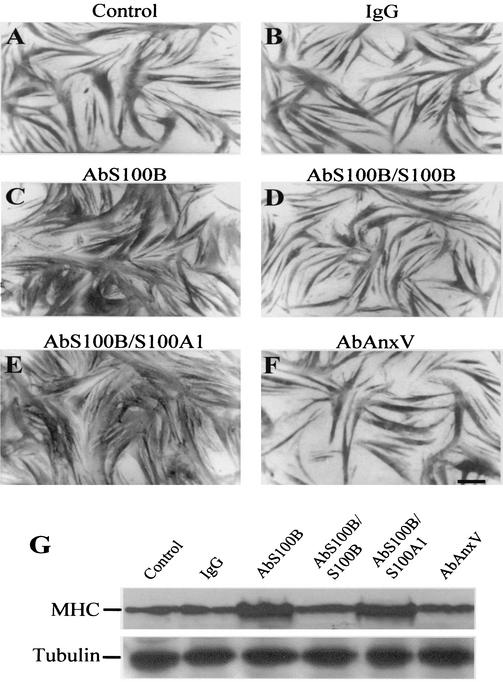
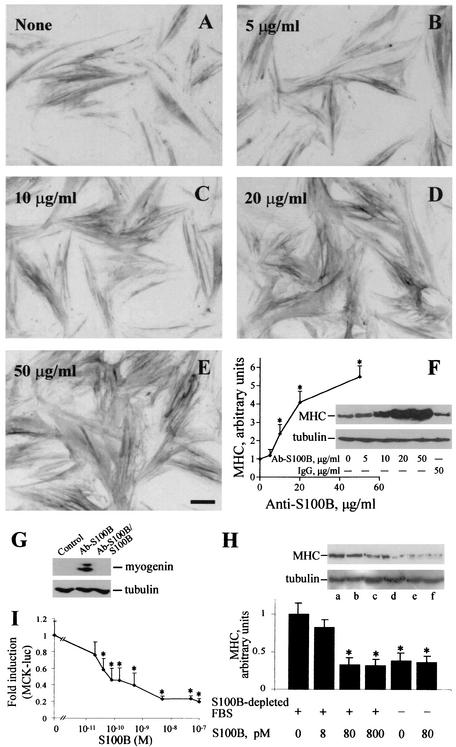
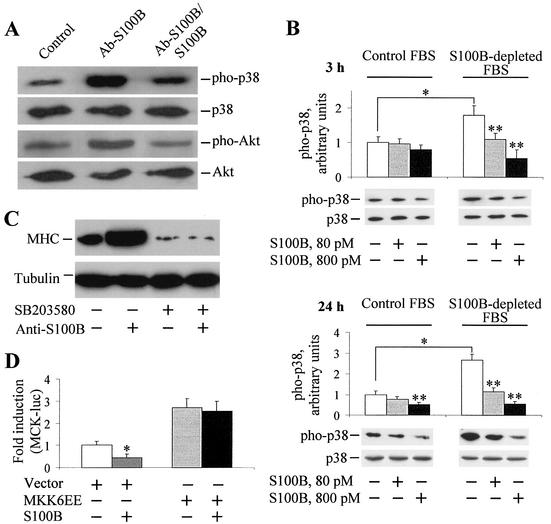
Since under the abovementioned culture conditions myoblasts were exposed to ∼80 pM S100B dimer (i.e., 2% of the total amount of S100B in FBS), in a second series of experiments we explored the possibility that serum S100B might have a role in the regulation of myogenic differentiation and/or myotube formation. To this end, a polyclonal anti-S100B antibody was added to myoblasts cultivated in 2% FBS to neutralize serum S100B, while parallel cells received identical amounts of nonimmune IgG, anti-S100B antibody preabsorbed with pure S100B, anti-S100B antibody preabsorbed with pure S100A1 (another member of the S100 protein family and the closest S100B homologue [12, 13, 36]), or an antibody against a protein unrelated to S100B (anti-annexin V antibody). Myoblasts were then analyzed for myotube formation and MHC content. We found that (i) the proportion of myotubes was higher following administration of anti-S100B antibody (Fig. 2E and F) compared with nonimmune IgG (Fig. 2C and D) or with no additions (Fig. 2A and B), irrespective of the absence (Fig. 2A, C, and E) or presence (Fig. 2B, D, and F) of insulin, as indicated by the myogenic index calculated for each condition (Fig. 2G and H, respectively); (ii) myotubes formed in the presence of anti-S100B antibody (Fig. 3C) or anti-S100B antibody preabsorbed with S100A1 (Fig. 3E) were larger and more numerous than those formed following no additions or the addition of nonimmune IgG, anti-S100B antibody preabsorbed with S100B, or an anti-annexin V antibody (Fig. 3A, B, D, and F, respectively), as also indicated by the MHC content under these conditions (Fig. 3G); and (iii) the ability of anti-S100B antibody to promote myotube formation was dose dependent (Fig. 4A to F). Myogenin, an early marker of myoblast differentiation (26), was detected in myoblasts exposed for 2 days to anti-S100B antibody but not in control myoblasts or myoblasts exposed to anti-S100B antibody preabsorbed with S100B (Fig. 4G). Collectively, these data suggested that neutralization of the picomolar amounts of S100B contained in FBS resulted in a remarkable acceleration of the myogenic program in vitro. To confirm that serum S100B inhibited myogenesis, myoblasts were cultivated in FBS that had been depleted of S100B by absorption to anti-S100B antibody and were analyzed for MHC content. These cells were found to express a larger amount of MHC compared with control cells (Fig. 4H). Also, on addition of picomolar amounts of S100B back to the S100B-depleted FBS, the MHC expression in myoblasts decreased to the levels found in matched myoblasts cultivated in FBS preabsorbed onto nonimmune IgG (Fig. 4H). Moreover, when added back to S100B-depleted FBS, S100B caused a dose-dependent inhibition of expression of MCK, another early marker of muscle differentiation, with half-maximal effect at ∼40 pM and maximal effect at ∼5 nM (Fig. 4I). This finding was in accordance with the observation that neutralization of FBS S100B resulted in a stimulation of myogenesis and, conversely, that reconstitution of S100B-depleted FBS with S100B caused inhibition of MHC expression (see Fig. 4H). The resulting inhibition curve displayed a rather steep phase between 0 and ∼150 pM S100B, accounting for ∼60% inhibition, that was followed by a slower phase in the nanomolar range of S100B concentration. Thus, the data in Fig. 4I also were consistent with the data in Fig. 1E, which showed that increasing to nanomolar levels the dose of S100B administered to myoblasts cultivated in complete (i.e., S100B-containing) FBS resulted in (a further) inhibition of myogenic differentiation. Collectively, these data suggested that minute (i.e., picomolar) amounts of S100B exerted a negative effect on myoblast differentiation and fusion and that accumulation of S100B in serum and/or the extracellular space might amplify such a negative effect.
FIG. 2.
Effects of neutralization of FBS S100B on myotube formation. Myoblasts were cultivated in 2% FBS without (A, C, and E) or with (B, D, and F) 10 nM insulin for 3 days with no additions (A and B), in the presence of 50 μg of nonimmune IgG/ml (C and D), or in the presence of 50 μg of anti-S100B antibody/ml (E and F). Bar = 100 μm. Cell morphology was analyzed by phase contrast, and the myogenic index was calculated for each condition, i.e., in the absence (G) or presence (H) of insulin. Neutralization of S100B in FBS by anti-S100B antibody results in stimulation of myotube formation both in the absence and the presence of insulin. Results shown are averages of three experiments plus SD. ∗, significantly different from control (P < 0.001).
FIG. 3.
Effects of neutralization of FBS S100B on myoblast differentiation and myotube formation as investigated by MHC immunocytochemistry. Myoblasts were cultivated in 2% FBS containing 10 nM insulin for 4 days with no additions (A) or in the presence of a 50-μg/ml concentration of nonimmune IgG (B), anti-S100B antibody (C), anti-S100B antibody preabsorbed with S100B (D), anti-S100B antibody preabsorbed with S100A1 (E), or anti-annexin V antibody (F) before immunocytochemistry with an anti-MHC antibody. The anti-S100B antibody and the anti-S100B antibody preabsorbed with S100A1, but not nonimmune IgG, the anti-S100B antibody preabsorbed with S100B, or the anti-annexin V antibody (AbAnxV), stimulate myotube formation. Undifferentiated myoblasts, which are MHC negative, are not seen. Bar = 100 μm. (G) Parallel myoblasts cultivated as described for panels A to F were solubilized and subjected to Western blotting with anti-MHC antibody. About 15 μg of protein was loaded in each lane. Tubulin was detected as described in the legend to Fig. 1. Note the larger amount of MHC in samples obtained from cells cultivated in the presence of the anti-S100B antibody and the anti-S100B antibody preabsorbed to S100A1 compared with each of the other conditions.
FIG. 4.
Dose dependence of the inhibitory effect of S100B on myogenic differentiation. Myoblasts were cultivated in 2% FBS containing 10 nM insulin for 3 days with no additions (A) or in the presence of 5 (B), 10 (C), 20 (D), or 50 (E) μg of anti-S100B antibody/ml before immunocytochemistry with anti-MHC antibody. Bar = 100 μm. (F) Parallel myoblasts cultivated and treated as described above or with 50 μg of nonimmune IgG/ml were solubilized and analyzed for MHC content by Western blotting (inset). MHC content was plotted versus anti-S100B antibody concentration. Tubulin was detected as described in the legend to Fig. 1 (inset). (G) Myoblasts were cultivated in 2% FBS for 2 days in the presence of anti-S100B antibody or anti-S100B antibody preabsorbed with S100B or with no additions before solubilization and Western blotting with anti-myogenin antibody. (H) Myoblasts were cultivated for 24 h in the presence of 10 nM insulin in FBS (2%) previously absorbed onto immobilized anti-S100B antiserum (lanes a to d) or immobilized nonimmune IgG (lanes e and f) in the absence (lanes a and e) or presence (lanes b to d and f) of 8 (b), 80 (c and f), or 800 (d) pM S100B. Cells were then analyzed for MHC and tubulin content by Western blotting (the inset shows the results of one representative experiment). In each lane, the amount of MHC was normalized to the tubulin content. Note the larger expressionexpression of MHC in myoblasts cultivated in S100B-depleted FBS compared with that in myoblasts cultivated in FBS preabsorbed onto nonimmune IgG. On addition of S100B back to preabsorbed FBS, the MHC concentration decreased dose dependently in the S100B concentration range tested, with maximal effect at ∼80 pM, while S100B (80 pM) did not influence MHC expression by myoblasts cultivated in FBS preabsorbed onto nonimmune IgG. Immune reaction was developed by ECL (SuperSignal West Pico). (I) Myoblasts cultured in 10% FBS without antibiotics were transfected with reporter gene MCK-luc. After 24 h, cells were washed extensively with DMEM and cultivated in 2% S100B-depleted FBS for an additional 24 h in the absence or presence of increasing concentrations of S100B, after which cells were harvested to measure luciferase activity. The data shown in panels H and I support the conclusion that picomolar amounts of S100B negatively regulate myogenesis and that S100B is one FBS factor implicated in inhibition of myogenic differentiation. Results shown in panels F, H, and I are averages of three experiments plus SD. ∗, significantly different from control (P < 0.001).
S100B inhibits myogenesis via inactivation of p38 kinase.
Myoblast differentiation has been shown to require activation of the p38 and Akt signaling pathways (33, 44, 48, 49). We found that the anti-S100B antibody, but not anti-S100B antibody preabsorbed with S100B, caused an increase in the extent of p38 phosphorylation compared with control cells (Fig. 5A), suggesting that picomolar amounts of S100B, like those found in FBS, might interfere with myogenic differentiation via inactivation of p38 kinase activity. To verify this possibility, three series of experiments were carried out. First, myoblasts were cultivated under the conditions described in the legend to Fig. 4H, that is, in the presence of S100B-depleted FBS, and the extent of p38 phosphorylation was measured (Fig. 5B). Phosphorylation of p38 was found to be higher in myoblasts cultivated in S100B-depleted FBS than in those in mock-absorbed FBS, and reconstitution of S100B-depleted FBS with S100B resulted in a dose-dependent decrease in the extent of p38 phosphorylation at 3 and 24 h after plating (Fig. 5B). Importantly, at 80 pM S100B the extent of p38 phosphorylation in myoblasts cultivated in S100B-depleted FBS (Fig. 5B, right panels) was nearly the same as that in myoblasts cultivated in complete FBS without other additions (Fig. 5B, left panels), in accordance with the data shown in Fig. 5A. The data in Fig. 5B also suggested that raising the S100B concentration to 800 pM caused a further reduction of the extent of p38 phosphorylation, particularly at 24 h after plating in both myoblasts cultivated in complete FBS and myoblasts cultivated in S100B-depleted FBS. This suggested that accumulation of S100B in serum might cause a strong and persistent inactivation of p38 kinase activity and consequent enhancement of inhibition of myogenic differentiation.
FIG. 5.
S100B inhibits myogenic differentiation via inactivation of p38 MAP kinase. (A) Myoblasts were cultivated in 2% FBS for 2 days in the presence of anti-S100B antibody or anti-S100B antibody preabsorbed with S100B or with no additions before solubilization and Western blotting with anti-phosphorylated p38 (pho-p38) or anti-phosphorylated Akt antibody (pho-Akt). Also shown in each lane are the total p38 and Akt. (B) Myoblasts were cultivated for 24 h in the presence of 10 nM insulin in FBS (2%) previously absorbed onto immobilized anti-S100B antiserum (lanes 1 to 3 from left) or immobilized nonimmune IgG (lanes 4 to 6 from left), in the absence (lanes 1 and 4) or presence of 80 (lanes 2 and 5) or 800 (lanes 3 and 6) pM S100B. After 3 or 24 h as indicated, cells were analyzed for phosphorylated and total p38 kinase content by Western blotting (shown are the results of one representative experiment). The amount of phosphorylated p38 was normalized to the total p38 content. Note the larger extent of p38 phosphorylation in myoblasts cultivated in S100B-depleted FBS compared with that in myoblasts cultivated in FBS preabsorbed onto nonimmune IgG. On the addition of S100B back to preabsorbed FBS, the extent of p38 phosphorylation decreased dose dependently in the S100B concentration range tested. Immune reaction was developed by ECL (SuperSignal West Pico). Results shown are averages of three experiments plus SD. ∗, significantly different from each other (P < 0.001). ∗∗, significantly different from the appropriate internal control. (C) Myoblasts were cultivated for 24 h in 10% FBS before switching to 2% FBS. At the time of FBS switching, cells received either the p38 inhibitor SB203580 (final concentration, 2 μM) or the SB203580 vehicle (dimethyl sulfoxide, final concentration, 0.02%) for 30 min and then anti-S100B antibody (50 μg/ml) as indicated. Myoblasts were cultivated for an additional 3 days under these conditions before solubilization and Western blotting with anti-MHC antibody. Immune reaction was developed by ECL (SuperSignal West Pico). Tubulin was detected as described in the legend to Fig. 1. The MHC blot was overexposed to detect a faint MHC immune band in lanes 3 and 4 from left. (D) Myoblasts cultured in 10% FBS without antibiotics were transfected with expression plasmid MKK6EE or empty vector and the reporter gene MCK-luc. After 24 h, cells were shifted to 2% FBS and cultivated for an additional 24 h in the absence or presence of 100 nM S100B, after which cells were harvested to measure luciferase activity. Results shown are averages of three experiments plus SD. ∗, significantly different from control (P < 0.001).
In a second series of experiments, myoblasts were treated with the p38 inhibitor, SB203580, and cultivated in the presence of anti-S100B antibody. SB203580 blocked myogenesis as expected (33, 48, 49), and administration of anti-S100B antibody failed to rescue the ability of myoblasts to differentiate under these conditions (Fig. 5C).
Lastly, to confirm that S100B was acting via inhibition of p38 kinase activity, we transfected myoblasts with MKK6EE, a constitutively active form of MKK6 (22), i.e., a kinase acting upstream of and activating p38. We found that administration of nanomolar doses of S100B failed to block the myogenic program (Fig. 5D). Collectively, these observations supported our conclusion that S100B might interfere with myoblast differentiation via inhibition of p38 activity.
Under the conditions used to analyze p38 phosphorylation, the administration of anti-S100B antibody also stimulated Akt phosphorylation to some extent (Fig. 5A).
L6 myoblasts express RAGE mRNA and protein, but RAGE does not transduce the S100B inhibitory effect on myoblast differentiation.
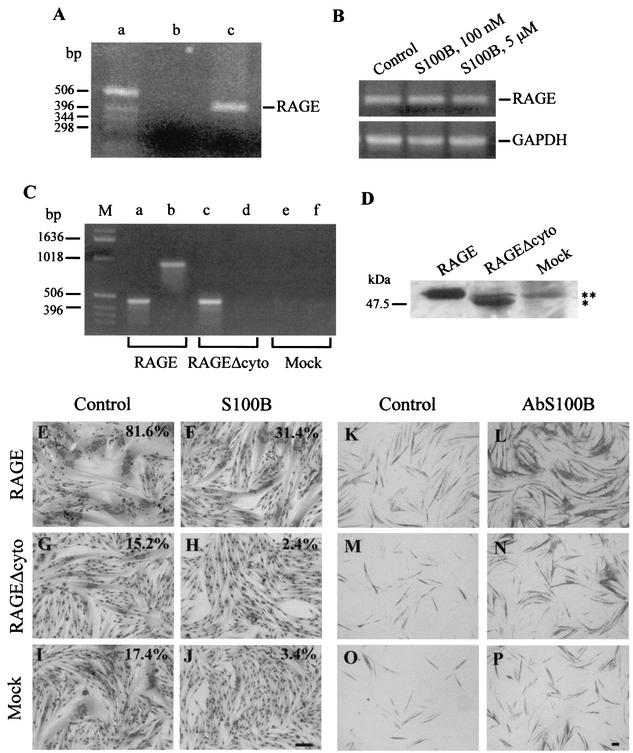
As the effects of S100B on neurons and other cells responsive to this protein were shown to depend on its interaction with RAGE (17, 20), we sought to determine whether RAGE might be implicated in the S100B effects on L6 myoblasts. Myoblasts were found to express RAGE mRNA (Fig. 6A) and protein (Fig. 6D). However, S100B did not up-regulate RAGE expression in myoblasts (Fig. 6B), which suggested the possibility that RAGE might not be implicated in the transduction of S100B effects on these cells. It is known, in fact, that RAGE ligands up-regulate RAGE expression (38). We also transfected L6 myoblasts with human RAGE cDNA or human RAGEΔcyto cDNA and tested S100B and anti-S100B antibody on myoblasts overexpressing RAGE or RAGEΔcyto. Expression of RAGE or RAGEΔcyto in myoblasts transfected with full-length human RAGE and RAGEΔcyto, respectively, was documented by RT-PCR and Western blotting (Fig. 6C and D, respectively). S100B inhibited myotube formation (Fig. 6E to L), and administration of anti-S100B antibody resulted in the stimulation of myoblast differentiation and fusion (Fig. 6K to P) in both myoblasts overexpressing full-length RAGE and myoblasts overexpressing RAGEΔcyto. These data suggested that RAGE was not implicated in the S100B effects on myoblasts and that S100B was interacting with another and as-yet-unknown receptor on myoblasts to negatively regulate their differentiation and fusion. We also speculate that this receptor avidly binds S100B and that S100B might be prevented from interacting efficiently with myoblast RAGE because of the presence of a strong competitor in FBS. Incidentally, differentiation and fusion were strongly enhanced in RAGE-transfected myoblasts (Fig. 6E and K) compared with RAGEΔcyto- and mock-transfected myoblasts (Fig. 6, panels G and M and panels I and O, respectively), suggesting the possibility that RAGE might play an important role in myogenesis, transducing a myogenic signal. A complete characterization of the properties exhibited by myoblasts overexpressing full-length RAGE and myoblasts overexpressing RAGEΔcyto will be presented elsewhere (unpublished data).
FIG. 6.
Detection of RAGE mRNA and protein in rat L6 myoblasts and effects of S100B on myoblasts overexpressing RAGE or RAGEΔcyto. (A) RT-PCR for RAGE. Lane a contained molecular weight markers, and lane b contained a sample in which cDNA mixture from the RT reaction was omitted. The molecular weight of RAGE PCR product (lane c) was compatible with the expected DNA fragment (453 bp). (B) RT-PCR for RAGE in myoblasts exposed to different doses of S100B. Shown are PCR products for RAGE (top gel) and GAPDH (bottom gel). Gels were analyzed by a densitometer. When normalized to GAPDH, the RAGE mRNA became appreciably constant irrespective of the absence or presence of S100B, suggesting that S100B does not up-regulate RAGE expression. (C) RT-PCR products for human RAGE and RAGEΔcyto subsequent to transfection of rat L6 myoblasts. Shown are PCR products obtained with human RAGE reverse primers 1 (lanes a, c, and e) and 2 (lanes b, d, and f). No PCR products could be obtained with reverse primer 2 in the case of RAGEΔcyto-transfected myoblasts (lane d) due to the specificity of this primer for a region present in full-length RAGE and absent from RAGEΔcyto. No PCR products could be obtained with either primer inthe case of mock-transfected myoblasts (lanes e and f). (D) Western blotting of RAGE and RAGEΔcyto in extracts from myoblasts transfected with human RAGE and RAGEΔcyto and mock-transfected myoblasts. Ten micrograms of protein was loaded in each lane. The RAGEΔcyto lane contains RAGEΔcyto (asterisk) plus endogenous full-length RAGE (double asterisk), while the mock-transfected lane contains endogenous full-length RAGE only. Note the lower molecular mass of human RAGEΔcyto compared with that of full-length human RAGE, as expected, and note that murine RAGE migrated slightly faster than human RAGE. (E to J) RAGE-transfected myoblasts (E and F), RAGEΔcyto-transfected myoblasts (G and H), and mock-transfected myoblasts (I and J) were cultivated in 2% FBS containing 10 nM insulin for 5 days in the absence (control [panels E, G, and I]) or presence (S100B [panels F, H, and J]) of 100 nM S100B and then fixed and stained with May-Grünwald Giemsa. The percentages shown refer to the myogenic index calculated in the different conditions. Note that S100B reduces the myogenic index irrespective of the transfection conditions. Also note the relatively high myogenic index in RAGE-transfected myoblasts (E) compared with the two other conditions (G and I), which suggests that overexpression of full-length RAGE results in an enhancement of myotube formation. (K to P) RAGE-transfected myoblasts (K and L), RAGEΔcyto-transfected myoblasts (M and N), and mock-transfected myoblasts (O and P) were cultivated in 2% FBS containing 10 nM insulin for 3 days in the absence (control [panels K, M, and O]) or presence (AbS100B [L, N, and P]) of 50 μg of a polyclonal anti-S100B antibody/ml and then fixed and immunostained for MHC detection. Note that blockade of FBS S100B by anti-S100B antibody results in stimulation of MHC expression. Again, note the relatively higher intensity of MHC immunoreactivity in RAGE-transfected myoblasts (K) compared with the two other conditions (M and O), which suggests that overexpression of full-length RAGE results in an enhancement of myoblast differentiation. Bars = 100 μm.
DISCUSSION
We identified myoblasts as a novel cell type responsive to extracellular S100B. Our data indicate that S100B reduces myoblast differentiation and fusion into myotubes via inactivation of p38 MAP kinase and that S100B brings about these effects at the very low levels found in FBS.
Several lines of evidence support this conclusion. First, neutralization of FBS S100B results in an acceleration of the expression of myogenin and MHC and of myoblast fusion into myotubes and in stimulation of the phosphorylation of p38, a kinase whose activity is crucial for myoblast differentiation and myotube formation (10, 33, 48, 49). Second, neutralization of FBS S100B fails to promote differentiation of myoblasts that had been treated with the p38 inhibitor SB203580. Third, myoblasts overexpressing a constitutively active form of MKK6, thus exhibiting a hyperactive p38, are insensitive to administration of nanomolar amounts of S100B. Fourth, myoblasts cultivated in FBS depleted of S100B express a larger amount of MHC and exhibit a higher level of phosphorylated p38 kinase compared with control cells, and addition of picomolar amounts of S100B back to the former cells results in a reduction of MHC expression and extent of p38 phosphorylation to the levels seen in myoblasts cultivated in control FBS. Fifth, at picomolar levels S100B causes a dose-dependent inhibition of MCK when added back to S100B-depleted FBS. Therefore, at the rather low concentration found in FBS, S100B reduces myogenesis in vitro, and S100B's ability to interfere with myoblast differentiation and myotube formation relies on its ability to inactivate p38 activity. Neutralization of FBS S100B also stimulates to a certain extent the phosphorylation of Akt, another kinase activated during myoblast differentiation (44), which adds to the possibility that S100B might play a regulatory role in myogenesis. Incidentally, these data suggest that S100B's inhibitory effect on myoblast differentiation and fusion is reversible, which points to S100B acting via a cell surface receptor.
As FBS contains ∼4 nM S100B dimer and myoblasts were cultivated in 2% FBS, our data suggest that an S100B concentration as low as 80 pM (i.e., the S100B dimer concentration in 2% FBS) negatively regulates L6 myoblast differentiation. Increasing the S100B concentration to nanomolar levels causes a further reduction of myoblast differentiation, suggesting that accumulation of the protein in serum, as observed under several pathological conditions (4, 8, 14, 24, 28, 29, 34, 35), might amplify the inhibitory effect of S100B on muscle differentiation. However, we cannot exclude the possibility that at ≥50 nM, S100B may cause some additional event that determines inhibition of myogenesis. The strong reduction in cell number observed in the presence of >100 nM S100B (Fig. 1C) suggests the possibility that at high levels S100B might cause myoblast death or interfere with myoblast proliferation, conditions that would result in the blockade of myogenesis. Data to be published elsewhere indicate that at submicromolar to micromolar levels S100B causes myoblast apoptosis via inhibition of ERK1/2 activity (unpublished data).
We show here for the first time that L6 myoblasts express RAGE mRNA and protein, a multiligand receptor of the immunoglobulin superfamily that binds S100B (17) and transduces both trophic and toxic effects of low and high doses of S100B, respectively, on a neuronal cell line (20). Yet our results suggest that RAGE is not implicated in the transduction of S100B effects on myoblasts because (i) S100B does not up-regulate RAGE expression in myoblasts and (ii) S100B inhibits myoblast differentiation and myotube formation and, conversely, an anti-S100B antibody stimulates myoblast differentiation and myotube formation in both myoblasts overexpressing full-length RAGE and myoblasts overexpressing RAGEΔcyto.
The present results are at variance with those obtained with neurons in that neuronal cell lines respond to nanomolar doses of S100B by extending neurites and surviving under stress conditions (3, 6, 20, 21, 47) whereas L6 myoblast differentiation and myotube formation are reduced under the same conditions. These differences might reflect differences in intrinsic properties of the two cell systems, likely related to the nature of the receptor that recognizes S100B. In any case, our data point to a different transduction mechanism of the S100B interaction with its cell surface receptor depending on the cell type considered. Our present data suggest that an as-yet-unknown receptor on myoblasts that recognizes S100B and transduces its regulatory effects binds the protein avidly, as inferred from, e.g., the S100B concentration (∼40 pM) at which half-maximal inhibitory effect of S100B on MCK expression is registered (Fig. 4I). Assuming that this value reflects the dissociation constant of S100B binding to myoblasts, it turns out that S100B binds to myoblasts with an affinity that is 1 to 2 orders of magnitude higher than the affinity with which S100B and other known RAGE ligands bind to RAGE (17, 20, 37, 38).
S100B is normally present in serum (13), and we measured ∼80 ng of S100B/ml of FBS (i.e., ∼4 nM S100B dimer), as mentioned earlier. Serum S100B should not come from astrocytes under normal conditions, as the protein should not be able to cross the blood-brain barrier, although it does so in the course of brain pathological conditions (8, 14, 24, 29, 34, 35). We do not know the source of normal serum S100B. While L6 myoblasts do not appear to release measurable amounts of S100B constitutively, any of the other cell types shown to express the protein (12, 13, 36) might release it, and S100B release might be enhanced by as-yet-unknown factors. It has been reported that activation of 5-HT1A, adenosine, or glutamate receptors on astrocytes and exposure of astrocytes to lysophosphatidic acid and forskolin result in an increased release of the protein (9, 32, 46) and that adipocytes release S100B upon stimulation with catecholamines (43). Thus, factors that remain to be identified might act on nonnervous cells expressing S100B to stimulate its release.
Collectively, our data suggest that at the very low concentrations found in serum S100B may act as a negative regulator of myogenesis by inactivating p38 in a receptor-mediated manner. Thus, S100B can be viewed as a physiological factor acting in conjunction and/or concert with other factors in the regulation of myoblast differentiation during development and/or muscle regeneration. Our data also suggest that the multiligand receptor, RAGE, which was shown to transduce S100B effects on neurons (20), has no role in the transduction of S100B effects on myoblasts. Future analyses should disclose the nature of the receptor implicated in the transduction of the S100B effects on myoblasts.
Acknowledgments
This work was supported by Telethon-Italy funds (Project 922) to R.D.
We thank Linda J. Van Eldik (Chicago, Ill.) and Volker Gerke (Münster, Germany) for providing us with the S100B and S100A1 constructs, respectively, Henri J. Huttunen (Helsinki, Finland) for providing us with the human RAGE and RAGEΔcyto constructs, and Pier Lorenzo Puri (La Jolla, Calif.) for providing us with the MKK6EE and MCK-luc constructs.
REFERENCES
- 1.Adami, C., G. Sorci, E. Blasi, A. L. Agneletti, F. Bistoni, and R. Donato. 2001. S100B expression in and effects on microglia. Glia 33:131-142. [PubMed] [Google Scholar]
- 2.Ahlemeyer, B., H. Beier, I. Semkova, C. Schaper, and J. Krieglstein. 2000. S100β protects cultured neurons against glutamate- and stauroporine-induced damage and is involved in the antiapoptotic action of the 5 HT1A-receptor agonist, Bay x 3702. Brain Res. 858:121-128. [DOI] [PubMed] [Google Scholar]
- 3.Alexanian, A. R., and J. R. Bamburg. 1999. Neuronal survival activity of S100 ββ is enhanced by calcineurin inhibitors and requires activation of NF-κB. FASEB J. 13:1611-1620. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, R. E., L. O. Hansson, O. Nilsson, R. Dijlai-Merzoug, and G. Settergren. 2001. High serum S100B levels for trauma patients without head injuries. Neurosurgery 48:1255-1258. [DOI] [PubMed] [Google Scholar]
- 5.Arcuri, C., I. Giambanco, R. Bianchi, and R. Donato. 2001. Annexin V, annexin VI, S100A1 and S100B in developing and adult avian skeletal muscles. Neuroscience 109:371-388. [DOI] [PubMed] [Google Scholar]
- 6.Barger, S. W., L. J. Van Eldik, and M. P. Mattson. 1995. S100β protects hippocampal neurons from damage induced by glucose deprivation. Brain Res. 677:167-170. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya, A., R. W. Oppenheim, D. Prevette, B. W. Moore, R. Brackenbury, and N. Ratner. 1992. S100 is present in developing chicken neurons and Schwann cells and promotes motor neuron survival in vitro. J. Neurobiol. 23:451-466. [DOI] [PubMed] [Google Scholar]
- 8.Büttner, T., S. Weyers, T. Postert, R. Sprengelmeyer, and W. Kuhn. 1997. S100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke 28:1961-1965. [DOI] [PubMed] [Google Scholar]
- 9.Ciccarelli, R., P. Di Iorio, V. Bruno, G. Battaglia, I. D'Alimonte, M. D'Onofrio, F. Nicoletti, and F. Caciagli. 1999. Activation of A1 adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S100β protein from cultured astrocytes. Glia 27:275-281. [PubMed] [Google Scholar]
- 10.Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase 2/p38 and rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274:4341-4346. [DOI] [PubMed] [Google Scholar]
- 11.Donato, R. 1988. Calcium-independent, pH-regulated effects of S100 proteins on assembly-disassembly of brain microtubule protein in vitro. J. Biol. Chem. 263:106-110. [PubMed] [Google Scholar]
- 12.Donato, R. 1999. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450:191-231. [DOI] [PubMed] [Google Scholar]
- 13.Donato, R. 2001. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 33:637-668. [DOI] [PubMed] [Google Scholar]
- 14.Fassbender, K., R. Schmidt, A. Schreiner, M. Fatar, F. Muhlhauser, M. Daffertshofer, and M. Hennerici. 1997. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J. Neurol. Sci. 148:101-105. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, W. S. T., L. C. Stanley, C. Ling, L. White, W. McLeod, L. J. Perrot, C. L. White III, and C. Araoz. 1989. Brain interleukin 1 and S100 immunoreactivity are elevated in Down's syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 86:7611-7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haglid, K. G., Q. Yang, A. Hamberger, S. Bergman, A. Widerberg, and N. Danielsen. 1997. S100β stimulates neurite outgrowth in the rat sciatic nerve grafted with acellular muscle transplants. Brain Res. 753:196-201. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann, M. A., S. Drury, C. Fu, W. Qu, A. Taguchi, Y. Lu, C. Avila, N. Kambham, A. Bierhaus, P. Nawroth, M. F. Neurath, T. Slattery, D. Beach, J. McClary, M. Nagashima, J. Morser, D. Stern, and A. M. Schmidt. 1999. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97:889-901. [DOI] [PubMed] [Google Scholar]
- 18.Hu, J., A. Ferreira, and L. J. Van Eldik. 1997. S100β induces neuronal cell death through nitric oxide release from astrocytes. J. Neurochem. 69:2294-2301. [DOI] [PubMed] [Google Scholar]
- 19.Hu, J., F. Castets, J. L. Guevara, and L. J. Van Eldik. 1996. S100β stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J. Biol. Chem. 271:2543-2547. [DOI] [PubMed] [Google Scholar]
- 20.Huttunen, H. J., J. Kuja-Panula, G. Sorci, A. L. Agneletti, R. Donato, and H. Rauvala. 2000. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through RAGE activation. J. Biol. Chem. 275:40096-40105. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki, Y., T. Shiojima, and M. Kinoshita. 1997. S100β prevents the death of motor neurons in newborn rats after sciatic nerve section. J. Neurol. Sci. 151:7-12. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, Y., C. Chen, Z. Li, W. Guo, J. A. Gegner, S. Lin, and J. Han. 1996. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271:17920-17926. [DOI] [PubMed] [Google Scholar]
- 23.Kligman, D., and D. R. Marshak. 1985. Purification and characterization of a neurite extension factor from bovine brain. Proc. Natl. Acad. Sci. USA 82:7136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, P., K. Dhital, M. Hossein-Nia, S. Patel, D. Holt, and T. Treasure. 1997. S100 protein release in a range of cardiothoracic procedures. J. Thorac. Cardiovasc. Surg. 113:953-954. [DOI] [PubMed] [Google Scholar]
- 25.Li, Y., S. W. Barger, L. Liu, R. E. Mrak, and W. S. T. Griffin. 2000. S100β induction of the proinflammatory cytokine interleukin-6 in neurons. J. Neurochem. 74:143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludolph, D. C., and S. T. Konieczny. 1995. Transcription factor families: muscling in on the myogenic program. FASEB J. 9:1595-1604. [DOI] [PubMed] [Google Scholar]
- 27.Mariggiò, M. A., S. Fulle, P. Calissano, I. Nicoletti, and G. Fanò. 1994. The brain protein S100ab induces apoptosis in PC12 cells. Neuroscience 60:29-35. [DOI] [PubMed] [Google Scholar]
- 28.Martenson, E. D., L. O. Hansson, B. Nilsson, E. von Schoultz, E. Mansson Brahme, U. Ringborg, and J. Hansson. 2001. Serum S-100b protein as a prognostic marker in malignant cutaneous melanoma. J. Clin. Oncol. 19:824-831. [DOI] [PubMed] [Google Scholar]
- 29.Otto, M., J. Wiltfang, E. Schutz, I. Zerr, A. Otto, A. Pfahlberg, O. Gefeller, M. Uhr, A. Giese, T. Weber, H. A. Kretzschmar, and S. Poser. 1998. Diagnosis of Creutzfeldt-Jakob disease by measurement of S100 protein in serum: prospective case-control study. BMJ 316:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peña, L. A., C. W. Brecher, and D. R. Marshak. 1995. β-Amyloid regulates gene expression of glial trophic substance S100β in C6 glioma and primary astrocyte cultures. Mol. Brain Res. 34:118-126. [DOI] [PubMed] [Google Scholar]
- 31.Petrova, T. V., J. Hu, and L. J. Van Eldik. 2000. Modulation of glial activation by astrocyte-derived protein S100B: differential responses of astrocyte and microglial cultures. Brain Res. 853:74-80. [DOI] [PubMed] [Google Scholar]
- 32.Pinto, S. S., C. Gottfried, A. Mendez, D. Goncalves, J. Karl, C. A. Goncalves, S. Wofchuk, and R. Rodnight. 2000. Immunocontent and secretion of S100B in astrocyte cultures from different brain regions in relation to morphology. FEBS Lett. 486:203-207. [DOI] [PubMed] [Google Scholar]
- 33.Puri, P. L., Z. Wu, P. Zhang, L. D. Wood, K. S. Bhakta, J. Han, J. R. Feramisco, M. Karin, and J. Y. J. Wang. 2000. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 14:574-584. [PMC free article] [PubMed] [Google Scholar]
- 34.Rosén, H., L. Rosengren, J. Herlitz, and C. Blonstrand. 1998. Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke 29:473-477. [DOI] [PubMed] [Google Scholar]
- 35.Rothoerl, R. D., C. Woertgen, M. Holzschuh, C. Metz, and A. Brawanski. 1998. S100 serum levels after minor and major head injury. J. Trauma 45:765-767. [DOI] [PubMed] [Google Scholar]
- 36.Schäfer, B. W., and C. W. Heizmann. 1996. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem. Sci. 21:134-140. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, A. M., O. Hori, J. Brett, S. D. Yan, J. L. Wautier, and D. Stern. 1994. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. 14:1521-1528. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt, A. M., S. D. Yan, S. F. Yan, and D. Stern. 2000. The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta 1498:99-111. [DOI] [PubMed] [Google Scholar]
- 39.Selinfreund, R. H., S. W. Barger, W. J. Pledger, and L. J. Van Eldik. 1991. Neurotrophic protein S100β stimulates glial cell proliferation. Proc. Natl. Acad. Sci. USA 88:3554-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng, J. G., R. E. Mrak, and S. W. T. Griffin. 1994. S100β protein expression in Alzheimer's disease: potential role in the pathogenesis of neuritic plaques. J. Neurosci. Res. 39:398-404. [DOI] [PubMed] [Google Scholar]
- 41.Sheng, J. G., R. E. Mrak, K. R. Bales, B. Cordell, S. M. Paul, R. A. Jones, S. Woodward, X. Q. Zhou, J. M. McGinness, and W. S. T. Griffin. 2000. Overexpression of the neurotrophic cytokine S100β precedes the appearance of neuritic β-amyloid plaques in APPV717F mice. J. Neurochem. 74:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorci, G., R. Bianchi, I. Giambanco, M. G. Rambotti, and R. Donato. 1999. Replicating myoblasts and fused myotubes express the calcium-modulated proteins S100A1 and S100B. Cell Calcium 25:93-106. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, F., K. Kato, and T. Nakajima. 1984. Hormonal regulation of adipose S-100 protein release. J. Neurochem. 43:1336-1341. [DOI] [PubMed] [Google Scholar]
- 44.Tamir, Y., and E. Bengal. 2000. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J. Biol. Chem. 275:34424-34432. [DOI] [PubMed] [Google Scholar]
- 45.Van Eldik, L. J., and D. B. Zimmer. 1987. Secretion of S100 from rat C6 glioma cells. Brain Res. 436:367-370. [DOI] [PubMed] [Google Scholar]
- 46.Whitaker-Azmitia, P. M., R. Murphy, and E. C. Azmitia. 1990. S100 protein is released from astroglial cells by stimulation of 5-HT1A receptors. Brain Res. 528:155-158. [DOI] [PubMed] [Google Scholar]
- 47.Winningham-Major, F., J. L. Staecker, S. W. Barger, S. Coats, and L. J. Van Eldik. 1989. Neurite extension and neuronal survival activities of recombinant S100β proteins that differ in the content and position of cysteine residues. J. Cell Biol. 109:3036-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, Z., P. J. Woodring, K. S. Bhakta, K. Tamura, F. Wen, J. R. Feramisco, M. Karin, J. Y. Wang, and P. L. Puri. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20:3951-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zetser, A., E. Gredinger, and E. Bengal. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274:5193-5200. [DOI] [PubMed] [Google Scholar]