Abstract
The intermediate filament protein nestin is characterized by its specific expression during the development of neuronal and myogenic tissues. We identify nestin as a novel in vivo target for cdk5 and p35 kinase, a critical signaling determinant in development. Two cdk5-specific phosphorylation sites on nestin, Thr-1495 and Thr-316, were established, the latter of which was used as a marker for cdk5-specific phosphorylation in vivo. Ectopic expression of cdk5 and p35 in central nervous system progenitor cells and in myogenic precursor cells induced elevated phosphorylation and reorganization of nestin. The kinetics of nestin expression corresponded to elevated expression and activation of cdk5 during differentiation of myoblast cell cultures and during regeneration of skeletal muscle. In the myoblasts, a disassembly-linked phosphorylation of Thr-316 indicated active phosphorylation of nestin by cdk5. Moreover, cdk5 occurred in physical association with nestin. Inhibition of cdk5 activity—either by transfection with dominant-negative cdk5 or by using a specific cdk5 inhibitor—blocked myoblast differentiation and phosphorylation of nestin at Thr-316, and this inhibition markedly disturbed the organization of nestin. Interestingly, the interaction between p35, the cdk5 activator, and nestin appeared to be regulated by cdk5. In differentiating myoblasts, p35 was not complexed with nestin phosphorylated at Thr-316, and inhibition of cdk5 activity during differentiation induced a marked association of p35 with nestin. These results demonstrate that there is a continuous turnover of cdk5 and p35 activity on a scaffold formed by nestin. This association is likely to affect the organization and operation of both cdk5 and nestin during development.
The intermediate filament (IF) protein nestin is expressed during the early stages of development in the CNS and in myogenic tissue (19, 35). Upon differentiation, nestin becomes down-regulated and is replaced by other tissue-specific IFs (19, 35). Interestingly, nestin expression is reinduced in the adult organism during pathological situations, such as the formation of the glial scar after injury to the CNS (6) and during regeneration of injured muscle tissue (6, 39, 40). Although very little is known about the functions or regulation of nestin, its specific expression is associated with morphologically dynamic cells such as proliferating and migrating cells. In addition, studies on the distribution and expression of nestin in denervated myofibers and in cultured myoblasts suggest that nestin has a close relationship with structures that participate in neural transmission (37, 39).
Cdk5 is critical for neuronal development, as demonstrated by abnormal corticogenesis and perinatal lethality in cdk5 knockout mice (27) and by abnormal neuronal migration, seizures, and adult lethality in mice deficient in p35, one of the protein activators of cdk5 (4). In addition, cdk5 is important in regulating differentiation and organization of muscle cells (16, 33). Moreover, the induction of cdk5 in rat skeletal muscle after nerve injury was recently reported, suggesting a role for cdk5 in muscle regeneration (8). In addition to its involvement in developing and regenerating muscle tissue, cdk5 is engaged in the neuregulin-mediated expression of AChRs at the NMJs (7). Cdk5 has multiple functions in neuronal tissues. It is involved in regulating neuronal survival, migration, neurite outgrowth, secretion, dopamine signaling, and cytoskeletal dynamics (reviewed in reference 5). However, the mechanisms underlying its regulatory functions in myogenic tissues—and its myogenic substrates—are poorly characterized.
Consistent with the proposed organizational functions of cdk5 in the nervous system, cdk5 is found to be associated with cytoskeletal components in neurons (11, 41). Several of its identified substrates are in fact cytoskeletal proteins, such as tau (12, 31), MAP1, and the NF proteins (17, 20, 21, 28, 36). The importance of cdk5 in the regulation of the cytoskeleton is also emphasized by recent findings that link disturbed regulation and hyperactivation of cdk5 to several neuronal diseases, including Alzheimer's disease (1, 18, 24, 32), Parkinson's disease (3), and amyotrophic lateral sclerosis (2, 25), and more specifically to the cytoskeletal disruptions that are typical of these diseases (28).
The close correspondence of cdk5 and nestin expression patterns at early developmental stages in neuronal and muscle cells, and the established role of cdk5 as an important regulator of cytoskeletal dynamics, led us to explore the possible interaction between nestin and cdk5 in these tissues. Our study shows that, both in vitro and in cultured cells, cdk5 phosphorylated nestin preferentially over its partner IF protein, vimentin, and induced subsequent reorganization of the cytoplasmic nestin network. Furthermore, we observed a spatiotemporal match between the expression and activation of cdk5 and nestin and an association between cdk5 and nestin during early stages of myogenic development, indicating that the interaction between cdk5 and nestin is a characteristic feature at stages of coexpression and that nestin may function as a scaffold for cdk5. Finally, we show that cdk5 is a modulator of nestin organization and dynamics in differentiating myoblasts and that the cdk5-specific phosphorylation of nestin could be involved in the scaffolding or targeting of cdk5 and p35 signaling.
MATERIALS AND METHODS
Abbrevations
The following abbreviations are used in this paper: cdk5, cyclin-dependent kinase 5; CNS, central nervous system; H1, histone 1; IF, intermediate filament; NF, neurofilament; NMJ, neuromuscular junction; AChRs, acetylcholine receptors; dncdk5, dominant-negative cdk5; DMEM, Dulbecco's modified Eagle's medium; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; BTX, biotinylated alpha-bungarotoxin; PBS, phosphate-buffered saline; DTT, dithiothreitol; IP, immunoprecipitation; PMSF, phenylmethylsulfonyl fluoride; IgG, immunoglobulin G; BL-I, butyrolactone I; MOPS, morpholinepropanesulfonic acid; pThr-316, phosphorylated Thr-316.
Cell cultures, plasmids, and transfections.
Immortalized CNS ST15A neuronal precursor cells were cultured as described previously (34). For transfection, 2 × 106 cells were grown to 70% confluency, harvested, and resuspended in 400 μl of OPTIMEM reduced-serum medium. Cells were transfected with wild-type cdk5, wild-type cdk5 and p35, dncdk5, dncdk5 and p35, and p35 alone, and a noncoding plasmid was used as a mock control to compensate for possible effects of transfection. The p35, cdk5, and dncdk5 constructs for cell transfections were in pcDNA3.1His vector expressing a six-His tag and an Express epitope. A total of 20 μg of plasmid DNA was used for transfections. Cells were transfected by electroporation at 220 V/cm and 975 μF. Transfected cells were plated on poly-l-lysine-coated coverslips or cell culture dishes and incubated for 48 to 60 h before experiments. Mouse C2C12 myoblasts were cultured in Ham's F12 and DMEM (ratio, 1:1) supplemented with 10% fetal calf serum and 2 mM glutamine. To induce differentiation, cells were plated at a density of 1 × 104 to 5 × 104 cells/cm2 on plastic dishes or coverslips and grown for 2 days before replacing growth medium with differentiation medium (DMEM supplemented with 2% fetal calf serum and 2 mM glutamine). Inhibition of cdk5 activity in differentiating C2C12 myoblasts was performed by culturing cells in differentiating medium in the presence of 10 μM of either of the cdk5 inhibitors, roscovitine or BL-I. The cells were incubated for 12 h in the presence of the inhibitor. For transfection, myoblasts were grown to 50 to 70% confluency, harvested, and resuspended in OPTIMEM—2 × 106 cells in 400 μl of OPTIMEM per sample. Cells were transfected with wild-type cdk5, wild-type cdk5 and p35, dncdk5, dncdk5 and p35, p35 alone (21), and empty plasmid as a control for the possible effects of transfection. At 24 h after transfection, the cells were induced to differentiate by replacing the growth medium with differentiation medium. The transfection efficiencies varied between 20 and 30% for ST15A cells and between 5 to 10% for C2C12 myoblasts.
Antibodies.
The nestin antibodies used were a monoclonal antibody (Invitrogen) and a polyclonal antibody against nestin (34) and a phosphopeptide antibody generated as described previously (34). Cdk5 was recognized by a polyclonal antibody (C-8) purchased from Santa Cruz Biotechnology. Three different antibodies were used to recognize p35—the polyclonal antibodies, N-20 and C-19 (Santa Cruz Biotechnology), and a monoclonal p35 antibody (clones ES19 and ES24) purchased from Sigma. Specific cdk5- and p35-blocking peptides, respectively, obtained from Santa Cruz Biotechnology were used to test for specificity of the immunoreactions. An anti-Express antibody recognizing the Express epitope expressed in the pcDNA3.1His vector was purchased from Santa Cruz Biotechnology. A monoclonal actin antibody was purchased from Sigma.
Separation of IF fractions following extraction with Triton X-100.
Triton X-100 buffer (25 mM HEPES [pH 7.6], 100 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 0.5% Triton X-100, and protease inhibitors) was added to cultured C2C12 cells at different time points of differentiation; cells were detached and collected, homogenized on ice, and centrifuged for 15,000 × g at 4°C for 45 min. The supernatant and the pellet fractions were dissolved in 3× Laemmli sample buffer for further SDS-PAGE and Western blotting analyses.
Animals and in situ injury.
A total of 30 13- to 14-week-old Sprague-Dawley rats weighing between 420 and 640 g were used. The in situ injury was induced by microsyringe injection of 2.0 μg of notexin toxin (phospholipase A2; volume, 20 μl) to the lateral gastrognemius muscle of the left hind limb. The right muscle was left to serve as an intact control. As a sham control, 0.9% NaCl was injected into the right gastrognemius in half of the rats. The injuries were induced under quick ether anesthesia. Animals were allowed to move freely in their cages after the injury. Animals were killed by neck dislocation at 6, 12, or 24 h or at 2, 3, 5, 7, 21, or 28 days after injury. After sacrifice, the traumatized muscle was divided sagittally across the lesion into two samples. One sample was immediately frozen in liquid nitrogen for biochemical analysis. The other sample was either fixed in 4% paraformaldehyde in 0.1 M phosphate buffer per liter and embedded in paraffin or else was fresh frozen in isopentane cooled with liquid nitrogen. Frozen samples were stored at −70°C until processed further.
The following antisera and antibodies were used: rabbit polyclonal antinestin antiserum 130 or mouse monoclonal antibody to rat nestin (Pharmingen, San Diego, Calif.). NMJs were demonstrated by toxin histochemistry, which was performed by incubating frozen sections overnight in 1:200 BTX (Molecular Probes, Eugene, Oreg.), which specifically binds to AChRs. The bound primary antibodies were visualized by using the appropriate avidin-biotin-peroxidase kit (Vector Laboratories, Burlingame, Calif.), which was also used for visualization of BTX.
IF preparations, in vitro phosphorylation of nestin, and phosphoamino acid analysis.
IFs were isolated from ST15A cells as described previously (34). For further cycles of disassembly and reassembly, reassembled IFs were recovered by centrifugation at 100,000 × g for 30 min. The filaments were solubilized in dissociation buffer containing urea (as described in reference 34). Following centrifugation at 14,000 × g for 30 min at 4°C, the supernatant was dialyzed into two changes of 500 volumes each of PBS containing 5 mM EDTA, 5 mM EGTA, and protease inhibitors. IF preparations were phosphorylated with the activated cdk5 and p35 complex. For the reconstitution assay, 10 μl of bacterially expressed p35 and cdk5 (21) (stored in 20 mM Tris [pH 7.4] and 5% glycerol at −70°C), was mixed with 6 μl of 10× PBS, 2 μl of 1 mM EDTA and 1 mM DTT, and H2O to make 60 μl of the mix. The mix was incubated at 30°C for 2 h. Twenty microliters of IF preparation (nestin, 100 μg/ml; vimentin, 1,000 μg/ml) was phosphorylated with 5 μl of the reaction mix, 20 μl of kinase buffer (50 mM MOPS [pH 7.4], 5 mM MgCl2), 100 μM ATP, 10 μCi of [γ-32P]ATP, protease inhibitors, and H2O to achieve a final volume of 50 μl. The kinase reaction was carried out at 30°C and stopped after 1 h by the addition of 3× Laemmli sample buffer. Phosphoamino acid analysis of the phosphorylated IF proteins was performed as described previously (34). In the text of this article, phosphorylation “in vitro” refers to phosphorylation of a purified substrate with purified kinase in the test tube. The term “in vivo” refers to metabolic phosphorylation inside the intact living cell. Therefore, in this context, “in vivo” does not refer to events in the intact animal.
Double-immunofluorescence confocal microscopy.
For double-label immunofluorescence experiments, cells were grown on coverslips. Cells were fixed and blocked as described previously (34) before incubation with monoclonal nestin antibody (Invitrogen) diluted 1:50 and either polyclonal cdk5 or p35 antibody (concentration, 4 μg/ml) for 30 min at room temperature, rinsed 3 times in PBS, and incubated with Alexa 488 goat anti-mouse Igs (Molecular Probes) and Alexa 546 anti-rabbit Igs (Molecular Probes). Cells were finally washed three times in PBS before being mounted in Mowiol 40-88 (32 459-0; Aldrich-Chemie, Steinheim, Germany) supplemented with 100 mg of 1.4.-diazabicyclo [2.2.2]-octan per ml (2780-2; Aldrich-Chemie). The fluorescence images were analyzed with a Leica TCS-SP confocal laser scanning microscope system (Leica, Heidelberg, Germany) equipped with an Ar-Kr laser (Omnichrome; Melels Griot, Carslbad, Calif.). Fluorophores were sequentially excited with wavelength peaks at 488 and 568 nm. Fluorescence detection windows were at ∼493 to 565 nm and ∼590 to 690 nm, respectively. Images stacks were acquired and processed into maximum projection images with Leica TCS NT/SP Scanware (version 1.6.587) software.
Western blotting.
For Western blotting of transfected cell samples, equal amounts of protein were loaded on SDS-10% PAGE under reducing conditions and transferred to nitrocellulose membranes. After blocking overnight at 4°C in 5% milk-PBS, membranes were probed at room temperature for 1 h with primary antibodies in 1% milk-PBS and 0.1% Tween 20; the membranes were then washed four times with PBS containing 0.1% Tween 20 and incubated with secondary antibodies coupled to horseradish peroxidase (at dilutions recommended by the manufacturer) and visualized by using an enhanced chemiluminescence Western blotting detection kit (Amersham, Little Chalfont, United Kingdom). For immunoblotting of muscle samples, protein extracts were prepared as described previously (40).
Immunocomplex kinase assay.
Transfected cells were washed twice with cold PBS. Cells (4 × 106 per sample) were lysed with 600 μl of IP buffer (PBS [pH 7.4]; 1% NP-40; 0.5% sodium deoxycholate; 1 mM EDTA; 1 mM EGTA; 20 mM NaF; 1 mM PMSF; 10 μg of aprotinin, 10 μg of leupeptin, and 10 μg of pepstatin; and 0.5 mM DTT) for 30 min on ice. The samples were homogenized eight times with a 26-gauge needle and centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration was determined by using the Bradford assay, and the samples were normalized accordingly. Five microliters of anti-cdk5 IgG was added to the cell lysates. The samples were incubated with rotation at 4°C for 1 h followed by 30 min with protein A Sepharose. Complexes were washed three times with kinase buffer (100 mM HEPES [pH 7.0], 10 mM MgCl2, 10 mM MnCl2, 10 mM EGTA, 1 mM DTT, 0.1 mM Na3VO4, 60 mM β-glycerophosphate, 30 mM p-nitrophenylphosphate). The Sepharose A pellets were resuspended in 20 μl of kinase buffer containing H1 (100 μg/ml) or 20 μl of IF preparation (nestin, 10 μg/ml) in IF kinase buffer (10 mM HEPES [pH 7.2], 60 mM NaCl, 0.5 mM CaCl2, 2.5 mM EGTA, 2 mM MgCl2). Five microliters of 5× ATP mix (1 mCi of [32P]ATP per ml and 125 μM cold ATP in kinase buffer) was added to start the reactions. Samples were incubated at 30°C for 10 min. The reaction was stopped by the addition of 3× sample buffer and boiled for 5 min. The samples were further centrifuged at 12,000 × g for 5 min and run on SDS-PAGE.
In vivo labeling and IP.
Control and cdk5- and p35-cotransfected ST15A cells were metabolically labeled with [32P]orthophosphate 48 h after transfection, and the cell lysate was prepared as described previously (34). Nestin was immunoprecipitated by dilution of the cell lysate with radioimmunoprecipitation assay buffer without SDS (20 mM HEPES [pH 7.4], 140 mM NaCl, 10 mM pyrophosphate, 5 mM EDTA, 0.4% NP-40, 100 mM PMSF, 10 μg of leupeptin per ml and 10 μg of antipain per ml) added to the polyclonal antinestin antibody 6 (34), and finally recovered with protein A Sepharose. Proteins were separated by SDS-PAGE. Gels were stained with either Coomassie brilliant blue or silver to control for equal loading of proteins; the gels were then dried and autoradiographed at −70°C by using Kodak X-Omat AR. For coimmunoprecipitation, cdk5- and p35-transfected or untransfected ST15A cells and differentiating C2C12 cells were lysed in IP buffer (20 mM HEPES [pH 7.4], 140 mM NaCl, 10 mM pyrophosphate, 5 mM EDTA, 0.4% NP-40, 100 mM PMSF, 10 μg of leupeptin per ml, and 10 μg of antipain per ml) for 30 min on ice. The lysate was lightly sonicated and centrifuged at 10,000 rpm (Eppendorf centrifuge 5417R) for 10 min. The supernatant was precleared with protein G and protein A Sepharose beads, respectively. Then, nestin and cdk5 were immunoprecipitated by monoclonal antinestin antibody or polyclonal cdk5 antibody and recovered with protein G Sepharose or protein A Sepharose. For negative controls, the lysate was incubated with Sepharose G beads or Sepharose A beads and mouse IgG or rabbit IgG, respectively. The immunoprecipitate was then immunoblotted for cdk5 or nestin.
RESULTS
Cdk5 is a potent nestin kinase in vitro.
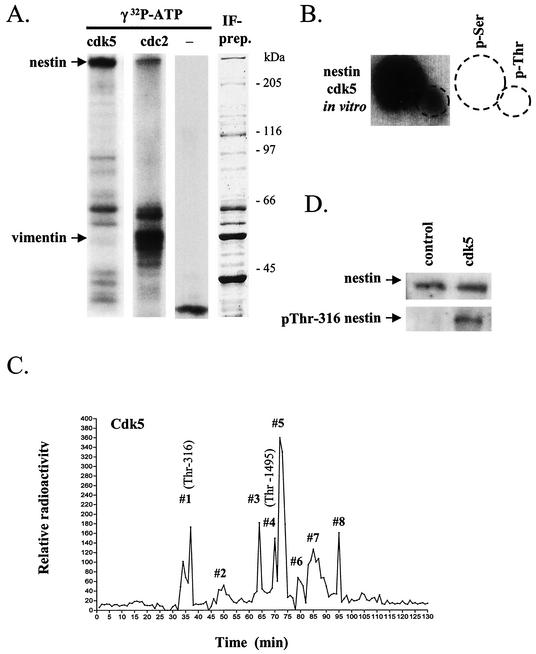
In vitro phosphorylation of IF preparations by cdk5 showed that nestin is efficiently phosphorylated by cdk5 (Fig. 1A, left lane) compared to vimentin, which showed only minor cdk5-mediated phosphorylation. In fact, vimentin seems to be a poor substrate for cdk5, considering that vimentin exists in a 10-fold excess in these IF preparations, as evidenced by Coomassie blue staining of SDS-PAGE gels (Fig. 1A, right lane). Interestingly, when compared to the phosphorylation of an IF preparation by cdc2 kinase, which we recently reported as a nestin kinase (34), the relative substrate specificity for vimentin versus nestin was entirely different, as cdc2 showed preferential phosphorylation of vimentin (Fig. 1A, second lane). The background phosphorylation of the IF preparation, i.e., in the absence of an added kinase is shown in the third lane. Phosphorylation of nestin by cdk5 occurred on both serine and threonine residues (Fig. 1B), with serine as the major target.
FIG. 1.
Cdk5 is a potent nestin kinase in vitro and phosphorylates nestin at Thr-316 and Thr-1495. (A) IF preparations isolated from ST15A cells were phosphorylated in vitro by cdk5 and cdc2 (left lane). The autoradiograph shows high phosphorylation of nestin by cdk5 in contrast to vimentin. The relative substrate specificity for vimentin versus nestin is completely different for cdc2 (second lane). The third lane from the left shows the background phosphorylation of IF preparations without the addition of a kinase. The right lane shows a Coomassie blue-stained SDS-polyacrylamide gel of the IF preparation used in the assays. (B) Phosphoamino acid analysis shows that the phosphorylation of nestin by cdk5 in vitro occurs on serine and threonine residues, with serine being the predominant target. (C) Tryptic nestin phosphopeptides, phosphorylated by cdk5 in vitro, were fractionated by reversed high-pressure liquid chromatography and sequenced. Sequencing of the corresponding 32P-containing fractions showed that cdk5 phosphorylates nestin at Thr-316 and Thr-1495 (shown in brackets). (D) Immunoblotting of in vitro cdk5-phosphorylated and unphosphorylated IF preparations with the pThr-316-nestin phosphospecific antibody shows that cdk5 increases phosphorylation at Thr-316.
Identification of Thr-316 as a cdk5-specific phosphorylation site on nestin.
In order to identify cdk5-specific phosphorylation sites on nestin, tryptic peptides of nestin phosphorylated in vitro by cdk5 were separated by using a C18 reversed high-pressure liquid chromatography column. The eluted fractions were collected, the radioactivity of each fraction was measured and plotted (Fig. 1C), and the radioactive peptides (radioactive peaks 1 to 8) were sequenced. The nestin peptide sequence 314-LQpTPGR (pT, phosphorylated Thr) was obtained from radioactive peak 1. Manual Edman degradation of the isolated peptide confirmed phosphorylation at Thr-316 (data not shown). Immunoblotting of nestin-containing IF preparations phosphorylated in vitro by cdk5 with a phosphospecific antibody against nestin phosphorylated at Thr-316 (34) further confirmed that cdk5 phosphorylates nestin at Thr-316 in vitro (Fig. 1D). In addition to Thr-316, we identified Thr-1495 as a cdk5-specific phosphorylation site. The sequence 1480-EESEADDLGETLPDSpTPLGLYL was obtained by sequencing radioactive peak 4. Sequencing of the other 32P peaks was not successful due to impurity of the fractions. Because nestin is a very large protein (>1,800 amino acids), it generates a large number of tryptic peptides, and the complete characterization of all phosphorylation sites was clearly beyond the scope of the present study. Instead, the main goal was to establish some bona fide in vivo phosphorylation sites that could be used as markers for cdk5-mediated nestin phosphorylation. In the following sections, pThr-316 was used as such a marker, as we had an antibody available to detect its phosphorylation.
Cdk5 is associated with nestin in neuronal progenitor ST15A cells.
Cdk5 was coimmunoprecipitated with nestin from ST15A cells, demonstrating that cdk5 is associated with the nestin network at endogenous protein levels (Fig. 2A). Interestingly, immunoblotting of IF preparations with antibodies against cdk5 showed the presence of cdk5 in these preparations (Fig. 2B). IFs that have been depolymerized by urea are able to spontaneously polymerize when the urea is removed and a small amount of salt is added. Cdk5 cocycled with the IFs through two repeated cycles of assembly-disassembly-reassembly (Fig. 2B). The cdk5 associated with the IFs is not active, as shown by the lack of background phosphorylation of IF preparations (Fig. 1A). The in vitro data on retained nestin-cdk5 complexes after disassembly-assembly cycles corroborates a firm association between nestin and cdk5.
FIG. 2.
Cdk5 is associated with nestin in ST15A neuronal progenitor cells. (A) Coimmunoprecipitation analysis shows that cdk5 is associated with nestin in ST15A cells. Nestin was immunoprecipitated from ST15A cells, and the precipitate was blotted against cdk5. As a negative control, the lysate was incubated with unspecific IgG prior to recovery with protein G Sepharose. (B) Immunoblotting of IF preparations prepared from ST15A cells with an antibody against cdk5 shows that cdk5 copurifies with intermediate filaments. The association persists through cycles of disassembly of the filaments in urea and reassembly of the filament upon removal of urea. The figure shows cdk5 immunblots of IF preparations subjected to two (labeled 1 and 2) subsequent cycles of disassembly and reassembly.
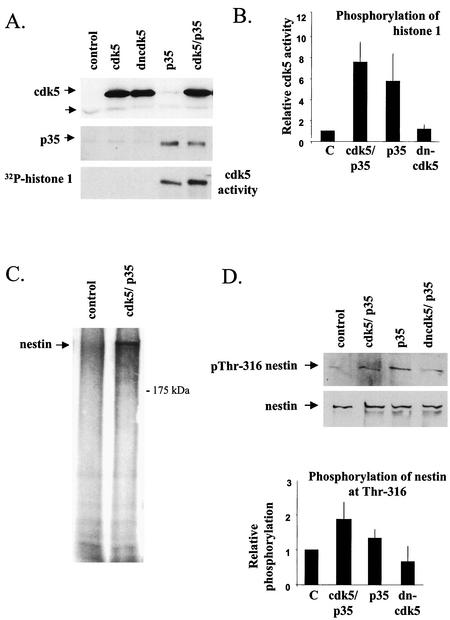
Overexpression of cdk5 and p35 in ST15A cells leads to elevated phosphorylation of nestin at Thr-316.
To determine the effects of the cdk5-specific phosphorylation of nestin in vivo, ST15A cells were cotransfected with the wild-type cdk5 or with dncdk5 together with the p35 activator protein or p35 alone. Successful transfection was confirmed by immunoblotting (Fig. 3A). The lower band on the cdk5 blot marked with an arrow demonstrates low expression of endogenous cdk5 in these cells. The higher molecular weight of the exogenous protein is due to a tag (see Material and Methods). The activity of cdk5 in untransfected versus transfected cells was analyzed by immunocomplex kinase assays with H1 used as a substrate. Cdk5 kinase activity was increased in cells cotransfected with cdk5 and p35 but was also elevated in cells transfected with p35 alone (Fig. 3A and B). The latter activation is likely to stem from p35-mediated activation of the endogenous cdk5. Cells transfected with the dncdk5 or cdk5 alone did not show any cdk5 activity (Fig. 3A and B). In order to determine the phosphorylation state of nestin in transfected cells, nestin was immunoprecipitated from in vivo 32P-labeled cells. Nestin showed increased phosphorylation in cdk5- and p35-cotransfected cells compared to nestin from control cells (Fig. 3C). To confirm that Thr-316 is involved in the cdk5-mediated phosphorylation, immunoblotting of whole-cell extracts from control and transfected cells was carried out with the pThr-316 phosphopeptide-specific antibody. The results show that phosphorylation at Thr-316 was increased in cells transfected with cdk5 and p35 or p35 alone and that this increase is abolished when p35 is cotransfected with dncdk5 (Fig. 3D), thus confirming Thr-316 as a bona fide cdk5 phosphorylation site.
FIG. 3.
Increased cdk5 activity in cdk5- and p35-cotransfected ST15A cells corresponds to elevated phosphorylation of nestin at Thr-316. (A) Immunoblotting of whole-cell extracts of ST15A cells transfected with cdk5, dncdk5, p35, and cdk5 and p35, all performed by using cdk5- (Santa Cruz; cdk5 antibody C-8) and p35-specific (Santa Cruz; p35 antibody C-19) antibodies. Immunoblots show increased expression of cdk5 and p35 in cells transfected with the respective protein (arrows in upper and middle panel). Endogenous cdk5 is present at low levels in these cells (lower arrow, upper panel). Immunocomplex kinase assays show increased cdk5 activity in cells transfected with cdk5 and p35 or p35 (lower panel). H1 was used as a substrate for the immunoprecipitated cdk5. (B) The graph shows the relative increase in phosphorylation of H1 by cdk5 immunoprecipitated from cdk5- and p35- or p35-transfected cells compared to the controls (analysis performed with with a phosphorimager), which have been given the value 1. The graphs show the mean values of three experiments. (C) Autoradiography of nestin immunoprecipitated from metabolically labeled control and cdk5- and p35-cotransfected ST15A cells. There is a marked increase in the phosphorylation level of nestin in transfected cells compared to that of control cells. (D) Immunoblotting of whole-cell extracts of ST15A cells transfected with cdk5 and p35, p35 alone, or p35 together with dncdk5, with the pThr-316 nestin antibody shows that nestin phosphorylation at Thr-316 is increased in cells overexpressing cdk5 and p35 and p35 alone. Cells coexpressing p35 and dncdk5 show no change in phosphorylation of Thr-316 compared to control cells. The graph shows the relative increase in phosphorylation of Thr-316 (analysis by densitometry) in transfected cells compared to control cells, which have been given the value 1. The graph shows the mean values of three experiments.
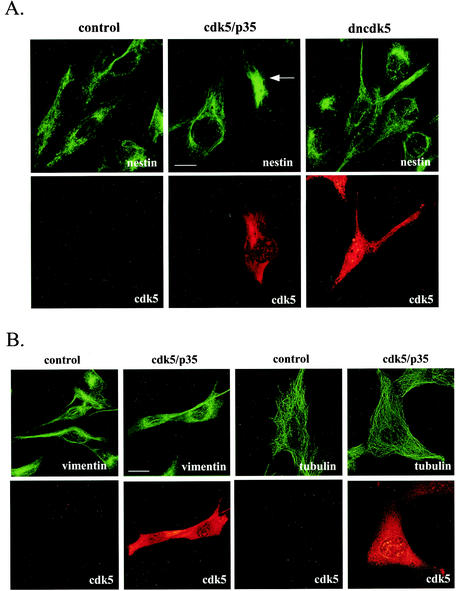
Increased cdk5 activity leads to reorganization of nestin.
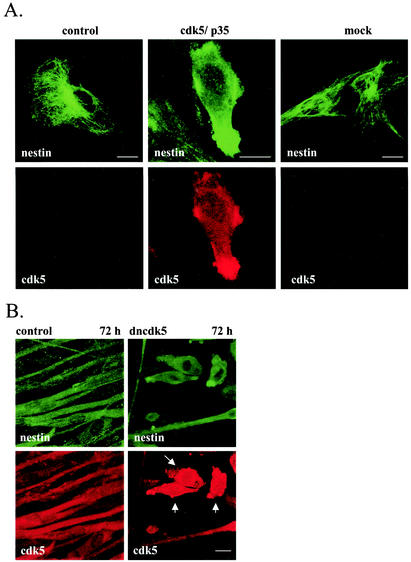
The effects of cdk5 and/or p35 overexpression on cytoskeletal organization was analyzed by immunofluorescence labeling of transfected ST15A cells 2 days after transfection. In untransfected cells, nestin formed a filamentous network extending from the nucleus to the plasma membrane (Fig. 4A). In cells overexpressing cdk5 and p35 or p35 alone (data not shown), nestin was retracted from the periphery of the cells and organized to a focal point close to the nucleus (Fig. 4A). No effect on nestin organization was observed in cells transfected with dncdk5 (Fig. 4A). We have previously shown that nestin and vimentin form indistinguishable structures in ST15A cells, indicating that nestin copolymerizes with vimentin into filaments (34). Interestingly, in contrast to nestin, the vimentin structure seemed to be largely unaffected by overexpression of cdk5 and p35 (Fig. 4B). As it has been shown that the microtubules interact with IFs, we wanted to examine if the reorganization of nestin in cdk5- and p35-transfected cells had any effect on the organization of microtubules. Tubulin filaments could be seen extending throughout the cytoplasm in untransfected cells, and the distribution of microtubules seemed to be unaffected by overexpression of cdk5 and p35 (Fig. 4B). In addition, the microfilament system appeared unaffected in cells overexpressing cdk5 and p35 (data not shown).
FIG. 4.
Reorganization of the nestin network in ST15A cells transfected with cdk5 and the p35 activator protein. Immunofluorescence confocal images of transfected and control ST15A cells show endogenous nestin, vimentin or tubulin (green), and cdk5 (red). Cdk5 was detected with antibodies against cdk5 (Santa Cruz; cdk5 antibody C-8). (A) Overexpression of cdk5 and p35 in ST15A cells induces reorganization of the nestin network. The network is retracted from the cell borders and concentrated at one pole of the nucleus (arrow). The nestin network appears unaffected in cells transfected with dominant negative cdk5. Bar, 10 μm. (B) Overexpression of cdk5 and p35 in ST15A cells does not affect the organization of vimentin and tubulin networks. Bar, 10 μm.
The expression pattern of nestin corresponds to expression and activation of cdk5 during myogenic differentiation and regeneration and at NMJs in adult muscle.
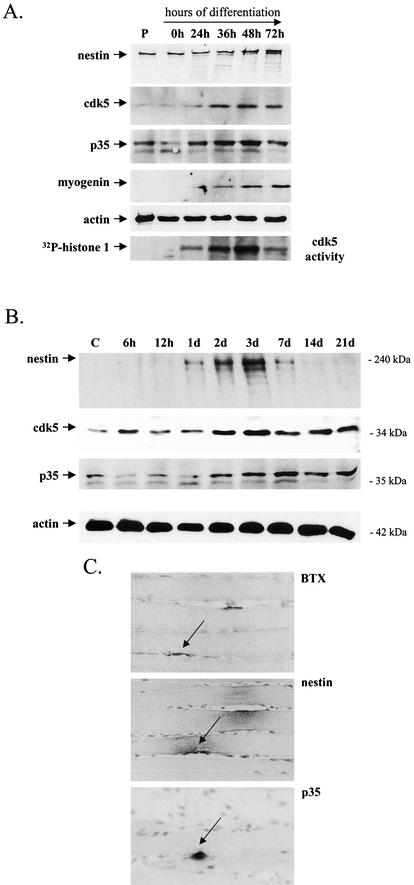
Our results indicate that cdk5 is involved in phosphorylation-mediated regulation of nestin and identify Thr-316 as a cdk5-specific phosphorylation site on nestin. Because in vitro phosphorylation assays and overexpression studies may not reflect a real interaction between two proteins, a myoblast C2C12 cell line was employed—which rapidly differentiates and produces contracting myotubes expressing characteristic muscle proteins in culture (42)—in order to characterize the relationship between nestin and cdk5 during early development. In addition, a muscle injury model was used, which triggers elevated nestin expression during the early regeneration phase of the muscle (39, 40). The regeneration of myofibers after necrotizing muscle injury mimics their formation during development and is, therefore, a versatile model for muscle differentiation (40).
Undifferentiated myoblasts expressed nestin and very low levels of cdk5 and p35, as shown by immunoblotting (Fig. 5A). Between 24 and 36 h of differentiation, cdk5 and p35 levels were elevated, corresponding to the increased expression of the muscle differentiation marker myogenin and a slight increase in nestin expression (Fig. 5A). The increasing expression of cdk5 and p35 was associated with increased kinase activity, as shown by in vitro kinase assays (Fig. 3A). At this time, the cells showed morphological changes that are typical of differentiating cells, i.e., they became elongated and were aligned in a parallel manner (data not shown). Cdk5 kinase activity peaked at 36 to 48 h of differentiation and was then reduced at 72 h (Fig. 5A), when multinucleated myotubes had formed (data not shown). Although the cdk5 activity had declined at 72 h of differentiation, the myotubes still expressed high levels of cdk5. The decrease in cdk5 activity at 72 h corresponded to down-regulation of the cdk5 activator, p35 (Fig. 5A).
FIG.5.
Nestin expression corresponds to the expression and activation of cdk5 during the early and late stages of myogenesis in regenerating muscle after necrotizing injury and at neuromuscular junctions. (A) C2C12 myoblasts were harvested at different times of differentiation and immunoblotted with antibodies against nestin, cdk5, p35 (Santa Cruz; p35 antibody N-20), and myogenin, which was used as a marker for muscle differentiation. P denotes proliferating myoblasts. At 24 to 36 h of differentiation, there is an increase in the expression of cdk5 and p35 that correlates with an increase in the expression of nestin and the differentiation marker myogenin. Immunoblotting with an actin antibody was used as a control for equal loading of proteins. Cdk5 kinase activity starts to increase at 24 h of differentiation and peaks at 48 h, as shown by the in vitro kinase assay, in which H1 was used as a substrate. At 72 h of differentiation, cdk5 activity is decreased, corresponding to a decrease of the activator, p35. (B) Immunoblotting of muscle samples, collected at different points in time after phospholipase A2-induced injury, with antibodies against nestin, cdk5, and p35 (N-20; Santa Cruz) shows that the increase in nestin expression correlates temporally with an increase in cdk5 and p35 expression during the regeneration process. d, days. (C) Immunohistochemical labeling of consecutive sections of an adult rat skeletal muscle sample shows specific expression of nestin and p35 at the NMJ. NMJs were identified by toxin immunohistochemistry by using BTX.
Protein extracts of rat muscle tissue samples, taken at different time points after phospholipase A-induced injury (39), were immunoblotted using nestin, cdk5, and p35 antibodies. Nestin was barely detected in control samples, and up to 12 h after injury, nestin expression remained unchanged (Fig. 5B). One day after injury, nestin expression was slightly increased and then rapidly elevated up to the 3-day time point. The expression remained high until 7 days postinjury, when it slowly attenuated and returned to the control levels at 21 days postinjury. Cdk5 kinase and p35 followed the same expression pattern as nestin (Fig. 5B). Their expression levels remained constant until 2 days after injury, when their levels were elevated. However, the expression of cdk5 remained elevated even when nestin expression started to decrease (Fig. 5B).
While nestin expression in the fully developed muscle is down-regulated to levels below the limit of immunoblot-based detection, nestin immunoreactivity remains at the NMJs in tissue sections of the adult rat muscle (39). Interestingly, the small amount of remaining nestin in adult muscle is coexpressed with p35 at the NMJs, as shown by immunohistochemical analysis (Fig. 5C). BTX, which binds specifically to AChRs, was used to identify NMJs (Fig. 5C). Similar results were obtained with cdk5 (data not shown). This analysis shows that the association between nestin and the cdk5 and p35 complex also remains during the adult stages of very low nestin expression.
Cdk5 is associated with nestin in differentiating myoblasts.
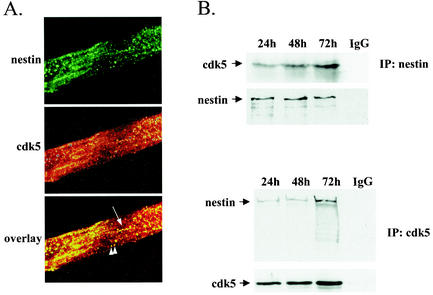
In addition to the temporal agreement of the expression profiles of cdk5 and nestin during cell culture and in situ myogenesis, we wanted to determine whether these two proteins were also physically associated with each other. To this end, we tested whether cdk5 would colocalize with nestin and whether it would be associated with nestin in coimmunoprecipitation analysis of differentiating myoblasts. The spatial coorganization of cdk5 and nestin was demonstrated by high magnification confocal microscopy of double-labeled myoblasts at 48 h of differentiation (Fig. 6A). Cdk5 colocalized with nestin along nestin filaments and in larger aggregate-like structures. Cdk5 also coimmunoprecipitated along with nestin and vice versa in C2C12 cells at 24, 48, and 72 h of differentiation, as demonstrated by immunoblotting of the precipitates with nestin and cdk5 antibodies (Fig. 6B).
FIG. 6.
Cdk5 is associated with nestin in differentiating C2C12 myoblasts. (A) Confocal microscopy demonstrates colocalization of cdk5 and nestin in C2C12 myotubes at 48 h of differentiation. At 48 h, nestin forms diffuse parallel arrays in the differentiating myotubes. Cdk5 colocalizes with nestin along nestin filaments (arrow) and in aggregate-like structures (arrowheads). Images are presented as max projections. (B) Coimmunoprecipitation analysis shows that cdk5 is associated with nestin in differentiating C2C12 myoblasts. Nestin and cdk5 (C-8; Santa Cruz) were immunoprecipitated from C2C12 cells, and the precipitate was blotted against cdk5 (upper panel, IP:nestin) and nestin (lower panel, IP:cdk5), respectively. The recovery of cdk5 and nestin is shown by immunoblotting with antibodies against the immunoprecipitated proteins. As a negative control, the lysates were incubated with unspecific IgG prior to recovery with protein G or A Sepharose, respectively.
Cdk5 activity is necessary for differentiation and the concomitant nestin reorganization in C2C12 myoblasts.
The involvement of cdk5 in regulating nestin organization in myogenic cells is supported by the disruption of the nestin network in C2C12 myoblasts overexpressing cdk5 and p35, as shown by immunofluorescence labeling (Fig. 7A). The effects of ectopic expression of cdk5 and p35 on nestin organization in C2C12 myoblasts were different from those observed in ST15A cells. Whereas nestin still appeared filamentous in ST15A cells, nestin showed a diffuse and spotty immunofluorescence pattern in C2C12 myoblasts overexpressing cdk5 and p35, indicating disassembly of nestin filaments (Fig. 7A). The transfected cells showed dramatic morphological changes with a fusiform appearance compared to the control cells. No effect on nestin organization was observed in mock-transfected cells.
FIG. 7.
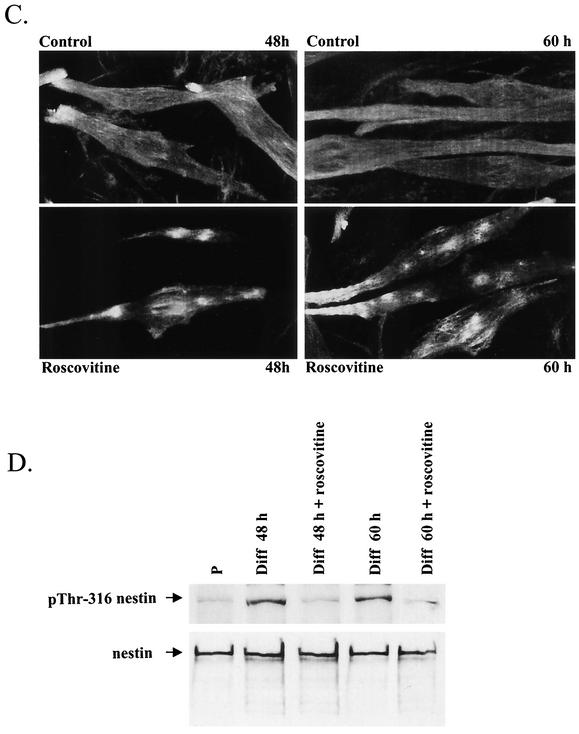
Cdk5 is a regulator of nestin phosphorylation and dynamics in differentiating myoblasts. (A) Images show double-immunofluorescence labeling of control and cdk5- and p35-transfected C2C12 cells with nestin and cdk5 antibodies. Overexpression of cdk5 together with p35 in C2C12 myoblasts leads to reorganization of nestin. Transfected cells show diffuse nestin immunoreactivity, indicating disassembly of nestin filaments. (B) Untransfected C2C12 myoblasts and myoblasts transfected with dominant-negative cdk5 were immunolabeled with nestin and cdk5 antibodies at 72 h of differentiation. At 72 h, untransfected myoblasts have fused and formed long multinucleated myotubes (control). Cells transfected with dominant-negative cdk5 remain unfused (arrows) and show a diffuse and spotty nestin immunoreactivity. (C) C2C12 myoblasts treated with the cdk5 inhibitor roscovitine for 12 h prior to fixation and labeling of the cells were immunolabeled with nestin antibodies at 48 and 60 h of differentiation. Nestin organization is disrupted in cells treated with roscovitine compared to control cells. In roscovitine-treated cells, nestin is concentrated into large condensed structures. (D) Immunoblots with nestin and pThr-316 antibodies of whole-cell extracts of proliferating (P), untreated, and roscovitine-treated C2C12 cells at 48 and 60 h of differentiation (Diff). The samples were normalized toward nestin. Nestin phosphorylation at Thr-316 is increased at 48 and 60 h of differentiation of untreated myoblasts. Immunoblots show decreased phosphorylation of nestin at Thr-316 in cells treated with the cdk5 inhibitor at 48 and 60 h of differentiation.
Cdk5 has previously been shown to be a positive modulator of myogenic differentiation of C2C12 cells (16). To examine the involvement of cdk5 in nestin reorganization during myogenic differentiation, C2C12 myoblasts were transfected with dominant-negative cdk5 and induced to differentiate for 48 and 72 h. Our results show that the expression of dncdk5 inhibited differentiation and the accompanying nestin reorganization of myoblasts. At 72 h of differentiation, control cells had fused into long multinucleated myotubes, in which nestin was reorganized into diffuse parallel fibers (Fig. 7B). The cells expressing the dominant-negative mutant remained unfused and lacked expression of myogenin (data not shown) but were morphologically different from undifferentiated myoblasts in that they were more contracted with a bipolar appearance and occasionally had thick protrusions (Fig. 7B). The nestin network in transfected myoblasts grown in differentiation medium for 72 h was disrupted, as was evident by diffuse nestin immunofluorescence labeling (Fig. 7B). The inability of myoblasts expressing dncdk5 to differentiate is not a consequence of transfection, as cells transfected with green fluorescent protein-tagged nestin differentiated normally (data not shown).
Successful transfection of C2C12 myoblasts was obtained only with undifferentiated proliferating cells. This limitation hampers detailed examination of causes and consequences, as the activation of cdk5 will be blocked prior to cdk5-mediated changes in nestin organization occurring later during differentiation. This implies that it is not possible to determine whether the loss of nestin organization is a specific effect of inhibited cdk5 or a secondary effect due to the abolished differentiation. In order to examine the effects on nestin organization induced by the inhibition of cdk5 activity at stages of differentiation when cells are already undergoing differentiation, we employed roscovitine and BL-I, both commonly used cdk5 inhibitors. At 48 and 60 h, control cells showed clear signs of differentiation, including elongated morphology and fusion of cells. At this stage, nestin showed a diffuse pattern with parallel arrays of filaments (Fig. 7C) compared to the extended network seen in undifferentiated myoblasts (Fig. 7A). In roscovitine-treated cells, the nestin network was condensed into large cytoplasmic aggregates (Fig. 7C). Treatment with BL-I yielded identical results (data not shown).
In order to confirm that the cdk5 inhibitor had affected nestin phosphorylation, we examined the phosphorylation of nestin at Thr-316 at these time points. Whole-cell extracts of roscovitine-treated and untreated C2C12 myoblasts were subjected to immunoblotting with antibodies against nestin phosphorylated at Thr-316. The samples were equalized with respect to nestin in order to determine changes in phosphorylation. Nestin showed very low constitutive phosphorylation at Thr-316 in proliferating C2C12 myoblasts. At 48 and 60 h of differentiation, phosphorylation at Thr-316 was increased (Fig. 7D). Treatment with the cdk5 inhibitor roscovitine led to decreased phosphorylation of Thr-316 at 48 and 60 h of differentiation, corresponding to the disruption of the nestin network (Fig. 7D). Identical results were obtained by using the other cdk5 inhibitor, BL-I (data not shown). These results further demonstrate the involvement of cdk5 in the phosphorylation-mediated regulation of nestin during differentiation of C2C12 cells.
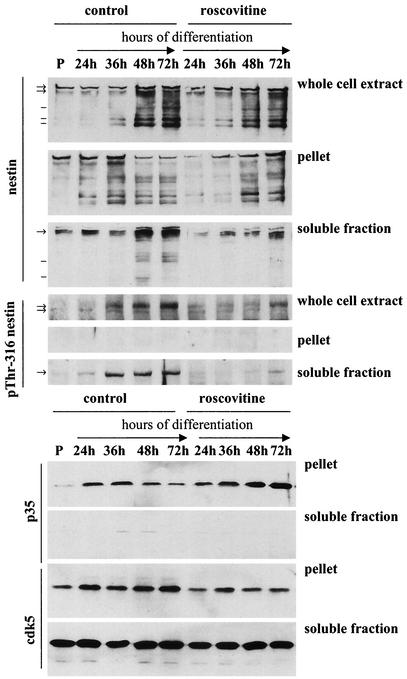
Nestin phosphorylation at Thr-316 is associated with the soluble fraction.
In order to examine the effect of nestin phosphorylation on the assembly and subcellular distribution of cdk5 and p35, Triton X-100 extraction of differentiating myoblasts was performed, and the soluble and pellet fractions were immunoblotted with nestin, pThr-316 nestin, and cdk5- and p35 antibodies (Fig. 8). The immunoblots showed an increase in nestin solubility during differentiation that was associated with phosphorylation of nestin at Thr-316 (Fig. 8). Phosphorylation of nestin at Thr-316 was exclusively associated with the soluble fraction (Fig. 8), indicating that phosphorylation of this site is involved in regulating the disassembly of nestin filaments. We have previously shown that mitotic reorganization of nestin is accompanied by increased phosphorylation at Thr-316 with subsequent disassembly of nestin (34).
FIG. 8.
The phosphorylation of nestin at Thr-316 is associated with nestin solubility. The soluble and insoluble fraction after detergent extraction of myoblasts at different time points of differentiation were immunoblotted with antibodies against nestin, pThr-316 nestin, cdk5, and p35 (N-20; Santa Cruz). Immunoblots show increases in nestin solubility during differentiation. Phosphorylation of nestin at Thr-316 is associated with the soluble pool. Roscovitine treatment inhibits Thr-316 phosphorylation and subsequent nestin solubilization. Arrows denote high-molecular-weight nestin bands that are recognized by the pThr-316 nestin antibody. Lines mark lower-molecular-weight nestin bands not recognized by the phosphospecific antibody. Cdk5 and p35 expression is increasing at 24 to 36 h of differentiation. Upon terminal differentiation at 72 h, p35 levels are decreased. Roscovitine treatment inhibits the decrease in p35 levels and the increase of cdk5. Cdk5 is associated with both the cytosolic, soluble fraction and the cytoskeletal, insoluble fraction, whereas p35 is present in the insoluble fraction.
Nestin phosphorylation at Thr-316 was diminished at 24, 48, and 72 h of differentiation upon roscovitine treatment, corresponding to a decrease in the amount of soluble nestin compared to that in the control cells (Fig. 8). In addition, treatment with the cdk5 inhibitor affected the protein levels of cdk5 and p35. The immunoblots showed that the increase in cdk5 expression was abolished in roscovitine-treated cells, indicating that cdk5 expression is regulated through a positive feedback loop by cdk5 activity (Fig. 8). Furthermore, p35 levels remained constant in treated cells, indicating that degradation of p35 was impaired (Fig. 8). p35 has been shown to be subjected to rapid turnover, and phosphorylation of p35 by cdk5 plays an autoregulatory role in p35 degradation (18, 24, 30). Hence, inhibiting cdk5 activity leads to stabilization of p35.
Interestingly, immunoblotting with antibodies against cdk5 and p35 showed that, whereas cdk5 is present in both the soluble and the insoluble fraction, p35 is present only in the insoluble fraction, demonstrating that p35 is not associated with nestin that is phosphorylated at Thr-316 (Fig. 8). During differentiation, the amount of cdk5 in the soluble pool remained constant, whereas the amount of cdk5 associated with the cytoskeletal fraction increased (Fig. 8).
The interaction between p35 and nestin is regulated by cdk5 activity.
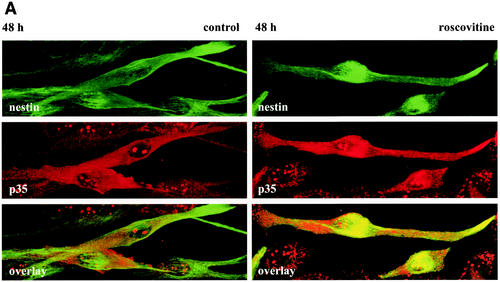
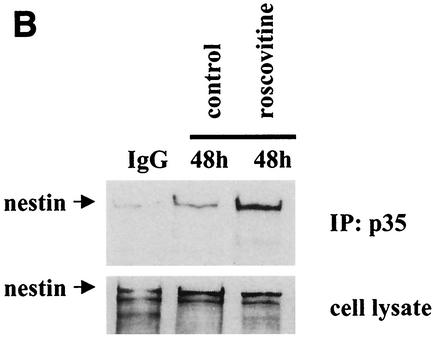
Cdk5 and protein activators of cdk5, such as p35, p25, and p39, have been shown to be associated with cytoskeletal components in neurons (12, 14, 17, 41). However, no significant association between p35 and nestin was seen in differentiating myoblasts at 48 h, as demonstrated by double-label immunofluorescence and coimmunoprecipitation analysis (Fig. 9). Interestingly, when cdk5 was inhibited with roscovitine, an increased association between p35 and nestin could be observed (Fig. 9), indicating that the interaction between p35 and nestin is regulated by cdk5 activity. In contrast, the interaction between cdk5 and nestin appears not to be dependent on the activity of cdk5, as cdk5 was associated with nestin in IF preparations from CNS progenitor cells (Fig. 2) exhibiting no detectable cdk5 activity (Fig. 1A).
FIG. 9.
The interaction between p35 and nestin is regulated by cdk5 activity. (A) Panel shows immunofluorescence confocal images of control and roscovitine-treated C2C12 myoblasts at 48 h of differentiation. Cells were labeled with antibodies against nestin shown in green and p35 (C-19; Santa Cruz) shown in red. Confocal images are presented as max projections. No significant colocalization between p35 and nestin is observed in untreated myoblasts (left panels). In roscovitine-treated cells, p35 is colocalized with nestin (right panels). (B) p35 was immunoprecipitated from control and roscovitine-treated C2C12 cells at 48 h of differentiation by using a monoclonal antibody against p35 (Sigma), and the precipitate was immunoblotted with an antibody against nestin. As a negative control, the lysates were incubated with unspecific IgG prior to recovery with protein G Sepharose. Immunoblotting shows that the interaction between nestin and p35 is enforced in roscovitine-treated cells compared to untreated cells. Immunoblotting of the cell lysate with a nestin antibody was used as a control for equal protein loading in the samples.
DISCUSSION
The protein nestin is specifically expressed during early stages of myogenic development, where it is coexpressed with vimentin. Upon myogenic differentiation, nestin and vimentin expression are down-regulated, whereas desmin expression continuously increases to become the predominant IF in mature myofibers (13, 35). The protein nestin forms indistinguishable filamentous networks with vimentin and desmin at stages of coexpression (35, 37). The intermediate filament network undergoes an extensive reorganization from the primarily perinuclear organization in myoblasts to long fibers in multinucleated myotubes. As the myotubes mature, desmin and nestin become localized to the Z bands, with a subsequent decrease in nestin expression (13, 35). Little is known about the regulation of nestin dynamics during myogenic differentiation. The dynamic organization and expression pattern of nestin calls for posttranslational regulation mechanisms that determine both the organization and, possibly, also the turnover and degradation of nestin. Here we identify nestin as a novel target for cdk5, a critical determinant for neuronal and muscular development, and we show that cdk5 is involved in regulating nestin dynamics in differentiating myoblasts. Furthermore, we demonstrate that cdk5 is associated with nestin in myoblasts at early stages of differentiation and at NMJs in the adult muscle. This association is a likely determinant of the organization and dynamics of nestin as well as of cdk5.
Cdk5 is a bona fide nestin kinase in vitro and in vivo.
Phosphorylation of IF preparations showed that cdk5 almost exclusively phosphorylates nestin over the partner IF protein, vimentin (Fig. 1). This was a surprising observation, since the cyclin-dependent serine and threonine kinases phosphorylate similar motifs on different IF proteins, and vimentin contains an SP consensus site (Ser-55) for proline-directed kinases, which could serve as a recognition site for cdk5. The specificity for nestin was further emphasized in vivo in transfected cells, in which overexpression of cdk5 and p35 in neuronal progenitor cells (Fig. 4A) and in myoblasts (Fig. 7) had prominent effects on nestin phosphorylation and organization, whereas the other major cytoskeletal components appeared to be unaffected (Fig. 4B). In view of the copolymerization of nestin and vimentin during filament formation, the disruption of nestin organization could have been anticipated to have effects on the vimentin network as well. On the other hand, based on the in vitro and in vivo phosphorylation data, cdk5 was not predicted to have a direct effect on vimentin, as it specifically phosphorylated nestin, whereas vimentin showed only minor phosphorylation by cdk5. Expression of a rod end deletion mutant nestin in vimentin-expressing nestin-free cells has been shown to affect the vimentin organization only mildly (22). The mutated nestin became incorporated into the vimentin network, albeit in an abnormal discontinuous fashion without altering vimentin organization, thus indicating that major organizational changes of nestin may occur without necessarily affecting its IF partner (22).
We have identified Thr-316 as an in vitro cdk5 phosphorylation site on nestin. The involvement of cdk5 in phosphorylating Thr-316 in vivo was supported by the increase in Thr-316 phosphorylation of native IF preparations phosphorylated by cdk5 in vitro (Fig. 1D), by the increase in phosphorylation of Thr-316 in cultured cells overexpressing cdk5 and p35, and by the decreased phosphorylation at this site in differentiating myoblasts treated with the cdk5 inhibitor roscovitine (Fig. 7D and 8). Thr-316 is a consensus site for cyclin-dependent kinases, and we have previously shown that the mitotic cdc2 kinase phosphorylates nestin at this site during mitosis with a subsequent increase in nestin solubility (34). The involvement of cdk5 in the regulation of nestin dynamics in vivo in differentiating myoblasts was followed by using the antibody specific for phospho-Thr-316. Cdk5 was demonstrated to phosphorylate nestin at Thr-316 during differentiation of myoblasts, and phosphorylation at this site was associated with increased nestin solubility. The diffuse nestin immunolabeling in cdk5- and p35-transfected C2C12 myoblasts indicates that the nestin network is disassembled, whereas nestin still appears to be filamentous in ST15A cells overexpressing cdk5 and p35. The difference of the in vivo filament organization in these cells is probably related to other posttranslational modifications, including phosphorylation at different sites and/or regulation by interactions with associated proteins. In addition to the used Thr-316 indicator site for cdk5 activity, the cdk5-mediated regulation of nestin is likely to be rather complex and involve several other phosphorylation sites, as has been shown for the NFs that contain numerous KSP repeats that are phosphorylated by cdk5 (28, 36). The identified phosphorylation sites, Thr-1495 and Thr-316, are not the only targets for cdk5, as demonstrated by phosphoamino acid analysis, which showed strong phosphorylation of serine residues on nestin phosphorylated in vitro by cdk5 and by the presence of additional tryptic phosphopeptides from nestin phosphorylated in vitro by cdk5 (Fig. 1B and C).
Association of nestin and cdk5—implications of nestin as a cdk5 and p35 scaffold.
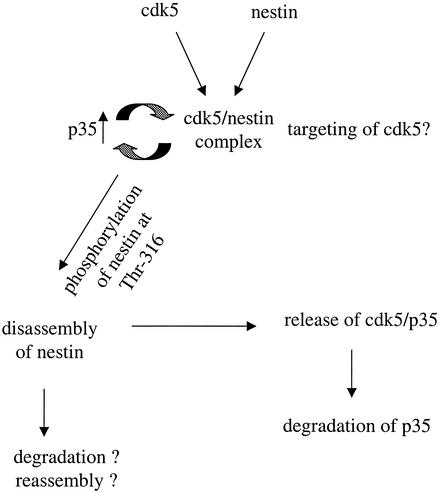
We show that cdk5 is physically associated with nestin in differentiating myoblasts, as demonstrated by immunocomplex assays and double-immunofluorescence labeling (Fig. 6). The close association between nestin and cdk5 raises the possibility that cdk5 regulates nestin; on the other hand, nestin could participate in targeting and regulation of cdk5 in myogenic cells. The intracellular localization of p35 also depends on factors regulating its degradation. Phosphorylation of p35 by the activated cdk5 plays an autoregulatory role in the ubiquitin-mediated proteolysis of p35 (29, 30). The rapid turnover of p35 may be a normal mechanism to prevent the formation of the more stable, abnormally active cdk5. The results on the interaction between nestin and p35 (Fig. 8 and 9) imply that a cdk5-mediated autoregulatory turnover loop is likely to take place on nestin. The observed association of p35 with nestin after cdk5 inhibition—and the fact that p35 was not associated with nestin phosphorylated at Thr-316—implies that there would be cdk5-dependent turnover of p35 on the nestin-cdk5 complex. In fact, the degradation of p35 in differentiating myoblasts is inhibited by roscovitine treatment. Although roscovitine and BL-I are inhibitors that could possibly also affect cdks other than cdk5, our collected data indicate that the effects of the inhibitors were indeed mediated by cdk5 inhibition. Taken together, the results regarding the interaction between nestin and p35 and cdk5 indicate that nestin could serve as an organizing molecule or scaffold for cdk5 and p35 signaling. A model for the interaction between cdk5 and nestin during myogenic development is shown in Fig. 10.
FIG. 10.
A tentative model for the role of nestin as a scaffold for cdk5 in myogenic tissues. There is a close relationship between the expression and organization of nestin and cdk5 during myogenic differentiation and regeneration. The cdk5-dependent phosphorylation of nestin at Thr-316 is associated with nestin disassembly. The disassembled nestin could be targeted for degradation or could be reassembled into filaments with other developmental-stage-specific IF proteins. In addition, there is cdk5-dependent turnover of p35 on nestin. Nestin could thereby act as a scaffold for cdk5 in myogenic tissue. The presence of a nestin-cdk5 signaling complex, with active turnover of associated p35, implies that nestin is likely to have a role in the regulation and/or targeting and compartmentalization of cdk5 and p35 activity.
In addition to the physical interactions between nestin and cdk5 in cultured cells, there is a close temporal association between nestin and cdk5 in vivo, in regenerating muscle tissue after injury, and at the NMJs in adult muscle. The closely corresponding nestin and cdk5 expression kinetics during early myogenic development and regeneration occur in cells that undergo active differentiation, migration, and fusion, all processes that are characterized by extensive reorganization of cells and cytoskeletal elements. The observed physical and spatiotemporal association of cdk5 with nestin is in agreement with the proposed role of nestin as a scaffold for cdk5 and p35 signaling. The association between nestin and cdk5 at NMJs further supports this hypothesis. It was recently suggested that cdk5 forms compartmentalized complexes with its target and regulatory molecules, such as cytoskeletal substrates, phosphatases, and kinases. The assembly of these complexes is supposed to facilitate the dynamic molecular interactions underlying cdk5-regulated functions (9). The factors that control when and where cdk5 forms complexes with its regulatory proteins regulate cdk5 activity. In this respect, nestin may serve as a scaffold for cdk5, targeting cdk5 to NMJs, where it is engaged in the neuregulin-induced expression of AChRs through modulation of the activation of neuregulin-specific ErbB receptors (7). Interestingly, nestin was recently reported to localize in growth cones of neurons, suggesting a role for nestin in growth cone guidance during axon elongation (43). Accordingly, a major proposed role for cdk5 during neuronal development is the regulation of neuritic development and axon guidance (26).
Physiological relevance of nestin phosphorylation by cdk5.
In addition to the suggested role in regulating nestin dynamics and cdk5 activity, the cdk5-mediated phosphorylation of nestin during myogenic differentiation may serve other functions as well. Our data show that phosphorylation of nestin by cdk5 is associated with increased solubility of nestin. In addition, cdk5 could be either directly or indirectly involved in regulating nestin turnover. Degradation via ubiquitination in a phosphorylation-modulated fashion was recently shown for keratins 8 and 18 (15). Nestin expression is down-regulated during differentiation, and nestin is replaced by other IF proteins in the adult organism. The lower-molecular-weight bands in the nestin immunoblots of differentiating C2C12 cells could reflect degradation of nestin. In fact, we have data that indicate that nestin is ubiquitinated in a phosphorylation-dependent manner during myogenic differentiation (T. He, C. Sahlgren, and J. E. Eriksson, unpublished observations). Cdk5 could also be involved in regulating the interaction between nestin and other IF proteins or other cytoskeletal components. A role for nestin as a linker protein interconnecting the cytoskeletal systems has recently been proposed (10, 38), and phosphorylation may be an important regulator of the dynamics of such interactions during myogenic development. The binding of NFs to microtubules has been shown to be inhibited by phosphorylation of NFs by cdk5 (23). These issues remain to be elucidated in forthcoming studies.
Although the exact physiological role of the interaction between cdk5 and nestin during the early stages of neuronal and myogenic development needs to be explored in greater detail, the results from this study demonstrate an important and novel link between cdk5 and nestin. The presence of a nestin-cdk5 complex is likely to have ramifications both in regulating nestin organization and in targeting the activity of p35 and cdk5 signaling. Taken together, we propose that the relationship between cdk5 and nestin is an intrinsic feature of myogenic differentiation and that cdk5 is involved in regulating nestin dynamics and organization during myogenic development.
Acknowledgments
We thank Ville Hietakangas, Ann-Sofi Härmälä-Braskén, Adolfo Rivero-Müller, Minna Poukkula, and Lea Sistonen for critical comments on this manuscript. We are also grateful to Kaija-Liisa Laine and Helena Saarento for expert technical assistance.
This study was supported by the Academy of Finland (Research Council for Environment and Natural Resources, grant 44191), the Erna and Victor Hasselblad Foundation, and the Sigrid Jusélius Foundation. C.M.S. was supported by the Turku Graduate School of Biomedical Sciences.
REFERENCES
- 1.Ahlijanian, M. K., N. X. Barrezueta, R. D. Williams, A. Jakowski, K. P. Kowsz, S. McCarthy, T. Coskran, A. Carlo, P. A. Seymour, J. E. Burkhardt, R. B. Nelson, and J. D. McNeish. 2000. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl. Acad. Sci. USA 97:2910-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajaj, N. P., S. T. al-Sarraj, P. N. Leigh, V. Anderson, and C. C. Miller. 1999. Cyclin dependent kinase-5 (CDK-5) phosphorylates neurofilament heavy (NF-H) chain to generate epitopes for antibodies that label neurofilament accumulations in amyotrophic lateral sclerosis (ALS) and is present in affected motor neurones in ALS. Prog. Neuropsychopharmacol. Biol. Psychiatry 23:833-850. [DOI] [PubMed] [Google Scholar]
- 3.Brion, J. P., and A. M. Couck. 1995. Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am. J. Pathol. 147:1465-1476. [PMC free article] [PubMed] [Google Scholar]
- 4.Chae, T., Y. T. Kwon, R. Bronson, P. Dikkes, E. Li, and T. H. Tsai. 1997. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18:29-42. [DOI] [PubMed] [Google Scholar]
- 5.Dhavan, R., and L. H. Tsai. 2000. A decade of cdk5. Nat. Rev. Mol. Cell. Biol. 2:749-759. [DOI] [PubMed] [Google Scholar]
- 6.Frisen, J., C. B. Johansson, C. Török, M. Risling, and U. Lendahl. 1995. Rapid, widespread and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J. Cell Biol. 131:453-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu, A. K., W. Y. Fu, J. Cheung, K. W. Tsim, F. C. Ip, J. H. Wang, and N. Y. Ip. 2001. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat. Neurosci. 4:374-381. [DOI] [PubMed] [Google Scholar]
- 8.Fu, W. Y., A. K Fu, K. C. Lok, F. C. Ip, and N. Y. Ip. 2002. Induction of Cdk5 activity in rat skeletal muscle after nerve injury. Neuroreport 13:243-247. [DOI] [PubMed] [Google Scholar]
- 9.Grant, P., P. Sharma, and H. C. Pant. 2001. Cyclin dependent protein kinase 5 (cdk5) and the regulation of neurofilament metabolism. Eur. J. Biochem. 268:1534-1546. [PubMed] [Google Scholar]
- 10.Herrmann, H., and A. Aebi. 2000. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr. Opin. Cell Biol. 12:79-90. [DOI] [PubMed] [Google Scholar]
- 11.Humbert, S., R. Dhavan, and L. Tsai. 2000. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell Sci. 113:975-983. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro, K., S. Kobayashi, A. Omori, M. Takamatsu, S. Yonekura, K. Anzai, K. Imahori, and T. Uchida. 1994. Identification of the 23 kDa subunit of tau protein kinase II as a putative activator of cdk5 in bovine brain. FEBS Lett. 342:203-208. [DOI] [PubMed] [Google Scholar]
- 13.Kachinsky, A. M., J. A. Dominov, and J. B. Miller. 1994. Myogenesis and the intermediate filament protein, nestin. Dev. Biol. 165:216-228. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, S., K. Ishiguro, A. Omori, M. Takamatsu, M. Arioka, K. Imahori, and T. Uchida. 1993. A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30 kDa subunit of tau protein kinase II, a proline-directed protein kinase associated with microtubule. FEBS Lett. 335:171-175. [DOI] [PubMed] [Google Scholar]
- 15.Ku, N. O., and M. B. Omary. 1996. Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J. Cell Biol. 149:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaro, J. B., M. Kitzmann, M. A. Poul, M. Vandromme, N. J. Lamb, and A. Fernandez. 1997. Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J. Cell Sci. 110:1251-1260. [DOI] [PubMed] [Google Scholar]
- 17.Lee, K. Y., and R. N. Johnston. 1997. Neurofilaments are part of the high molecular weight complex containing neuronal cdc2-like kinase (nclk). Brain Res. 773:197-202. [DOI] [PubMed] [Google Scholar]
- 18.Lee, M. S., Y. T. Kwon, M. Li, J. Peng, R. M. Friedlander, and L. H. Tsai. 2000. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405:360-364. [DOI] [PubMed] [Google Scholar]
- 19.Lendahl, U., L. Zimmerman, and R. D. G. McKay. 1990. CNS stem cells express a new class of intermediate filament proteins. Cell 60:585-595. [DOI] [PubMed] [Google Scholar]
- 20.Lew, J., R. Winkfein, H. Paudel, and J. H. Wang. 1992. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J. Biol. Chem. 267:25922-25926. [PubMed] [Google Scholar]
- 21.Li, B., S. L. Zhang, J. Gu, N. D. Amin, and H. C. Pant. 2000. Integrin α1/β1-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J. Neurosci. 20:6055-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marvin, M. J., J. Dahlstrand, U. Lendahl, and R. D. McKay. 1998. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J. Cell Sci. 111:1951-1961. [DOI] [PubMed] [Google Scholar]
- 23.Miyasaka, H., S. Okabe, K. Ishiguro, T. Uchida, and N. Hirokawa. 1993. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by τ protein kinase II. J. Biol. Chem. 268:22695-22702. [PubMed] [Google Scholar]
- 24.Nath, R., M. Davis, A. W. Probert, N. C. Kupina, X. Ren, G. P. Schielke, and K. K. Wang. 2000. Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem. Biophys. Res. Commun. 274:16-21. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen, M. D., R. C. Lariviere, and J. Julien. 2001. Deregulation of cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30:135-147. [DOI] [PubMed] [Google Scholar]
- 26.Nikolic, M., H. Dudek, Y. T. Kwon, Y. F. Ramos, and L. H. Tsai. 1996. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 10:816-825. [DOI] [PubMed] [Google Scholar]
- 27.Ohshima, T., J. M. Ward, C. G. Huh, G. Longenecker, Veeranna, H. C. Pant, R. O. Brady, L. J. Martin, and A. B. Kulkarni. 1996. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 93:11173-11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pant, A. C., Veeranna, H. C. Pant, and N. Amin. 1997. Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5). Brain Res. 765:259-266. [DOI] [PubMed] [Google Scholar]
- 29.Patrick, G. N., P. Zhou, Y. T. Kwon, P. M. Howley, and L. H. Tsai. 1998. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway J. Biol. Chem. 273:24057-24064. [DOI] [PubMed] [Google Scholar]
- 30.Patrick, G. N., L. Zukerberg, M. Nikolic, S. de la Monte, P. Dikkes, and L. H. Tsai. 1999. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402:615-622. [DOI] [PubMed] [Google Scholar]
- 31.Paudel, H. K. 1997. Phosphorylation by neuronal cdc2-like protein kinase promotes dimerization of τ protein in vitro. J. Biol. Chem. 272:28328-28334. [DOI] [PubMed] [Google Scholar]
- 32.Pei, J. J., I. Grundke-Iqbal, K. Iqbal, N. Bogdanovic, B. Winblad, and R. F. Cowburn. 1998. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Res. 797:267-277. [DOI] [PubMed] [Google Scholar]
- 33.Philpott, A., E. B. Porro, M. W. Kirschner, and L. H. Tsai. 1997. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev. 11:1409-1421. [DOI] [PubMed] [Google Scholar]
- 34.Sahlgren, C. M., A. Mikhailov, J. Hellman, Y.-H. Chou, U. Lendahl, R. D. Goldman, and J. E. Eriksson. 2001. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2-kinase. J. Biol. Chem. 276:16456-16463. [DOI] [PubMed] [Google Scholar]
- 35.Sejersen, T., and U. Lendahl. 1993. Transient expression of the intermediate filament nestin during skeletal muscle development. J. Cell Sci. 106:1291-1300. [DOI] [PubMed] [Google Scholar]
- 36.Sharma, P., J. J. Barchi, X. Huang, N. D. Amin, H. Jaffe, and H. C. Pant. 1998. Site-specific phosphorylation of Lys-Ser-Pro repeat peptides from neurofilament H by cyclin-dependent kinase 5: structural basis for substrate recognition. Biochemistry 37:4759-4766. [DOI] [PubMed] [Google Scholar]
- 37.Sjoberg, G., W. Q. Jiang, N. R. Ringertz, U. Lendahl, and T. Sejersen. 1994. Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp. Cell Res. 214:447-458. [DOI] [PubMed] [Google Scholar]
- 38.Steinert, P. M., Y. H. Chou, V. Prahlad, D. A. Parry, L. N. Marekov, K. C. Wu, S. I. Jang, and R. D. Goldman. 1999. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV α-internexin. J. Biol. Chem. 274:9881-9890. [DOI] [PubMed] [Google Scholar]
- 39.Vaittinen, S., R. Lukka, C. M. Sahlgren, J. Rantanen, T. Hurme, U. Lendahl, J. E. Eriksson, and H. Kalimo. 1999. Specific and innervation-regulated expression of the intermediate filament nestin at neuromuscular and myotendinous junctions in skeletal muscle. Am. J. Pathol. 154:590-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaittinen, S., R. Lukka, C. M. Sahlgren, T. Hurme, J. Rantanen, U. Lendahl, J. E. Eriksson, and H. Kalimo. 2001. The expression of intermediate filament nestin as related to vimentin and desmin in regenerating skeletal muscle. J. Neuropathol. Exp. Neurol. 60:588-597. [DOI] [PubMed] [Google Scholar]
- 41.Veeranna, G. J., K. T. Shetty, M. Takahashi, P. Grant, and H. C. Pant. 2000. Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Brain Res. Mol. Brain Res. 76:229-236. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe, D., and O. Saxel. 1977. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725-727. [DOI] [PubMed] [Google Scholar]
- 43.Yan, Y., J. Yang, W. Bian, and N. Jing. 2001. Mouse nestin protein localizes in growth cones of P19 neurons and cerebellar granule cells. Neurosci. Lett. 302:89-92. [DOI] [PubMed] [Google Scholar]