Abstract
Inhibition of Na+,K+-ATPase (NKA) activity in renal epithelial cells by activation of G protein-coupled receptors is mediated by phosphorylation of the catalytic α-subunit followed by endocytosis of active molecules. We examined whether agonists that counteract this effect do so by dephosphorylation of the α-subunit or by preventing its internalization through a direct interaction with the endocytic network. Oxymetazoline counteracted the action of dopamine on NKA activity, and this effect was achieved not by preventing α-subunit phosphorylation, but by impaired endocytosis of α-subunits into clathrin vesicles and early and late endosomes. Dopamine-induced inhibition of NKA activity and α-subunit endocytosis required the interaction of adaptor protein 2 (AP-2) with the catalytic α-subunit. Phosphorylation of the α-subunit is essential because dopamine failed to promote such interaction in cells lacking the protein kinase C phosphorylation residue (S18A). Confocal microscopy confirmed that oxymetazoline prevents incorporation of NKA molecules into clathrin vesicles by inhibiting the ability of dopamine to recruit clathrin to the plasma membrane. Dopamine decreased the basal levels of inositol hexakisphosphate (InsP6), whereas oxymetazoline prevented this effect. Similar increments (above basal) in the concentration of InsP6 induced by oxymetazoline prevented AP-2 binding to the NKA α-subunit in response to dopamine. In conclusion, inhibition of NKA activity can be reversed by preventing its endocytosis without altering the state of α-subunit phosphorylation; increased InsP6 in response to G protein-coupled receptor signals blocks the recruitment of AP-2 and thereby clathrin-dependent endocytosis of NKA.
The Na+,K+-ATPase (NKA) activity in polarized epithelia provides the motive force for vectorial transport of sodium (1). In renal (2) and jejunal (3, 4) epithelia, locally produced dopamine (DA) regulates NKA activity and thereby sodium excretion during high-salt diet. Furthermore, acute increments in arterial blood pressure increase urinary sodium excretion (“pressure natriuresis”) without significant changes in glomerular hemodynamics (5). This phenomenon is associated with decreased apical Na+/H+-exchanger and basolateral NKA activity and redistribution of these transporters in renal proximal tubule cells (6).
Elucidation of the mechanisms by which the traffic of ion transport proteins is regulated by G protein-coupled receptor (GPCR) signals is central to understanding pressure natriuresis, as well as other forms of altered sodium transport, e.g., those engendered by catecholamines and other hormones. The latter are also important modulators of NKA in lung alveolar cells, where stimulation of its activity promotes the clearance of excess alveolar fluid in congestive heart failure and acute respiratory distress syndrome (7–9).
We have previously investigated possible mechanisms by which GPCRs regulate NKA activity in epithelia (10) and found that inhibition was associated with removal of active molecules from the plasma membrane in renal tubule cells (11). Endocytosis of NKA molecules is initiated by phosphorylation of the Ser18 residue in the catalytic α-subunit (12, 13). Although phosphorylation of the α-subunit is necessary for internalization, it does not affect per se the enzymatic activity of NKA while it resides in the plasma membrane, i.e., it is the subunits' removal from the membrane that causes the decreased activity in intact cells, whereas phosphorylation serves as the triggering signal in this process (12).
In the present study, we evaluated whether agonists that counteract inhibitory effects on NKA activity do so by preventing α-subunit phosphorylation (or promoting its dephosphorylation through the action of protein phosphatases), or by regulation of other targets responsible for NKA endocytosis. In addition, we examined the linking mechanisms within the endocytic network that are triggered by phosphorylation of the NKA α-subunit leading to its endocytosis.
Materials and Methods
Experiments were performed in renal proximal convoluted tubule (PCT) cells obtained from Sprague–Dawley rats (11–13) and in opossum kidney (OK) cells transfected with either the full-length cDNA NKA α1 subunit or the Ser18 → Ala mutant (12–14) as previously reported. Microdissected PCTs were isolated from Sprague–Dawley rats as described (15).
NKA Activity in Isolated Proximal Tubules.
Tubule segments were transferred in 1 μl of Hanks' medium into individual BSA-coated wells of 96-well plates (Nunclon, Naperville, IL). After an equilibration period of 10 min at room temperature, the agonists (1 μl) were added and samples were incubated for 2.5 min (this incubation time was found to be optimal for clathrin vesicle incorporation of NKA α-subunit during endocytosis; ref. 11) at room temperature in Hanks' medium. The incubation was terminated by placing the samples on ice and adding 5 μl of cold 10 mM Tris⋅HCl (pH 7.4). Thereafter, the samples were placed for 10 min on dry ice to ensure cell permeabilization. We have previously determined that, in PCT cells, this procedure allows measurement of NKA activity originated mostly from the plasma membrane and not from intracellular organelles (13). Samples were thawed before addition of 7 μl of NKA assay medium (final concentration, mM: NaCl 100, KCl 5, MgCl2 10, EGTA 1, Tris⋅HCl 100, Mg-ATP 10, [γ-32P]ATP 50 nCi). The assay was carried out for 15 min at 37°C, and the reaction was stopped by cooling the samples on ice and adding 50 μl of 5% trichloroacetic acid. The samples (64 μl) were then transferred into 2 ml of 10% activated charcoal and centrifuged, and the radioactivity in an aliquot (500 μl) from the supernatant was measured. NKA activity (defined as the difference between activities determined in the presence or absence of 5 mM ouabain) was measured in four replicates of four to five tubules for each group.
Phosphorylation and Immunoprecipitation of NKA in Intact Cells.
Renal PCT cells (4–6 mg of protein in 3 ml) were labeled during 2 h at 32°C with 250 μCi/ml [32P]orthophosphate (NEN Life Science Products) as previously described (12, 13). The incubation with different agonists was terminated by removing the medium, adding buffer [100 mM NaCl, 50 mM Tris⋅HCl, 2 mM EGTA, 30 mM NaF, 30 mM Na4O7P2, 1 mM Na3VO4, 1 mM PMSF, 10 μg/ml leupeptin, 4 μg/ml aprotinin, and 1% Triton X-100 (pH 7.45)], and placing the samples on ice. The cells were disrupted by homogenization. Immunoprecipitation of the NKA α-subunit and adaptor proteins was performed as described (12, 13). Proteins were analyzed by SDS/PAGE using the Laemmli buffer system (16) and by Western blotting. Protein content was determined according to Bradford (17).
Preparation of Clathrin-Coated Vesicles (CCV).
Isolation of CCV was performed as described by Hammond and Verroust (18). Briefly, after preincubation protocols, PCT cells were homogenized by using a motor pestle homogenizer (Kimble–Kontes, Vineland, NJ) in 1 mM EGTA, 0.5 mM MgCl2, 0.1 M 2-(N-morpholino)ethanesulfonic acid, and 0.2 mg/ml NaN3, titrated to pH 6.5 with NaOH. The homogenate was centrifuged at 85,000 × g for 1 h, and the pellet was resuspended in the same buffer and applied to a discontinuous sucrose gradient (wt/vol): 60%, 50%, 40%, 10%, and 5%. Samples were then centrifuged at 80,000 × g for 75 min and collected from the 10–40% interface; they were washed in homogenization buffer and pelleted at 85,000 × g for 1 h. Wheat germ agglutinin was added to a concentration of 1 mg:10 mg protein and incubated overnight at 4°C. The agglutinated material was sedimented at 20,000 × g for 15 min.
Preparation of Endosomes.
After incubation with different agonists, the samples were transferred to ice and cold homogenization buffer containing 250 mM sucrose and 3 mM imidazole (pH 7.4) was added. The cells were gently homogenized (15–20 strokes) to minimize damage of the endosomes by using a motor pestle homogenizer (Kimble–Kontes), and the samples were subjected to a brief centrifugation (3,000 × g at 4°C). Endosomes were fractionated on a flotation gradient, as described (11–13), by using the technique described by Gorvel et al. (19).
Confocal Microscopy.
The preincubation with different agonists was terminated by fixation of the cells with 4% formaldehyde in PBS for 10 min at room temperature. After rinsing twice with PBS, cells were incubated in acetone at −20°C for 5 min and then quenched with PBS containing 1% BSA for 30 min. Staining with primary antibodies [against NKA α-subunit (20) and adaptor protein 2 (AP-2) α-subunit] was performed at room temperature for 1 h; incubation with secondary antibodies against the NKA α-subunit (anti-rabbit rhodamine-labeled) and clathrin heavy chain (anti-mouse Oregon green-labeled) were performed at room temperature for 1 h as well. After rinsing with PBS, the coverslips were mounted (SlowFade light; Molecular Probes) and examined with a confocal laser scanning microscope (Leica TCS NT; Leica Lasertechnik, Heidelberg, Germany). Excitation wavelengths 488 nm for Oregon green and 568 nm for rhodamine were used. The confocal microscope was equipped with an Ar/Kr laser, a double dichroic mirror for rhodamine/fluorescein, and a 63× lens (Leica PL APO 63×/1.32–0.6 oil). Analyses of the data were performed with imaris and colocalisation software (Bitplane, Zurich, Switzerland) (21).
Determination of Phosphatidylinositol 3-Kinase (PI3-K) Activity.
Cells were homogenized in 400 μl of lysis buffer [140 mM NaCl, 10 mM Hepes, 10 mM sodium pyrophosphate, 10 mM NaF, 1 mM CaCl2, 1 mM MgCl2, 2 mM Na3VO4, 10% (vol/vol) glycerol, 1% Nonidet P-40, 10 μg/ml aprotinin, 50 μM leupeptin, 2 mM PMSF (pH 8.1)] and solubilized by continuous stirring for 1 h at 4°C. After centrifugation, the supernatant was collected and 500 μg of protein (in 1 ml) was incubated with an anti-PI3-K p85α antibody (Santa Cruz Biotechnology). After overnight incubation with the antibody, protein A-Sepharose was added and the immune complex (p85α antibody coupled to protein A-Sepharose) was washed four times with buffer C [100 mM NaCl, 1 mM Na3VO4, 20 mM Hepes (pH 7.5)] and resuspended in 40 μl of buffer [180 mM NaCl, 20 mM Hepes (pH 7.5)]. PI3-K activity was assessed directly on the protein A-Sepharose beads as described (22).
Determination of Inositol Polyphosphates in Renal Epithelial Cells.
Growing OK cells were labeled with myo-[3H]inositol to isotopic equilibrium over a period of 144 h, so that >90% of the cellular material would have incorporated myo-[3H]inositol to the same specific activity as the medium. The cells were washed once in Hanks' phosphate buffer and preincubated for 5 min at 25°C. The buffer was aspirated and an identical buffer containing the agonists was added (incubations up to 2.5 min). Thereafter, the buffer was aspirated and 0.5 ml of 5% (wt/vol) trichloroacetic acid containing 125 μg of phytic acid as an unlabeled carrier was added. Subsequent extraction and neutralization by ether washing of the inositol phosphates were carried out as described previously (23). Samples were then stored at −20°C until subjected to HPLC. Separation was achieved by using a simple phosphate gradient (23). Concentrations of inositol polyphosphates were determined by measuring the diameters of trypsinized spherical cells (to determine volume), and then calculating the concentration based on the known specific activity of the labeling medium and the fact that >90% of the cell inositol phosphates would have been synthesized with this medium.
Statistics.
Comparison between two experimental groups was made with the nonpaired Student's t test. For multiple comparisons, one-way ANOVA with Sheffe's correction was used; P < 0.05 was considered significant.
Results
Effect of DA and Oxymetazoline (Oxy) on NKA Activity, α-Subunit Phosphorylation, and Endocytosis.
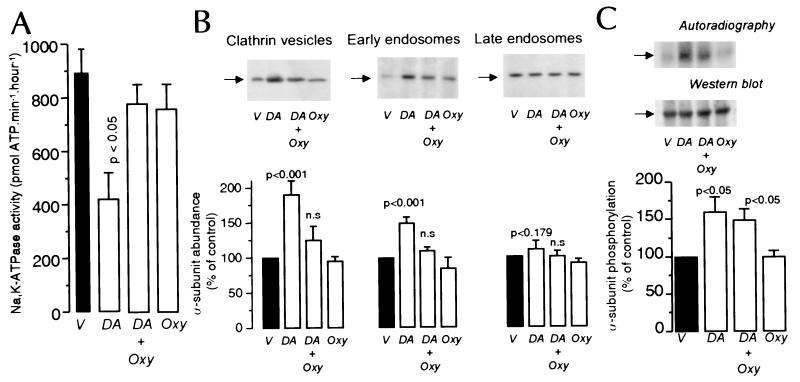
Because endocytosis of NKA molecules in response to DA is responsible for the decreased activity in intact PCT cells, we have now examined whether receptor agonists that counteract the effect of DA on NKA activity do so by preventing NKA subunit endocytosis. We chose to study the effect of Oxy because it had been shown to prevent the inhibitory effect of DA on NKA activity (24). PCT cells incubated with DA (2.5 min at 23°C) exhibited decreased (50 ± 16%, n = 7; P < 0.05) NKA activity (Fig. 1A), and this effect was blocked by coincubation with 1 μM Oxy (97 ± 21%; n = 7; not significant); Oxy alone had no significant effect on NKA activity examined under Vmax condition for its major substrates, as reported by others (24). Incubation of PCT cells with 1 μM DA was associated with a significant increase in NKA α-subunit abundance in CCV and early endosomes (Fig. 1B). The abundance did not change in late endosomes, consistent with our previous observation (11) that within this time frame (2.5 min) the subunits have not yet reached the late endosomal compartment. The presence of 1 μM Oxy, however, abolished the DA effect, whereas Oxy alone did not change the α-subunit distribution among these organelles.
Figure 1.
Effect of DA and Oxy on NKA activity, α-subunit phosphorylation and its abundance in clathrin vesicles, early and late endosomes. PCT cells were incubated with or without 1 μM DA (2.5 min at 23°C) in the presence or absence of 1 μM OXY. (A) NKA activity in microdissected PCT is the mean + SE of seven independent experiments performed in triplicate. (B) Effect of DA and Oxy on NKA α-subunit abundance in CCV, early endosomes, and late endosomes. A representative experiment (Upper) and the quantitative data of five experiments expressed as percent of control mean + SE (Lower) are shown. (C) NKA α-subunit phosphorylation. A representative experiment (Upper) indicating the state of α-subunit phosphorylation, and the quantitative data of five experiments expressed as percent of control mean + SE are shown in Lower.
Because phosphorylation of NKA α-subunit constitutes a trigger in this process (12, 13), we next evaluated whether Oxy counteracts the effect of DA by interfering with phosphorylation or by promoting the dephosphorylation of the α-subunit (Fig. 1C). The increased phosphorylation of immunoprecipitated NKA α-subunit induced by 1 μM DA was not significantly affected by Oxy. Basal phosphorylation of the α-subunit was also not altered by Oxy alone.
Effect of DA and Oxy on AP-2 Recruitment and PI3-K Activity During NKA Endocytosis.
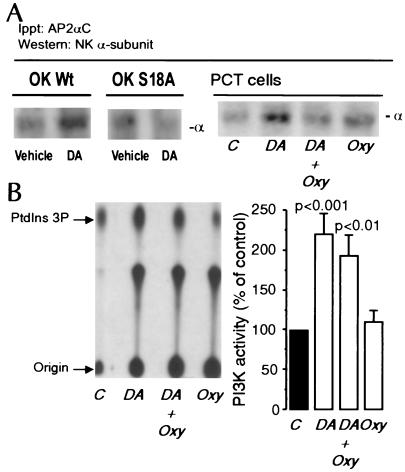
Because adaptor proteins such as AP-2 mediate the assembly of clathrin through recognition signals present within the cytoplasmic domain of the target protein (25–28), we tested whether activation of GPCRs by DA promotes the interaction of AP-2 with NKA during its endocytosis, and, if present, whether this effect requires protein kinase C (PKC)-dependent phosphorylation of its α-subunit. For this purpose, we used OK cells, an established cell line of PCT origin, in which the DA signaling involved in the regulation of NKA activity, α-subunit phosphorylation, and endocytosis is similar to that of rat PCT cells (12, 13). OK cells were incubated with 1 μM DA for 2.5 min at 23°C, and the incubation was terminated by homogenization and immunoprecipitation of AP-2 by using an antibody against the AP-2αC subunit (Upstate Biotechnology, Lake Placid, NY). The presence of NKA in the immunoprecipitate was analyzed by Western blot using a monoclonal antibody against the α-subunit. Fig. 2A indicates that, in DA-treated cells, the NKA α-subunit coimmunoprecipitated with the AP-2. Because internalization of NKA molecules does not occur in cells expressing the cDNA α1-subunit carrying a point mutation (Ser18) in the PKC phosphorylation site or a truncated variant (deletion of first 31 amino acids) that excludes the PKC target residue (12, 13), we next examined whether absence of the phosphorylation site in the α-subunit would impair its ability to bind AP-2. In OK cells expressing the S18A mutant, DA failed to promote the interaction between AP-2 and NKA (Fig. 2A).
Figure 2.
Effects of DA and Oxy on AP-2 recruitment and PI3-K activity. (A) OK cells wild type (OK Wt), cells expressing the S18A mutant (OK S18A), and PCT cells were incubated with or without DA (2.5 min at 23°C). The immunoprecipitated material with an AP2αC antibody was analyzed by Western blotting using an antibody against the NKA α1-subunit. The figures shown are representatives of four replicate experiments. (B) PI3-K was determined in cells incubated with 1 μM DA in the presence or absence of 1 μM Oxy (2.5 min at 23°C). A representative TLC separation of phosphatidylinositol 3-phosphate (Left) and the quantitative data of four experiments expressed as percent of control of the mean + SE are shown in Right.
We next examined whether Oxy could prevent internalization of the subunits by blocking the interaction of AP-2 with the NKA α-subunit. Similarly to OK cells, in PCT cells incubated with 1 μM DA (2.5 min at 23°C), the AP-2 coimmunoprecipitated with the NKA α-subunit, and this association was blocked by Oxy (Fig. 2A Right). Because AP-2 recruitment depends on PI3-K activity (G.A.Y., R. Efendiev, C.H.P., A.I.K., P.-O.B., and A.M.B., unpublished observations), we also examined the effect of DA and Oxy on PCT PI3-K activity. DA (1 μM; 2.5 min at 23°C) increased PI3-K activity in PCT cells, and the magnitude of this effect was not significantly altered by Oxy (Fig. 2B).
Effect of DA and Oxy on Clathrin Recruitment During NKA Endocytosis.
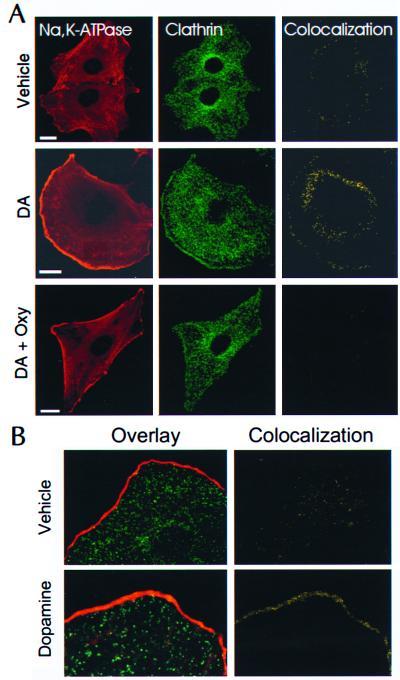
Confocal images of OK cells demonstrate a discrete background staining of NKA (rhodamine) with increased fluorescence at the edge of the cells (Fig. 3A Left). This pattern was maintained in all of the different protocols. Clathrin staining (Fig. 3A Middle), represented by punctate structures of homogeneous distribution, was particularly intense around the nuclei (representing traffic of newly synthesized proteins) and at the periphery of the cell treated with DA; this distribution was blocked by treatment with Oxy. Although the resolution of this technique does not allow the visualization of NKA subunit retrieval from the plasma membrane, colocalization analysis of the data (yellow staining, Fig. 3A Right) revealed that DA increases the colocalization of clathrin and NKA molecules, and that this effect is blocked by coincubation with Oxy. Oxy alone has no effect on NKA/clathrin colocalization (not shown). In addition, Fig.3B further suggests that the NKA α-subunit colocalizes with clathrin at the plasma membrane or in vesicles that are beneath the plasma membrane.
Figure 3.
Visualization of clathrin and NKA α1-subunit in OK cells. (A) Cells were plated onto glass coverslips and incubated with or without 1 μM DA (2.5 min at 23°C) in the presence or absence of 1 μM OXY. OK cells were fixed and stained with secondary fluorescent-labeled antibodies (Rhodamine, NKA; Oregon green, clathrin). Confocal images are representatives of three to eight experiments. (Bar = 2 μm.) (B) Enlarged view of clathrin and NKA α1-subunit interaction in OK cells incubated with or without DA as indicated above. Cells were processed as described in A. (Left) Overlay images of NKA (Rhodamine) and clathrin (Oregon green) staining. (Right) Colocalization (yellow) analysis of the data.
Effect of DA and Oxy on Inositol Polyphosphate Metabolism in Renal Cells.
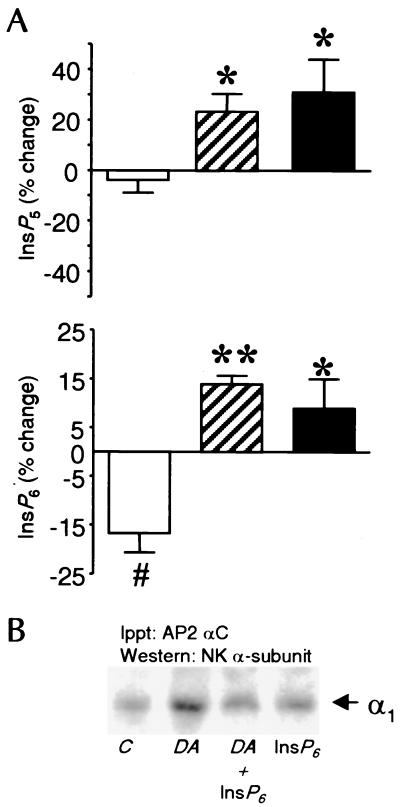
OK cells, instead of isolated PCT cells, were chosen for these experiments because cells have to be labeled with myo-[3H]inositol to isotopic equilibrium over a period of 144 h. Under these conditions, >90% of the cellular material would have incorporated myo-[3H]inositol to the same specific activity as in the medium. Thus, changes in the radioactivity level of the inositol polyphosphates represent changes in mass. DA, by itself, had no significant effect on 1,3,4,5,6-pentaphosphate (InsP5) levels, whereas stimulation of OK cells with either DA plus Oxy or Oxy alone resulted in a rapid (within 30 s) transient increase in both InsP5 and inositol hexakisphosphate (InsP6) compared with both control and DA-treated cells (Fig. 4A). The increase was reversed by 1 min, and no further increases were detected after up to 2.5 min of stimulation with Oxy (data not shown). The InsP6 concentration was significantly (P < 0.05, n = 4) decreased in DA-treated cells.
Figure 4.
(A) Effects of DA and Oxy on the InsP5 and InsP6 concentrations in OK cells. Cells were stimulated with the appropriate agonists (DA, open bars; DA + Oxy, hatched bars; Oxy, filled bars) for up to 2.5 min. The 30-s time point is illustrated. (A) InsP5 (Upper) and InsP6 (Lower) are shown as percent of control + SEM for four separate experiments, each carried out in triplicate. Differences were examined with instat 1.0 (GraphPad, San Diego) using a two-tailed t test. *, P < 0.05; **, P < 0.01, both vs. DA. #, P < 0.05 vs. control, using a one sample, two paired t test. (B) Effect of exogenous InsP6 on DA-induced coprecipitation of NKA α-subunit and AP-2. Cells were incubated with DA (1 μM) in the presence of 10 μM InsP6 (n = 4). Digitonin was used as previously described (13) to permit access of InsP6 to the interior of the cells, and was present in all experimental conditions (vehicle, DA, DA + InsP6, and InsP6).
Effect of InsP6 on AP-2 Recruitment.
The effect of exogenous InsP6 was examined in isolated PCT cells incubated with or without DA (Fig. 4B). For InsP6 to gain access to the cells' interior, permeabilization was performed as described previously (13, 29). Digitonin, which was present in all experimental groups, did not affect the ability of 1 μM DA to promote the colocalization of AP-2 and the NKA α-subunit, whereas the presence of 10 μM InsP6 prevented this effect. InsP6 alone was without effect.
Discussion
Short-term regulation of NKA activity could be mediated by altering the characteristics (e.g., the turnover rate) of the enzyme present in the plasma membrane, as well as by endocytosis from (inhibition; refs. 11–13) or recruitment of new molecules into (stimulation; refs. 8 and 15) the plasma membrane. This study demonstrates that phosphorylation of the NKA α-subunit in response to DA triggers the association of AP-2 with the α-subunit and recruitment of clathrin during endocytosis. Agonists that counteract the inhibitory action of DA do so by blocking endocytosis rather than by modifying the state of phosphorylation of the catalytic α-subunit. Moreover, such effect is accomplished by increasing the cellular levels of InsP6 and inhibition of AP-2 recruitment.
Because phosphorylation of the NKA α-subunit is critical for subunit internalization (12, 13), we sought to determine whether agonists that counteract the effect of DA on NKA activity do so by preventing phosphorylation (or promoting dephosphorylation) of the catalytic α-subunit, thereby preventing endocytosis. We chose to study in renal epithelial cells the effect of Oxy, a compound postulated to counteract the effect of DA on NKA activity by activating protein phosphatases (24). Oxy action is mediated by activation α1 and α2 adrenergic receptors, and the cellular responses to it has been associated, depending on the tissue, with increased intracellular calcium (30) and with negative coupling to adenylyl cyclase. (31, 32). Whereas, in that study (24), the effects of DA and Oxy were determined after 30 min of incubation, we examined the effect of these agonists during 2.5 min, because within this time frame the NKA activity is significantly inhibited by DA and the α-subunit remains phosphorylated (after 10 min the α-subunit is no longer phosphorylated while being removed from the basolateral membrane) (12). Although Oxy blocked the effect of DA on NKA activity, our results show that this effect was not mediated by affecting the level of phosphorylation of the α-subunit, but instead, Oxy prevented the incorporation of NKA α-subunits into CCV and endosomes. That Oxy did not block the increased phosphorylation induced by DA suggests that its mechanism of action does not involve interference with the DA-receptor signaling or activation of protein phosphatases.
It has been previously reported (24) that Oxy stimulates NKA activity. The fact that incubation with Oxy in this study did not result in stimulation of NKA could be because of different incubation times (2.5 vs. 30 min), and/or because of measurement of NKA under different Na+ concentrations (20 vs. 100 mM) in the two studies. Despite this discrepancy, our data indicating that incubation of PCTs with Oxy during 2.5 min did not stimulate NKA activity but blocked the inhibitory action of DA are in agreement with the observation that Oxy did not increase the number of NKA units at the plasma membrane, but blocked the endocytosis of such molecules induced by DA.
Endocytosis of NKA molecules, like with other integral membrane proteins (33, 34), occurs through their stepwise translocation within CCV into endosomal compartments. Clathrin adaptors select the membrane protein to be internalized by direct interaction through specific motifs present in such proteins. Our results demonstrate that DA promotes the interaction of AP-2 with the NKA α-subunit. Moreover, such interaction was absent in OK cells expressing the S18A mutant, indicating that AP-2 recruitment is directly related to the state of α1-subunit phosphorylation. The effects of DA and Oxy on AP-2 and clathrin recruitment were studied in OK cells in culture. The presence of DA (35, 36) as well as α-adrenergic (32) receptors, in this cell line of renal proximal tubule origin has been well documented. The mechanism of NKA regulation as well as the signaling mechanisms involved (12, 13) described so far are comparable to those operating in isolated rat PCT cells, and other intracellular signals unrelated to pump regulation are also similar (37–39). As demonstrated in OK cells, in isolated PCT cells, the NKA α1-subunit coprecipitated with AP-2 in response to DA, and this “binding” was blocked by Oxy. Whereas the NKA α1-subunit possesses several putative internalization motifs, the signal recognized by AP-2 remains to be identified.
Using a different methodology (confocal microscopy), we confirmed that another component of the endocytic network is regulated by DA and Oxy. Because the resolution of this technique did not allow direct visualization of the changes in the number of NKA molecules within the plasma membrane, we determined whether they colocalize with clathrin during the formation of coated pits and vesicles. DA clearly promoted a redistribution of clathrin (enriched at the edges of the cells) and colocalization with NKA α-subunit molecules. This colocalization was absent when cells were incubated simultaneously with DA + Oxy, and Oxy alone did not affect the distribution of clathrin or its association with NKA molecules. These results add further support to the hypothesis that regulation of NKA activity by different agonists could be mediated by controlling the number of enzyme units at the plasma membrane.
DA-induced NKA endocytosis requires activation of PI3-K (22). Several functions have been ascribed to PI3-K during endocytosis, among them recruitment of AP-2 (40), facilitating endosomal traffic by targeting rab5 (41), and regulation of cytoskeleton dynamics (42). Because the time course of activation of PI3-K coincided with the increased abundance of NKA α-subunits within CCV, it is possible that activation of PI3-K by DA would be affected by Oxy, thereby blocking the sequential activation of different signaling molecules involved in NKA traffic. Because DA in the presence of Oxy still significantly increased PI3-K activity, it is likely that other factors preventing NKA internalization are regulated by Oxy. In addition, the results suggest the existence of other intracellular messengers triggered by DA that are responsible for AP-2/clathrin recruitment during NKA endocytosis.
It has been reported (in in vitro studies) that PI3-K products could either favor (40) or antagonize (26, 43) recruitment of adaptor proteins to the plasma membrane. Also, the interaction of clathrin adaptors with cargo molecules is negatively regulated by inositol polyphosphates (44, 45). Because the latter are potent inhibitors of clathrin assembly, we explored in this study whether DA and Oxy modulate the metabolism of inositol polyphosphates (InsP5 and InsP6) in cultured renal epithelial cells in a way that could explain their opposite effects during NKA endocytosis. Interestingly, the InsP6 concentration was significantly (P < 0.05, n = 4) decreased in DA-treated cells, as if the basal InsP6 levels have to be reduced because they represent constitutively a force opposing endocytosis. In OK cells, the basal concentration of InsP6 is about 5- to 7-fold that of InsP5, and InsP6 is also a more potent inhibitor of clathrin assembly than InsP5 (45). Therefore, in intact cells, the smaller InsP6 changes may be more relevant than the larger InsP5 increases, and the decrement of basal InsP6 concentrations by DA may actually remove an inhibitory molecule, thus promoting endocytosis. This concept is strengthened by observations that InsP6 binds to membranes (44), and therefore the changes may occur locally at the internalization site. No changes were seen in the concentration of pyrophosphate derivatives of InsP6, which are in a state of rapid equilibrium with InsP6, indicating that these increments represented a specific effect on InsP5 and InsP6. The increases in polyphosphate concentration in response to Oxy were relatively small, 30–40% for InsP5 and 10–15% for InsP6. However, using cell volume measurements and the specific radioactivity of the labeling medium, we have estimated the basal cellular concentration of InsP6 to be approximately 68.5 μM, with a range from 57.5 to 77.4 μM for DA-treated vs. DA + Oxy-treated cells, respectively. Interestingly, 10 μM InsP6 (similar to the increased concentration induced by oxymetazoline in intact cells) blocked the ability of DA to promote the interaction of AP-2 and NKA α-subunits. Therefore, although the precise mechanism of action of InsP6 has yet to be resolved, it is likely that this compound may interfere with the mechanisms that promote AP-2 recruitment.
The results of this study indicate that physiological regulation of NKA activity in renal tubule, and presumably other epithelial cells, can be achieved by varying the abundance of active molecules within the basolateral membrane. Although this process requires phosphorylation of Ser18 in the rat α-subunit, it does not exclude the possibility that, in other species lacking this residue, DA may regulate NKA activity and endocytosis through phosphorylation of the Ser11 residue (another consensus site within the α-subunit phosphorylated by PKC). Although phosphorylation of the α-subunit constitutes a triggering signal for endocytosis, as it favors the recruitment of AP-2 and clathrin, impaired phosphorylation is not the mechanism by which endocytosis is prevented in response to a receptor signal. Thus, the intracellular traffic of NKA subunits in response to GPCRs signals is probably regulated by interactions at other stages of endocytosis, such as adaptor protein recruitment and/or CCV vesicle formation, through a distinct intracellular signaling network, such as InsP6.
Acknowledgments
We thank A. V. Chibalin for assistance with experiments involving intact cell phosphorylation. This study was supported in part by funds from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, Karolinska Institutet, Novo Nordisk Foundation, and by National Institutes of Health Grant DK53460 (to C.H.P).
Abbreviations
- NKA
Na+,K+-ATPase
- CCV
clathrin-coated vesicles
- GPCR
G protein-coupled receptor
- PCT
proximal convoluted tubule
- OK
opossum kidney
- DA
dopamine
- Oxy
oxymetazoline
- PKC
protein kinase C
- AP-2
adaptor protein 2
- PI3-K
phosphatidylinositol 3-kinase
- InsP5
inositol 1,3,4,5,6-pentaphosphate
- InsP6
inositol hexakisphosphate
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060025597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060025597
References
- 1.Skou J C, Esmann M. J Bioenerg Biomembr. 1992;24:249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- 2.Bertorello A, Hökfelt T, Goldstein M, Aperia A. Am J Physiol. 1988;254:F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- 3.Finkel Y, Eklöf A C, Granquist L, Soares-da-Silva P, Bertorello A M. Gastroenterology. 1994;107:675–679. doi: 10.1016/0016-5085(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 4.Vieira-Coelho M A, Teixeira V L, Finkel Y, Soares-Da-Silva P, Bertorello A M. Am J Physiol. 1998;275:G1317–G1323. doi: 10.1152/ajpgi.1998.275.6.G1317. [DOI] [PubMed] [Google Scholar]
- 5.Guyton A C. Hypertension. 1992;19, Suppl. I:I-2–I-8. doi: 10.1161/01.hyp.19.1_suppl.i2. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Mircheff A K, Hensley C B, Magyar C E, Warnock D G, Chambrey R, Yip K-P, Marsh D G, Holstein-Rathlou N-H, McDonough A A. Am J Physiol. 1996;270:F1004–F1014. doi: 10.1152/ajprenal.1996.270.6.F1004. [DOI] [PubMed] [Google Scholar]
- 7.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe H A, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder J I. J Clin Invest. 1998;102:1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertorello A M, Ridge K, Chibalin A V, Katz A I, Sznajder J I. Am J Physiol. 1999;276:L20–L27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 9.Saldias F, Lecuona E, Friedman E, Barnard M L, Ridge K M, Sznajder J I. Am J Physiol. 1998;274:L694–L701. doi: 10.1152/ajplung.1998.274.5.L694. [DOI] [PubMed] [Google Scholar]
- 10.Bertorello A M, Katz A I. Am J Physiol. 1993;265:F743–F755. doi: 10.1152/ajprenal.1993.265.6.F743. [DOI] [PubMed] [Google Scholar]
- 11.Chibalin A V, Katz A I, Berggren P-O, Bertorello A M. Am J Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- 12.Chibalin A V, Pedemonte C H, Katz A I, Féraille E, Berggren P-O, Bertorello A M. J Biol Chem. 1998;273:8814–8819. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- 13.Chibalin A V, Ogimoto O, Pedemonte C H, Pressley T A, Katz A I, Féraille E, Berggren P-O, Bertorello A M. J Biol Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- 14.Pedemonte C H, Pressley T A, Cinelli A R, Lokhandwala M F. Mol Pharmacol. 1997;52:88–97. doi: 10.1124/mol.52.1.88. [DOI] [PubMed] [Google Scholar]
- 15.Carranza M L, Rousselot M, Chibalin A V, Bertorello A M, Favre H, Féraille E. J Physiol (London) 1998;511:235–243. doi: 10.1111/j.1469-7793.1998.235bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M M. Anal Biochem. 1974;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Hammond T G, Verroust P G. Am J Physiol. 1994;266:F554–F562. doi: 10.1152/ajprenal.1994.266.4.F554. [DOI] [PubMed] [Google Scholar]
- 19.Gorvel J-P, Chavrier P, Zerial M, Gruenberg J. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 20.Carranza M L, Féraille E, Favre H. Am J Physiol. 1996;271:C136–C143. doi: 10.1152/ajpcell.1996.271.1.C136. [DOI] [PubMed] [Google Scholar]
- 21.van Steensel B, van Binnendijk E P, Hornsby C D, van der Voort H T M, Krozowski Z S, de Kloet E R, van Driel R. J Cell Sci. 1996;109:787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- 22.Chibalin A V, Zierath J R, Katz A I, Berggren P-O, Bertorello A M. Mol Biol Cell. 1998;9:1209–1220. doi: 10.1091/mbc.9.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson O, Barker C J, Sjöholm Å, Carlqvist H, Mitchel R H, Bertorello A M, Nilsson T, Honkanen R, Mayr G W, Zwiller J, Berggren P-O. Science. 1997;278:471–474. doi: 10.1126/science.278.5337.471. [DOI] [PubMed] [Google Scholar]
- 24.Aperia A, Ibarra F, Svensson L-B, Klee C, Greengard P. Proc Natl Acad Sci USA. 1992;89:7394–7397. doi: 10.1073/pnas.89.16.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhausen T, Bonifacino J S, Riezman H. Curr Opin Cell Biol. 1997;9:488–494. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 26.Ohno H, Stewart J, Fournier M-C, Bosshart H, Thee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley L C, Shoelson S, Kirchhausen T. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boll W, Ohno H, Songyang Z, Rapoport I, Cantley L C, Bonifacino J S, Kirchhausen T. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- 29.Erusalimsky J D, Friedberg I, Rozengurt E. J Biol Chem. 1988;263:19188–19194. [PubMed] [Google Scholar]
- 30.Minneman K P. Pharmacol Rev. 1988;40:87–119. [PubMed] [Google Scholar]
- 31.Murphy T G, Bylund D B. Mol Pharmacol. 1988;34:1–7. [PubMed] [Google Scholar]
- 32.Blaxall H S, Cerutis D R, Hass N A, Iversen L J, Bylund D B. Mol Pharmacol. 1994;45:176–181. [PubMed] [Google Scholar]
- 33.Pearse B M F, Robinson M S. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 34.Schmid S L. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 35.Bates M D, Caron M G, Raymond J R. Am J Physiol. 1991;260:F937–F945. doi: 10.1152/ajprenal.1991.260.6.F937. [DOI] [PubMed] [Google Scholar]
- 36.Nash S R, Godinot N, Caron M G. Mol Pharmacol. 1993;44:918–925. [PubMed] [Google Scholar]
- 37.Bates M D, Olsen C L, Becker B N, Alberts F J, Middleton J P, Mulheron J G, Jin S L, Conti M, Raymond J R. J Biol Chem. 1993;268:14757–14763. [PubMed] [Google Scholar]
- 38.Perrichot R, Garcia-Ocaña A, Covette S, Comoy E, Amiel C, Friedlander G. Biochem J. 1995;312:433–437. doi: 10.1042/bj3120433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lederer E D, Sohi S S, McLeish K R. J Am Soc Nephrol. 1998;9:975–985. doi: 10.1681/ASN.V96975. [DOI] [PubMed] [Google Scholar]
- 40.Wursmer A E, Emr S D. EMBO J. 1998;17:4930–4942. doi: 10.1093/emboj/17.17.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonsen A, Lippé R, Christoforidis S, Gaullier J-M, Brech A, Callaghan J, Toh B-H, Murphy C, Zerial M, Stenmark H. Nature (London) 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 43.Gaidarov I, Chen Q, Falck J R, Reddy K K, Keen J H. J Biol Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- 44.Chang M P, Mallet W G, Mostov K E, Brodsky F M. EMBO J. 1993;12:2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye W, Ali N, Bembenek M E, Shears S B, Lafer E M. J Biol Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]