Abstract
Mutations of NPHS1 or NPHS2, the genes encoding nephrin and podocin, as well as the targeted disruption of CD2-associated protein (CD2AP), lead to heavy proteinuria, suggesting that all three proteins are essential for the integrity of glomerular podocytes, the visceral glomerular epithelial cells of the kidney. It has been speculated that these proteins participate in common signaling pathways; however, it has remained unclear which signaling proteins are actually recruited by the slit diaphragm protein complex in vivo. We demonstrate that both nephrin and CD2AP interact with the p85 regulatory subunit of phosphoinositide 3-OH kinase (PI3K) in vivo, recruit PI3K to the plasma membrane, and, together with podocin, stimulate PI3K-dependent AKT signaling in podocytes. Using two-dimensional gel analysis in combination with a phosphoserine-specific antiserum, we demonstrate that the nephrin-induced AKT mediates phosphorylation of several target proteins in podocytes. One such target is Bad; its phosphorylation and inactivation by 14-3-3 protects podocytes against detachment-induced cell death, suggesting that the nephrin-CD2AP-mediated AKT activity can regulate complex biological programs. Our findings reveal a novel role for the slit diaphragm proteins nephrin, CD2AP, and podocin and demonstrate that these three proteins, in addition to their structural functions, initiate PI3K/AKT-dependent signal transduction in glomerular podocytes.
The renal glomerulus is the site of a number of disorders that lead to proteinuria and chronic renal failure (19, 25). Recent studies of the hereditary nephrotic syndrome have emphasized the role of the glomerular podocytes in generating a size-selective filtration barrier and have provided a new understanding of the mechanisms leading to proteinuria, in both inherited and acquired diseases. The hereditary nephrotic syndrome is a heterogeneous disease characterized by heavy proteinuria and renal failure. Mutations of NPHS1 (20) or NPHS2 (4, 13), the genes encoding the glomerular podocyte proteins nephrin and podocin, as well as the targeted disruption of the CD2-associated protein (CD2AP) in mice (43), lead to a severe nephrotic syndrome, suggesting that these proteins are indispensable for an intact glomerular filter. All three proteins localize at opposing sites of the secondary foot processes formed by podocytes, specialized epithelial cells that ensure size- and charge-selective ultrafiltration (14, 15, 39, 41, 42). This specialized cell-cell contact between opposing secondary foot processes is called the slit diaphragm (38). Nephrin is a member of the immunoglobulin superfamily and appears to form a zipper-like filter structure within the ∼40-nm-wide slit between two foot processes (reviewed in reference 48). It was recently shown that nephrin is a signaling protein that interacts with podocin, a podocyte protein of the stomatin family, facilitating nephrin signaling (18) and regulating its recruitment to lipid raft microdomains (41, 45). CD2AP is an adaptor molecule that was first identified as an SH3-containing protein that binds to the cytoplasmic domain of CD2 (8). Mice lacking CD2AP exhibit a nephrotic syndrome that resembles that caused by NPHS1 mutations (43), suggesting that nephrin and CD2AP participate in a common signaling pathway.
Phosphorylated lipids, such as PI(4,5)P2 and PI(3,4,5)P3, are key mediators in diverse intracellular signaling pathways controlling cell growth, cell migration, endocytosis, and cell survival (reviewed in reference 5). The conversion of PI(4,5)P2 to PI(3,4,5)P3 at the inner leaflet of the plasma membrane is catalyzed by phosphoinositide 3-OH kinases (PI3Ks). Class Ia PI3Ks are heterodimers of regulatory and catalytic subunits. The regulatory subunit p85 mediates the activation of the p110 catalytic counterpart by direct interaction with phosphotyrosine residues of activated transmembrane receptors or adaptor proteins. Signaling proteins with pleckstrin homology domains then accumulate at sites of PI3K activation by directly binding to newly generated PI(3,4,5)P3. A major downstream mediator of PI3K activity is the serine-threonine kinase AKT. Other PH domain-containing proteins that are activated by PI(3,4,5)P3 include GDP-GTP exchange factors for Rac and ARF6 and protein tyrosine kinases of the Bruton's tyrosine kinase (Btk) and Tec family. These proteins play a major role in remodeling the actin cytoskeleton, the control of endocytosis and protein trafficking, and cell survival (5). The binding of PI3K-generated phospholipids to the PH domain of AKT leads to the translocation of AKT to the inner surface of the plasma membrane and induces conformational changes that are required for the proper phosphorylation and activation of AKT. Relocalization of AKT to the plasma membrane allows the close proximity of AKT to regulatory kinases that phosphorylate AKT at two regulatory sites, threonine-308 and serine-473, resulting in AKT activation (7). Among a wealth of effects, AKT activity was found to be required for the growth factor-dependent survival of a wide variety of cell types, ranging from fibroblasts to neurons, and it blocks apoptosis induced by toxic stimuli (reviewed in reference 6).
It has been speculated that nephrin, podocin, and CD2AP participate in common signaling pathways; however, which signaling proteins may interact at the cytosolic surface of the slit diaphragm protein complex is still not clear. Here, we identify p85, the regulatory subunit of PI3K, as the first signaling intermediate recruited by the slit diaphragm protein complex. Using human embryonic kidney (HEK) 293T cells, a cell line that lacks nephrin, podocin, and CD2AP, and cultured differentiated mouse podocytes, we demonstrate that nephrin, podocin, and CD2AP stimulate PI3K-dependent signaling pathways. Our findings suggest that the activation of canonical kinase cascades, such as PI3K/AKT signaling, represents an essential component to maintain the functional integrity of podocytes in vivo.
MATERIALS AND METHODS
Plasmids and antibodies.
Nephrin, CD2AP, and podocin plasmids have been described previously (18). Richard Mulligan (Harvard Medical School, Boston, Mass.) and Dusty Miller (Fred Hutchinson Cancer Institute, Seattle, Wash.) kindly provided the retroviral constructs pMD-G, pMD-gp, and pLXSN. To generate retroviral gene transfer vectors, human nephrin and mouse CD2AP were subcloned into pLXSN. The control vector was identical but did not contain nephrin or CD2AP. All cloning steps were verified by automated sequencing. FLAG-tagged regulator of G protein signaling 3 (RGS3) has been described (3). HA.AKT wild type (pCMV-HA-AKT) and a dominant-negative mutant (pCMV-HA-AKT K179 M) were kindly provided by Alex Toker (Boston, Mass.) and Philip Tsichlis (Philadelphia, Pa.), pcDNA3-HA-AKT was a gift from Silvio Gutkind (Bethesda, Md.), PI3K cDNA (pCMV6-p85α-mycC) was kindly provided by Lewis Cantley and David Fruman (Boston, Mass.), pcDNA3-HA-GSK-3β was provided by Jim Woodgett (Toronto, Canada), v-ras was provided by Vikas Sukhatme (Boston, Mass.), and constitutively active AKT in pMX was provided by Norihisa Masuyama (Tokyo, Japan). ntibodies were obtained from Cell Signaling Technologies (anti-AKT, pSer473-AKT, GSK-3β, pSer9-GSK-3β, 14-3-3 phospho-binding motif, pSer112-Bad, and pSer136-Bad), Sigma (antiactin and M2 anti-FLAG), Santa Cruz (anti-myc and antihemagglutinin [anti-HA]), and Upstate Biotechnologies (anti-p85, anti-p110, and anti-PY 4G10). Generation of an anti-CD2AP antiserum has been described (8). Immunoprecipitating nephrin antiserum was kindly provided by Lawrence B. Holzman (Ann Arbor, Mich.).
Cell culture and retrovirus production.
MDCK cells were grown in Dulbecco's modified Eagle medium-F-12 supplemented with 10% fetal calf serum. Conditionally immortalized mouse podocytes were generated as described previously (30, 36) and grown at permissive temperature in the presence of 10 U of gamma interferon/ml. To induce differentiation, the cells were maintained on type I collagen at 37°C without gamma interferon for at least 14 days. cd2ap−/− podocytes have been described previously (42). Conditionally immortalized cd2ap−/−podocytes were derived from cd2ap−/+ mice crossed with a transgenic mouse that expresses a temperature-sensitive form of simian virus 40 large T antigen under the control of an interferon-inducible promoter. At the permissive temperature, these cells grow continuously in culture. At the nonpermissive temperature and after interferon removal, the cells stop growing, differentiate, and acquire a podocyte-like morphology. Retrovirus for gene transfer was produced by cotransfection of HEK 293T cells with 2.5 μg of pMD-G, 7.5 μg of pMD-gp, and 10 μg of the retroviral transfer vector. The supernatant was harvested, centrifuged to remove cellular debris, and filtered. MDCK cells and mouse podocytes were transduced three times and selected in G418 (200 μg/ml) as described previously (2).
Generation and purification of a nephrin-specific antiserum.
A recombinant, gel-purified nephrin fragment (amino acids 108to 1241)fused to the maltose-binding protein (MBP) was used to immunize rabbits (Cocalico Biologicals, Reamstown, Pa.), following a standard immunization protocol (2). Protein A columns were used to affinity purify a nephrin-specific antiserum. Specificity was verified by using bacterially expressed recombinant proteins, cell lysates from transfected cells,and homogenized renal tissue.
Subcellular fractionation.
HEK 293T cells were transiently transfected with the appropriate constructs, serum starved overnight, and harvested and lysed at 4°C in a glass-glass homogenizer in 1 ml of homogenization buffer (250 mM sucrose- 1 mM EDTA-20 mM Tris [pH 7.5]-44 μg of phenylmethylsulfonyl fluoride/ml-protease inhibitors). Nuclei were removed by centrifugation at 1,000 × g for 10 min at 4°C. The postnuclear supernatant was centrifuged at 100,000 × g for 30 min at 4°C. The supernatant (S100; soluble fraction) was collected, and the pellet (P100; membrane fraction) was washed once in homogenization buffer and then resuspended in lysis buffer containing 1% Triton X-100 and 0.2% sodium dodecyl sulfate (SDS). The protein concentration was determined, and equal amounts of protein were separated by SDS-10% polyacrylamide gel electrophoresis.
Coimmunoprecipitation.
Coimmunoprecipitations were performed as described previously (3). Briefly, HEK 293T cells were transiently transfected using the calcium phosphate method. After incubation for 24 h, the cells were washed twice and lysed in 1% Triton X-100 lysis buffer. After centrifugation at 15,000 × g (15 min; 4°C) and ultracentrifugation at 100,000 × g (30 min; 4°C), cell lysates containing equal amounts of total protein were precleared with protein G-Sepharose and then incubated for 1 h at 4°C with the appropriate antibody, followed by incubation with 40 μl of protein G-Sepharose beads for ∼3 h. The beads were washed extensively with lysis buffer, and bound proteins were resolved by SDS-10% polyacrylamide gel electrophoresis. In kidney cortex and cultured podocytes, nephrin, podocin, and CD2AP are contained within lipid raft microdomains and are therefore insoluble in nonionic detergents, such as Triton X-100. Therefore, the lysis buffer was supplemented with 20 mM CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}. Sufficient solubilization was monitored by Western blotting of different fractions during the preparation. Before immunoprecipitation, cellular lysates were extensively precleared by ultracentrifugation and adsorption to protein G beads. All kidneys were freshly prepared and perfused in situ with ice-cold phosphate-buffered saline before lysis.
2-D gel analysis.
Equal amounts of protein from cells transfected with nephrin, CD2AP, podocin, or control plasmid in combination with wild-type AKT or a dominant-negative mutant were separated on a two-dimensional (2-D) gel. In the first dimension, proteins were separated by isoelectric focusing (Bio-Rad, Munich, Germany). The second dimension separated the proteins according to their molecular weights. After 2-D electrophoresis, proteins were blotted onto polyvinylidene difluoride (PVDF) membranes, blocked with 5% bovine serum albumin, and stained with antibodies as appropriate.
Protein phosphorylation.
To determine the phosphorylation status of AKT, GSK-3β, and Bad, specifically phosphorylated residues were visualized by Western blot analysis, using phosphospecific antisera (Cell Signaling Technologies). Equal loading was confirmed by reprobing the membrane with the non-phospho-specific antibodies and by amido black staining. The degrees of phosphorylation of AKT, GSK-3β, and Bad were quantified by densitometry of nonsaturated radiographs with NIH Image software.
Anoikis assays, DNA laddering, caspase 3 activity assays, and annexin V binding.
Anoikis assays, as well as DNA laddering, were performed essentially as described previously (21). Caspase 3 activity was monitored in a quantitative fluorescence assay using a commercially available caspase 3 substrate. Briefly, ∼3 × 106 cells, retrovirally transduced to stably express the appropriate transgenes, were detached from cell culture dishes, and equal numbers of cells were maintained in suspension for 2 to 12 h. Thereafter, cells were harvested and lysed in a glass-glass homogenizer at 4°C in 200 μl of 30 mM NaCl-50 mM NaF-25 mM Na4P2O7-0.1 mM EDTA-20 mM Tris [pH 7.5]-44 μg of phenylmethylsulfonyl fluoride/ml-protease inhibitors. The lysate was centrifuged at 100,000 × g for 30 min at 4°C, and the supernatant (cytosolic fraction) was collected. Caspase 3 activity was determined as described previously (32). Briefly, 6 ng of fluorogenic caspase 3 substrate, Ac-DEVD-AMC (Alexis Biochemicals, San Diego, Calif.), was added to 100 μl of the cytosolic extract in a 96-well plate. Fluorescence was determined (excitation at 380 nm and emission at 420 to 460 nm) at 30°C for 30 min. The slope of the linear curve obtained over 30 min of registration was used as a measure for enzyme activity. Quantitation of apoptosis by annexin V binding was performed as described elsewhere (10a). Briefly, differentiated podocytes were detached and kept in suspension culture for various times, washed once in phosphate-buffered saline, and resuspended in annexin V binding buffer (R&D Systems). Fluorescein isothiocyanate-conjugated annexin V was added, and the samples were analyzed by flow cytometry with a FACScan (Becton Dickinson). Cells were plated at identical initial densities, and quantitation was done with Cell Quest software. All experiments were done in triplicate. Statistical analysis was performed using the statistical software Instat2 (GraphPad) and SigmaPlot (Jandel Scientific).
Statistical analysis.
Data are expressed as means ± standard errors of the mean (SEM) of n experiments. Statistical evaluation was performed using Student's t test or analysis of variance for repeated measures, followed by a Bonferroni test (SigmaPlot and Instat2). P values of <0.05 were considered to be statistically significant.
RESULTS
Nephrin and CD2AP associate with the p85 regulatory subunit of PI3K.
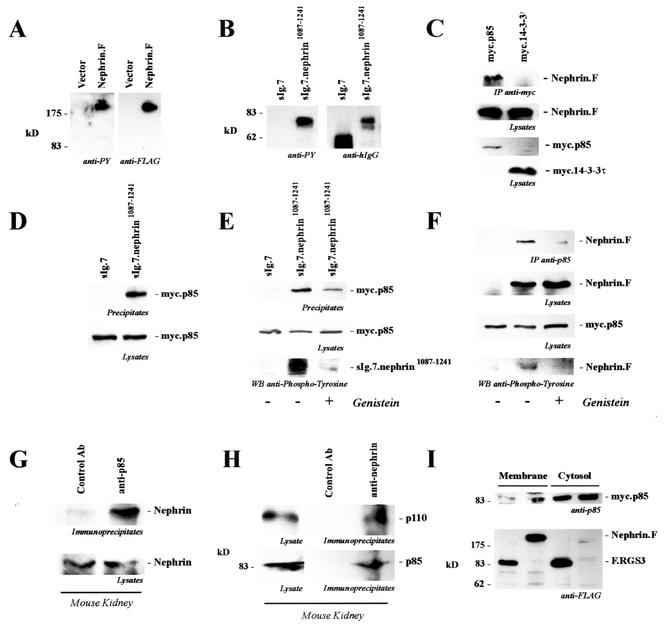
The immunoglobulin family member nephrin is a critical structural component of the podocyte slit diaphragm. Based on the structure of the glomerular slit diaphragm (38) and the electron microscopic localization of nephrin (39), it has been suggested that the N-terminal six immunoglobulin repeats of nephrin form interdigitating zipper-like homo- and heterophilic interactions (39, 47). Using HEK 293T cells as a model system, it was recently demonstrated that nephrin stimulates cellular signaling that is augmented by podocin (18). It has been speculated that nephrin, podocin, and CD2AP participate in common signaling pathways; however, it is still not clear which signaling proteins may be recruited by the slit diaphragm protein complex. We therefore started to search for nephrin-interacting cytosolic proteins. The carboxy-terminal domain of nephrin is relatively short but contains several potential tyrosine phosphorylation sites. In particular, Y1176, Y1193, and Y1210 are predicted to serve as potential docking sites for SH2 domain-containing adaptor and signaling molecules (53; http://scansite.mit.edu/). As demonstrated in Fig. 1A, nephrin was heavily tyrosine phosphorylated in transiently transfected HEK 293T cells. Identical results were obtained when the cytoplasmic tail of nephrin was fused to the CH2 and CH3 domain of human immunoglobulin G1 (IgG1), followed by the transmembrane region of CD7 (Fig. 1B). Interestingly, the tyrosine-phosphorylated carboxy-terminal domain of nephrin associated with p85 (Fig. 1C and D), the regulatory subunit of PI3K, whereas no binding of other SH2 domain-containing proteins, including Grb2, Shc, and CrkII, was observed (data not shown). This interaction could also be demonstrated for endogenous proteins precipitated from mouse kidney lysates (Fig. 1G and H), confirming that the interaction occurs in vivo. Pretreatment of HEK 293T cells with genistein, a tyrosine kinase inhibitor with some preference for the src family of nonreceptor protein tyrosine kinases, inhibited tyrosine phosphorylation of the cytoplasmic tail of nephrin and the interaction with p85 (Fig. 1E and F), suggesting that the binding of p85 requires nephrin tyrosine phosphorylation. Finally, expression of nephrin in serum-starved HEK 293T cells resulted in the association of p85 with the plasma membrane, demonstrating that nephrin recruits PI3K to the plasma membrane (Fig. 1I).
FIG. 1.
The carboxy-terminal cytoplasmic tail of nephrin interacts with p85. (A) C-terminally FLAG-tagged full-length nephrin (Nephrin.F) was expressed in HEK 293T cells and immunoprecipitated with anti-FLAG antibody. Tyrosine phosphorylation was detected with the specific antiphosphotyrosine antibody 4G10 (anti-PY; left). On the right is shown a reprobe with anti-FLAG antibody. (B) The cytoplasmic tail of nephrin was fused to the CH2 and CH3 domains of human IgG1, followed by the transmembrane region of CD7 (sIg.7.nephrin1087-1241), and precipitated with protein G-Sepharose beads. The CH2 and CH3 domains of human IgG1, followed by the transmembrane region of CD7 without a cytoplasmic tail, served as the control (sIg.7). Tyrosine phosphorylation was detected with the antiphosphotyrosine-specific antibody 4G10 (anti-PY; left). On the right are shown expression levels of the constructs (reprobe with anti-human IgG antibody). (C) C-terminally FLAG-tagged full-length nephrin (Nephrin.F) was coexpressed with myc-tagged p85 or 14-3-3τ. After immunoprecipitation (IP) with the anti-myc antibody, the immobilized nephrin was detected with the anti-FLAG antibody (top). The middle and lower blots show expression of the proteins in cell lysates. (D and E) myc.p85 was coexpressed with the constructs described for panel B. After precipitation with protein G, immobilized p85 was detected with a specific anti-p85 antibody. Prior to lysis and immunoprecipitation, cells were treated with solvent (−) or genistein (50 μM; 30 min) (+) as indicated. (D) Top, precipitates; bottom, lysates stained for p85. (E) Top, precipitates stained for p85; middle, lysates stained for p85; bottom, nephrin in precipitates stained for phosphotyrosine. (F) myc.p85 was coexpressed with C-terminally FLAG-tagged full-length nephrin (Nephrin.F). After precipitation of p85, nephrin was detected with anti-FLAG antibody and tyrosine phosphorylation of nephrin was detected with antiphosphotyrosine antibody. Cells were treated with solvent or genistein (50 μM, 30 min) prior to lysis and immunoprecipitation. Top, precipitates stained for nephrin; upper middle, lysates stained for nephrin; lower middle, lysates stained for p85; bottom, nephrin in precipitates stained for phosphotyrosine. (G) To show endogenous interaction, mouse kidneys were freshly isolated and perfused in situ with ice-cold phosphate-buffered saline and subjected to glass-glass homogenization in a CHAPS lysis buffer. The precleared lysates were prepared by extensive centrifugation and preabsorption and subsequently divided into two portions. One half was immunoprecipitated with a control antibody (Ab) (anti-HA antibody), and the other half was precipitated with an anti-p85 antibody. Immobilized nephrin was detected with a nephrin-specific antiserum. (H) To confirm endogenous interaction, precleared lysates of mouse kidney were divided into two portions. One half was immunoprecipitated with a control antibody (anti-HA antibody), and the other half was precipitated with an antinephrin antibody. Immobilized p85 was detected with a p85-specific antiserum (bottom). On top is shown the coprecipitating enzymatically active p110 subunit of PI3 kinase, indicating that nephrin interacts with the active heterodimer of PI3K. (I) myc.p85-expressing HEK 293T cells were transfected with FLAG-tagged nephrin or with FLAG-tagged RGS3 (F.RGS3) as a control protein. Recruitment of myc.p85 to the membrane compartment was monitored by preparing a 100,000 × g pellet (P100 [Membrane]) and a 100,000 × g supernatant fraction (S100 [Cytosol]).
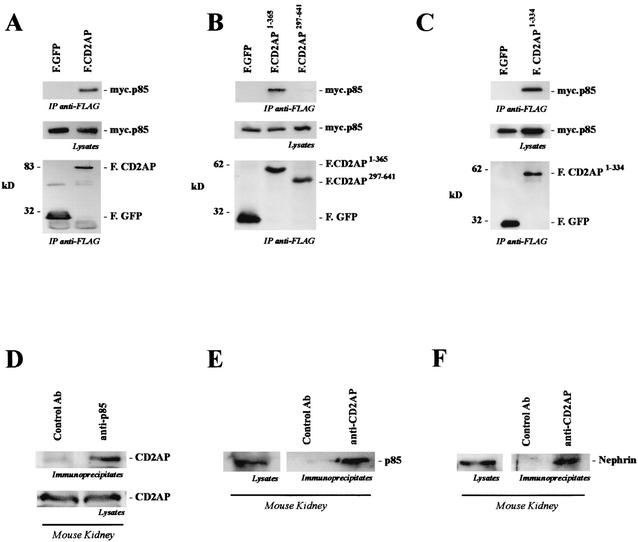
CD2AP was originally cloned as a CD2-interacting protein (8). Targeted disruption of the cd2ap gene in mice causes a severe nephrotic syndrome 3 to 4 weeks after birth (43). CD2AP contains three consecutive SH3 domains at the amino terminus, followed by a proline-rich domain, a coiled-coiled domain, and a potential monomeric actin binding domain. CD2AP has been shown to bind to the cytoplasmic tail of nephrin via a novel carboxy-terminal domain (27, 42). Interestingly, CD2AP also interacted with p85 (Fig. 2A). This interaction was mediated by the N-terminal portion (amino acids 1 to 334) of CD2AP, containing the three SH3 domains (Fig. 2B and C), and was detectable in vivo between endogenous proteins (Fig. 2D and E). As described previously, CD2AP not only interacted with p85 but also coprecipitated nephrin in vivo (Fig. 2F). These data suggested that nephrin and CD2AP may indeed participate in a common signaling pathway.
FIG. 2.
CD2AP interacts with p85. (A) myc.p85 was coexpressed with FLAG-tagged CD2AP (F.CD2AP) or the control protein GFP. After immunoprecipitation (IP) with anti-FLAG antibody, coprecipitating myc.p85 was detected with a p85-specific antibody (top). The middle and lower gels show protein expression in the lysates. (B and C) The interaction of p85 occurs with the amino-terminal portion of CD2AP, a region containing all three SH3 domains and a highly tyrosine-phosphorylated segment (top). The middle and lower gels show protein expression in the lysates. (D and E) To demonstrate endogenous interaction, freshly isolated kidneys were lysed in CHAPS lysis buffer and subjected to immunoprecipitation with control antibody (Ab) (anti-HA antibody) and anti-85 polyclonal antibody or anti-CD2AP polyclonal antibody. Prior to immunoprecipitation, the lysates were precleared extensively to ensure specific coprecipitation. Coprecipitating CD2AP (D) or p85 (E) protein was detected with a specific antiserum. (F) As previously shown, endogenous nephrin coprecipitated with CD2AP.
Nephrin and CD2AP induce phosphorylation and activation of the PI3K target protein kinase, AKT.
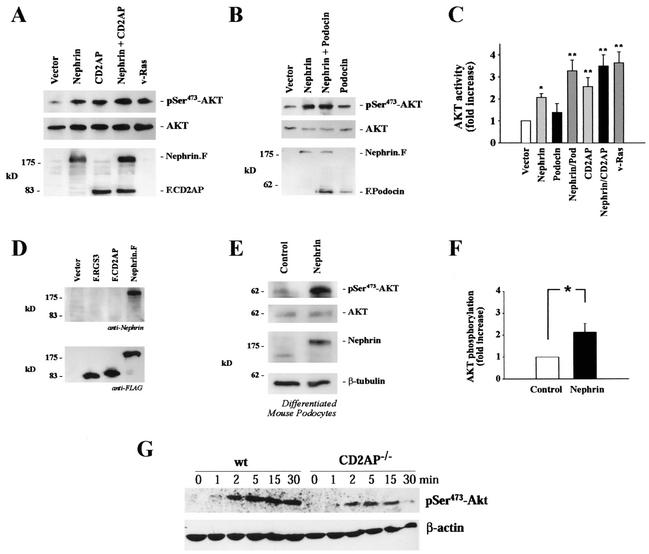
PI3K can initiate cellular signaling through activation of a number of cellular intermediates, including tyrosine kinases, GTPase activating proteins for small G proteins, and a variety of serine-threonine kinases, such as the atypical PKC isoforms, pp70 S6 kinase, and c-AKT. Recent findings have revealed a critical role for AKT as a mediator of PI3K-dependent cellular events (6, 7). Since overexpression of cell surface receptors (51, 58) and adaptor proteins (16, 17, 33) can initiate signaling in a ligand-independent fashion, we determined whether expression of nephrin, CD2AP, and/or podocin triggers PI3K-dependent signaling in HEK 293T cells. Figure 3A demonstrates that nephrin triggers phosphorylation of AKT on serine 473. CD2AP stimulated AKT with similar potency (Fig. 3A). In contrast, little if any activation was detectable in HEK 293T cells expressing podocin (Fig. 3B and C). However, both podocin and CD2AP strongly augmented the nephrin-induced AKT activation (Fig. 3C) without affecting the expression levels of nephrin (data not shown). The nephrin- and CD2AP-induced AKT phosphorylation was completely inhibited by the PI3K inhibitor wortmannin, demonstrating that the phosphorylation and activation of AKT is PI3K dependent (data not shown).
FIG. 3.
Nephrin and CD2AP stimulate phosphorylation of AKT at serine-473. (A and B) HEK 293T cells were transfected with HA.AKT (5 μg) together with vector (10 μg; left lane), nephrin (5 μg plus 5 μg of vector; second lane from left), CD2AP (5 μg plus 5 μg of vector; third lane from left), nephrin and CD2AP (5 plus 5 μg; fourth lane from left), or v-ras (5 μg plus 5 μg of vector; fifth lane from left) as indicated (A) or together with vector (10 μg; left lane), nephrin (5 μg plus 5 μg of vector, second lane from left), nephrin and podocin (5 plus 5 μg; third lane from left), or podocin (5 μg plus 5 μg of vector; fourth lane from left) as indicated (B). Phosphorylation of AKT was monitored with a specific anti-phospho-serine-473 antibody (top and middle). Equal loading and comparable expression of AKT was confirmed by reprobing the blots with a non- phosphospecific anti-AKT antiserum (middle). Equal expression levels of nephrin, CD2AP, and podocin were demonstrated by using the respective antibodies (bottom). F, FLAG. (C) The degree of phosphorylation of AKT was quantified by densitometry of nonsaturated radiographs with NIH Image software. Depicted is the statistical analysis of 10 independent experiments (*, P < 0.05; **, P < 0.01; n = 10). The error bars indicate SEM. (D) To generate a nephrin-specific antiserum, recombinant MBP-tagged nephrin (amino acids 1087 to 1241) was bacterially expressed, affinity purified by fast protein liquid chromatography, and used for the immunization of rabbits. The purified antiserum specifically recognizes nephrin but does not cross-react with other proteins. (E) To test whether nephrin induces AKT activation in podocytes, conditionally immortalized mouse podocytes were retrovirally transduced with nonexpressing retrovirus (Control) or to stably express nephrin (Nephrin), differentiated at 37°C, and assayed for AKT phosphorylation (phospho-serine-473 [pSer473] antibody; top), expression of endogenous AKT (upper middle), and nephrin expression (lower middle). Equal loading was confirmed by reprobing the membrane with anti-β-tubulin antibody (bottom). (F) Densitometric analysis of five independent experiments in podocytes. The degree of phosphorylation of AKT was quantified by densitometry of nonsaturated radiographs with NIH Image software (*, P < 0.05; n = 5). (G) To further substantiate the role of CD2AP in AKT activation in podocytes, we examined AKT phosphorylation in podocytes derived from cd2ap knockout mice. The loss of CD2AP strongly inhibited AKT activation induced by EGF, whereas EGF-induced ERK1/2 activation was not reduced (not shown). These findings further substantiate the role of CD2AP in PI3K/AKT activation. Top, AKT phosphorylation of wild type (wt) and CD2AP knockout (CD2AP−/−) podocytes. Bottom, actin level as a loading control.
To demonstrate that nephrin triggers AKT activation in podocytes, we generated pools of conditionally immortalized mouse podocytes stably expressing nephrin. These podocytes were generated as previously described (30, 36) and have been extensively characterized (H. Pavenstädt, unpublished data). Mouse podocytes were transduced repeatedly at the permissive temperature of 33°C with an ecotropic retrovirus containing the nephrin cDNA (nephrin) or the control vector (2). After selection in neomycin, the podocytes were incubated at 37°C in the absence of gamma interferon to induce differentiation. While conditionally immortalized differentiated podocytes contain only low levels of nephrin protein (42, 55), the retrovirus-mediated nephrin expression resulted in activation of AKT, monitored with the phosphoserine-473-specific AKT antiserum (Fig. 3E). Equal loading was confirmed by reprobing the membrane with anti-β-tubulin and with anti-AKT antibodies. Nephrin expression was monitored with an affinity-purified antiserum that specifically recognized nephrin, as shown in Fig. 3D. The nephrin-induced AKT activation was confirmed in five independent experiments (Fig. 3F), confirming the results obtained in transiently transfected HEK 293T cells. To further substantiate the role of CD2AP in AKT activation in podocytes, we examined AKT phosphorylation in podocytes derived from cd2ap knockout mice. As demonstrated in Fig. 3G, the loss of CD2AP strongly inhibited AKT activation induced by epidermal growth factor (EGF), while EGF-induced ERK1/2 activation was not reduced (data not shown). These findings suggest that both nephrin and CD2AP are involved in PI3K and AKT activation.
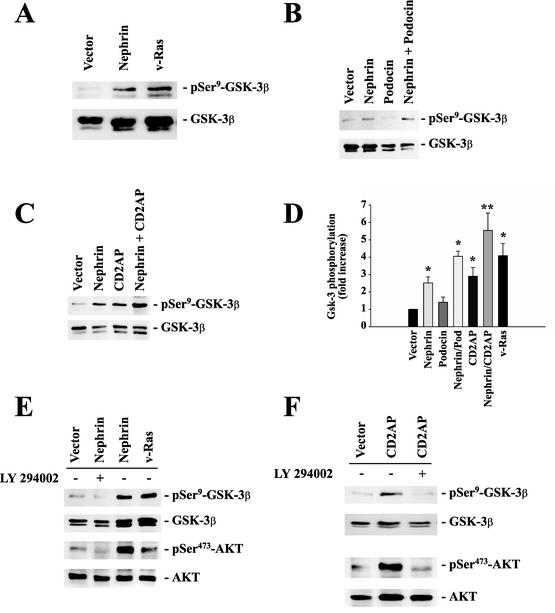
To confirm the nephrin-mediated AKT activation, we monitored the phosphorylation of glycogen synthase kinase 3β (GSK-3β) in HEK 293T cells expressing nephrin. GSK-3β is a ubiquitously expressed serine-threonine protein kinase that phosphorylates and inactivates glycogen synthase, a critical downstream element of the PI3K/AKT pathway. Its activity can be inhibited by AKT-mediated phosphorylation at serine-9. Both nephrin and CD2AP strongly increased phosphorylation of GSK-3β (Fig. 4A to C). Statistical analysis revealed that the nephrin-mediated effect was strongly augmented by podocin and by CD2AP (Fig. 4D). Both AKT phosphorylation and activation could be completely blocked by the highly selective PI3K inhibitor LY 294002 (Fig. 4E and F), confirming that the nephrin- and CD2AP-mediated stimulation of AKT requires activation of PI3K. In summary, these data demonstrate that both nephrin and CD2AP stimulate PI3K-dependent signaling in HEK 293T cells, as well as in podocytes.
FIG. 4.
Nephrin, CD2AP, and podocin cooperatively activate the PI3K/AKT pathway. To monitor AKT activity, phosphorylation of GSK-3β at serine-9 was measured in HEK 293T cells transfected with identical amounts of plasmid DNA, as indicated (A, B, C, E, and, F, top). The cells were cotransfected with 4 μg of myc.AKT and 4 μg of GSK-3β; with 8 μg of vector for vector alone and 4 μg of expression plasmid plus 4 μg of vector for nephrin, podocin, CD2AP, or v-Ras alone; and with 4 plus 4 μg of plasmid DNA for nephrin plus CD2AP or nephrin plus podocin. This approach ensured equal expression levels of the relevant cDNAs and allowed statistical analysis of several independent experiments. Equal loading and comparable expression of AKT and GSK-3β were confirmed by reprobing the blots with nonphosphospecific anti-AKT and anti-GSK-3β antisera (middle and bottom) and with the relevant antisera against podocin, nephrin, and CD2AP (not shown). (D) The degree of phosphorylation was quantified by densitometry of nonsaturated radiographs with NIH Image software. Depicted is the statistical analysis of 10 independent experiments (*, P < 0.05; **, P < 0.01; n = 10). The error bars indicate SEM. (E and F) Incubation of cells with (+) the specific PI3K inhibitor LY 294002 (25 μM; 30 min) abrogated nephrin-CD2AP-stimulated AKT activation and AKT-mediated GSK-3β phosphorylation.
Identification of downstream target proteins of nephrin-induced PI3K/AKT signaling.
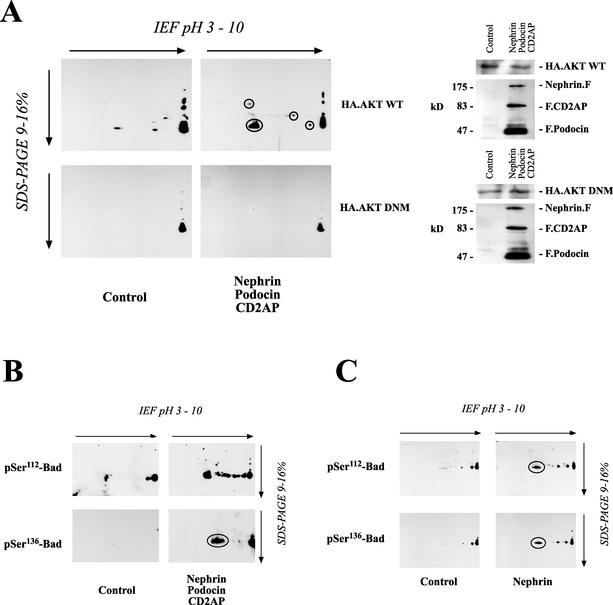
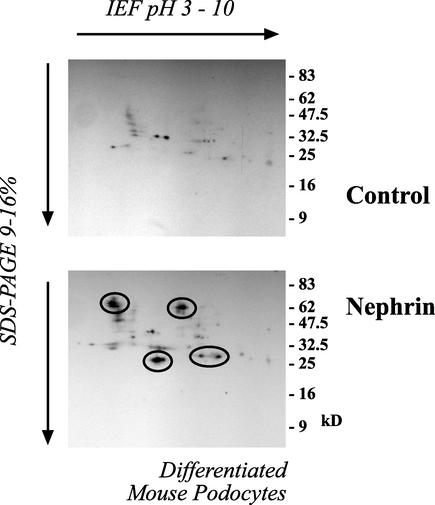
To further substantiate the relevance of our finding that nephrin and CD2AP interact with PI3K and stimulate PI3K-dependent signaling, we started to search for downstream target proteins of the nephrin-CD2AP-mediated PI3K/AKT activity. The search for substrates of AKT has been facilitated by the definition of a consensus sequence of the preferred AKT in vitro phosphorylation site, RXRXXS/T (1, 34). After phosphorylation, several AKT targets bind to the 14-3-3 family of adaptor proteins, resulting in their cellular redistribution and/or inactivation (49). The 14-3-3 proteins are a highly conserved family of proteins involved in the regulation of cell survival, apoptosis, proliferation, and cell cycle control. Regulation by 14-3-3 is mediated through phosphorylation-dependent protein-protein interactions (37, 52, 54). Thus, phosphorylation-dependent binding of 14-3-3 to target proteins is a critical event in AKT-mediated cellular programs (7). To analyze the set of target proteins phosphorylated in nephrin-induced AKT activation, we therefore used a phosphospecific antiserum raised against the 14-3-3 mode 1 binding motif (31, 54) in combination with 1- and 2-D gel analysis. Equal amounts of protein from cells expressing or lacking nephrin, CD2AP, and podocin in combination with wild-type AKT or a dominant-negative AKT mutant were separated on 1- or 2-D gels. 1-D gel electrophoresis showed a large number of proteins being phosphorylated at putative 14-3-3 binding sites (data not shown). Since the resolution was insufficient, 2-D gel electrophoresis was utilized for further analysis. As depicted in Fig. 5A, the three podocyte proteins stimulated the phosphorylation of several target proteins, thereby creating a putative 14-3-3 binding site. Depicted in Fig. 5A are the most prominent spots. This phosphorylation was strictly dependent on AKT activity and was abolished by the coexpression of the dominant-negative AKT mutant. Interestingly, a prominent protein detected by the phosphospecific antiserum had a molecular mass of ∼23 kDa and a pI of ∼6. Additional experiments identified this protein as Bad (Fig. 5B and C). Bad is a proapoptotic Bcl-2 family member that interacts via its BH3 domain with prosurvival Bcl-2 family members, such as Bcl-XL, to inhibit their prosurvival activity and promote apoptosis (56). Bad is regulated by AKT-dependent phosphorylation on serine-112 and serine-136 (6). Phosphorylation of either of these sites causes Bad to dissociate from Bcl-XL and to associate with 14-3-3, thereby promoting cell survival at a postmitochondrial level (59). Nephrin, podocin, and CD2AP induced the phosphorylation of Bad on both regulatory sites (Fig. 5B and C). These data suggested that the nephrin-induced PI3K/AKT activation may trigger antiapoptotic signaling and confirmed that Bad is a major substrate for AKT-dependent 14-3-3 binding in HEK 293T cells. Identical results were obtained in differentiated podocytes (Fig. 6). Nephrin expression was associated with the phosphorylation of several target proteins with molecular masses of ∼70, 65, 30, and 23 kDa; the last spot was confirmed as phospho-Bad (not shown).
FIG. 5.
Identification of downstream target proteins of nephrin-CD2AP-podocin-stimulated AKT activity. (A) Equal amounts of protein from HEK 293T cells transfected with either a vector control (9 μg of plasmid) or nephrin (3 μg), CD2AP (3 μg), and podocin (3 μg) in combination with either wild-type (WT) AKT or a dominant-negative mutant (DNM) were separated on a 2-D gel. To analyze the formation of active 14-3-3 binding sites associated with the nephrin-CD2AP-podocin-stimulated AKT activity, cell lysates were transferred onto PVDF membranes and immunoblotted with a phosphospecific antiserum raised against the 14-3-3 mode 1 binding motif (left). Lysates from these cells showed comparable AKT expressions (right). F, FLAG; IEF, isoelectric focusing. (B and C) The proapoptotic Bcl2 family member Bad is regulated by AKT-dependent phosphorylation on serine-112 (pSer112) and serine-136. Equal amounts of protein from HEK 293T cells expressing either a vector control (9 μg of plasmid) or nephrin (3 μg), CD2AP (3 μg), and podocin (3 μg) in combination with wild-type AKT were separated on a 2-D gel. Nephrin, podocin, and CD2AP induced the phosphorylation of endogenous Bad on both regulatory sites, serine-112 and serine-136, as demonstrated by the phosphospecific antiserum (B), whereas AKT expression was not influenced (not shown). The same result, although with a weaker effect, could be demonstrated with nephrin alone (C). Circles indicate spots identified with phosphospecific antiserum against 14-3-3 mode 1 binding motif (A) or with phosphospecific antisera against Bad (B and C).
FIG. 6.
Nephrin expression induces phosphorylation of several target proteins at putative 14-3-3 binding sites in differentiated mouse podocytes. Equal amounts of protein from differentiated mouse podocytes transduced with empty retroviral vector (Control) or to stably express nephrin (Nephrin) were separated on a 2-D gel. To analyze the phosphorylation of target proteins associated with the nephrin-stimulated AKT activation, cell lysates were transferred onto PVDF membranes and immunoblotted with a phosphospecific antiserum raised against the 14-3-3 mode 1 binding motif (circles indicate spots identified with that antiserum). IEF, isoelectric focusing.
Nephrin-mediated activation of the PI3K/AKT pathway regulates complex biological programs.
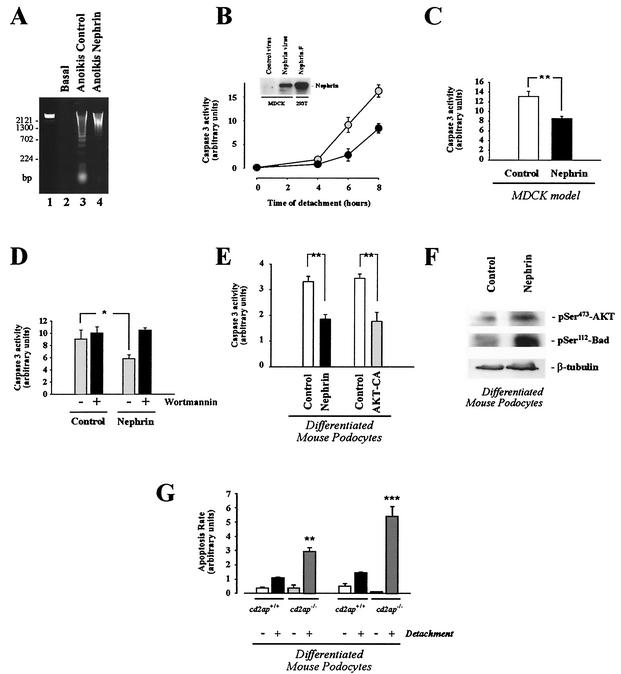
Among a wealth of effects of PI3K/AKT activity, the PI3K-induced AKT signaling pathway is one of the main antiapoptotic cell survival pathways known. AKT-mediated phosphorylation of Bad has been shown to prevent different modes of apoptosis. To demonstrate that nephrin-mediated PI3K activation can regulate complex cellular programs, we investigated whether nephrin protects cells against apoptosis. Detachment of epithelial cells, such as MDCK cells, from the extracellular matrix triggers programmed cell death, a phenomenon known as anoikis (12), while integrin-mediated binding of MDCK cells to the matrix leads to rapid stimulation of the PI3K/AKT signaling pathway that prevents apoptosis (21). Thus, the loss of PI3K/AKT activity after detachment appears to induce cell death. We tested whether expression of nephrin in this well-defined model of anoikis compensates for the loss of integrin-mediated AKT activation. MDCK cells were transduced to express nephrin (Fig. 7B). Apoptosis rates were monitored by DNA laddering, as well as by caspase 3 activation. Detachment of MDCK cells from the matrix induced a time-dependent dramatic increase in apoptosis, as demonstrated by DNA laddering (Fig. 7A) and caspase 3 activity (Fig. 7B). Expression of nephrin significantly inhibited apoptosis compared to that in control cells (Fig. 7A to C). The survival benefit of nephrin-expressing cells was completely reversed by wortmannin, indicating that nephrin exerts its survival effect via the PI3K/AKT pathway (Fig. 7D). To show that regulation of cell viability may be an important aspect of PI3K signaling in podocytes, we subjected control and nephrin-expressing podocytes to anoikis. Interestingly, cultured differentiated podocytes are surprisingly resistant to a variety of apoptotic stimuli (T. B. Huber, B. Hartleben, M. Schmidts, G. Walz, and T. Benzing, unpublished data), but are exquisitely sensitive to detachment. In perfect agreement with the MDCK model of anoikis, the expression of nephrin in differentiated mouse podocytes strongly inhibited detachment-induced apoptosis in podocytes (Fig. 7E). Interestingly, the nephrin-mediated survival benefit was comparable to the expression of a constitutively active mutant of AKT (Fig. 7E), and nephrin expression facilitated the maintenance of AKT and BAD phosphorylation after the induction of apoptosis (Fig. 7F), further supporting the concept that nephrin-mediated AKT activation can protect podocytes from undergoing apoptosis. Conditionally immortalized differentiated podocytes contain only low levels of nephrin protein but relatively large amounts of CD2AP. To demonstrate that CD2AP-mediated AKT activation also participates in a pathway that safeguards podocytes against apoptotic signals, we examined detachment-induced apoptosis in podocytes derived from cd2ap knockout mice. As shown in Fig. 7G, the loss of CD2AP dramatically increased susceptibility to detachment-induced apoptosis compared to wild-type podocytes. However, there was no difference between the spontaneous apoptosis rates of cd2ap−/− and wild-type podocytes. These findings suggest that both nephrin and CD2AP participate in PI3K/AKT signaling that safeguards podocyte viability and provide proof that nephrin-CD2AP-mediated PI3K activation can regulate complex biological programs.
FIG. 7.
Nephrin-mediated PI3K/AKT activation is associated with inhibition of apoptotic cell death. (A to D) The MDCK cell model of anoikis was used to test whether nephrin-stimulated AKT activity was associated with an inhibition of apoptosis. MDCK cells were retrovirally transduced to express nephrin and subjected to apoptosis assays (DNA laddering [A] and quantitative caspase 3 activity [B to D]) as described in Materials and Methods. (A) DNA-laddering assay. Lane 1, DNA marker; lane 2, control cells without detachment; lane 3, anoikis in cells transduced with control virus; lane 4, anoikis in cells transduced with nephrin retrovirus. (B) Time course of quantitative caspase 3 activity in cells transduced with control virus (n = 3; shaded circles) and nephrin-transduced cells (n = 3; solid circles). The inset shows that these cells express nephrin. FLAG-tagged full-length nephrin (Nephrin.F), expressed in 293T cells, served as a positive control for the blot. The error bars indicate SEM. (C) Statistical analysis of the nephrin effect on quantitative caspase 3 activity after induction of anoikis (n = 6; **, P < 0.01). (D) Effect of wortmannin treatment (100 nM; 120 min) (+) on quantitative caspase 3 activity in control and nephrin-expressing cells (n = 4; *, P < 0.05). (E) Nephrin expression and constitutively active AKT inhibit detachment-induced apoptosis in differentiated mouse podocytes. Mouse podocytes were retrovirally transduced to stably express nephrin or a constitutively active mutant of AKT. The differentiated mouse podocytes were detached and kept in suspension culture for 4 h. Equal amounts of protein from cell lysates were subjected to caspase 3 activity assays as described in Materials and Methods. Depicted is a statistical analysis of six (control [open column] versus nephrin [solid column]) and four (control [open column] versus constitutively active AKT [shaded column]) independent experiments (**, P < 0.01). (F) Nephrin expression maintains AKT and Bad phosphorylation after induction of anoikis. Differentiated control or nephrin-expressing podocytes were detached and kept in suspension culture for 4 h. Phosphorylation of AKT at serine-473 (pSer473; top) and Bad at serine-112 (middle) were monitored with phosphospecific antibodies; equal loading of proteins was confirmed by reprobing the membrane with an antitubulin antibody (bottom). (G) Increased susceptibility of cd2ap−/− podocytes to detachment-induced apoptosis. cd2ap+/+ and cd2ap−/− podocytes were differentiated, detached from the cell culture dish, and kept in suspension culture (2 h [left]; 4 h [right]). Programmed cell death was evaluated by annexin V binding as described in Materials and Methods (**, P < 0.01, and ***, P < 0.001 compared to cd2ap+/+ cells; n = 3). +, present; −, absent.
DISCUSSION
Recent studies have emphasized the role of the podocyte slit diaphragm as a crucial size-selective filtration barrier in the kidney and have revealed novel aspects of the mechanisms leading to proteinuria in both inherited and acquired diseases (19, 46). However, it is unclear how the loss of either nephrin, podocin, or CD2AP causes the functional changes that abolish the formation of slit diaphragms, alter the morphology of podocytes, and eventually result in podocyte depletion. It has been speculated that these proteins participate in common signaling pathways; however, it has remained unclear which signaling proteins are recruited by the slit diaphragm protein complex in vivo.
We found that the carboxy-terminal cytoplasmic tail of nephrin and CD2AP interact with the p85 regulatory subunit of PI3K. This could be shown with endogenous proteins from mouse kidney cortex. Thus, PI3K is the first protein demonstrated to interact with the cytoplasmic surface of the slit diaphragm protein complex in vivo. The interaction of nephrin with PI3K was phosphotyrosine dependent and susceptible to genistein, a broad-spectrum tyrosine kinase inhibitor with preference for protein tyrosine kinases of the src family. Two other observations suggest that src family members mediate the downstream signaling of nephrin: first, the carboxy-terminal cytoplasmic tail of nephrin is phosphorylated by src kinases in vivo (50), and second, targeted disruption of the src family member fyn in mice results in massive proteinuria (57). The renal phenotype is preserved in fyn−/− rag1−/− double-mutant mice, which lack functional B or T lymphocytes, suggesting that the kidney manifestations of fyn−/− mice are due to an intrinsic glomerular defect. Fyn deficiency results in podocyte foot process fusion and eventually podocyte loss, a result almost identical to that of nephrin deficiency in both mice (35) and humans (20). These observations suggest that regulated tyrosine phosphorylation of the cytoplasmic tail of nephrin by src family members, and the phosphorylation-dependent interaction with SH2 domain-containing signaling molecules, such as p85, are critical for the integrity of the glomerular filter. Interaction of p85 with nephrin and CD2AP was associated with a strong activation of AKT. It has been shown that nephrin interacts with podocin and that this interaction facilitates nephrin signaling (18). Data from several laboratories suggest that podocin functions to recruit nephrin and CD2AP to lipid rafts localized at the slit diaphragm (18, 41, 42, 45). Our results demonstrate that both nephrin and CD2AP induce PI3K-dependent signaling. Hence, the slit diaphragm proteins nephrin, CD2AP, and podocin appear to serve at least two functions. First, these proteins are indispensable for the structural organization of the slit diaphragm. Second, nephrin interacts with podocin, CD2AP, and cytoplasmic adaptor proteins, such as the p85 subunit of PI3K, to initiate signaling from the slit diaphragm. This nephrin-induced signaling cascade was established in three independent models, HEK 293T cells, MDCK cells, and in vitro-differentiated podocytes. In addition, the functional consequences of the loss of CD2AP for AKT activation were validated in podocytes derived from CD2AP-deficient mice. Hence, both tyrosine phosphorylation of nephrin and the stimulation of PI3K appear to represent important aspects of the signaling events that originate at the slit diaphragm of glomerular podocytes. PI3K and AKT control complex cellular programs, including regulation of gene expression, migration, remodeling of the actin cytoskeleton, endocytosis, and the control of cell viability and growth (5). Interestingly, AKT is also required to repress collagenase expression and to induce the synthesis of laminin and type IV collagen chains (28, 29); both are key components of the glomerular basement membrane. Since basement membrane abnormalities are involved in the development of several forms of proteinuria, AKT activation may contribute to the synthesis and/or maintenance of an intact glomerular basement membrane. In addition, AKT signaling transactivates cell surface receptors (26) and modifies cellular metabolism (5, 9, 23, 44). Moreover, it has been shown that AKT provides a strong survival signal that protects cells from death induced by various stresses (7). Podocyte death and podocyte depletion have been proposed as hallmarks of both primary and secondary forms of glomerulosclerosis for many years and are now considered key steps in the development of progressive renal disease (10, 11, 22, 24, 25, 40). Nephrin-podocin-CD2AP-induced AKT activation is associated with a strong inhibition of detachment-induced apoptosis (anoikis) in podocytes. In contrast, podocytes that lack CD2AP are highly susceptible to anoikis. Furthermore, the targeted disruption of cd2ap results in apoptotic cell death of podocytes in vivo (E. Böttinger, personal communication). Thus, the structural and functional integrity of the slit diaphragm proteins is required for the control of complex biological programs, i.e., inhibition of apoptosis and cell survival.
When nephrin was first isolated in 1998, the authors speculated that the protein might initiate signaling within the podocyte (20). Our findings suggest that the PI3K/AKT pathway represents an important signaling target of slit diaphragm proteins in several different model systems, including cultured podocytes. Additional studies need to address the questions of how nephrin-mediated PI3K/AKT activation is regulated in vivo and which other PI3K-dependent pathways may be important for podocyte function and viability. A detailed understanding of the signaling events that occur at the cell-cell contacts of glomerular podocytes will help us to understand the pathogenesis of proteinuria.
Acknowledgments
We thank Christina Engel, Birgit Schilling, and Stefanie Keller for excellent technical assistance and members of the Walz laboratory for helpful discussions. We are grateful to Lawrence B. Holzman for helpful discussions and providing immunoprecipitating nephrin antisera. We thank Karl Tryggvason, Lewis Cantley, David Fruman, Philip Tsichlis, Alex Toker, Silvio Gutkind, Brian Seed, Dusty Miller, Richard Mulligan, Jim Woodgett, and Roland Schüle for providing cDNAs.
This study was supported by DFG grants Be2212 and Wa517 and the Deutsche Nierenstiftung (T.B.H.).
REFERENCES
- 1.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 2.Benzing, T., P. Gerke, K. Hopker, F. Hildebrandt, E. Kim, and G. Walz. 2001. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc. Natl. Acad. Sci. USA 98:9784-9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benzing, T., M. B. Yaffe, T. Arnould, L. Sellin, B. Schermer, B. Schilling, R. Schreiber, K. Kunzelmann, G. G. Leparc, E. Kim, and G. Walz. 2000. 14-3-3 interacts with regulator of G protein signaling proteins and modulates their activity. J. Biol. Chem. 275:28167-28172. [DOI] [PubMed] [Google Scholar]
- 4.Boute, N., O. Gribouval, S. Roselli, F. Benessy, H. Lee, A. Fuchshuber, K. Dahan, M. C. Gubler, P. Niaudet, and C. Antignac. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24:349-354. [DOI] [PubMed] [Google Scholar]
- 5.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. [DOI] [PubMed] [Google Scholar]
- 6.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 7.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 8.Dustin, M. L., M. W. Olszowy, A. D. Holdorf, J. Li, S. Bromley, N. Desai, P. Widder, F. Rosenberger, P. A. van der Merwe, P. M. Allen, and A. S. Shaw. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94:667-677. [DOI] [PubMed] [Google Scholar]
- 9.Edinger, A. L., and C. B. Thompson. 2002. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 13:2276-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elger, M., and W. Kriz. 1998. Podocytes and the development of segmental glomerulosclerosis. Nephrol. Dial. Transplant. 13:1368-1373. [DOI] [PubMed] [Google Scholar]
- 10a.Ernst, J. D., L. Yang, J. L. Rosales, and V. C. Broaddus. 1998. Preparation and characterization of an endogenously fluorescent annexin for detection of apoptotic cells. Anal. Biochem. 260:18-23. [DOI] [PubMed]
- 11.Fries, J. W., D. J. Sandstrom, T. W. Meyer, and H. G. Rennke. 1989. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab. Investig. 60:205-218. [PubMed] [Google Scholar]
- 12.Frisch, S. M., and E. Ruoslahti. 1997. Integrins and anoikis. Curr. Opin. Cell Biol. 9:701-706. [DOI] [PubMed] [Google Scholar]
- 13.Fuchshuber, A., G. Jean, O. Gribouval, M. C. Gubler, M. Broyer, J. S. Beckmann, P. Niaudet, and C. Antignac. 1995. Mapping a gene (SRN1) to chromosome 1q25-q31 in idiopathic nephrotic syndrome confirms a distinct entity of autosomal recessive nephrosis. Hum. Mol. Genet. 4:2155-2158. [DOI] [PubMed] [Google Scholar]
- 14.Holthofer, H., H. Ahola, M. L. Solin, S. Wang, T. Palmen, P. Luimula, A. Miettinen, and D. Kerjaschki. 1999. Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am. J. Pathol. 155:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzman, L. B., P. L. St John, I. A. Kovari, R. Verma, H. Holthofer, and D. R. Abrahamson. 1999. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56:1481-1491. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 17.Hu, W. H., H. Johnson, and H. B. Shu. 2000. Activation of NF-κB by FADD, Casper, and caspase-8. J. Biol. Chem. 275:10838-10844. [DOI] [PubMed] [Google Scholar]
- 18.Huber, T. B., M. Kottgen, B. Schilling, G. Walz, and T. Benzing. 2001. Interaction with podocin facilitates nephrin signaling. J. Biol. Chem. 276:41543-41546. [DOI] [PubMed] [Google Scholar]
- 19.Kerjaschki, D. 2001. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J. Clin. Investig. 108:1583-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kestila, M., U. Lenkkeri, M. Mannikko, J. Lamerdin, P. McCready, H. Putaala, V. Ruotsalainen, T. Morita, M. Nissinen, R. Herva, C. E. Kashtan, L. Peltonen, C. Holmberg, A. Olsen, and K. Tryggvason. 1998. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1:575-582. [DOI] [PubMed] [Google Scholar]
- 21.Khwaja, A., P. Rodriguez-Viciana, S. Wennstrom, P. H. Warne, and J. Downward. 1997. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16:2783-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, Y. H., M. Goyal, D. Kurnit, B. Wharram, J. Wiggins, L. Holzman, D. Kershaw, and R. Wiggins. 2001. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 60:957-968. [DOI] [PubMed] [Google Scholar]
- 23.Kozma, S. C., and G. Thomas. 2002. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. Bioessays 24:65-71. [DOI] [PubMed] [Google Scholar]
- 24.Kriz, W., N. Gretz, and K. V. Lemley. 1998. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 54:687-697. [DOI] [PubMed] [Google Scholar]
- 25.Kriz, W., and K. V. Lemley. 1999. The role of the podocyte in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 8:489-497. [DOI] [PubMed] [Google Scholar]
- 26.Lee, M. J., S. Thangada, J. H. Paik, G. P. Sapkota, N. Ancellin, S. S. Chae, M. Wu, M. Morales-Ruiz, W. C. Sessa, D. R. Alessi, and T. Hla. 2001. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 8:693-704. [DOI] [PubMed] [Google Scholar]
- 27.Li, C., V. Ruotsalainen, K. Tryggvason, A. S. Shaw, and J. H. Miner. 2000. CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am. J. Physiol. Renal Physiol. 279:F785-F792. [DOI] [PubMed] [Google Scholar]
- 28.Li, X., U. Talts, J. F. Talts, E. Arman, P. Ekblom, and P. Lonai. 2001. Akt/PKB regulates laminin and collagen IV isotypes of the basement membrane. Proc. Natl. Acad. Sci. USA 98:14416-14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mawal-Dewan, M., A. Lorenzini, L. Frisoni, H. Zhang, V. J. Cristofalo, and C. Sell. 2002. Regulation of collagenase expression during replicative senescence in human fibroblasts by Akt-forkhead signaling. J. Biol. Chem. 277:7857-7864. [DOI] [PubMed] [Google Scholar]
- 30.Mundel, P., J. Reiser, A. Zuniga Mejia Borja, H. Pavenstadt, G. R. Davidson, W. Kriz, and R. Zeller. 1997. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236:248-258. [DOI] [PubMed] [Google Scholar]
- 31.Muslin, A. J., J. W. Tanner, P. M. Allen, and A. S. Shaw. 1996. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84:889-897. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson, D. W., A. Ali, N. A. Thornberry, J. P. Vaillancourt, C. K. Ding, M. Gallant, Y. Gareau, P. R. Griffin, M. Labelle, Y. A. Lazebnik, et al. 1995. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37-43. [DOI] [PubMed] [Google Scholar]
- 33.Nishitoh, H., M. Saitoh, Y. Mochida, K. Takeda, H. Nakano, M. Rothe, K. Miyazono, and H. Ichijo. 1998. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell 2:389-395. [DOI] [PubMed] [Google Scholar]
- 34.Obata, T., M. B. Yaffe, G. G. Leparc, E. T. Piro, H. Maegawa, A. Kashiwagi, R. Kikkawa, and L. C. Cantley. 2000. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 275:36108-36115. [DOI] [PubMed] [Google Scholar]
- 35.Putaala, H., R. Soininen, P. Kilpelainen, J. Wartiovaara, and K. Tryggvason. 2001. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum. Mol. Genet. 10:1-8. [DOI] [PubMed] [Google Scholar]
- 36.Reiser, J., W. Kriz, M. Kretzler, and P. Mundel. 2000. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 11:1-8. [DOI] [PubMed] [Google Scholar]
- 37.Rittinger, K., J. Budman, J. Xu, S. Volinia, L. C. Cantley, S. J. Smerdon, S. J. Gamblin, and M. B. Yaffe. 1999. Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol. Cell 4:153-166. [DOI] [PubMed] [Google Scholar]
- 38.Rodewald, R., and M. J. Karnovsky. 1974. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 60:423-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruotsalainen, V., P. Ljungberg, J. Wartiovaara, U. Lenkkeri, M. Kestila, H. Jalanko, C. Holmberg, and K. Tryggvason. 1999. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA 96:7962-7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiffer, M., M. Bitzer, I. S. Roberts, J. B. Kopp, P. ten Dijke, P. Mundel, and E. P. Bottinger. 2001. Apoptosis in podocytes induced by TGF-beta and Smad7. J. Clin. Investig. 108:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarz, K., M. Simons, J. Reiser, M. A. Saleem, C. Faul, W. Kriz, A. S. Shaw, L. B. Holzman, and P. Mundel. 2001. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Investig. 108:1621-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih, N. Y., J. Li, R. Cotran, P. Mundel, J. H. Miner, and A. S. Shaw. 2001. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am. J. Pathol. 159:2303-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih, N. Y., J. Li, V. Karpitskii, A. Nguyen, M. L. Dustin, O. Kanagawa, J. H. Miner, and A. S. Shaw. 1999. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286:312-315. [DOI] [PubMed] [Google Scholar]
- 44.Shioi, T., J. R. McMullen, P. M. Kang, P. S. Douglas, T. Obata, T. F. Franke, L. C. Cantley, and S. Izumo. 2002. Akt/protein kinase B promotes organ growth in transgenic mice. Mol. Cell. Biol. 22:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons, M., K. Schwarz, W. Kriz, A. Miettinen, J. Reiser, P. Mundel, and H. Holthofer. 2001. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am. J. Pathol. 159:1069-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somlo, S., and P. Mundel. 2000. Getting a foothold in nephrotic syndrome. Nat. Genet. 24:333-335. [DOI] [PubMed] [Google Scholar]
- 47.Tryggvason, K. 1999. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J. Am. Soc. Nephrol. 10:2440-2445. [DOI] [PubMed] [Google Scholar]
- 48.Tryggvason, K., and J. Wartiovaara. 2001. Molecular basis of glomerular permselectivity. Curr. Opin. Nephrol. Hypertens. 10:543-549. [DOI] [PubMed] [Google Scholar]
- 49.Tzivion, G., and J. Avruch. 2001. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 277:3061-3064. [DOI] [PubMed] [Google Scholar]
- 50.Verma, R. K., B. Wharram, I. A. Kovari, R. Kunkel, D. Nihalani, K. K. Wary, R. C. Wiggins, P. Killen, and L. B. Holzman. 2003. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 278:20716-20723. [DOI] [PubMed]
- 51.Weng, L. P., J. Yuan, and Q. Yu. 1998. Overexpression of the transmembrane tyrosine phosphatase LAR activates the caspase pathway and induces apoptosis. Curr. Biol. 8:247-256. [DOI] [PubMed] [Google Scholar]
- 52.Yaffe, M. B., and A. E. Elia. 2001. Phosphoserine/threonine-binding domains. Curr. Opin. Cell Biol. 13:131-138. [DOI] [PubMed] [Google Scholar]
- 53.Yaffe, M. B., G. G. Leparc, J. Lai, T. Obata, S. Volinia, and L. C. Cantley. 2001. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 19:348-353. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe, M. B., K. Rittinger, S. Volinia, P. R. Caron, A. Aitken, H. Leffers, S. J. Gamblin, S. J. Smerdon, and L. C. Cantley. 1997. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91:961-971. [DOI] [PubMed] [Google Scholar]
- 55.Yan, K., J. Khoshnoodi, V. Ruotsalainen, and K. Tryggvason. 2002. N-linked glycosylation is critical for the plasma membrane localization of nephrin. J. Am. Soc. Nephrol. 13:1385-1389. [DOI] [PubMed] [Google Scholar]
- 56.Yang, E., J. Zha, J. Jockel, L. H. Boise, C. B. Thompson, and S. J. Korsmeyer. 1995. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80:285-291. [DOI] [PubMed] [Google Scholar]
- 57.Yu, C. C., T. S. Yen, C. A. Lowell, and A. L. DeFranco. 2001. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr. Biol. 11:34-38. [DOI] [PubMed] [Google Scholar]
- 58.Zhou, B. P., M. C. Hu, S. A. Miller, Z. Yu, W. Xia, S. Y. Lin, and M. C. Hung. 2000. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-κB pathway. J. Biol. Chem. 275:8027-8031. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, H., X. M. Li, J. Meinkoth, and R. N. Pittman. 2000. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 151:483-494. [DOI] [PMC free article] [PubMed] [Google Scholar]