Abstract
To investigate mechanisms of apical sorting in the secretory pathway of epithelial cells, we expressed varying amounts of the 165 amino acid isoform of vascular endothelial growth factor (VEGF165) and transforming growth factor β1 (TGF-β1) via replication defective adenoviruses. Apical sorting of both proteins was efficient at low expression levels but saturated or was reversed at high expression levels. High expression levels of TGF-β1 were effective at competing VEGF165 out of the apical pathway; however, VEGF165 did not compete out TGF-β1. Tunicamycin inhibition experiments showed that the apical polarity of VEGF165 was independent of N-glycosylation. We conclude that the apical sorting of these two molecules is a saturable, signal-mediated process, involving competition for apical sorting receptors. The sorting of the two proteins does not appear to involve N-glycans as sorting signals, or lectin sorters. The observations are particularly relevant to gene therapy because they demonstrate that overexpression of a transgene can result in undesirable missorting of the encoded protein.
Epithelial cells possess a highly polarized structure, which is responsible for a myriad of vectorial functions performed by these cells (1). One of these vectorial functions is the polarized transport and secretion of proteins into apical or basolateral compartments. The sorting process is believed to take place at the level of the trans-Golgi network, a distal Golgi compartment, by incorporation of apical and basolateral proteins into distinct populations of post-Golgi vesicles (2–5). Segregation is directed by domain-specific sorting signals, which have been identified in both soluble membrane proteins destined for secretion, as well as in membrane proteins with final residence in apical or basolateral regions of the plasma membrane (3, 5, 6). Dominant basolateral signals, frequently containing critical tyrosine residues, are located in the cytoplasmic domain of basolateral plasma membrane proteins. In LDL receptor, two basolateral signals have been described: one acting exclusively as a basolateral signal, functional even when the protein is expressed at relatively high levels (strong signal), the other one doubling as an endocytic signal, which is saturated at moderate expression levels (weak signal) (7, 8). Apical signals of various types have been reported: glycosyl phosphatidylinositol anchors (9–11), transmembrane domains, N-glycans (12), O-glycans (13), and unidentified proteinaceus “patches” (14). Two major scenarios are envisioned for how these signals mediate apical sorting. In the first scenario, they interact with “apical sorting receptors.” In the second scenario, they interact with specialized lipid microdomains (or “rafts”) (15) that have the ability to segregate themselves into apical transport vesicles. Both scenarios invoke a transmembrane linkage to the vesicle assembly machinery in the cytosol.
For soluble secreted proteins, N-glycans and O-glycans have been postulated as apical sorting signals, but no basolateral signals have yet been identified. Scheiffele et al. (12) have shown that the addition of N-glycans to rat growth hormone, normally unsorted, results in the apical sorting of this hormone in MDCK cells, which led them to postulate the existence of “lectin sorters.” A growing body of evidence supports the existence of nonglycan apical sorting signals, imagined as three-dimensional proteinaceous patches, as certain proteins are sorted and secreted preferentially from the apical surface in the absence of any glycan (14, 16, 17). If receptors for either glycans or proteinaceous signals are involved in apical sorting, it would be expected that the apical sorting mechanism would be saturable and highly dependent on secretory protein expression levels. In addition, apical proteins using similar receptors would compete with each other for the limited receptor population. On the other hand, if apical sorting depends on direct interaction of the signals with lipid microdomains or rafts (as postulated for glycosyl phosphatidylinositol-anchored proteins and certain apical transmembrane proteins) that segregate themselves to the apical pathway, it would be expected that the high binding capacity of such a system would result in saturation kinetics that are at best difficult to observe. In this second scenario, no competition would be observed between two apically secreted proteins. To date, no information is available on this important aspect of the protein sorting machinery in epithelial cells.
In this manuscript, we use adenovirus-mediated gene transfer to regulate the expression of two apically secreted glycoproteins over a wide range of concentration. For these studies we used a polarized epithelial cell line, RPE-J, which can be infected by adenoviruses at high multiplicities of infection (mois) without signs of cytotoxicity. The reason for the high receptivity of RPE-J cells for adenoviruses is not known. By measuring the polarized release of transduced proteins at increasing levels of expression we show that the apical pathway is saturable. Furthermore, by varying the expression of one protein while keeping the other one constant in double adenovirus transduction experiments, dramatic competition was observed. Tunicamycin inhibition studies are consistent with proteinaceous patches, rather than glycans, mediating the apical sorting of one of the proteins. Taken together, the experiments support the hypothesis that both of these glycoproteins are sorted by interaction with a limited number of nonlectin apical sorting receptors.
Materials and Methods
Replication-Defective Adenovirus Vectors.
Adenoviral vectors for expression of transforming growth factor β1 (TGF-β1) containing mutations of cysteine to serine at positions 223 and 225 (18) and β-galactosidase were generated by using the shuttle vector pAdCMV as described (19). Recombinant virus was identified by dot blot hybridization with the cDNA and was further amplified in 293 cells as described (20). The adenoviral vector encoding the 165 amino acid isoform of vascular endothelial growth factor (VEGF165) was provided by GenVec (Rockville, MD). All adenoviral vectors used were human type 5 serotype E-1 deleted and were generated to titers of 1 × 1011-1 × 1012 plaque-forming units/ml. Vectors were found free of contaminating wild-type adenoviral recombinants.
Cell Culture and Transduction with Adenovirus Vectors.
All cell culture reagents were obtained from GIBCO/Life Technologies (Grand Island, NY) unless otherwise indicated. RPE-J cells were maintained in DMEM supplemented with 4% CELLECT Gold FBS (ICN), nonessential amino acids, glutamine, and penicillin/streptomycin at 32°C in a 95% air/5% CO2 environment as described (21, 22). All assays were carried out on cells seeded at a density of 3.5 × 105 cells/cm2 on Matrigel-coated 1.2-cm-diameter Transwell filters. Transduction with adenoviruses was performed 6 days after plating as follows. Cells were rinsed in 20 mM Hepes-buffered DMEM containing 0.2% BSA (transduction medium). One hundred microliters of the appropriate vector diluted in transduction medium was added to the apical chamber of the Transwell, and the cells were returned to the 32°C incubator with no fluid in the basal chamber. After 2 h, 1 ml of culture medium was added to the basal chamber and 0.5 ml was added to the apical chamber. For assays requiring a second transduction, this was repeated exactly as described 16 h after the initial transduction.
Antibodies, β-Galactosidase, and ELISA Assays.
All reagents were purchased from Sigma except antibodies against VEGF and TGF, and recombinant human VEGF and TGF standards for ELISA were purchased from R & D Systems. Antibodies conjugated to alkaline phosphatase were obtained from Jackson ImmunoResearch. β-galactosidase was assayed as described elsewhere (23). VEGF and TGF were assayed as follows. Twenty-four hours after transduction, RPE-J monolayers were washed three times with transduction medium. After the last wash, the cells were returned to the incubator. After 14–16 h, media were collected from the apical and basal compartment, and the volume was measured. Cells were lysed in 1 ml of 1% Triton X-100 in 50 mM Tris (pH8.0), 150 mM NaCl, 5 mM EDTA, and 1 mM PMSF for 30 min at 4°C and then were centrifuged for 10 min at 13,000 × g. The supernatant was collected, and the insoluble pellet was discarded. Triton X-100 was added to the apical and basal samples to a final concentration of 1%. Samples were then assayed for TGF by ELISA according to the method of Danielpour (24). Capture antibodies in both assays were mouse monoclonals diluted 1:500 in PBS and immobilized overnight in 96-well ELISA plates. Second antibodies were either goat anti-VEGF of chicken anti-TGF-β1 diluted 1:1,000. Secondary antibodies were detected by using either donkey anti-goat IgG or donkey anti-chicken IgY antibodies conjugated to alkaline phosphatase at a dilution of 1:500. The TGF assay was sensitive in the range of 10–1,000 pg. The VEGF assay was sensitive in the range of 30–1,000 pg.
Immunohistochemistry.
RPE-J cells were growth on 8-well chamber slides (Lab-Tek) and were transduced as previously described (26). Sixteen to eighteen hours after the second transduction, cells were incubated with brefeldin A (Sigma), 1 μg/ml for one hour, were fixed in acetone:methanol 50:50 (vol/vol) at −20°C for 10 min, and were air dried and blocked in 1% BSA in PBS saline (pH 7.4) containing Triton X-100 0.6% for one hour at room temperature. Slides were incubated with a rabbit polyclonal anti-TGFb1 (Santa Cruz Biotechnology) and goat anti-VEGF (Santa Cruz Biotechnology) diluted in 1% BSA in PBS (pH 7.4) containing Triton X-100 0.6% for 1 h at room temperature. After washing, slides were incubated with FITC-conjugated donkey anti-rabbit antibody (The Jackson Laboratory) and with a CY-3-conjugated donkey anti-goat (The Jackson Laboratory) and were viewed with an epifluorescence microscope (model BX50, Olympus, Melville, NY) equipped with a cooled charge-coupled device camera. Images were digitally acquired by using National Institutes of Health image 1.52 software and were recompiled in Adobe photoshop 5.0 (Adobe Systems, Mountain View, CA).
Pulse–Chase Assays.
RPE-J cells were transduced with the appropriate adenoviral vector as described above. After 24 h, the cells were labeled with 80 μCi each of [35S]express and [35S]cysteine (NEN) as described (25, 26) except that the cells were chased in transduction medium. At various times of chase, the apical and basal medium was collected and VEGF or TGF were immunoprecipitated by using the corresponding polyclonal antibodies described above. The immunoprecipitates were resolved by SDS/PAGE. The gels were dried and analyzed by using a STORM 860 PhosphorImager (Molecular Dynamics) and uncoated phosphor screen as described (25, 26).
Results
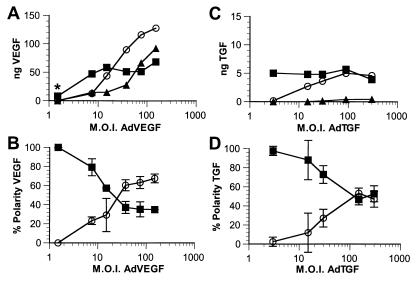
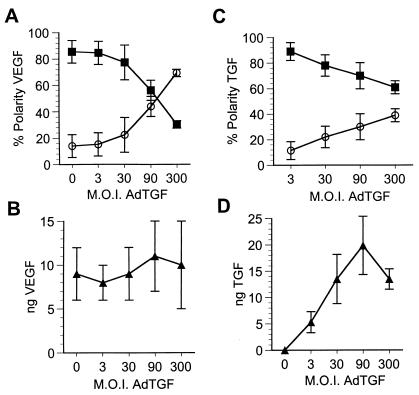
To test the saturability of the apical secretory pathway, E1-deficient replication-defective adenovirus vectors encoding VEGF (AdVEGF) or TGF-β1 (AdTGF) were transduced into polarized monolayers of RPE-J cells, grown on Matrigel-coated Transwell filters, at mois from 1.5 to 300 (Fig. 1). Twenty-four hours later, the cells were switched to serum-free medium, and the VEGF or TGF content of the apical or basal media, or of the cells and filter, was determined by ELISA. The synthesis of TGF was significantly lower than the synthesis of VEGF even though the expression of both genes was driven by the same CMV 1e promoter. Both VEGF and TGF were secreted nearly 100% apically at low mois. Increasing mois of AdVGF and AdTGF resulted in increased synthesis of the encoded proteins; however, the amount of VEGF or TGF measured in the apical medium did not increase proportionately. Rather, the additional protein produced was detected in the basal medium or, at higher mois, was retained in the cells. When analyzed as a percentage of protein in the apical or basolateral medium, we found that the polarity of VEGF had reversed from near 100% apical to as much as 70% basal at a moi of 150. At the highest expression levels, even the amount of VEGF entering the basal medium began to plateau, and further increases in total VEGF production were measured as cell/filter-associated protein. Similar results were observed for TGF with the primary difference being that TGF secretion did not reverse in polarity; rather, it became nonpolar at moi of 90–300. Saturation of the apical transport for VEGF occurs at secretion levels of ≈26,000 monomeric VEGF molecules⋅h−1⋅cell−1. In contrast, apical transport of TGF saturated at much lower secretion levels: 800 monomeric prepromolecules⋅h−1⋅cell−1.
Figure 1.
Saturation of apical VEGF and TGF secretion on transduction via adenovirus vectors. RPE-J cells grown on porous filter supports were transduced with AdVEGF (A and B) or AdTGF (C and D) at increasing mois. The amounts of VEGF (A and B) or TGF (C and D) secreted into the apical (■) or basal (○) media, or retained in the cells (▴) were determined by ELISA. A and C show representative experiments demonstrating the amount of VEGF or TGF actually detected in the samples after adjusting for volume. B and D illustrate the percentage of total secreted VEGF or TGF (polarity) released into the apical (■) or the basal (○) media. Note that, although at low mois nearly 100% of both VEGF and TGF are secreted into the apical medium, increasing mois result in large increases in the production of VEGF or TGF with progressively larger fractions secreted basally or retained by the cells. Data in C and D are represented as mean ± SD (n ≥ 3). *, this point is from a separate experiment.
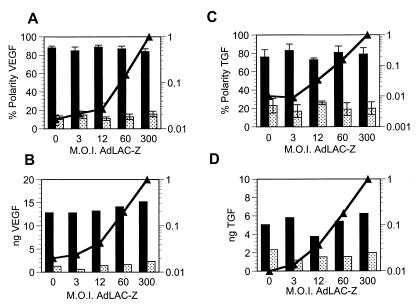
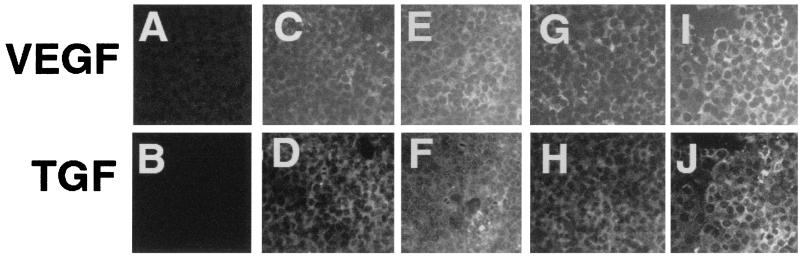
To verify that the alterations of polarity that we observed were not an artifact of adenovirus infection or a general effect of protein overexpression, we performed the following control experiments. RPE-J monolayers were transduced with a constant low level of either AdVEGF or AdTGF; the respective mois were selected (according to the data in Fig. 1) to provide ≈80% apical polarity. After inoculation and subsequent overnight incubation, the same monolayers were transduced a second time with increasing mois of AdCMV-LacZ, an E1-deficient replication-defective adenovirus vector encoding β-galactosidase with a nuclear localization signal. The polarity of VEGF (Fig. 2 A and B) or TGF (C and D) secretion, as well as the β-galactosidase activity levels (solid line), were then determined after a second overnight incubation in serum-free medium. As shown in Fig. 2, neither the secretion levels nor the polarized release of VEGF or TGF were altered by increasing mois of AdCMV-LacZ, even at the highest β-galactosidase expression levels tested. Microscopic examination of the monolayers indicated homogeneous expression of the enzyme (data not shown). Consistently, immunofluorescence experiments demonstrated that the expression of VEGF and TGF throughout the infected monolayers was homogeneous, even at low mois, and that the levels of expression of each protein were not affected by simultaneous overexpression of the other one (Fig. 3).
Figure 2.
Expression of a nonsecretory protein via an adenovirus vector does not alter the synthesis or the polarized secretion of VEGF and TGF. RPE-J cells grown on porous filter supports were transduced with AdVEGF (A and B) or AdTGF (C and D) at mois estimated from Fig. 1 to result in an ≈80% apical secretion of the gene product. Sixteen hours later, the cells were transduced with AdCMV-LacZ, expressing the β-galactosidase gene, at mois between 0 and 300. Eight hours later, the media were changed. After 14–16 h, the media and the cells were assayed for VEGF or TGF. Increasing mois of AdCMV-LacZ as assayed by β-galactosidase activity (▴-▴) had no effect on the polarized secretion or secretion levels of VEGF (A and B) or TGF (C and D). In A and C, the results from four separate experiments are shown as mean ± SEM. B and D are representative experiments. Dark bars, apical secretion; light bars, basal secretion.
Figure 3.
Expression of VEGF and TGF is homogeneous throughout the monolayer and is not affected when both proteins are transduced simultaneously. Monolayers of RPE-J cells, grown on glass coverslips, were transduced with AdVEGF moi = 15 (C and D) or moi = 50 (E and F) followed 16 h later by transduction with AdTGF moi = 5 (C and D) or moi = 150 (E and F). Alternatively, cells were transduced initially with AdTGF moi = 9 (G and H) or 50 (I and J) followed 16 h later by transduction with AdVEGF moi = 15 (G and H) or moi = 150 (I and J). All cells with treated with brefeldin A (1 mM) for 3 h followed by staining for either TGF (upper row) or VEGF (lower row). Control cells without transduction are shown in A and B.
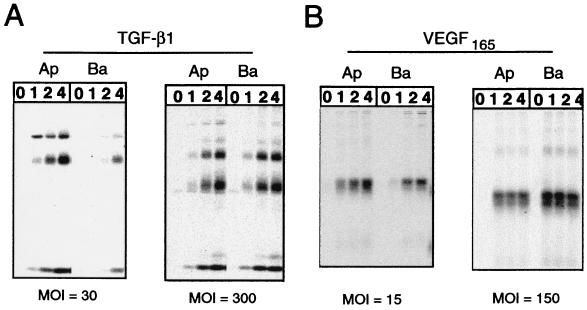
To examine the kinetics of protein synthesis and transport through the secretory pathway for VEGF and TGF, and to verify that we were indeed examining vectorially secreted protein rather than protein leaked during the overnight incubation period, we performed pulse–chase analysis for a shorter (4–6 h) time period (Fig. 4). In RPE-J cells transduced with a low moi of AdVEGF (Fig. 4B), VEGF was found to rapidly transit through the secretory pathway with a half-time of less than 1 h. In contrast TGF (Fig. 4A) traveled through the secretory pathway significantly more slowly, with a half-time of 4–6 h. Although the half-times of secretion were not substantially altered at higher mois, the changes in polarity of secretion observed by ELISA (Fig. 1) were faithfully reproduced for both TGF and VEGF.
Figure 4.
Pulse–chase analysis of VEGF and TGF secretion. RPE-J cells, grown on Transwell chambers, were transduced with AdVEGF or AdTGF at either a low or high moi. Twenty-four hours later, the cells were pulsed with [35S]express and [35S]cysteine for 20 min. At different chase times, the amount of radioactive TGF (A) or VEGF (B) released into apical or basal media was assessed by SDS/PAGE and phosphorimaging. VEGF was analyzed on nonreducing gels and appears primarily as an ≈45-kDa dimer. Its secretion is predominantly apical at a moi ≈ 15 but reverses to a basal polarity at a moi ≈ 150. TGF, analyzed on reducing gels, appears as three bands: an uncleaved 55 kDa-precursor, a 45-kDa latent chain, and the 17-kDa bioactive peptide. TGF secretion is primarily into the apical medium at a moi of 30 whereas it becomes nonpolar at a moi of 300. The data agree with and confirm the Elisa results shown in Fig. 1.
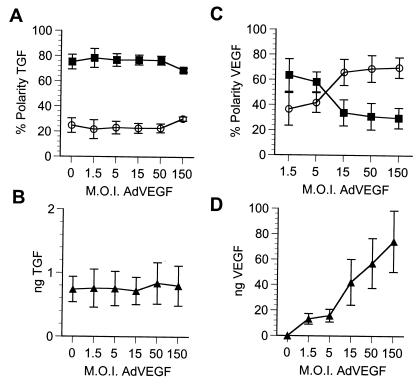
Molecules using the same signal-mediated sorting pathway are expected to compete for components of the pathway. To test this hypothesis, we carried out competition experiments that tested the ability of VEGF and TGF to compete with each other for space or receptors in the apical pathway. Doses of AdVEGF and AdTGF were deliberately chosen to provide ≈80% apical polarity to facilitate detection of competition. Interestingly, cells initially infected with AdVEGF at a moi of 15 that were secondarily (16 h later) transduced with increasing mois of AdTGF exhibited a considerable decrease in the fidelity of apical VEGF sorting with a polarity reversal at the highest AdTGF mois (Fig. 5A). The reversal of polarity of VEGF, from 82% apical to 30% apical, was observed at the same high TGF expression levels that abolished polarized secretion of TGF (Fig. 5 C and D). Control experiments demonstrated that the secretion of VEGF remained constant over the entire range of AdTGF mois (Fig. 5B), indicating that the effects observed were not caused by a toxic effect of increasing viral mois.
Figure 5.
TGF effectively competes VEGF out of the secretory pathway. Monolayers of RPE-J cells, grown on porous filter supports, were transduced with a constant dose of AdVEGF (moi ≈ 15), estimated to result in at least 80% apical secretion of VEGF and, 16 h later, with increasing mois of AdTGF. The amount of VEGF or TGF secreted into the apical (■) or basal (○) media were assayed by ELISA. The polarized secretion of VEGF was reversed from 83% apical to 70% basolateral (A) without changes in the secreted levels of VEGF (B). Control experiments showed that increased levels of AdTGF resulted in loss of the polarized secretion of TGF (C and D). Results are expressed as mean ± SD (n = 3).
In contrast, the apically polarized secretion of TGF (moi of AdTGF = 9) was not affected by VEGF at any dose, with the possible exception of the highest moi (Fig. 6A). Control experiments detected no interference of VEGF with TGF synthesis over the entire range of AdVEGF mois (Fig. 6B) and reproduced the reversal of polarity of VEGF at high expression levels of this protein (Fig. 6 C and D), exactly as in the experiments described in Fig. 2. Note that the low apical polarity level of VEGF (62%) at low moi of AdVEGF (1.5) in this experiment is caused by the expression of TGF in the same cells. In brief, TGF easily competed VEGF out of the apical pathway whereas VEGF did not compete TGF at practically all expression levels tested.
Figure 6.
VEGF does not effectively compete TGF out of the apical pathway. Monolayers of RPE-J cells, grown on Transwells, were transduced with a constant dose of AdTGF (moi ≈ 9), estimated to result in at least 80% apical secretion of TGF and, 16 h later, with increasing mois of AdVEGF. The amount of VEGF or TGF secreted into the apical (■) or basal (○) media were assayed by ELISA. Neither the polarized secretion of TGF nor its secreted levels were affected by high mois of AdVEGF, even when control experiments showed that identical mois of AdTGF resulted in very high rates of secretion and loss of the polarized secretion of VEGF (C and D). Results are expressed as mean ± SD (n = 3).
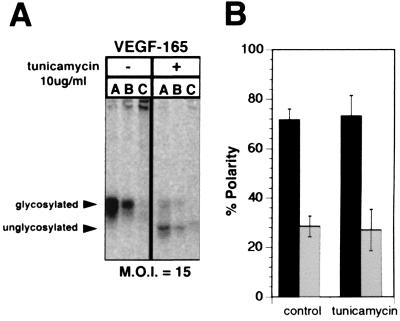
To examine the potential role of the single N-glycan in VEGF as a sorting signal, we examined the polarity of secretion of VEGF in RPE-J cells transduced with AdVEGF at a moi of 15 in the presence or absence of tunicamycin, an inhibitor of N-glycosylation (Fig. 7). Although a reduced expression of VEGF was noticed in tunicamycin-treated cells, no difference in polarity of secretion of the unglycosylated protein was observed, suggesting that the N-glycan in VEGF does not play a role in its sorting. Similar experiments performed with TGF were inconclusive because, as observed by others (27, 28), TGF was not secreted by tunicamycin treated cells.
Figure 7.
N-glycans are not necessary for apical secretion of VEGF. The secretion of VEGF by RPE-J cells transduced with AdVEGF at a moi of 15 was examined by pulse–chase analysis as in Fig. 2. Twenty-four hours after transduction with AdVEGF, cells were pretreated for 30 min with 0 or 10 μg/ml tunicamycin (an inhibitor of N-glycosylation), were starved for 30 min in cysteine- and methionine-free medium, and then were labeled with [35S]express and [35S]cysteine for 20 min. After a 3-h chase in medium containing 0 or 10 μg/ml tunicamycin, cells were lysed and VEGF was immunoprecipitated from the apical or basal compartments, or the cells. A shows a representative experiment in which VEGF was immunoprecipitated from the apical medium (A), basal medium (B), or cell lysates (C) and was analyzed by SDS/PAGE and phosphorimaging. B shows the quantification of three experiments, presenting the data as mean ± SD (■, apical medium; □, basal medium). Note that VEGF appears to be delivered predominantly to the apical surface of both treated and untreated control cells. That tunicamycin successfully inhibited the glycosylation of VEGF is demonstrated by the increased mobility of VEGF apparent in A. Quantitation of the apical and basal VEGF is indicated a nearly identical 75% apical polarity of secretion of VEGF by tunicamycin treated and untreated cells.
Discussion
Our results show, for two different proteins, that apical secretion is a saturable process. In the case of VEGF, apical saturation was reached at a secretion level of ≈26,000 VEGF molecules⋅cell−1⋅h−1 obtained when confluent monolayers were infected with AdVEGF at a moi as low as 7. Importantly, a linear increase in the synthesis of VEGF was observed throughout practically the entire range of moi and was not accompanied by significant changes in the half-time of secretion, suggesting that no key biosynthetic or processing process was saturated. Consistent with saturation of an apical sorting step, VEGF that could not enter the apical pathway appeared in the basolateral medium; at the highest synthetic rates (moi ≈150), basolateral secretion surpassed by a factor of 2.2 the maximal apical secretion of VEGF. Beyond moi ≈ 30, intracellular VEGF levels started to rise and continued to increase linearly almost up to the maximum virus levels tested (moi ≈ 150). At the maximum synthetic rates, both the basolateral and the intracellular VEGF started to plateau. Our results indicate that the capacity of the basolateral pathway for VEGF is ≈2.2-fold the capacity of the apical pathway for this molecule, which is approximately similar to the basolateral:apical secretion ratio observed for secreted proteins presumed to be devoid of trafficking signals in MDCK cells (29). The simplest interpretation of the data are that an apical sorting mechanism became saturated at the highest secretion rates, resulting in diversion of the excess secreted molecules into a default basolateral pathway, presumably as soluble molecules. We do not have any other data on the default pathway of soluble secretory proteins in RPE-J cells; however, a membrane protein whose basolateral signal is not recognized by RPE-J cells is targeted without any polarity to the cell surface during biosynthesis (26). Alternatively, excess VEGF molecules might be interacting with a basolateral sorting mechanism with lower affinity for VEGF than the apical sorting mechanism.
Interestingly, apical TGF release saturated even at the lowest moi tested (≈3), which results in infection of most of the cells in the monolayer. At moi ≈ 3, most of the TGF was secreted apically, but, at higher multiplicities, the levels of TGF expression continued to rise linearly, with most of the excess synthesized protein secreted basolaterally. As in the case of VEGF, this behavior is consistent with the saturation of an apical sorting step. However, no crossover between the apical and basolateral secretion was observed. Maximum basolateral levels of TGF, observed at mois of ≈300, were 800 molecules⋅cell−1⋅h−1, nearly identical to the apical secretion levels. In this case, the apical:basolateral secretion ratio was ≈50%. No intracellular accumulation of TGF was observed after both apical and basolateral pathways were saturated; because TGF was processed normally at high moi (Fig. 3), the results suggest that the accumulation of the protein in the secretory pathway never exceeded the exocytic capacity of the cell.
The saturation of apical TGF secretion occurred at secretion levels approximately 33 times lower than the levels required for saturation of apical VEGF secretion. Furthermore, TGF efficiently competed VEGF out of the apical pathway, leading to its quantitative diversion to the basolateral pathway (Fig. 4). In contrast, VEGF did not effectively compete TGF out of the apical pathway (Fig. 5). As the synthesis of neither of the two proteins was affected by high expression levels of the other one, the results are consistent with the two proteins competing for an apical sorting receptor, rather than for a biosynthetic or processing step before the sorting step. That overexpression of VEGF or TGF may compete with a sorting step common to both apical and basolateral pathways is unlikely because the protein excluded from the apical pathway was quantitatively redistributed to the basal pathway; however, we cannot formally exclude this possibility because well characterized basolateral secretory markers were not available. The data are compatible with a model in which TGF has a longer occupancy time for the putative apical sorting receptor than VEGF. Indeed, TGF has a much slower half-time of secretion than VEGF (Fig. 4), which is consistent with the more complex processing of this molecule (30).
The ability of TGF to successfully compete VEGF out of the apical pathway and to readily saturate the apical sorting pathway provides essential information for future attempts to identify proteins sorters involved in apical transport. The nature of the apical sorting receptor whose existence is supported by the data in this report remains unknown. Given the suggested involvement of N-glycans as apical sorting signals (12), we tested the hypothesis that it might be a lectin sorter using tunicamycin as an inhibitor of N-glycosylation. Interestingly, the apical polarity of VEGF was not altered in the presence of this inhibitor, even when VEGF showed a clear decrease in apparent molecular weight, consistent with its single glycosylation site not being occupied. These results suggest that N-glycans are not necessary for the apical sorting of VEGF and, by extension, imply that the common sorting receptor also used by TGF is not a lectin. Unfortunately, and consistent with previous reports (28, 31), the secretion of TGF (which has three N-glycan residues) was blocked by tunicamycin, indicating that this growth factor belongs to the group of proteins whose folding/transport along the secretory pathway requires the participation of N-glycans (32–34). However, the secretion of VEGF, as previously reported (35, 36), was not affected by the removal of its single N-glycan. These results support the existence of at least two pathways to the apical surface, one mediated by glycans and a second one mediated by nonglycan signals.
The results reported here have some important practical consequences for gene therapy. The advent of replication defective viruses has provided an efficient vehicle for the transfer of genes into mammalian cells. Many of the genes currently under consideration for the treatment of various diseases encode secreted proteins (37–40). Natural targets to act as factories for the in vivo production of secretory transgenes are simple epithelia. Our results indicate that excessive expression of a secreted protein may result in its presence in an undesired body compartment. As the secretion of a transduced protein into the wrong compartment may result in loss of biological activity or in undesired biological effects, understanding the signals that drive the sorting of membrane and secreted proteins is imperative to being able to control their final destination.
Acknowledgments
The authors are grateful to Imre Kovesdi (Genvec) for providing AdVEGF and Anita Roberts (National Institutes of Health) for providing the TGF-β1 ser223, 225 cDNA. This work was supported by National Institutes of Health Grant R01 EY08538 and a Jules and Doris Stein Professorship awarded by Research to Prevent Blindness Foundation to E.R.-B., National Institutes of Health Grant F32 EY06669 to A.D.M, and a Fight for Sight Grant in Aid awarded to F.R.
Abbreviations
- VEGF
vascular endothelial growth factor
- TGF
transforming growth factor
- moi
multiplicity of infection
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070049497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070049497
References
- 1.Rodriguez-Boulan E, Nelson W J. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 2.Keller P, Simons K. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- 3.Mostov K E. Histol Histopathol. 1995;10:423–431. [PubMed] [Google Scholar]
- 4.Hunziker W, Fumey C, Honing S, Kamel L C. Cell Biol Int. 1994;18:321–325. doi: 10.1006/cbir.1994.1081. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Boulan E, Zurzolo C. J Cell Sci Suppl. 1993;17:9–12. doi: 10.1242/jcs.1993.supplement_17.2. [DOI] [PubMed] [Google Scholar]
- 6.Le Gall A H, Yeaman C, Muesch A, Rodriguez-Boulan E. Semin Nephrol. 1995;15:272–284. [PubMed] [Google Scholar]
- 7.Hunziker W, Harter C, Matter K, Mellman I. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- 8.Matter K, Hunziker W, Mellman I. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 9.Brown D A, Crise B, Rose J K. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- 10.Lisanti M P, Rodriguez-Boulan E. Trends Biochem Sci. 1990;15:113–118. doi: 10.1016/0968-0004(90)90195-h. [DOI] [PubMed] [Google Scholar]
- 11.Lisanti M P, Rodriguez-Boulan E. Cell Biol Int Rep. 1991;15:1023–1049. doi: 10.1016/0309-1651(91)90054-m. [DOI] [PubMed] [Google Scholar]
- 12.Scheiffele P, Peranen J, Simons K. Nature (London) 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 13.Yeaman C, Le Gall A H, Baldwin A N, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Boulan E, Gonzalez A. Trends Cell Biol. 1999;9:291–294. doi: 10.1016/s0962-8924(99)01595-0. [DOI] [PubMed] [Google Scholar]
- 15.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez A, Rizzolo L, Rindler M, Adesnik M, Sabatini D D, Gottlieb T. Proc Natl Acad Sci USA. 1987;84:3738–3742. doi: 10.1073/pnas.84.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzolo M P, Bull P, Gonzalez A. Proc Natl Acad Sci USA. 1997;94:1834–1839. doi: 10.1073/pnas.94.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner A M, Marquardt H, Malacko A R, Lioubin M N, Purchio A F. J Biol Chem. 1989;264:13660–13664. [PubMed] [Google Scholar]
- 19.Sullivan D M, Chung D C, Anglade E, Nussenblatt R B, Csaky K G. Invest Ophthalmol Visual Sci. 1996;37:766–774. [PubMed] [Google Scholar]
- 20.Graham F L, Prevec L. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 21.Marmorstein A D, Bonilha V L, Chiflet S, Neill J M, Rodriguez-Boulan E. J Cell Sci. 1996;109:3025–3034. doi: 10.1242/jcs.109.13.3025. [DOI] [PubMed] [Google Scholar]
- 22.Nabi I R, Rodriguez-Boulan E. Mol Biol Cell. 1993;4:627–635. doi: 10.1091/mbc.4.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1998. [Google Scholar]
- 24.Danielpour D. J Immunol Methods. 1993;158:17–25. doi: 10.1016/0022-1759(93)90254-5. [DOI] [PubMed] [Google Scholar]
- 25.Marmorstein A D, Zurzolo C, Le Bivic A, Rodriguez-Boulan E. In: Cell Biology: A Laboratory Handbook. Celis J, editor. Vol. 4. London: Academic; 1998. pp. 341–350. [Google Scholar]
- 26.Marmorstein A D, Gan Y C, Bonilha V L, Finnemann S C, Csaky K G, Rodriguez-Boulan E. J Cell Biol. 1998;142:697–710. doi: 10.1083/jcb.142.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner A M, Lioubin M N, Marquardt H, Malacko A R, Wang W C, Shapiro R A, Neubauer M, Cook J, Madisen L, Purchio A F. Mol Endocrinol. 1992;6:1691–1700. doi: 10.1210/mend.6.10.1448117. [DOI] [PubMed] [Google Scholar]
- 28.Xue S, Brunner A M, Purchio A F, Gentry L E. Mol Endocrinol. 1989;3:1090–1098. doi: 10.1210/mend-3-7-1090. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb T A, Beaudry G, Rizzolo L, Colman A, Rindler M, Adesnik M, Sabatini D D. Proc Natl Acad Sci USA. 1986;83:2100–2104. doi: 10.1073/pnas.83.7.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazono K, Thyberg J, Heldin C. J Biol Chem. 1992;267:5668–5675. [PubMed] [Google Scholar]
- 31.Brunner A M, Lioubin M N, Marquardt H, Malacko A R, Wang W C, Shapiro R A, Neubauer M, Cook J, Madisen L, Purchio A F. Mol Endocrinol. 1992;6:1691–1700. doi: 10.1210/mend.6.10.1448117. [DOI] [PubMed] [Google Scholar]
- 32.Hebert D N, Zhang J X, Chen W, Foellmer B, Helenius A. J Cell Biol. 1997;139:613–623. doi: 10.1083/jcb.139.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J X, Braakman I, Matlack K E, Helenius A. Mol Biol Cell. 1997;8:1943–1954. doi: 10.1091/mbc.8.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond C, Braakman I, Helenius A. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claffey K P, Senger D R, Spiegelman B M. Biochim Biophys Acta. 1995;1246:1–9. doi: 10.1016/0167-4838(94)00144-6. [DOI] [PubMed] [Google Scholar]
- 36.Peretz D, Gitay-Goren H, Safran M, Kimmel N, Gospodarowicz D, Neufeld G. Biochem Biophys Res Commun. 1992;182:1340–1347. doi: 10.1016/0006-291x(92)91880-y. [DOI] [PubMed] [Google Scholar]
- 37.Svensson E C, Schwartz L B. Curr Opin Cardiol. 1998;13:369–374. doi: 10.1097/00001573-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Middleton P G, Alton E W. Thorax. 1998;53:197–199. doi: 10.1136/thx.53.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang M W, Leiden J M. Semin Interventional Cardiol. 1996;1:185–193. [PubMed] [Google Scholar]
- 40.Reichel M B, Ali R R, Hunt D M, Bhattacharya S S. Ophthalmic Res. 1997;29:261–268. doi: 10.1159/000268024. [DOI] [PubMed] [Google Scholar]