Abstract
Far-UV irradiation of DNA leads to the dimerization of pyrimidine bases, resulting in the formation of cyclobutane type dimers and (6–4) photoproducts. In the dry state, an additional thymine dimeric photolesion, the spore photoproduct, is also generated. While most photoproducts are expected to be produced between adjacent pyrimidines, little attention has been paid to lesions involving bases located on different DNA strands. Using HPLC– mass spectrometry analysis of enzymatically digested DNA, we observed that, in the dry state, inter-strand dimeric photoproducts represented 30% of the total yield of dimeric thymine lesions. The major inter-strand damage was found to be the spore photoproduct. Formation of inter-strand lesions in significant yield could be obtained in solution upon modification of the DNA conformation as the result of the addition of large amounts of ethanol. In both cases, DNA is in the A-form, which is characterized by a high compaction, likely to favor inter-strand photoreactions. Since the latter DNA conformation is also predominant in bacterial spores, the formation and repair of dimeric photoproducts involving thymine bases located on different DNA strands may thus be relevant in terms of deleterious effects of UV radiation to the latter microorganisms.
INTRODUCTION
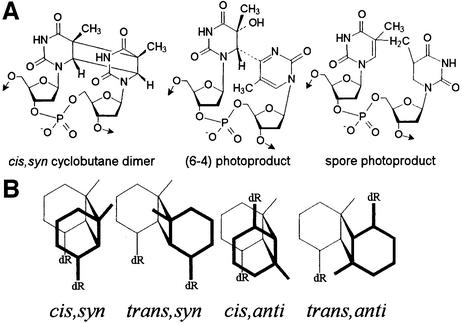
UV radiation is a well known mutagen that induces the formation of a series of lesions within DNA. In the UVC and UVB range, damage is triggered by the direct absorption of the incident light by DNA bases. Reactions of the resulting excited species lead mostly to the dimerization of pyrimidine bases. UV-induced photoproducts exhibit a large variety of chemical structures (1,2). Indeed, cyclobutane type dimers, pyrimidine (6–4) pyrimidone photoproducts and their related Dewar valence isomers may be produced at each of the four bipyrimidine sites with a strong sequence dependence (3). Under anhydrous conditions, such as within bacterial spores, 5,6-dihydro-5-(α-thyminyl)-thymine, the so-called ‘spore photoproduct’ (Fig. 1) is additionally generated by a photoreaction involving two thymine moieties (4–6). Moreover, all these photoproducts exhibit asymmetric centers in their base moiety. Consequently, several isomers may be produced. For instance, the position of the pyrimidine bases with respect to the cyclobutane ring defines the cis and trans isomers of the cyclobutane dimers. In addition, the latter lesions may be either syn or anti, depending on whether the C5–C6 bonds of the two pyrimidines are parallel or antiparallel, respectively. However, while all the different isomers may be isolated following irradiation of free bases and nucleosides, the configuration of the DNA helix makes the UV-induced dimerization of adjacent pyrimidine bases very stereospecific. For a given bipyrimidine sequence, only one diastereoisomer of the (6–4) photoproduct is produced, together with the corresponding cis,syn cyclobutane dimer. Under specific conditions, one of the diastereoisomers of the thymine trans,syn cyclobutane dimer may also be obtained in low yield (7,8). The variety of photoproducts within double-stranded DNA might be even more complex than it may be anticipated from the chemical structure of their base moiety. Indeed, pyrimidine dimerization is usually discussed in terms of photoreaction between adjacent bases but photoproducts may also be formed between non-adjacent pyrimidines. This has been proposed to occur in single-stranded poly(dG·dT) (9,10) and in DNA hairpins (11). The resulting photoproducts have been shown to induce frameshift mutations upon replication of synthetic oligonucleotides (12). In the latter works, the modified bases were supposed to be located on the same DNA strand. In contrast, very few data are available on photoproducts between pyrimidines of different DNA strands.
Figure 1.
UV-induced thymine dimeric lesions within DNA. (A) Chemical structure of the photoproducts involving adjacent bases. The stereochemistry of the spore photoproduct formed within DNA has not yet been determined. (B) Schematic representation of the different isomers of the thymidine cyclobutane dimer. dR, 2-deoxyribose. cis,syn and trans,anti cyclobutane dimers are meso forms and thus exist as a single isomer.
We presently report that inter-strand thymine dimeric photoproducts constitute a major class of UVC-induced lesions in dry DNA. The photochemistry of DNA in the dry state has received much less attention (13) than in aqueous solution, for obvious reasons of extrapolation of the results to the photochemistry of DNA in a cellular environment. However, UV irradiation of dehydrated DNA has recently regained attention. First, UV-irradiated dry films of DNA have been proposed to be used as a new material (14). A possible application could be the trapping of substances exhibiting toxic properties through interaction with cellular DNA (15,16). Photochemistry of DNA in the dry state is also widely studied in relation to the behavior of bacterial spores exposed to extraterrestrial solar radiation (17,18). In addition to its formation, the spore photoproduct is the topic of extensive investigations in terms of repair. Indeed, several groups are studying the spore photoproduct lyase, a DNA repair enzyme that is able to catalyze reversion of the spore photoproduct to the original unmodified thymines (19–21). Even though this activity is well known, the detailed enzymatic process remains to be completely elucidated (22–24). Radiolabeled plasmid DNA exposed to UV-radiation in the dry state is often used as substrate for these studies (24,25). Altogether, the exhaustive distribution of photo-induced lesions within UV-irradiated dry DNA is information of interest in several fields.
We have recently reported the distribution of photoproducts involving adjacent pyrimidine bases in UVC-irradiated films of DNA (26). The main differences with results obtained in aqueous solution were the formation of the spore photoproduct and the main trans,syn diastereoisomer of the TT cyclobutane dimer, together with an increase in the ratio between the yield of (6–4) adduct and cyclobutane dimer at TC sites. The study of the photochemistry of DNA in the dry state is presently extended to the formation of inter-strand dimeric lesions. This investigation required the development of a highly specific assay for the quantification of these photoproducts based on the use of HPLC coupled to tandem mass spectrometry following appropriate DNA hydrolysis. Emphasis was also placed on the role of the conformational differences between DNA in the dry state, on the one hand, and in aqueous solution, on the other, that may explain the formation of inter-strand photoproducts. For this purpose, experiments involving irradiation of DNA in the presence of a large amount of ethanol and studies on the UVC-photolysis of thymidine (Thd) under different conditions were carried out. Altogether, it could be concluded that DNA in the A-form favors the formation of both inter-strand thymine dimer lesions and the spore photoproduct.
MATERIALS AND METHODS
Chemicals
Calf thymus DNA, phosphodiesterase I, phosphodiesterase II and nuclease P1 were purchased from Sigma (St Louis, MO). Ethanol was analytical grade (Prolabo, France). Calibrated solutions of the cyclobutane dimers, spore photoproduct, (6–4) photoproduct and Dewar valence isomer of thymidylyl-(3′-5′)-thymidine (TpT) were prepared as previously reported (7,8). Thd dimeric photoproducts were synthesized and isolated as previously described (27,28). The concentration of the solutions of Thd cyclobutane dimers was determined following photoreversion of the compounds to Thd and subsequent measurement of the UV absorption at 267 nm. The concentration of the solutions of (6–4) adducts and spore photoproducts was determined spectrophotometrically using reported UV absorption features (29). Thymine cis,syn cyclobutane dimer and spore photoproduct were prepared by hot formic acid hydrolysis (88%, 135°C, 6 h) of the corresponding Thd derivatives. Solutions of resulting photoproducts were then calibrated by UV spectrophotometry, using reported molecular absorption coefficients (29).
HPLC–mass spectrometry analyses
Samples were injected onto an Agilent 1100 series chromatographic system equipped with an Uptisphere ODB (Montluçon, France) octadecylsilyl silica gel column (3 µm particle size, 150 × 2 mm i.d.). The column oven was set at 28°C and the flow rate was 200 µl min–1. The mobile phase was a gradient of acetonitrile (maximum proportion 20% v/v) in either 2 mM ammonium formate or 2 mM triethylammonium acetate. Methanol was added at a flow rate of 100 µl min–1 at the outlet of the UV detector set at 280 nm. Then, the resulting mixture was directed towards an API 3000 triple quadrupolar mass spectrometer (Perkin-Elmer/Sciex, Thornhill, Canada). The latter apparatus was used either in the product ion scan or the multiple reaction monitoring (MRM) mode. In the former configuration, the first quadrupole is used to isolate the pseudo-molecular ion to be characterized prior to fragmentation in the collision cell. The spectrum of resulting daughter ions is determined by scanning of the third quadrupole. In the MRM mode, pseudo-molecular ions of a given mass are isolated in the first quadrupole and fragmented. Daughter ions specific to the compound of interest are then quantified following isolation in the third quadrupole.
Irradiation of DNA
The UVC source used was a 2 × 15 W germicidal lamp (VL 215G; Bioblock Scientific, Illkirch, France). A stock solution of isolated calf thymus DNA (1 mg ml–1 in deionized water) was prepared. Irradiation of DNA in solution was performed with aliquots of 3 ml under magnetic stirring in a 4.5 cm diameter Petri dish. The lamp was placed above the sample. The fluence was 2100 J m–2 min–1 and irradiation times ranged between 5 and 60 min. Dry DNA films were prepared by lyophilization of 2 ml of solution in a 4.5 cm diameter Petri dish. Following freeze-drying, the film was allowed to equilibrate with ambient atmosphere for 1 h. It was then exposed to the UVC light (fluence 2400 J m–2 min–1) for 1 min. The film was then solubilized in 2 ml of water and an aliquot (150 µl) was collected. The remaining solution was freeze-dried. The same irradiation protocol was repeated three times (overall exposure times 1, 2, 3 and 4 min). Irradiations were performed in triplicate. Following exposure to the lowest dose (2100 and 2400 J m–2 for dry film and aqueous solution, respectively), melting temperature (Tm) of calf thymus DNA was determined and compared with that of a non-irradiated sample. For this purpose, the concentrated DNA solution (1 mg ml–1) was diluted 30 times in 50 mM NaCl. The thermal denaturation was followed between 20 and 90°C by monitoring the UV absorption at 260 nm on a 8453 Hewlett-Packard spectrophotometer equipped with a 89090A thermostated cell. Another series of experiments involved irradiation of DNA solutions containing increasing concentrations of ethanol. For that purpose, 200 µl of a 1 mg ml–1 DNA solution was mixed with the appropriate volume of deionized water and ethanol in order to obtain 1 ml of solution containing 0, 20, 40, 60 or 80% (v/v) of the latter alcohol. Samples were exposed, under magnetic stirring in a watch glass, to increasing doses of UVC (800, 1500 and 3100 J m–2). Ethanol was then removed under vacuum and the resulting aqueous phase was freeze-dried. The obtained residue was solubilized in 200 µl of deionized water.
Quantification of photoproducts
Following any type of irradiation, fractions of 50 µl of DNA solution were digested as previously reported (8) by nuclease P1 and phosphodiesterase I at pH 5.5 for 2 h at 37°C. The pH was then adjusted to 8, and alkaline phosphatase and phosphodiesterase II were added. The sample was left at 37°C for 2 h. Then, the pH was set at 6.5 by addition of 0.1 N HCl. The samples were analyzed by HPLC–MS/MS for their content of TpT lesions as previously described (8). Thd dimeric photoproducts were quantified in the same samples. The chromatographic conditions were very similar to those used for TpT lesions, with the exception that the aqueous mobile phase was a 2 mM solution of ammonium formate instead of triethylammonium acetate. Thd photoproducts were quantified by MRM mass spectrometry detection in both the positive and negative electrospray modes. Chromatographic features of the photoproducts and transitions used for their detection are listed in Table 1. The difference between values obtained in the negative and positive modes was below 5% and results were thus averaged. The contents of thymine spore photoproduct and cis,syn cyclobutane dimer were also determined following hot 88% formic acid hydrolysis of DNA. The respective transitions used were 253→236 and 253→210 in the positive mode, and 251→208 and 251→125 in the negative mode. The HPLC conditions were the same as for the detection of Thd lesions. The retention times of the thymine spore photoproduct and cis,syn cyclobutane dimer were 14.7 and 10.9 min, respectively. In all cases, the amount of photoproduct detected was determined by external calibration. The amount of analyzed DNA was inferred from the area of the peak of 2′-deoxyguanosine and adenine of the HPLC-UV chromatogram of enzymatically digested and chemically hydrolyzed samples, respectively.
Table 1. Parameters of the HPLC–MS/MS detection of Thd dimeric photoproducts in the positive (ESP+) and negative (ESP–) electrospray ionization modes.
| Product | ||||||
|---|---|---|---|---|---|---|
| <>c,s | <>t,s | <>c,a | <>t,a | (6–4) | Spore | |
| Retention time (min) | 16.8 | 17.1/17.6 | 18.9/19.2 | 23.4 | 23.0/25.4 | 25.6/27.6 |
| ESP+ | 253 | 253 | 127 | 127 | 235 | 253 |
| ESP– | 350 | 350 | 125 | 241 | n.d.a | 279 |
Parent ions were set at m/z = 485 and 483, respectively. Detection in the multiple reaction monitoring mode involved the daughter ions listed. Retention times are provided for the single or the (+) and (–) diastereoisomers of each photoproduct. <>c,a, <>c,s, <>t,s and <>t,a, cis,anti, cis,syn, trans,syn and trans,anti cyclobutane dimers; (6–4), (6–4) photoproduct; spore, spore photoproduct.
aThymidine (6–4) photoproducts are ionized in very low yield in the negative mode and cannot be quantified under these conditions.
Irradiation of thymidine
UVC irradiation of Thd was performed in a 10 cm diameter Petri dish using the UVC source described above. First, 15 ml of a 1 mM Thd solution was frozen at –80°C and then exposed to UVC light on dry ice (overall dose 100 000 J m–2). Another sample was prepared by lyophilization of 15 ml of a 1 mM aqueous solution of Thd followed by exposure of the resulting film to 40 000 J m–2 UVC light. A similar experiment was performed with a Thd film prepared by overnight evaporation of a 10 mM solution (15 ml) of Thd in ethanol in a fume hood. Following irradiation, the films prepared for the latter two experiments were solubilized in 15 ml of deionized water. All irradiated samples were analyzed by HPLC–MS/MS in the product ion scan mode. Fragmentation of either protonated ([M+H]+, m/z = 485) or deprotonated ([M–H]–, m/z = 483) pseudo-molecular ions of the dimeric photoproducts was monitored. The HPLC UV detector was set at 260 nm.
RESULTS
Analytical approach
Pyrimidine dimeric photoproducts were quantified by HPLC coupled to tandem mass spectrometry following hydrolysis of DNA. The latter step was carried out under different conditions. First, hot acidic hydrolysis was used to release the photoproducts as modified bases through the cleavage of the N-glycosidic bonds. This DNA hydrolysis procedure has been widely applied to irradiated radiolabeled DNA in order to quantify cyclobutane dimers (7,30,31) and spore photoproducts (32–34). However, this approach failed to accurately quantify the labile (6–4) photoproducts and their related Dewar valence isomers. Digestion of DNA by exonucleases was used as an alternative hydrolysis technique. The latter enzymes have been found to be unable to cleave the intra-dimer phosphodiester bonds (35,36). The photoproducts involving adjacent pyrimidines are thus released as modified dinucleoside monophosphates that are readily quantified by HPLC–MS/MS.
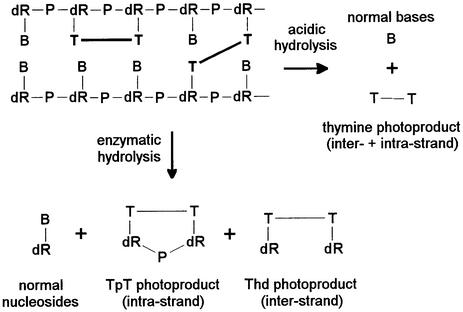
In the present work, enzymatic digestion of DNA was also applied to the quantification of dimeric photoproducts involving thymine bases located on different DNA strands. This approach was based on the assumption that, as far as the DNA digestion was concerned, inter-strand photoproducts could be considered as two monomeric lesions. Therefore, the enzymes used for the digestion should release the latter class of lesions as nucleoside derivatives (Fig. 2). One limitation might have been the bulky nature of the photoproducts that may reduce the activity of the enzymes. However, in contrast to lesions involving adjacent pyrimidines, inter-strand damage is unlikely to strongly distort the short pieces of DNA produced during the digestion process. In addition, the combination of endo- and exonucleases used has been shown to allow the release of digestion-resistant lesions such as thymidine glycols and 5-formyl-2′-deoxyuridine (37).
Figure 2.
Principle of the enzymatic and acidic release of inter- and intra-strand photoproducts from DNA. B, normal base; dR, 2-deoxyribose; P, phosphate.
HPLC–MS/MS was then used to discriminate between the inter- and intra-strand photoproducts in the enzymatically digested samples; the molecular weight of TpT photoproducts is 546 while that of the corresponding Thd lesions is 484. In addition, we previously reported that the structure of Thd dimeric photoproducts exhibited a drastic effect on their collision-induced fragmentation pathways in electrospray mass spectrometry in the positive mode (28). In the course of the present work, similar observations were made in the negative mode (data not shown). This data allowed the setting-up of highly specific MRM methods in both the negative and positive modes. In addition, the specificity of the detection was improved by the efficient separation of all of the Thd photoproducts on the reverse-phase HPLC column.
UVC irradiation of thymidine
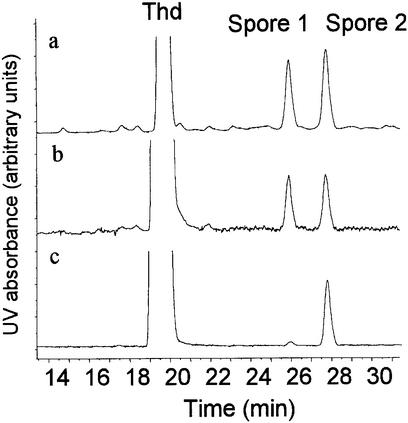
HPLC–MS/MS was found to be a straightforward technique to determine the distribution of photoproducts within UVC-irradiated Thd samples. Indeed, combination of HPLC separation and analysis of the fragmentation mass spectra made possible the identification and determination of the relative yield of all possible photoproducts in a single injection. Therefore, the latter assay allowed the accurate comparison of the effect of exposure of Thd to UVC under various conditions, as an extension of early works (29,38,39). A first observation was the similarity in the relative yields of the different photoproducts in frozen and lyophilized aqueous solution (Table 2). In contrast, the distribution of dimeric lesions was drastically different in dry Thd depending on the preparation of the solid film. Indeed, when an ethanolic solution was dried, spore photoproducts, and mostly one of the two diastereoisomers (Fig. 3), were the major UV-induced lesions. In contrast, irradiation of a freeze-dried aqueous solution yielded all photoproducts in significant yields (Table 2). Under the latter conditions, the two diastereoisomeric spore photoproducts were generated in similar amounts.
Table 2. Distribution of dimeric photoproducts (expressed as a percentage of the combined yield of quantified lesions) upon exposure of Thd to UVC light under different conditions.
| Condition | Product | |||||
|---|---|---|---|---|---|---|
| <>c,s | <>t,s | <>c,a | <>t,a | Spore | (6–4) | |
| Frozen aqueous solution | 10 | 12 | 24 | 3 | 38 | 13 |
| Lyophilized aqueous solution | 12 | 22 | 28 | 7 | 27 | 4 |
| Air-dried ethanolic solution | 5 | 6 | 8 | 1 | 78 | 2 |
<>c,a, <>c,s, <>t,s and <>t,a, cis,anti, cis,syn, trans,syn and trans,anti cyclobutane dimers; (6–4), (6–4) photoproduct; spore, spore photoproduct. For the trans,syn and cis,anti cyclobutane dimers, the (6–4) and the spore photoproducts reported values represent the sum of the yield of the two diastereoisomers.
Figure 3.
Chromatograms recorded upon analysis of Thd exposed to UVC radiation in (a) frozen aqueous solution, (b) lyophilized aqueous solution and (c) air-dried ethanolic solution. An octadecylsilyl silica gel column (3 µm particle size, 150 × 2 mm i.d.) was used at a temperature of 28°C. The mobile phase (flow rate 200 µl min–1) was a gradient of acetonitrile (maximum proportion 20% v/v) in 2 mM ammonium formate. The elution was monitored by a UV spectrophotometer set at 260 nm. Other dimeric photoproducts are not detected due to their low absorption at the latter wavelength.
Quantification of inter-strand photoproducts in UVC-irradiated DNA
The formation of inter-strand photoproducts released as Thd lesions and intra-strand dimeric lesions hydrolyzed as modified dinucleoside monophosphates was quantified within UVC-irradiated isolated DNA. Only traces of the inter-strand cis,syn cyclobutane dimer and, to a lesser extent, of the trans,anti related lesion were detected within DNA exposed to UVC in aqueous solution. The respective yields were 0.022 and 0.004 lesions 108 bases–1 J–1 m–2, while that of the intra-strand cis,syn thymine cyclobutane dimer was 8.4 lesions 108 bases–1 J–1 m–2. It should be mentioned that reported yields for the latter photoproduct are often in the range of 1 lesion 105 bases–1 J–1 m–2. This apparent discrepancy with our results is accounted for by the fact that we used concentrated DNA solutions in order to limit the level of photoproducts and prevent secondary photoreactions. We have previously shown that the quantum yield obtained under our conditions is in close agreement with available data (40).
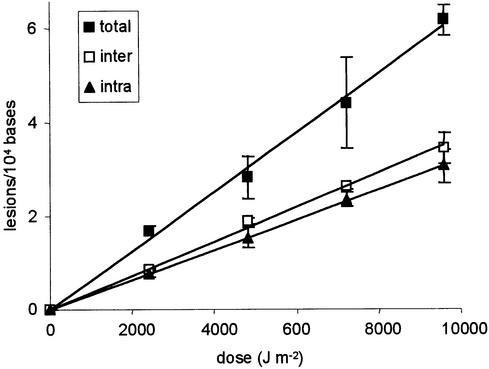
In contrast to results obtained with aqueous solutions, photoproducts involving non-adjacent thymine residues were detected in high yields in UVC-irradiated dry DNA. The two diastereoisomers of the Thd spore photoproduct were detected in almost equal amounts in enzymatically digested DNA samples. Their level was found to be proportional to the applied UVC dose and their combined yield was slightly higher than that of the corresponding intra-strand lesion (Fig. 4). Quantification of the spore photoproduct was also performed as a thymine dimeric lesion following acidic hydrolysis. The level of spore photoproduct measured under the latter conditions was found to be equal to the combined amount of TpT and Thd spore photoproducts detected in enzymatically digested DNA samples (Fig. 4). Similar observations were made for the cis,syn cyclobutane dimer. Its formation was found to be linear with respect to the dose. As for the spore photoproduct, the sum of the yield of inter-strand and intra-strand cis,syn thymine cyclobutane dimer determined in enzymatically digested DNA was identical to that of the related thymine lesion quantified following acidic hydrolysis. The yields of formation were found to be 2.9, 9.7 and 13.6 lesions 108 bases–1 J–1 m–2 for the Thd, TpT and Thy derivatives, respectively.
Figure 4.
Formation of the spore photoproduct within UV-irradiated freeze-dried DNA. The lesion was quantified as dinucleoside monophosphate (TpT, intra-strand) or thymidine (Thd, inter-strand) dimeric photoproduct in enzymatically digested DNA. The reported level of Thd spore photoproducts represents the sum of the amount of the two diastereoisomers. The total level of spore photoproduct, released as a thymine lesion, was measured in acid-hydrolyzed DNA samples. Results represent the mean ± SD of three independent experiments.
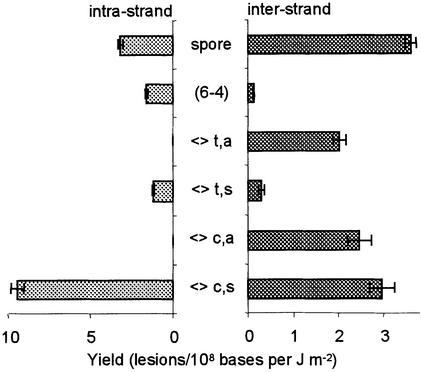
Actually, all types of Thd photoproducts resulting from the formation of dimeric lesions between non-adjacent thymine bases were readily detected within UVC-irradiated dry DNA (Fig. 5). The spore photoproduct was the main thymine inter-strand dimeric lesion. The cis,syn, cis,anti and trans,anti isomers of the cyclobutane dimers were also produced in significant yields. The level of inter-strand (6–4) photoproduct and trans,syn cyclobutane dimer was significantly lower. The yield of inter-strand lesions could be compared with those of photoproducts involving adjacent thymine bases (26). Altogether, inter-strand lesions represented 31% of the quantified thymine photoproducts. This high yield of inter-strand photoproducts resulted in a drastic stabilization of the DNA duplex by formation of covalent bonds between bases located on opposite strands. Indeed, the Tm value for calf thymus DNA exposed to UVC light in the dry state was found to be 84.8°C while that of freeze-dried non-irradiated DNA was 78.8°C. In contrast, calf thymus DNA exposed to UVC radiation in aqueous solution exhibited a Tm value of 75.4°C. The latter result is in agreement with a destabilization of the double helix by intra-strand bipyrimidine photoproducts, as previously suggested by the formation of trans,syn cyclobutane dimer between adjacent thymine bases upon exposure of aqueous solution of DNA to high doses of UVC light (8).
Figure 5.
Distribution of intra- and inter-strand thymine photoproducts within UVC-irradiated freeze-dried DNA (<>c,a, <>c,s, <>t,s and <>t,a, cis,anti, cis,syn, trans,syn and trans,anti cyclobutane dimers). Yields of formation of intra-strand photoproducts were taken from Douki and Cadet (26). Results represent the yield of formation ± SE obtained by linear regression of the level of lesion with respect to the applied dose (n = 15). For the trans,syn and cis,anti cyclobutane dimers, the (6–4) and the spore photoproducts, reported values represent the sum of the yield of the two diastereoisomers.
Effect of the DNA conformation on the photoproduct distribution
Modification of the DNA conformation was induced by addition of ethanol to an aqueous solution, using the protocol described by Patrick and Gray (7). Both inter- and intra-strand photoproducts were quantified. Three increasing doses of UVC radiation were applied to the samples. A linear dose–course relationship was observed for all photoproducts and all ethanol concentrations. Linear regression of the latter data allowed determination of the yield of each of the studied lesions. As previously reported, addition of ethanol resulted in a decrease in the yield of intra-strand cis,syn cyclobutane dimer (7). Indeed, the yield of the latter lesion in the presence of 80% ethanol represented only 64% of that formed in pure aqueous solution. A similar observation was made for the (6–4) photoproduct. It may be added that, in order to prevent DNA precipitation, these experiments were performed at low ionic strength. As previously observed (40), significant formation of the main trans,syn isomer of the intra-strand thymine cyclobutane dimer was observed under these conditions. The conformational changes associated with the addition of ethanol had a much more drastic effect on the formation of the latter photoproduct than on the other intra-strand lesions since its yield upon UVC irradiation of DNA in 80% ethanol represented only 11% of that generated in pure water.
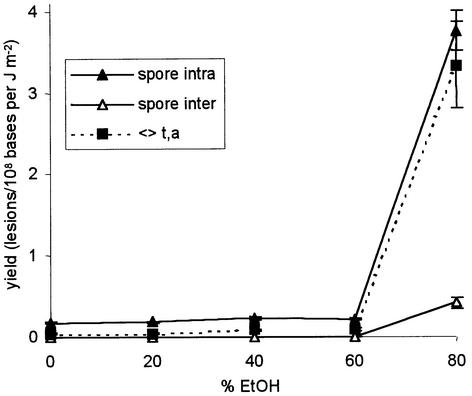
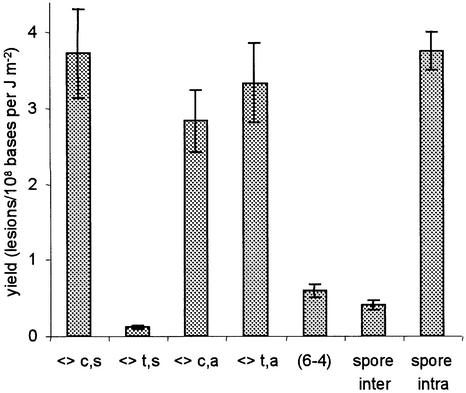
In contrast to the three above-mentioned lesions involving adjacent thymines, the spore photoproduct was detected in larger amounts in DNA samples irradiated in the presence of 80% ethanol. As previously reported (7), the increase in the yield of spore photoproduct was not linear with respect to the ethanol concentration but occured abruptly for alcohol concentrations between 60 and 80% (Fig. 6). Interestingly, the same trend was observed for the inter-strand thymine dimer photoproducts. The cis,syn, cis,anti and trans,anti thymine cyclobutane dimers were the major inter-strand lesions, while the (6–4) photoproduct and the trans,syn cyclobutane dimer were detected in lower amounts (Fig. 7). The main difference between DNA exposed to UV light in the dry state and in 80% ethanolic solution was the proportion of inter-strand spore photoproduct. Indeed, the ratios between the yield of intra- and inter-strand spore photoproduct were 0.9 and 9.1, respectively.
Figure 6.
Effect of the presence of ethanol on the yield of formation of intra- and inter-strand spore photoproducts and inter-strand trans,anti thymine cyclobutane dimer. Each point represents, for a defined ethanol concentration, the yield of formation (± SE) obtained by linear regression of the level of lesion with respect to the applied dose (n = 12).
Figure 7.
Distribution of inter-strand lesions and spore photoproducts within isolated DNA exposed to UVC light in aqueous solution containing 80% ethanol. Each value represents the yield of formation (± SE) obtained by linear regression of the level of lesion with respect to the applied dose (n = 12).
DISCUSSION
The far-UV photochemistry of DNA components is now for the most part well established. Identification of the major lesions and determination of their physical and chemical properties has been extensively carried out on model systems such as bases, nucleosides and dinucleoside monophosphates (for reviews see 1,2). However, the distribution of UV-induced photoproducts within DNA has long remained to be completely determined. Indeed, most of the biochemical assays used for the quantification of the latter lesions do not exhibit a high specificity. First, methods based on the use of antibodies raised against a given class of photolesions (41–43) do not allow the individual measurement of the photoproducts at each of the four possible bipyrimidine sites. This limitation is partly avoided in assays involving sequencing following conversion of the lesions into strand breaks (44–46). However, the sensitivity of such techniques is often poor. In addition, (6–4) photoproducts are mostly revealed following alkaline treatment, which is known to degrade only a minor fraction of the latter lesion (47–49). HPLC associated with tandem mass spectrometry represents an interesting alternative to achieve a more specific quantification of UV-induced DNA photoproducts. In particular, we applied the latter approach to the determination of the distribution of each of the four possible cyclobutane dimers, (6–4) photoproducts and Dewar valence isomers within isolated and cellular DNA (3,8,28). The assay was also recently applied to 2′-deoxyuridine hydrates (50) and the spore photoproduct involving adjacent thymine bases (26).
In the present work, HPLC–MS/MS was used to quantify the formation of inter-strand thymine photoproducts, based on the assumption that the latter type of lesions would be released as nucleoside dimers following cleavage of the phosphodiester linkage by phosphodiesterases. A possible flaw in the latter approach could be the cleavage, by the digestion enzymes, of the intra-dimer phosphate group of photoproducts involving adjacent pyrimidines. However, this has not been observed upon incubation of trinucleotides carrying cyclobutane dimers and (6–4) photoproducts with phosphodiesterases (35,36). In addition, in contrast to early reports (35), we (36) and others (51–53) did not observe any hydrolysis of the intra-dimer phosphate group by nuclease P1. The latter observations of the resistance of the phosphodiester linkage of bipyrimidine photoproducts to enzymatic hydrolysis are presently confirmed by the negligible amount of cyclobutane dimer and (6–4) photoproduct detected as Thd derivatives in digests of DNA exposed to UV light in aqueous solution. Moreover, the ratio between intra- and inter-strand spore photoproducts was 10 times lower in DNA exposed to UV light in solution containing 80% ethanol than upon irradiation of dry DNA films. This shows that the large proportion of inter-strand spore photoproduct detected under the latter conditions was not artifactual and that the phosphodiester group of the TpT derivative of the spore photoproduct was also resistant to exonuclease activities. These observations are in agreement with time–course studies of the enzymatic hydrolysis of the latter lesion from DNA as modified TpT (26). The quantitative aspect of the release of the inter-strand dimers as nucleoside photoproducts was also inferred from the identity of the amount of spore photoproduct and cis,syn thymine cyclobutane dimer quantified as base derivatives, on the one hand, and as modified dinucleoside monophosphates and Thd lesions, on the other. Indeed, the acidic hydrolysis aimed at releasing the modified bases is expected to allow the quantification of the total amount of photoproducts. Therefore, since the release of intra-strand lesions as dinucleoside monophosphate is quantitative, it may be concluded that all inter-strand photolesions are quantitatively detected as modified nucleosides. Altogether, this series of control experiments established the reliability of the proposed analytical approach for the separate quantification of inter- and intra-strand UV-induced photoproducts.
The induction of inter-strand dimeric lesions was found to be a major feature of the photochemistry of DNA in the dry state, in addition to the established formation of the spore photoproduct (4,13). Indeed, the global yield of inter-strand photoproducts represented one-third of that of overall thymine dimer lesions. This was confirmed by thermal denaturation studies showing that, in contrast to irradiation in aqueous solution, exposure of DNA to UVC light in the dry state results in the stabilization of the double helix. One likely explanation for the latter observation is the induction of covalent bonds, i.e. photoproducts, between the two DNA strands. In contrast, photoproducts involving non-adjacent thymine bases were barely detected in UVC-irradiated DNA solutions. Interestingly, not all inter-strand lesions were equally produced in dry DNA films. Inter-strand (6–4) photoproducts, eluted as two separated diastereoisomers, were detected in relatively low amounts, as well as the (+) and (–) trans,syn diastereoisomers of the cyclobutane dimer. The corresponding cis isomer of the latter photoproduct was produced in a 10 times higher yield. Both the cis,anti and trans,anti isomers of cyclobutadithymidine were also obtained in large amounts. The latter result provided further support for the fact that the quantified lesions were actually inter-strand photoproducts. Indeed, while one can argue that the syn isomers of thymidine cyclobutane dimer may involve non-adjacent bases located on the same strand of the duplex in unusual conformations or in pieces of single-stranded DNA, anti cyclobutane dimers may only be obtained between thymine residues with their respective C5=C6 double bond in an antiparallel orientation. Such a conformation cannot be obtained between pyrimidines covalently linked to the same DNA strand. Among all lesions involving thymine residues located on different strands, the spore photoproduct was produced in the highest yield. The level of the latter lesion was even slightly higher than that of the corresponding photoproduct involving adjacent thymines. Interestingly, the two diastereoisomers of the spore photoproduct of Thd were detected in approximately equal amounts. In contrast, recent observations suggested that the spore photoproduct was induced between adjacent thymines as a single diastereoisomer (26). These results strengthen the proposal that inter-strand lesions are produced in the dry state.
We then tried to establish whether the formation of inter-strand dimeric lesions was either specific to dry DNA as the result of dehydration or explained by the conformation encountered under the latter conditions. A similar issue has previously been discussed for the formation of the spore photoproduct (7,13). Experiments involving UVC irradiation of free Thd showed the predominant effect of the stacking between thymine moieties on the distribution of UV-induced photoproducts. Indeed, when a dry film was prepared by lyophilization of an aqueous solution, all types of photoproducts were produced while one of the two diastereoisomers of the spore photoproduct was the major lesion upon irradiation of a Thd film prepared by evaporation of an ethanolic solution. In both cases, Thd was in the dry state but the arrangement of the molecules differed due to differences in film preparation. In contrast, similar results were obtained with frozen and freeze-dried aqueous solution, indicative of a minor effect of the presence of water molecules. Further evidence for the major role played by the conformation was obtained from experiments involving exposure of DNA to UVC light in aqueous solution containing a high concentration of ethanol. Indeed, it has been reported that for a proportion of ethanol higher than 60–70% (7,54), DNA exhibits a conformation similar to the A-form adopted in the dry state (55). Interestingly, the formation of spore photoproduct is still favored in such ethanolic solutions in spite of the presence of water (7). Using HPLC–MS/MS, we observed that the number of inter-strand dimeric lesions and spore photoproduct within irradiated DNA remained very low for ethanol concentrations below 60%. A sharp increase in the yield of both types of photoproducts was observed when the proportion of ethanol was 80%. The remarkable similarity in the distribution of inter-strand thymine dimer lesions in 80% ethanolic solution and in dry films of DNA strongly suggests that conformational features are involved in the formation of non-adjacent thymine photoproducts.
The fact that dry DNA (55,56) and DNA in 80% ethanol (7,54) are both mostly in the A-form, rather than in the B-form as in aqueous solution, provides further support for the major role of the conformation in the formation of inter-strand lesions. However, the analytical approach used does not allow definitive conclusions to be drawn on whether the involved thymine bases are located on opposite strands of the same DNA duplex or on different DNA fibers. The latter proposal would provide an explanation for the insolubility of highly UVC-irradiated DNA films (14), which is expected to be extensively reticulated. However, formation of photoproducts between different DNA fibers is hampered by the low accessibility of the C5=C6 double bond of thymine bases. In addition, the observation that inter-strand photoproducts are produced in solution in the presence of 80% ethanol also suggests that thymines of a same duplex are involved. Indeed, even though DNA is partly aggregated under the latter conditions (54,57), interaction between DNA fibers is likely to be much weaker than in the dry state. Moreover, A-form DNA is characterized by a much higher compaction than the B-conformation. This is likely to explain the favored inter-strand photoreactions within DNA in the dry state and in 80% ethanolic solution.
Interestingly, A-form DNA is largely predominant in bacterial spores following complexation of small acid-soluble proteins (25,58). Therefore, it would be interesting to check whether inter-strand spore photoproducts are produced therein and, if so, if the spore photoproduct lyase is able to restore the chemical integrity of DNA at such damaged sites. More generally, the formation and repair of inter-strand UV-induced lesions by either nucleotide excision repair (59), photolyases (60), UV endonuclease (61) or the system involved in the removal of chemical crosslinks (62) would also be interesting to investigate. In that respect, the analytical tools developed in the present study would be useful to monitor the rate of elimination of the photoproducts.
Acknowledgments
ACKNOWLEDGEMENT
Partial support for this work was provided by the Centre National d’Etudes Spatiales (France) and the European Space Agency.
REFERENCES
- 1.Cadet J. and Vigny,P. (1990) Photochemistry of nucleic acids. In Morrison,H. (ed.), Bioorganic Photochemistry. Wiley, New York, NY, Vol. 1, pp. 1–272.
- 2.Douki T. and Cadet,J. (1995) UV and nucleic acids. In Jornvall,H. and Jollès,P. (eds), Interface between Chemistry and Biochemistry. Birkhauser Verlag, Basel, Switzerland, pp. 173–197.
- 3.Douki T. and Cadet,J. (2001) Individual determination of the yield of the main-UV induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions. Biochemistry, 40, 2495–2501. [DOI] [PubMed] [Google Scholar]
- 4.Donnellan J.E. and Setlow,R.B. (1965) Thymine photoproducts but not thymine dimers found in ultraviolet-irradiated bacterial spores. Science, 149, 308–310. [DOI] [PubMed] [Google Scholar]
- 5.Varghese A.J. (1970) 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem. Biophys. Res. Commun., 38, 484–490. [DOI] [PubMed] [Google Scholar]
- 6.Setlow P. (2001) Resistance of spores of Bacillus subtilis to ultraviolet light. Environ. Mol. Mutagen., 38, 97–104. [DOI] [PubMed] [Google Scholar]
- 7.Patrick M.H. and Gray,D.M. (1976) Independence of photoproduct formation on DNA conformation. Photochem. Photobiol., 24, 507–513. [DOI] [PubMed] [Google Scholar]
- 8.Douki T., Court,M., Sauvaigo,S., Odin,F. and Cadet,J. (2000) Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by HPLC-MS/MS. J. Biol. Chem., 275, 11678–11685. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen H.T. and Minton,K.W. (1988) Ultraviolet-induced dimerization of non-adjacent pyrimidines. A potential mechanism for the targeted –1 frameshift mutation. J. Mol. Biol., 200, 681–693. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen H.T. and Minton,K.W. (1989) Extensive photodimerization of non-adjacent pyrimidines. J. Mol. Biol., 210, 869–874. [DOI] [PubMed] [Google Scholar]
- 11.Todd P.A. and Glickman,B.W. (1982) Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc. Natl Acad. Sci. USA, 79, 4123–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingbeck J.M. and Taylor,J.-S. (1999) Preparation and characterization of DNA containing a site-specific nonadjacent cyclobutane thymine dimer of the type implicated in UV-induced –1 frameshift mutagenesis. Biochemistry, 38, 13717–13724. [DOI] [PubMed] [Google Scholar]
- 13.Rahn R.O. and Hosszu,J.L. (1969) Influence of relative humidity on the photochemistry of DNA films. Biochim. Biophys. Acta, 190, 126–131. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M., Kato,K., Nomizu,M., Sakairi,N., Ohkawa,K., Yamamoto,H. and Nishi,N. (2002) Preparation and characterization of DNA film induced by UV irradiation. Chem. Eur. J., 8, 1407–1412. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M., Kato,K., Nomizu,M. and Haruki,M. (2002) UV-irradiated DNA matrix selectively accumulates heavy metal ions. Bull. Chem. Soc. Jpn, 75, 1627–1632. [Google Scholar]
- 16.Yamada M., Kato,K., Nomizu,M., Ohkawa,K., Yamamoto,H. and Nishi,N. (2002) UV-irradiated DNA matrixes selectively bind endocrine disruptors with a planar structure. Environ. Sci. Technol., 36, 949–954. [DOI] [PubMed] [Google Scholar]
- 17.Horneck G. (1995) Exobiology, the study of the origin, evolution and distribution of life within the context of cosmic evolution: a review. Planet Space Sci., 43, 189–217. [DOI] [PubMed] [Google Scholar]
- 18.Horneck G. (1981) Survival of microorganisms in space: a review. Adv. Space Res., 1, 39–48. [DOI] [PubMed] [Google Scholar]
- 19.Munakata N. and Rupert,C.S. (1972) Genetically controlled removal of “spore photoproduct” from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J. Bacteriol., 111, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Wang T.S. and Rupert,C.S. (1977) Evidence for the monomerization of spore photoproduct to two thymines by the light independent “spore repair” process in Bacillus subtilis. Photochem. Photobiol., 25, 123–127. [DOI] [PubMed] [Google Scholar]
- 21.Fajardo-Cavazos P., Salazar,C. and Nicholson,W.L. (1993) Molecular cloning and characterization of the Bacillus subtilis spore photoproduct lyase (spl) gene, which is involved in repair of UV radiation-induced DNA damage during spore germination. J. Bacteriol., 175, 1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehl R.A. and Begley,T.P. (1999) Mechanistic studies on the repair of a novel DNA photolesion: the spore photoproduct. Org. Lett., 1, 1065–1066. [DOI] [PubMed] [Google Scholar]
- 23.Rebeil R. and Nicholson,W.L. (2001) The subunit structure and catalytic mechanism of Bacillus subtilis DNA repair enzyme spore photoproduct lyase. Proc. Natl Acad. Sci. USA, 98, 9038–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheek J. and Broderick,J.B. (2002) Direct H atom abstraction from spore photoproduct C-6 initiates DNA repair in the reaction catalyzed by spore photoproduct lyase: evidence for a reversibly generated adenosyl radical intermediate. J. Am. Chem. Soc., 124, 2860–2861. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson W.L., Setlow,B. and Setlow,P. (1991) Ultraviolet irradiation of DNA complexed with α/β small, acid soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc. Natl Acad. Sci. USA, 88, 8288–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douki T. and Cadet,J. (2003) Formation of the spore photoproduct and other dimeric lesions between adjacent pyrimidines in UVC-irradiated dry DNA. Photochem. Photobiol. Sci., 2, 433–436. [DOI] [PubMed] [Google Scholar]
- 27.Ravanat J.-L., Douki,T., Incardona,M.-F. and Cadet,J. (1993) HPLC separations of normal and modified nucleobases and nucleosides on an amino silica gel column. J. Liquid Chromatogr., 16, 3185–3202. [Google Scholar]
- 28.Douki T., Court,M. and Cadet,J. (2000) Electrospray-mass spectrometry characterization and detection of far-UV induced thymine photoproducts. J. Photochem. Photobiol. B Biol., 54, 145–154. [DOI] [PubMed] [Google Scholar]
- 29.Varghese A.J. (1970) Photochemistry of thymidine on ice. Biochemistry, 9, 4781–4787. [DOI] [PubMed] [Google Scholar]
- 30.Niggli H.J. and Cerutti,P.A. (1983) Cyclobutane-type pyrimidine dimer formation and excision in human skin fibroblasts after irradiation with 313 nm ultraviolet light. Biochemistry, 22, 1390–1395. [DOI] [PubMed] [Google Scholar]
- 31.Cadet J., Gentner,N.E., Rozga,B. and Paterson,M.C. (1983) Rapid quantitation of ultraviolet induced thymine-containing dimers in human cell DNA by reverse-phase high performance liquid chromatography. J. Chromatogr., 280, 99–108. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera-Juarez E. and Setlow,J.K. (1977) Formation of a thymine photoproduct in transforming DNA by near ultraviolet irradiation. Arch. Biochem. Biophys., 475, 315–322. [DOI] [PubMed] [Google Scholar]
- 33.Lindberg C. and Horneck,G. (1991) Action spectra for survival and spore photoproduct formation of Bacillus subtilis irradiated with short-wavelength (200–300 nm) UV at atmospheric pressure. J. Photochem. Photobiol. B Biol., 11, 69–80. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., Palasingam,K. and Nicholson,W.L. (1994) High-pressure liquid chromatography assay for quantitatively monitoring spore photoproduct repair mediated by spore photoproduct lyase during germination of UV-irradiated Bacillus subtilis spores. Anal. Biochem., 221, 61–65. [DOI] [PubMed] [Google Scholar]
- 35.Liuzzi M., Weinfeld,M. and Paterson,M.C. (1989) Enzymatic analysis of isomeric trithymidilates containing ultraviolet light-induced cyclobutane pyrimidine dimers. I. Nuclease P1 mediated hydrolysis of the intradimer phosphodiester linkage. J. Biol. Chem., 264, 6355–6363. [PubMed] [Google Scholar]
- 36.Douki T., Zalizniak,T. and Cadet,J. (1997) Far-UV-induced dimeric photoproducts in short oligonucleotides: sequence effects. Photochem. Photobiol., 66, 171–179. [DOI] [PubMed] [Google Scholar]
- 37.Frelon S., Douki,T., Ravanat,J.-L., Tornabene,C. and Cadet,J. (2000) High performance liquid chromatography—tandem mass spectrometry measurement of radiation-induced base damage to isolated and cellular DNA. Chem. Res. Toxicol., 13, 1002–1010. [DOI] [PubMed] [Google Scholar]
- 38.Rahn R.O. and Hosszu,J.L. (1969) Photochemical studies of thymine on ice. Photochem. Photobiol., 10, 131–137. [DOI] [PubMed] [Google Scholar]
- 39.Fisher G.J. and Johns,H.E. (1970) Ultraviolet photochemistry of thymine in aqueous solution. Photochem. Photobiol., 11, 429–444. [DOI] [PubMed] [Google Scholar]
- 40.Douki T., Angelov,D. and Cadet,J. (2001) UV laser photolysis of DNA: effect of duplex stability on charge-transfer efficiency. J. Am. Chem. Soc., 123, 11360–11366. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell D.L. and Clarkson,J.M. (1984) Use of synthetic polynucleotides to characterize an antiserum made against UV-irradiated DNA. Photochem. Photobiol., 40, 743–748. [DOI] [PubMed] [Google Scholar]
- 42.Mori T., Nakane,M., Hattori,T., Matsunaga,T., Ihara,M. and Nikaido,O. (1991) Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem. Photobiol., 54, 225–232. [DOI] [PubMed] [Google Scholar]
- 43.Matsunaga T., Hatakeyama,Y., Ohta,M., Mori,T. and Nikaido,O. (1993) Establishment and characterization of a monoclonal antibody recognizing the Dewar valence isomer of (6-4) photoproducts. Photochem. Photobiol., 57, 934–940. [DOI] [PubMed] [Google Scholar]
- 44.Doetsch P.W., Chan,G.L. and Haseltine,W.A. (1985) T4 DNA polymerase (3′-5′) exonuclease, an enzyme for the detection and quantification of stable DNA lesions: the ultraviolet example. Nucleic Acids Res., 13, 3285–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sage E., Cramb,E. and Glickman,B.W. (1992) The distribution of UV damage in the lacI gene of Escherichia coli: correlation with mutation spectrum. Mutat. Res., 269, 285–299. [DOI] [PubMed] [Google Scholar]
- 46.Pfeifer G.P., Drouin,R. and Holmquist,G.P. (1993) Detection of DNA adducts at the DNA sequence level by ligation-mediated PCR. Mutat. Res., 288, 39–46. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell D.L. (1988) The induction and repair of lesions produced by the photolysis of (6-4) photoproducts in normal and UV-hypersensitive human cells. Mutat. Res., 194, 227–237. [DOI] [PubMed] [Google Scholar]
- 48.Kan L.-S., Voituriez,L. and Cadet,J. (1992) The Dewar valence isomer of the (6-4) photoadduct of thymidylyl-(3′-5′)-thymidine monophosphate: formation, alkaline lability and conformational properties. J. Photochem. Photobiol. B Biol., 12, 339–357. [DOI] [PubMed] [Google Scholar]
- 49.Smith C.A. and Taylor,J.-S. (1993) Preparation and characterization of a set of deoxyoligonucleotide 49-mers containing site-specific cis-syn, trans-syn I, (6-4) and Dewar photoproducts of thymidylyl(3′-5′)-thymidine. J. Biol. Chem., 268, 11143–11151. [PubMed] [Google Scholar]
- 50.Douki T., Vadesne-Bauer,G. and Cadet,J. (2002) Formation of 2′-deoxyuridine hydrates upon exposure of nucleosides to gamma radiation and UVC-irradiation of isolated and cellular DNA. Photochem. Photobiol. Sci., 1, 565–569. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Taylor,J.-S. and Gross,M.L. (1999) Nuclease P1 digestion combined with tandem mass spectrometry for the structure determination of DNA photoproducts. Chem. Res. Toxicol., 12, 1077–1082. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Rempel,D.L., Taylor,J.-S. and Gross,M.L. (2001) A method for quantification from composite spectra: application to the determination of isomeric DNA photoproducts by tandem mass spectrometry. Anal. Chem., 73, 185–191. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Gross,M.L. and Taylor,J.-S. (2001) Use of a combined enzymatic digestion/ESI mass spectrometry assay to study the effect of TATA-binding protein on photoproduct formation in a TATA box. Biochemistry, 40, 11785–11793. [DOI] [PubMed] [Google Scholar]
- 54.Girod J.C., Johnson,W.C., Hunnington,S.K. and Maestre,M.F. (1973) Conformation of deoxyribonucleic acid in alcohol solutions. Biochemistry, 12, 5092–5096. [DOI] [PubMed] [Google Scholar]
- 55.Lindsay S.M., Lee,S.A., Powell,J.W., Weidlich,T., DeMarco,C., Lewen,G.D. and Tao,N.J. (1988) The origin of the A to B transition in DNA fibers and films. Biopolymers, 27, 1015–1043. [DOI] [PubMed] [Google Scholar]
- 56.Cooper P.J. and Hamiton,L.D. (1966) The A-B conformational change in the sodium salt of DNA. J. Mol. Biol., 16, 562–563. [DOI] [PubMed] [Google Scholar]
- 57.Herbeck R., Yu,T.-J. and Petitcolas,W.L. (1976) Effects of cross-linking on the secondary structure of DNA I. Cross-linking by photodimerization. Biochemistry, 15, 2656–2660. [DOI] [PubMed] [Google Scholar]
- 58.Mohr S.C., Sokolov,N.V.H.A., He,C. and Setlow,P. (1991) Binding of small acid-soluble proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc. Natl Acad. Sci. USA, 88, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sancar A. and Tang,M.-S. (1993) Nucleotide excision repair. Photochem. Photobiol., 57, 905–921. [DOI] [PubMed] [Google Scholar]
- 60.Sancar A. (1994) Structure and function of DNA photolyase. Biochemistry, 33, 2–9. [DOI] [PubMed] [Google Scholar]
- 61.Takaio M., Yonemasu,R., Yamamoto,K. and Yasui,A. (1996) Characterization of a UV endonuclease gene from the fission yeast Schizosaccharomyces pombe and its bacterial homolog. Nucleic Acids Res., 24, 1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dronkert M.L.G. and Kanaar,R. (2001) Repair of DNA interstrand cross-links. Mutat. Res., 486, 217–247. [DOI] [PubMed] [Google Scholar]