Abstract
In Rhizobium leguminosarum, NodD can activate nodA transcription in response to inducer flavonoids. Here, we show that the inducible nodA promoter contains an intrinsic part through which NodD can activate nodA transcription in an inducer-independent manner. Evidence was provided that NodD binds to target DNA through anchoring the two half-sites of the nod box as a tetramer. An imperfect inverted repeat AT-N10-GAT was found in each half-site and is critical for NodD binding. Mutation of the inverted repeat of the nod box distal half-site allowed NodD to activate nodA transcription in an inducer-independent manner in vivo, and to modulate the DNA bending of the NodD–nod box complex in the absence of inducer in vitro.
INTRODUCTION
In nitrate-poor soils, strains of Azorhizobium, Bradyrhizobium, Mesorhizobium and Rhizobium (collectively known as rhizobia), form nitrogen-fixing symbiosis with leguminous plants in a host-specific way (1,2). In compatible interactions, invading rhizobia and infected roots differentiate into nitrogen-fixing bacteroid in the nodules. Flavonoids released by legume roots are amongst the first signals exchanged in the molecular dialog between the two symbionts. Through rhizobial regulators of the NodD family, inducer flavonoids trigger the expression of nodulation genes (nod, noe and nol). In turn, most nodulation genes participate in the synthesis or secretion of a family of lipochito-oligosaccharide molecules, the Nod factors, which are required for bacterial entry into root hairs. Thus, NodD is of central importance during the nodulation process between rhizobia and host plants.
Some rhizobia species, e.g. Bradyrhizobium japonicum, Rhizobium sp. NGR234, Rhizobium meliloti and Rhizobium tropici harbor two to five copies of nodD genes, whereas Rhizobium leguminosarum bv. viciae and R.leguminosarum bv. trifolii have only one nodD gene (3). In the symbiotic plasmid pRL1JI of R.leguminosarum bv. viciae, the nodD gene is transcribed divergently from the nodABCIJ operon (4). Besides the nodABCIJ operon, NodD also activates the transcription of all three other identified nodulation operons (nodFEL, nodMNT and nodO) in response to inducer (5–7). NodD autoregulates its own nodD operon (8). The minimal inducible nod promoters contain two highly conserved DNA elements. One is a highly conserved DNA motif (47 bp nod box), and the other is a conserved –10 sequence, which is ∼15 bp downstream of the nod box consensus (Fig. 1A) (9). The 47-bp nod boxes share 32 highly conserved consensus nucleotides. From the comparison of various nod box sequences of (brady)rhizobia and Azorhizobium caulinodans, Holsters and Goethals have proposed that a so-called NodD box with the panlindromic structure ATC-N9-GAT is the primary NodD-binding element (10).
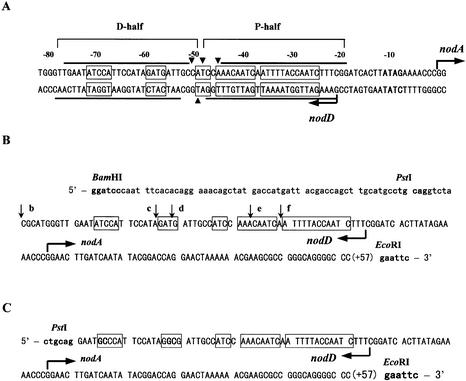
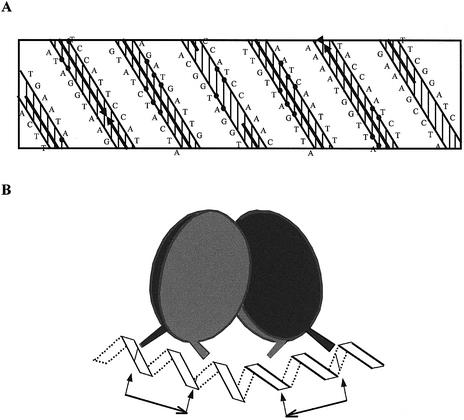
Figure 1.
The nodA promoter of the symbiotic plasmid pRL1JI. The transcription start sites for the nodA and nodD genes are indicated by arrows (8,9). The nodA transcription start site is numbered +1. The consensus sequence of the canonical nod box is boxed (9,14). (A) The minimal inducible nodA promoter. The conserved sequence ATAG, which is part of the nodA promoter, is in bold (9). Lines indicate the DNase I footprints of NodD, and triangular arrowheads denote hypersensitive sites (8). The two protected half-sites of the nod box separated by the hypersensitive sites are named D-half and P-half, respectively. (B) The nodA promoter deletions cloned between the BamHI–EcoRI sites of pBluescriptKSII-derived plasmids pBSb, pBSc, pBSd, pBSe and pBSf (see Materials and Methods). The 5′ ends of the deletions are different and marked by the vertical arrows, and the 3′ ends are all at position +57. The upper case letters represent the nodA promoter region, and the lower case letters are related to relevant plasmids. The BamHI, PstI and EcoRI sites are in bold. (C) The nodA promoter (–75 to +57) with an O13 mutant D-half cloned in the PstI–EcoRI sites of pUO13 (see Materials and Methods). The four mutated residues in the O13 D-half are in bold. The PstI and EcoRI sites are also in bold.
NodD shows extensive amino acid similarity with the LysR-type transcriptional regulators (LTTRs) and has properties similar to many LTTRs (11). These proteins have attracted a considerable amount of attention for a number of reasons. First, the LysR family is likely to be the largest family of prokaryotic regulatory proteins (a recent database search revealed over 200 members). Secondly, these proteins appear to function in ways that are somewhat atypical for classical prokaryotic regulators. Although most LTTRs are activated by small specific molecules, these small molecules generally do not greatly affect the binding affinity of the proteins to their target promoters. The regions of DNA protected by these proteins from DNase I digestion are usually 50–60 bp long, much longer than those protected by most other known transcriptional regulators. Mutational studies and analysis of amino acid sequence similarity between LTTRs have identified three basic functional domains: an N-terminal DNA-binding domain, a coinducer recognition/response domain and a conserved C-terminal domain (11,12). The LTTRs bear no obvious activation domains. DNase I footprints show that NodD specifically protects the nod box region (13,14). Based on analysis of the symmetry and phasing of the cis elements, NodD is predicted to bind to target DNA as a dimer or tetramer (10,14). Genetic evidence indicates that NodD interacts directly with inducer flavonoids to activate transcription (15,16), but direct proof of this is lacking (17). Several lines of evidence support the model that the NodD–nod box binding alters upon addition of appropriate flavonoids: these are increased binding to nod box sequences in Sinorhizobium meliloti and A.caulinodans (10,18) and changes in DNase I footprints (18). However, in other cases, the affinity and/or binding of NodD for nod boxes seem not to be affected (19).
The mechanism by which LTTRs switch on and off their target promoters in response to coinducer is not fully understood. Recently, several clues indicate that LTTRs may regulate transcription through modulating the DNA structure (20–22). For example, in vitro, the ligand of OccR octopine can relax the OccR-incited DNA bend (20). The wild-type OxyR-wt causes a sharper DNA bend on the oxySRS promoter than the positive mutant OxyR-C199S, which is locked in the activated conformation (22). The DNA bending by CatR is also inducer CCM-responsive (23). These LTTRs protect their regulated operators in a similar position (approximately –75 to –25), and incite a DNA bend. The protected region is long and can be divided into two halves or subsites. Sinorhizobium meliloti NodD also binds to two distinct sites on the same face of the nod box helix and induces a bend in the DNA (14). In this work, we demonstrated in R.leguminosarum how tetrameric NodD binds to the nod box through anchoring its two tandem half-sites. In an effort to understand the role of such double-site interactions between tetrameric NodD and the two nod box half-sites in the NodD–nod box-mediated transcriptional control, the nod box distal half-site (D-half) was mutated and inactivated.
MATERIALS AND METHODS
Microbiological techniques
Bacterial strains and plasmids are listed in Table 1 or in the text. Media and general growth conditions were as described by Hu et al. (8). Diparental conjugation was performed to mobilize broad host range plasmids from Escherichia coli to R.leguminosarum as described by Simon et al. (24).
Table 1. Bacterial strains, plasmids and DNA fragments.
| Relevant characteristics | Reference or source | |
|---|---|---|
| Rhizobium | ||
| 8401 | Rhizobium leguminosarum bv. viciae cured of its symbiotic plasmid, Strr | (44) |
| Escherichia coli | ||
| S17-1 | 294 recA, chrom, RP4 derivative | (24) |
| Plasmids | ||
| pKT230 | IncQ broad-host-range plasmid; Strr, Kanr | (45) |
| pIJ1518 | 1.8 kb BclI fragment with nodD in pKT230; Kanr | (4) |
| pMP220 | lacZ downstream of a multi-cloning site; IncP broad-host-range plasmid; Tcr | (9) |
| pMP221 | Opposite multi-cloning site in pMP220 | Stored in this laboratory |
| pUW | nodA promoter (–75 to +57) cloned into the EcoRI–PstI sites of pUC119 | This study |
| pUO13 | nodA promoter (–75 to +57) with the O13 D-half cloned into the EcoRI–PstI sites of pUC119 | This study |
| pMP221O13 | EcoRI–PstI fragment cloned from pUO13 into pMP221 | This study |
| Fragments | ||
| TnodboxT | 5′-TTGAATATCCATTCCATAGATGATTGCCATCCAAACAATCAATTTTACCAATCTTTCGG-3′ | This study |
| 3′-CTTATAGGTAAGGTATCTACTAACGGTAGGTTTGTTAGTTAAAATGGTTAGAAAGCCTT-5′ | ||
| TDhalfT | 5′-TTGAATATCCATTCCATAGATGATTGCCATACTGACTGACTGACTGACTGACTGACTGA-3′ | This study |
| 3′-CTTATAGGTAAGGTATCTACTAACGGTATGACTGACTGACTGACTGACTGACTGACTTT-5′ | ||
| TPhalfT | 5′-TTTGCATGCATGCATGCATGCATGCATGATCCAAACAATCAATTTTACCAATCTTTCGG-3′ | This study |
| 3′-ACGTACGTACGTACGTACGTACGTACTAGGTTTGTTAGTTAAAATGGTTAGAAAGCCTT-3′ |
Enzymes and chemicals
Restriction endonucleases and DNA ligase were purchased from Promega. Bio-Rad Protein Assay Kit I based on the Bradford method was from Bio-Rad; [α-32P]dATP was from Amersham; SDS–PAGE low molecular weight protein marker was from Shanghai Lizhu Dongfeng Biotech. Co.; HiFi-Bst DNA polymerase was produced in our own laboratory (25); other chemical reagents were above analytical grade.
Plasmids and DNA fragments
Three short DNA fragments TnodboxT, TDhalfT and TPhalfT were prepared by directly annealing complementary synthesized single-strand oligonucleotides (Table 1). We had previously constructed deletions of the nodA–nodD promoter region from two ends and obtained two series of clones (stored in this laboratory). From five clones of one series, nodA promoter deletions (∼200 bp) were cloned into pBluescriptIIKS, resulting in plasmids pBSb, pBSc, pBSd, pBSe and pBSf (Fig. 1B). DNA fragments EbB, EcB, EdB, EeB and EfB were the EcoRI–BamHI fragments digested from plasmids pBSb, pBSc, pBSd and pBSe, respectively. Plasmids pMP221b, pMP221c, pMP221d and pMP221e were constructed by cloning the EcoRI–PstI fragments from plasmids pBSb, pBSc, pBSd and pBSe to pMP221, respectively. Plasmids pMP220b, pMP220c, pMP220d and pMP220e were constructed by cloning the EcoRI–PstI fragments from plasmids pBSb, pBSc, pBSd and pBSe to pMP220, respectively. Three primers were synthesized: P1, AGTGAATTCG GGCCCCTGCCCG GCGCTTCG; P2, GCACTGCAGG AATATCCATT CCATAGATG; P3, GCACTGCAGG AATGCCCATT CCATAGGCGA TTGCCATCC. With pBSb as template and P1/P2 and P1/P as primer, PCR was used to produce two nodA promoter fragments with a wild-type D-half and an O13 mutant D-half. pUO13 was constructed by cloning the nodA promoter (–75 to +57) with the O13 D-half into the EcoRI–PstI sites of pUC119. In contrast, pUW was constructed by cloning the nodA promoter (–75 to +57) with a wild-type D-half into the EcoRI–PstI sites of pUC119 (Fig. 1C). pMP221O13 and pMP220O13 were constructed by cloning the same nodA promoter (–75 to +57) with the O13 D-half into the EcoRI–PstI sites of pMP221 and pMP220, respectively.
Purification of NodD protein and determination of the active NodD concentration
NodD was prepared as described before (26). The concentration of active NodD was determined by measuring the stoichiometry of DNA bound when DNA was in high molar excess over NodD. The first titration test in gel mobility shift assays was used to choose an appropriate concentration of NodD, which was 100-fold that which could shift ∼95% of the labeled TnodboxT (10–12 M). In the second titration test, the chosen NodD concentration and the labeled TnodboxT concentration (10–12 M) were fixed, and unlabeled TnodboxT was added and increased gradually in the reaction system of gel mobility shift assays. When the ratio of shifted fragment to free fragment was decreased to 1/1, the molar concentration of TnodboxT was increased to 2-fold that of the active tetrameric NodD. The molar concentration of active NodD subunit was 4-fold that of tetrameric NodD on this condition.
Gel mobility shift assay and DNase I footprinting
Gel mobility shift assays were performed as described (27). HiFi-Bst DNA polymerase was used to blunt the ends of the annealed fragments or the EcoRI-digested fragments with [α-32P]dATP. In the assays to determine apparent equilibrium association constants, no non-specific competitor DNA was added. In other assays, 200 ng of calf thymus DNA (ctDNA) was added as competitor in the 10 µl reaction volume.
DNase I footprinting reactions were performed as described (27). The A+G ladder was produced by the method of Liu and Hong (28). Plasmids pBSb, pBSc, pBSd and pBSe were digested by EcoRI, the EcoRI end blunted with [α-32P]dATP, and then digested by BamHI. NodD (0.4 ng/µl), radiolabeled DNA fragment (1 × 10–11 M) and ctDNA (20 ng/µl) were incubated at 28°C for 30 min in a 30 µl volume, then 2 µl of DNase I (2 µg/ml, 4 units/µg; Boehringer Mannheim) was added to digest for 30 s. The reaction was terminated, and then extracted with phenol–chloroform. The aqueous phase was precipitated with ethanol, and the samples were analyzed on 6% denaturing polyacrylamide gel.
Binding site selection
The following procedure was according to the SELEX procedure (29). The random oligonucleotides were synthesized: a 72 base oligonucleotide 5′-TTC GAG CTC CAC CGC GGT (A/G)(T/C)C CAT TCC (A/G)(T/C)A (A/G)(A/G)(T/C) GAT TGC C(A/G)(T/C) CCT GCA GGA TCC GGC CGG TAC CCA GCT TGA-3′ (D-N9) with nine residues mutated and its corresponding primers 5′-GGC GAA TTC GAG CTC CAC CGC GG-3′ and 5′-AGG GAA TTC AAG CTG GGT ACC GGC CG-3′. The second strand of the oligonucleotides D-N9 was synthesized by primer extension in the first PCR cycle, and then the double-stranded product was amplified by PCR (five cycles). The PCR product was digested with EcoRI and radiolabeled with [α-32P]dATP by HiFi-Bst DNA polymerase. Purified NodD (1 ng/µl) was used in gel mobility shift assays to shift or select target oligonucleotides from the others. The position of the shifted fragments was measured by autoradiography. Gel slices were excised from such position of the gel run on the same conditions, and ground in 1.5-ml Eppendorf tubes. TE buffer was added to rinse the selected probe out of the macerated gel by incubating the tube at 37°C for 4 h in a hybridization incubator. The extracted DNA was then amplified by PCR (30 cycles). Gel slices from the same position of the parallel lanes (two lanes apart) without NodD added were taken as the negative control. In the second and third cycles of selection, unlabeled DNA probe (200 ng) was added to make DNA probe molar excessive. The final PCR product was directly cloned into pUCm-T-vector. Twenty-nine such clones sequenced were finished (pUT-DI and pUT-DII). The control oligonucleotides from the preselection pools of D-N9 were also cloned into pUCm-T vector, and the resulting clones were named pUT-O. Twenty such clones were also sequenced (pUT-O).
β-Galactosidase assays
Rhizobium was incubated at 28°C under aeration in TY medium (8) until the A600 value increased to 0.5. The β-galactosidase assays were performed as described (30). Naringenin was added to a final concentration of 10 µM as the induction condition. Assays of β-galactosidase activities were performed in triplicate and were reproducible within 15% from experiment to experiment.
DNA bending by circular permutation assay
The EcoRI site of pUW and pUO13 was removed by digesting the plasmids with EcoRI, blunting the EcoRI site with Pfu DNA polymerase and then ligating the resulting product. With the resulting plasmids as templates, PCR was used to generate five pairs of 226-bp fragments with the following five pairs of primers: BP1, 5′-TTG AAT ATC CAT TCC ATA GAT GAT TGC CAT CCA AAC-3′ and BP2, 5′-GAA GAA TTC ATG TGC TGC AAG GCG ATT AAG-3′; BP3, 5′-CAG GAA TTC GCT ATG ACC ATG ATT AC-3′ and BP4, 5′-GTT TTC CCA GTC ACG ACG TTG-3′; BP5, 5′-TTG TGT GGA ATT GTG AGC GGA-3′ and BP6, 5′-TCT GAA TTC ATT CGG GCC CCT GCC CGG C-3′; BP7, 5′-TAG GCA CCC CAG GCT TTA CAC TTT A-3′ and BP8, 5′-TTC GAA TTC CTG ATA TTG ATC AAG TTC-3′; BP9, 5′-CGG GAA TTC AGC GCA ACG CAA TTA ATG TG-3′ and BP10, 5′-ATC CGA AAG ATT GGT AAA ATT GAT TGT TTG GAT GGC. The NodD-binding sites are distributed from the ends to the middle of the 226-bp fragments. These fragments were digested by EcoRI and radiolabeled by blunting the ends with [α-32P]dATP to produce five pairs of 222-bp probes. Gel mobility shift assays were performed to detect the mobility shift of the free fragments and the NodD–fragment complexes.
RESULTS
Oligomeric form of NodD at target DNA
The stoichiometry of NodD and target DNA is an important detail to reveal the mechanism underlying the transcriptional regulation of inducible nod genes. Fisher and Long have reported that R.meliloti NodD binds to the nodH nod box through two distinct subsites on the same face of the DNA helix (14). We wished to establish the oligomerization degree of NodD on the intact R.leguminosarum nodA nod box and two mutant nod boxes with only one half-site.
Three 60-bp DNA fragments, named TnodboxT, TDhalfT and TPhalfT, were prepared by directly annealing synthetic complementary single strain oligonucleotides (Table 1). TnodboxT contains a wild-type nodA nod box. TDhalfT contains only the D-half of the nod box. TPhalfT contains only the proximal half (P-half) of the nod box. The DNA sequence of the other half in TDhalfT and TPhalfT is randomly given.
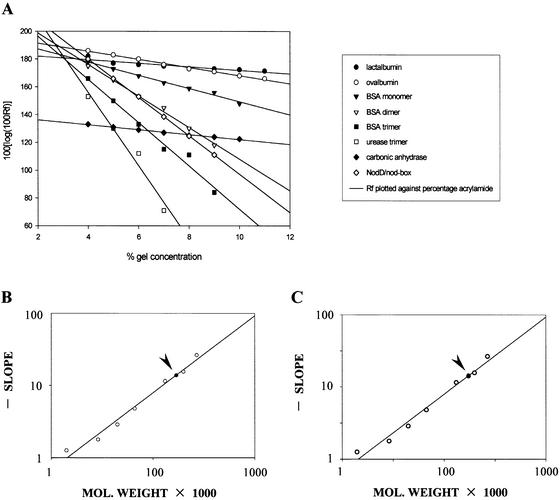
A method described by Ferguson is often used to determine the molecular weight of proteins through non-denaturing polyacryamide gel electrophoresis. Such a method is also valid to determine the molecular weight of protein–DNA complexes (31). In order to calculate the oligomerization degree of NodD, this method was used to determine the molecular weight of the NodD–DNA complexes. Several different concentrations of gel were used for gel mobility shift assays as well as the electrophoresis of molecular weight standards. After electrophoresis, the standards were located by Coomassie blue staining while the protein–DNA complex was detected by autoradiography. Rf values were determined and the molecular weight of the complex was estimated according to a calibration curve obtained through plotting twice, as in Figure 2. Then, the molecular weight of the protein component of the complex could be determined by subtracting the contribution of DNA from the total value.
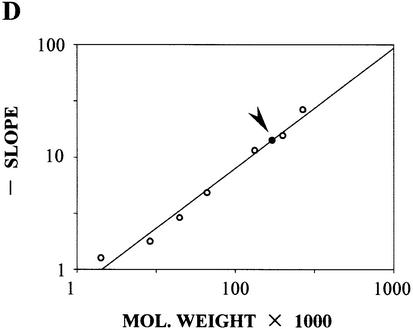
Figure 2.
The molecular weight of the NodD–DNA complex NodD–TnodboxT, NodD–TDhalfT and NodD–TPhalfT. (A) Calibration curves. Logarithm of relative mobility (Rf) plotted against percentage acrylamide, showing the relationship between each species’ mobility and the gel concentration: lactalbumin, carbonic anhydrase, ovalbumin, BSA monomer, BSA dimer, BSA trimer, urease trimer, NodD–TnodboxT. (B) Ferguson plot. The gradient of each line in (A) (Kf) plotted against the molecular weight of the standards (open circles), generating a standard curve from which the molecular weight of the NodD–TnodboxT complex (closed circle, also indicated by arrowhead) can be determined. (C) Ferguson plot to determine the molecular weight of the NodD–TDhalfT complex. (D) Ferguson plot to determine the molecular weight of the NodD–TPhalfT complex.
A value of 170 kDa was obtained for the NodD–TnodboxT complex (Fig. 2B), 174 kDa for the NodD–TDhalfT (Fig. 2C) and 171 kDa for the NodD–TPhalfT (Fig. 2D). Subtracting 36 kDa to allow for the contribution of the DNA, yields an estimate of 134, 138 and 135 kDa for the molecular weight of the protein component of the NodD–TnodboxT complex, the NodD–TDhalfT complex and the NodD–TPhalfT complex, respectively. The calculated molecular weight is in good agreement with the value expected for binding of the homotetrameric NodD, the molecular weight of the monomer being reported as 34 kDa.
Thus, NodD appears to bind to the two ‘half-boxes’, with one intact half, as well as the intact nod box as a tetramer. This suggests that tetramer is the DNA-bound form of NodD at least for the uninduced NodD because the mobility shift assay patterns to generate the data in Figure 2 lack an intermediate band corresponding to dimeric NodD bound to DNA fragments TDhalfT and TPhalfT (data not shown).
Binding affinity of NodD for the intact nod box and the two half-boxes
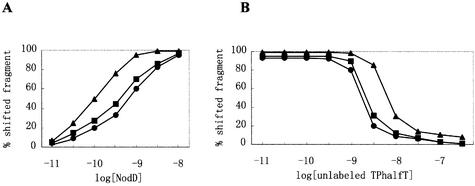
To quantify NodD binding to the intact nod box and the two half-boxes, the purified NodD protein was titrated against DNA fragments TnodboxT, TDhalfT and TPhalfT in gel mobility shift assays. These assays were conducted using concentrations of DNA probes (1 × 10–13 M) well below the effective binding dissociation constant for the intact nod box to evaluate the apparent equilibrium association constant governing the assembly of NodD–DNA complex. The apparent equilibrium association constant (Ka) was determined from the averages of at least three separate experiments with each fragment. As shown in Figure 3A, Ka for the NodD–TnodboxT interaction, the NodD–TDhalfT interaction and the NodD–TPhalfT interaction was calculated to be 1 × 1010, 2.4 × 109 and 1.5 × 109, respectively. Binding competition experiments with unlabeled DNA fragment TPhalfT were also performed (Fig. 3B). The competition curves are consistent with the binding curves. In Figure 3 and other cases of this study, the concentration of NodD refers to that of the active NodD subunit. We determined the concentration of the active NodD subunit by measuring the stoichiometry of DNA bound when DNA was in high molar excess over NodD (see Materials and Methods). In Figure 3, the percent shifted fragment refers to the ratio of shifted fragment to the sum of shifted and free fragments.
Figure 3.
Binding of NodD to DNA fragments TnodboxT, TDhalfT and TPhalfT. (A) NodD–DNA binding isotherm curves as a function of the increasing concentration of NodD. The labeled DNA fragments TnodboxT (triangle), TDhalfT (square) and TPhalfT (circle) were added in at a final concentration of 1 × 10–13 M. (B) Competition titration of NodD–TnodboxT (triangle), NodD–TDhalfT (square) and NodD–TPhalfT (circle) with added unlabeled TPhalfT. NodD was added at a final concentration of 0.4 ng/µl, and the labeled DNA fragments at 1 × 10–12 M.
Specific NodD-binding determinant of the D-half and the P-half
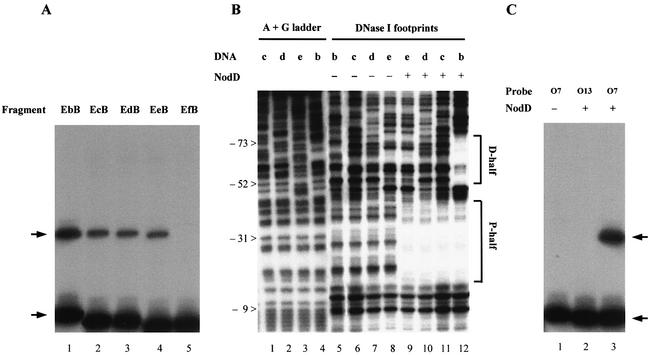
To localize the specific NodD-binding determinant of the nod box more precisely, we used nod box deletions in gel mobility shift assays. The fragments, named EbB, EcB, EdB, EeB and EfB, were the EcoRI–BamHI fragments digested from plasmids pBSb, pBSc, pBSd, pBSe and pBSf, respectively (Fig. 1B). In gel mobility shift assays, the deletions from the D-half to the P-half twice obviously impaired the NodD-binding affinity (Fig. 4A). The first interval at which there was a significant step down occurred between –85 and –60 (Fig. 4A, lanes 1 and 2), and the second between –42 and –37 (Fig. 4A, lanes 4 and 5). DNA fragment EeB bound to NodD (Fig. 4A, lane 4), indicating that the minimal 18-bp sequence (–42 to –25) contains a basal NodD-binding determinant. Further deletion (–42 to –37) made the shifted band disappear (Fig. 4A, lane 5). DNase I footprinting showed the corresponding specific NodD protection on these fragments. The deletion up to –60 made NodD lose all its specific footprints on the left sequence of the D-half (Fig. 4B, lane 11). The 18-bp sequence (–42 to –25) allowed NodD to form a similar protection pattern on the P-half region (Fig. 4B, lane 9).
Figure 4.
NodD binding to the wild-type nodA nod box and the mutants. (A) Gel mobility shift assay shows how the deletions impair the NodD binding affinity. Radiolabeled fragments EbB, EcB, EdB, EeB and EfB were used as probes in lanes 1–5, respectively. NodD was added at a final concentration of 4 pg/µl, probe at 1 × 10–12 M and ctDNA at 20 ng/µl. Arrows indicate the free probe band and the shifted band. (B) DNase I footprinting shows the ‘footprints’ of NodD on fragments EbB, EcB, EdB and EeB. The assays were performed on the sense strain of the nodA promoter. The sequences are numbered relative to the nodA transcriptional start site. The D-half and P-half regions are marked. (C) The radiolabeled EcoRI–HindIII fragments from pUT-O7 and pUT-O13 were used as probes in gel mobility shift assay. pUT-O7 contains TATCCATTCCGCAGATGATTGCCGCCC, and pUT-O13 contains TGCCCATTCCATAGGCGATTGCCATCC. NodD was added at a final concentration of 40 pg/µl, probe at 1 × 10–12 M and ctDNA at 20 ng/µl.
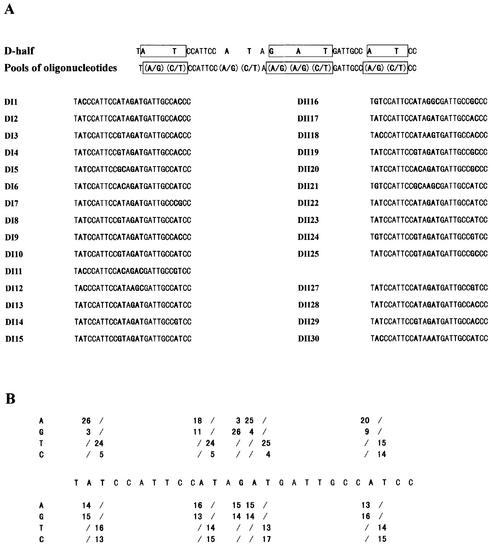
We aligned the D-half and the inverted P-half sequences. The consensus sequence is ATN3TN3ATNGATN2TTN3A, and the conserved consensus sequence AT-N10-GAT-N7-A. In order to test whether these nucleotides are critical for NodD binding, we selected synthetic binding sites from pools of oligonucleotides. We synthesized oligonucleotides carrying nine mutated bases (D-N9) in the context of the D-half sequence (Fig. 5). Synthetic sites bound by NodD were sequestered away from the pool of oligonucleotides by a shift in gel mobility. The bound sequences were then amplified by PCR, and the selection was repeated. In the selection assays, the purified NodD used is predicted to assume uninduced conformation. In D-N9, A is randomly mutated to A or G, T to T or C, and G to A or G.
Figure 5.
NodD-binding sites selected from pools of oligonucleotides carrying nine mutated nucleotides in the context of the D-half sequence. (A) Sequences of 29 selected probes bound specifically by NodD. The wild-type D-half sequence context is given in the first line, with the consensus conserved-sequence boxed. The mutated nucleotides are in bold: nucleotide A was randomly mutated to A or G, nucleotide T was mutated to T or C, and nucleotide G to A or G in a 1:1 ratio. Pools of 512 species of mutant oligonucleotides are denoted in the second line. The oligonucleotides selected from two independent selections (DI and DII) were aligned, with the mutant nucleotides in bold. (B) Summary of frequencies of nucleotide residues for selected (top bases) and unselected (bottom bases) oligonucleotides. The nucleotides of the consensus AT-N10-GAT-N7-A are clearly biased for NodD binding. Even the ‘control’ nucleotides A and T of the non-conserved consensus sequence are also biased. Out of 29 selected oligonucleotides, there are 21 with the sequence AT-N10-GAT selected.
Two independent selections (DI and DII), each with three rounds of binding and amplification, were carried out with D-N9. In order to select the strongest binding sites, the DNA fragment was molar excessive in the last two rounds of binding. The selected oligonucleotides were cloned into plasmid pUCm-T vector, and the resulting clones were named pUT-DI and pUT-DII. The control oligonucleotides from the preselection pools of D-N9 were also cloned into pUCm-T vector, and the resulting clones were named pUT-O. pUT-DI(1–15), pUT-DII(16–25), pUT-DII(27–30) and pUT-O(1–29) were successfully sequenced. The 29 sequenced selected oligonucleotides were aligned (Fig. 5A). The frequencies of such site-directed mutated nucleotides for the selected oligonucleotides were then compared with those for the oligonucleotides from the D-N9 preselection pool (Fig. 5B). All the six nucleotides of the conserved consensus AT-N10-GAT-N7-A were clearly biased for the specific NodD binding. Even the ‘control’ nucleotides A and T of the non-conserved consensus sequence were also biased. The last T belonging to the other half was not biased. In 21 out of 29 sequenced binding sites, AT-N10-GAT was selected, indicating that these nucleotides might be cooperative and more important for NodD binding. pUT-O7 and pUT-O13 were two clones of the sequenced ‘control’ clones pUT-O(1–29). pUT-O7 contained a D-half sequence TATCCATTCCGCAGATGATTGCCGCCC, and pUT-O13 TGCCCATTCCATAGGCGATTGCCATCC. In order to testify if the nucleotides at the AT-N10-GAT are critical for NodD binding, the two EcoRI–HindIII fragments of pUT-O7 and pUT-O13 were further used in the gel mobility shift assay. As expected, when the AT at positions 2/3 and 15/16 were both changed to GC, the specific NodD binding was abolished (Fig. 4C, lane 2), whereas when the AT at positions 11/12 and 24/25 were both changed to GC, the specific NodD binding was hardly impaired (Fig. 4C, lane 3).
Effects of the D-half inactivation on nodA transcription
NodD, as well as many other LTTRs, binds unusually long DNA sequences (8,14,19,20,22,23,32–36). These long DNA sequences almost always contain two protein-binding subsites or half-sites. Through anchoring the two half-sites, these LTTRs can cause a DNA bend on the DNA target. It is an emerging theme that such DNA bending is involved in the transcriptional control by LTTRs. Here, in an effort to understand the role of the double-site interaction between tetrameric NodD and the two half-sites of the nod box in the transcriptional regulation and the DNA structure modulation, we chose to inactivate the D-half-site of the nod box.
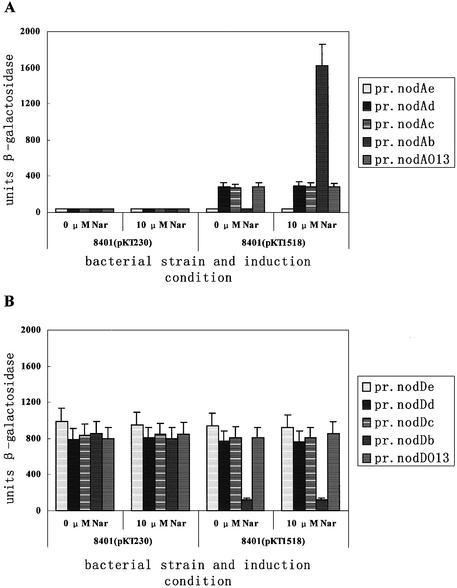
Plasmids pMP221e, pMP221d, pMP221c and pMP221b were constructed by fusing nodA promoter deletions to the lacZ gene in plasmid pMP221 (see Materials and Methods). In pMP221b, the nodA promoter has a wild-type D-half. In pMP221c and pMP221d, the D-half is partially deleted. In pMP221e, the nodA promoter is further deleted to position –43. From the above results, we had known that the O13 mutant D-half was inactive for the specific NodD binding (Fig. 4C, lane 2). Thus, a nodA promoter derivative (–75 to +57) with an O13 mutant D-half was also cloned into pMP221 to construct pMP221O13 (see Materials and Methods). These nodA promoter derivatives, named pr.nodAe, pr.nodAd pr.nodAc, pr.nodAb and pr.nodAO13, were tested in 8401(pKT230) and 8401(pIJ1518) for induction. Rhizobium leguminosarum strain 8401(pKT230) is a nodD– strain, while 8401(pIJ1518) is a nodD+ strain (Table 1).
The results are shown in Figure 6. In 8401(pKT230), all of the nodA promoter derivatives had very low promoter activity (∼35 units) in the absence and presence of inducer 10 µM naringenin. In 8401(pIJ1518), in the presence of inducer, pr.nodAb was activated (∼1600 units), and pr.nodAc, pr.nodAd and pr.nodAO13 were also activated (∼285 units). pr.nodAc, pr.nodAd and pr.nodAO13 were only partially activated by NodD when compared to pr.nodAb. In 8401(pIJ1518), in the absence of inducer, it is intriguing that pr.nodAc, pr.nodAd and pr.nodAO13 were still similarly activated (∼285 units). In contrast, pr.nodAb was switched off (∼35 units). On all the conditions, pr.nodAe had very low promoter activity (∼35 units), which indicated that the 5′ deletion to position –43 might start to destroy the RNA polymerase recruiting site of the ‘real’ deficient nodA promoter.
Figure 6.
Inactivation of the nod box D-half allows NodD to partially activate nodA transcription in a naringenin-independent manner. The height of the bars indicates the measured β-galactosidase level of various promoter derivatives. (A) pr.nodAe, pr.nodAd, pr.nodAc, pr.nodAb and pr.nodAO13 were assayed for nodA promoter activity in R.leguminosarum 8401(pKT230) and 8401(pIJ1518) in the presence and absence of 10 µM naringenin. (B) pr.nodDe, pr.nodDd, pr.nodDc, pr.nodDb and pr.nodDO13 were assayed for nodD promoter activity in R.leguminosarum strain 8401(pKT230) and 8401(pIJ1518) in the presence and absence of 10 µM naringenin.
Considering the above deletions are from position +65 to position +23 relative to the nodD transcriptional start site, we also determined their effects on nodD transcription. The same PstI–EcoRI fragments were cloned from plasmids pBSb, pBSc, pBSd and pBSe into pMP220, which has a reverse multi-cloning site to that of pMP221. In 8401(pKT230), the β-galactosidase level of pr.nodDb, pr.nodDc, pr.nodDd, pr.nodDe and pr.nodDO13 varied between 760 and 990 units. The results indicate that 5′ deletions up to position +23 have not destroyed the constitutive nodD promoter. In 8401(pIJ1518), the β-galactosidase levels of pr.nodDc, pr.nodDd, pr.nodDe and pr.nodDO13 did not obviously change, and that of pr.nodDb was reduced to 120 units. The results indicate that the nodD negative autoregulation needs an intact nod box.
In conclusion, the inducible nodA promoter in essence contains an intrinsic part (approximately +1 to –56) through which NodD can activate or at least partially activate nodA transcription in an inducer-independent manner. Such an intrinsic part contains only one intact half-site of the nod box, the P-half. It is worth highlighting that on this condition the uninduced NodD is also able to activate nodA transcription. In the absence of inducer, a functional D-half appears essential for NodD to intrinsically repress such NodD-mediated activation. Inactivation of the D-half through partial deletion or site-directed mutation of the AT-N10-GAT can abolish such intrinsic repression.
Effect of the O13 mutant D-half on the tetrameric NodD-induced DNA bending
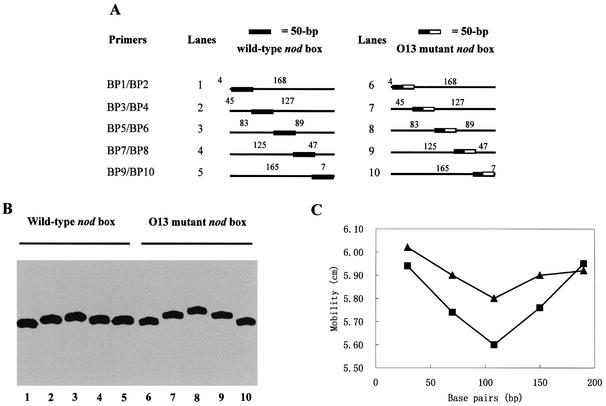
From the above results, we knew that the O13 mutant D-half was inactive for its specific NodD binding, and that the O13 mutant D-half allowed tetrameric NodD to activate nodA transcription constitutively. Then, we were interested to see if the D-half inactivation and the resulting NodD-mediated transcriptional activation occurred with a DNA structural modulation. Circular permutation assays were performed to detect the modulation of DNA bend. These assays are based upon the observation that a bend at the middle of a DNA molecule affects the mobility of that fragment more severely than the same bend at the end of the molecule (37). Five pairs of 226-bp fragments were produced by PCR. The nod box region was distributed from the ends to the middle of these DNA fragments. The only divergence between every pair of DNA fragments was that four base pairs of the wild-type D-half are mutated as those of the O13 mutant D-half.
The NodD–DNA complexes that were formed exhibited a position-dependent mobility (Fig. 7). Such large alternations in mobility are generally interpreted as being due to a DNA bend (37). Complexes formed between NodD and wild-type nod box fragments showed a weaker position-dependent mobility (compare lanes 1–5 to lanes 6–10, Fig. 7A), indicating the O13 mutant D-half allows NodD to cause a sharper DNA bend at the nod box region. The free 222-bp DNA fragments did not exhibit any position-dependent mobility (data not shown), indicating that all the created sequences do not have a significant intrinsic sequence-directed curvature. The position of the bend can be found by identifying the slowest migrating complex since such a complex will have the bend at the middle of its DNA fragment (37). Therefore, the distance migrated was plotted against the number of bases between the fragment left end and the –49 A of the nod box. The mobility distances of the complexes were determined by measuring the distance traveled from the well during the electrophoresis. The slowest migrating band was found to have its center between the D-half and the P-half (Fig. 7B). The bend angles can be estimated by using the formula µm/µe = cos(α/2), where µm is the mobility of the protein– DNA complex with a bend at the center of the DNA fragment, and µe is the mobility of the protein–DNA complex with a bend at the end of the DNA fragment (38). This formula would predict that complexes with the intact D-half have a 30° bend, while complexes with the mutant D-half have a 44° bend (Fig. 7). However, the fastest migrating fragment has its bend site rather far away from either end (∼30 bp). Therefore, assuming that this formula is valid for the gel system used in this study, these calculated bend angles probably underestimate the true values.
Figure 7.
Determination of NodD-induced DNA bending on the wild-type nod box and the O13 mutant nod box by circular permutation. (A) Five pairs of 222-bp radiolabeled DNA fragments used in (B) indicating the relative position of the NodD-binding site in relation to the ends of the fragments. (B) The mobility of NodD–DNA complexes using the DNA fragments listed in (A). Gel mobility shift assays were done with 1 pM DNA (lanes 1–10), 20 ng/µl ctDNA (lanes 1–10), 4 pg/µl (lanes 1–5) and 40 pg/µl (lanes 6–10) NodD. (C) Graphical representation of NodD-induced DNA bending on the wild-type nodA nod box (triangle) and the O13 mutant nod box (square). The mobility (in cm) of the NodD–DNA complexes is plotted against the number of bases between the left end of each fragment and the midpoint of the 50 bp nod box region. The bending angle by which DNA deflects from linearity is measured as described in Materials and Methods. The complexes with the intact D-half are calculated to have a 30° bend, while the complexes with the mutant D-half have a 44° bend according to an experiential formula.
DISCUSSION
Tetrameric NodD binds to promoter through anchoring the two half-sites of nod box
We propose that the most important nucleotides for the specific NodD binding are located in a 2-fold inverted repeat AT-N10-GAT-N16-ATC-N10-AT. This proposition has derived from the analysis of the symmetry of the NodD footprints on the nod box (Fig. 8A), the NodD affinity of various nod box deletions (Fig. 4) and the in vitro selection experiments (Fig. 5). Similarly, Toledano et al. have reported that the oxidized form of another LTTR OxyR also recognizes a 2-fold inverted repeat ATAGntnnnanCTATnnnnnnn ATAGntnnnanCTAT in vitro (22). Such a proposed binding motif gives a clue to the general binding motif of the LysR family. In fact, the unusually long DNA targets of many LTTRs share common features in length, sequence and even location (19,20,22,23,32–36). These target sites can usually be divided into two binding subsites or half-sites, and are often located at a similar position, from approximately –75 to –25 relative to the transcriptional start site of LTTR-activated promoters. The D-half-site (–75 to –50) almost always consists of an imperfect inverted repeat, which often shares the common LysR motif T-N11-A (10). The P-half (–50 to –25) is superimposed on the –35 region for RNA polymerase (33–36). The potential inverted repeat in this half-site is usually not obvious, likely due to possible dual roles of this region as target sites for both NodD and RNA polymerase. Besides the conserved nucleotides in the proposed motif, the other nucleotides of the nod box region also appear to be required for the natural optimal NodD binding, but not critical to a basal NodD binding. Supporting evidence comes from our in vitro selection experiments. The four mutated A and T nucleotides, which do not belong to the inverted repeat AT-N10-GAT, are also biased (Fig. 5). However, mutations of the three out of four nucleotides at the same time do not obviously impair NodD binding (Fig. 4C, lane 3).
Figure 8.
Model for tetrameric NodD binding on the nod box region. (A) Planar representation of the nodA nod box sequence. The positions of the residues are projected onto the surface of a cylinder that is then unrolled onto a flat surface. The DNA is assumed to adopt the B-form conformation, 10.4 bp per helical turn. The solid arrows indicate the regions protected from DNase I digestion. Closed circles denote the consensus sequence of the D-half (left) and P-half (right). (B) Tetrameric NodD assumes a cyclically symmetric dimer of NodD dimers. Each dimer is also cyclically symmetric and binds to one half-site with the basic motif AT-N10-GAT. Arrows and short lines indicate the exact position of the binding motif T-N11-A-N18-T-N11-A.
In Figure 8B, we propose a model for the arrangement of the NodD–nod box complex. In this model, tetrameric NodD is postulated to be a cyclically symmetric dimer of NodD dimers. Each NodD dimer is also suggested to be cyclically symmetric and bind to one inverted half-site with the central major groove unprotected. Many other LTTRs such as CysB, OxyR, TrpI and NahR are also reported to be tetrameric (12,22,39). The crystal structure of a dimeric CysB (88–324) has been solved (39). The dimer itself is cyclically symmetric, and the tetramer is proposed to be a dimer of dimers. We failed to detect the hypothetical NodD dimer band either by adding naringenin in vitro to the purified NodD or using NodD crude extract from induced Rhizobium cells (data not shown). This indicates that tetramer may be a functional unit of NodD at target DNA. The proposition that tetrameric NodD assumes a cyclic symmetric dimer of NodD dimers is consistent with the suggested 2-fold inverted repeat of the DNA binding motif.
In addition, we find that the two sub-halves of each half-site AT-N10-GAT bind to NodD in a highly cooperative manner. In DNase I digestion, NodD loses its specific protection on the left D-half sequence when one half of the D-half is deleted (Fig. 5B). Consistently, nod box deletions and its concomitant NodD affinity changes also support such a conclusion (Fig. 5A).
The inducible nodA promoter contains a NodD-dependent constitutive ‘promoter’
It is usually thought that only the activated form or coinducer-bound form of positive transcriptional factors can rescue the deficiency of those inducible promoters (40). However, it is intriguing that we find that the inducible nodA promoter contains an intrinsic part through which NodD can activate nodA transcription in an inducer-independent manner (Fig. 7). Rather, the uninduced tetrameric NodD can also activate nodA transcription at least partially. This is a challenge to the classical point of view. The reason why such activation is partial may lie in that pr.nodAc, pr.nodAd and pr.nodAO13 have weaker NodD affinity than pr.nodAb. Figure 3 shows that without a specific D-half, the nod box region has an ∼7-fold lower NodD affinity. However, the possibility still cannot be excluded that the NodD–pr.nodA complex with the mutant D-half is only partially competent for nodA transcription.
In another perspective, we suggest that in the absence of coinducer, an intact D-half is required for NodD to intrinsically repress the NodD-mediated partial activation (Fig. 6). Such intrinsic transcriptional repression requires the intact D-half or perhaps only the specific NodD binding ability of the D-half. Most recently, we have found that such intrinsic repression is dependent on the distance between the D-half and P-half. When the –54 nucleotide T was resected, NodD, whether induced or not, completely repressed the transcription. When a nucleotide G was inserted to position –54, NodD could not completely repress the transcription in the absence of inducer. In the control experiment, in which the D-half (–75 to –54) was entirely resected, NodD, whether induced or not, partially activated the transcription (J. Feng, X.-C. Chen, B.-H. Hou, F. Q. Li and G.-F. Hong, unpublished data). Insight into the mechanism underlying such intrinsic D-half-relevant transcriptional repression cannot directly explain but does give a clue to the induction mechanism for the wild-type nodA promoter.
DNA-bend modulation in the D-half-relevant transcriptional repression
In the absence of inducer, tetrameric NodD induces a sharper DNA bend on pr.nodAO13 than on the wild-type nodA promoter (Fig. 7). On pr.nodAO13 and pr.nodAb, tetrameric NodD-induced DNA bending is similar in position, which is near the midpoint of the nod box region (Figs 2 and 7). Since the D-half in pr.nodAO13 is inactive for its specific NodD anchoring (Fig. 5), the P-half is expected to decide the orientation of tetrameric NodD alone. Wang and Winans have demonstrated that the D-half-site of a natural OccR binding site can itself also decide the orientation of tetrameric OccR (21). Though the O13 mutant D-half does not change the primary structure of the NodD–nod box complex and may also not destroy the ‘real’ deficient nodA promoter, it does cause a large DNA conformational change, which may account for the NodD-mediated activation.
Several lines of evidence indicate that activator proteins can act as switch factors as a result of their DNA bending properties. The only effect cAMP·CRP is known to have on the DNA binding site is inducing a bend from linearity (37). An intrinsic DNA curvature is universally found in the 5′-upstream regions of the psbA family, and is important for basal transcription (42). Transcriptional factor MerR can relax or twist DNA upon ligand mercuric ion binding (43,44). Recent progress on the mechanism of transcriptional regulation controlled by LTTRs argues that the induction mechanism correlates with DNA bending (20,22). IlvY mediates transcriptional regulation in a DNA supercoiling-dependent manner (41).
Fisher and Long have shown that in vitro addition of flavonoids does not change the DNase I footprints of NodD (19). In all our attempts, we have failed to detect the hypothetical binding of NodD dimer. Therefore, it is less likely that NodD regulates nodA transcription through oligomerization. Most likely, tetrameric NodD only changes its conformation in response to inducer. However, since both induced and uninduced NodD can similarly activate the nodA promoter derivatives pr.nodAc, pr.nodAd and pr.nodAO13, it is hard to imagine that NodD conformational change itself serves as the induction trigger.
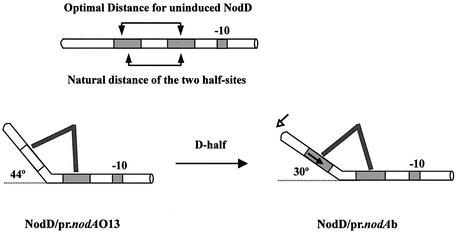
In Figure 9, we suggest a model to understand how the D-half inactivation allows uninduced NodD to induce a sharper DNA bend. It is necessary first to highlight the importance of the non-sequence-specific affinity between NodD and DNA in the NodD binding to target DNA (21). Though such non-sequence-specific binding does not leave ‘footprints’ on the additional vector DNA in place of the missing half region (Fig. 4), it does allow NodD to form a weak protection on a weakened digestion condition with a lower concentration of DNase I and especially with a shorter digestion time (15 s) (data not shown). Such non-sequence-specific binding for OccR has been reported to cause obvious protection on the vector DNA in DNase I digestion (21). In our model, DNA is hypothesized to have an optimal distance to interact with uninduced NodD. The natural distance between the two NodD-binding half-sites is slightly shorter than the optimal distance. For the O13 mutant nod box, one dimer of NodD is fixed to the P-half while the other can move freely on DNA. However, the steric effect eventually forces that dimer to move to its optimal position, allowing the NodD–nod box complex to assume an optimal DNA bend (ΔGP = – RT ln KP = –52.4 kJ/mol). For the wild-type nod box, the preferential binding to the specific D-half allows NodD dimer to shift its binding site to further reduce the Gibbs free energy of the whole complex (ΔGW = – RT ln KW = – 57.1 kJ/mol). Such a shift will result in a DNA bend modulation considering the rigidity of NodD protein. Part of the Gibbs free energy might be stored in the DNA bend modulation. We suggest that the conformational alternation of the NodD–nod box complex, especially the promoter DNA part, accounts for the intrinsic D-half-relevant transcriptional repression. The altered conformation is proposed to be not competent for RNA polymerase any more. Our model indicates that the physiological advantage of the long target site of LTTRs might be that LTTRs could transfer the conformational change from protein to DNA through bending or twisting DNA in response to inducer. Tetrameric NodD might decrease the converging angle of its two dimers to shorten its optimal distance in response to inducer.
Figure 9.
Model of D-half-relevant NodD-induced DNA bending. The –10 conserved sequence and the two half-sites are indicated by shadowed rectangles. Tetrameric NodD is simply represented by two converging lines. Combination of steric effect and the O13 D-half inactivation allows the NodD–nod box complex to adopt an optimal DNA bend. The preferential binding to the specific wild-type D-half forces NodD dimer to shift its contact position (marked by the solid arrow), resulting in a DNA bend modulation.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Dr Feng-Qing Li and Bo Zhou for helpful discussion in this work. This work was supported by Pan-Deng Plan of China to G.-F.H.
REFERENCES
- 1.Schultze M. and Kondorosi,A. (1998) Regulation of symbiotic root nodule development. Annu. Rev. Genet., 32, 33–57. [DOI] [PubMed] [Google Scholar]
- 2.Broughton W.J., Jabbouri,S. and Perret,X. (2000) Keys to symbiotic harmony. J. Bacteriol., 182, 5641–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rhijn P.J., Feys,B., Verreth,C. and Vanderleyden,J. (1993) Multiple copies of nodD in Rhizobium tropici CIAT899 and BR816. J. Bacteriol., 175, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossen L., Shearman,A.W.B. and Downie,J.A. (1985) The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA, B, C genes. EMBO J., 4, 3369–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shearman C.A., Rossen,L., Johnston,A.W.B. and Downie,J.A. (1986) The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by NodD plus a factor in pea root exudate. EMBO J., 5, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surin B.P. and Downie,J.A. (1988) Characterization of the Rhizobium leguminosarum genes nodLMN involved in efficient host-specific nodulation. Mol. Microbiol., 2, 173–183. [DOI] [PubMed] [Google Scholar]
- 7.Maagd R.A.D., Wijfjies,A.H.M. and Lugtenberg,B.J.J. (1989) nodO, a new nod gene of the Rhizobium leguminosarum Biovar viciae Sym plasmid pRL1JI, encodes a secreted protein. J. Bacteriol., 171, 6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H.L., Liu,S.T., Yang,Y., Chang,W.Z. and Hong,G.F. (2000) In Rhizobium leguminosarum, NodD represses its own transcription by competing with RNA polymerase for binding sites. Nucleic Acids Res., 28, 2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaink H.P., Okker,R.J.H., Wijffelman,C.A., Pees,E. and Lugtenberg,B.J.J. (1987) Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol., 9, 27–39. [DOI] [PubMed] [Google Scholar]
- 10.Goethals K., van Montagu,M. and Holsters,M. (1992) Conserved motifs in a divergent nod box of Azorhizobiuim caulinodans ORS571 reveal a common structure in promoters regulated by the LysR-type protein. Proc. Natl Acad. Sci. USA, 89, 1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schell M.A. (1993) Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol., 47, 597–626. [DOI] [PubMed] [Google Scholar]
- 12.Lochowska A. and Hryniewicz,M.M. (2001) Functional dissection of the LysR-type CysB transcriptional regulator. J. Biol. Chem., 276, 2098–2107. [DOI] [PubMed] [Google Scholar]
- 13.Hong G.F., Burn,J.E. and Johnston,A.W.B. (1987) Evidence that DNA involved in the expression of nodulation (nod) genes in Rhizobium leguminosarum binds to the product of the regulatory gene nodD. Nucleic Acids Res., 15, 9677–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher R.F. and Long,S.R. (1993) Interaction of NodD at the nod box: NodD binds to two distinct sites on the same face of the helix and induces a bend in the DNA. J. Mol. Biol., 233, 336–348. [DOI] [PubMed] [Google Scholar]
- 15.Spaink H.P., Wijffelman,C.A., Pees,E., Okker,R.J.H. and Lugtenberg,B.J.J. (1987) Rhizobium nodulation gene nodD as a determinant of host specificity. Nature, 328, 337–339. [Google Scholar]
- 16.Burn J.E., Wootton,H.J.C. and Johnston,A.W.B. (1989) Single and multiple mutations affecting properties of the regulatory gene nodD of Rhizobium. Mol. Microbiol., 3, 1567–1577. [DOI] [PubMed] [Google Scholar]
- 17.Schlaman H.R.M., Phillips,D.A. and Kondorosi,E. (1998) Genetic organization and transcriptional regulation of rhizobial nodulation genes. In Spaink,H.P., Kondorosi,A. and Hooykaas,P.J.J. (eds), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, Boston, London, pp. 361–386.
- 18.Kondorosi E., Gyuris,J., Schmidt,J., John,M., Duda,E., Hoffman,B., Schell,J. and Kondorosi,A. (1989) Positive and negative control of nod gene expression in Rhizobium meliloti is required for optimal nodulation. EMBO J., 8, 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher R.F. and Long,S.R. (1989) DNA footprinting analysis of the transcriptional activator proteins NodD1 and NodD3 on inducible nod gene promoters. J. Bacteriol., 171, 5492–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L., Helmann,J.D. and Winans,S.C. (1992) The A. tumefaciens transcriptional activator OccR causes a bend at a target promoter, which is partially relaxed by a plant tumor metabolite. Cell, 69, 659–667. [DOI] [PubMed] [Google Scholar]
- 21.Wang L. and Winans,S.C. (1995) The sixty nucleotide OccR operator contains a subsite essential and sufficient for OccR binding and a second subsite required for ligand-responsive DNA bending. J. Mol. Biol., 253, 691–702. [DOI] [PubMed] [Google Scholar]
- 22.Toledano M.B., Kullik,I., Trinh,F., Baird,P.T., Schneider,T.D. and Storz,G. (1994) Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell, 78, 897–909. [DOI] [PubMed] [Google Scholar]
- 23.Mcfall S.M., Klem,T.J., Fujita,N., Ishihama,A. and Chakrabarty,A.M. (1997) DNase I footprinting, DNA bending and in vitro transcription analyses of ClcR and CatR interactions with the clcABD promoter: evidence of a conserved transcriptional activation mechanism. Mol. Microbiol., 24, 965–976. [DOI] [PubMed] [Google Scholar]
- 24.Simon R., Priefer,V. and Puhler,A. (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology, 1, 784. [Google Scholar]
- 25.Huang W.H., Zhai,F. and Hong,G.F. (1999) Cloning, expression, characterization and application of Bst DNA polymerase Large Fragment. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai), 31, 379–384. [PubMed] [Google Scholar]
- 26.Feng J., Li,F.Q., Hu,H.L., Li,Q. and Hong,G.F. (2002) Expression and purification of Rhizobium leguminosarum NodD. Protein Exp. Purif., 26, 321–328. [DOI] [PubMed] [Google Scholar]
- 27.Liu S.T., Chang,W.Z., Cao,H.M., Hu,H.L., Chen,Z.H., Ni,F.D. and Hong,G.F. (1998) A HU-like protein binds to specific sites within nod promoters of Rhizobium leguminosarum. J. Biol. Chem., 273, 20568–20574. [DOI] [PubMed] [Google Scholar]
- 28.Liu S.T. and Hong,G.F. (1998) Three-minute G+A specific reaction for DNA sequencing. Anal. Biochem., 255, 158–159. [DOI] [PubMed] [Google Scholar]
- 29.Oliphant A.R., Brandl,C.L. and Struhl,K. (1989) Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Mol. Cell. Biol., 9, 2944–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J. Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Orchard K. and May,G.E. (1993) An EMSA-based method for determining the molecular weight of a protein–DNA complex. Nucleic Acids Res., 21, 3335–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee K.Y., Senear,D.F. and Hatfield,G.W. (1998) Activation of gene expression by a ligand-induced conformational change of a protein–DNA complex. J. Biol. Chem., 273, 11257–11266. [DOI] [PubMed] [Google Scholar]
- 33.Coco W.M., Parsek,M.R. and Chakrabarty,A.M. (1994) Purification of the LysR family regulator, ClcR and its interaction with the Pseudomonas putida clcABD chlorocatechol operon promoter. J. Bacteriol., 176, 5530–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jourlin-Castelli C., Mani,N., Nakano,M.M. and Sonenshein,A.L. (2000) CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J. Mol. Biol., 295, 865–878. [DOI] [PubMed] [Google Scholar]
- 35.Lamblin A.J. and Fuchs,J.A. (1994) Funtional analysis of the Eshcherichia coli K-12 cyn operon transcriptional regulation. J. Bacteriol., 176, 6613–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang M. and Crawford,I. (1990) The roles of indoleglycerol phosphate and the TrpI protein in the expression of trpBA from Pseudomonas aeruginosa. Nucleic Acids Res., 18, 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H.-M. and Crothers,D.M. (1984) The locus of sequence-directed and protein induced DNA bending. Nature, 308, 509–513. [DOI] [PubMed] [Google Scholar]
- 38.Thompson J.F. and Landy,A. (1988) Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res., 16, 9687–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyrrell R., Verschueren,K.H., Dodson,E., Murshudov,G.N., Addy,C. and Wilkinson,A.J. (1997) The structure of the cofactor-binding fragment of the LysR family member, CysB: a family fold with a surpring subunit arrangement. Structure, 5, 1017–1031. [DOI] [PubMed] [Google Scholar]
- 40.Schell M.A., Brown,P.H. and Raju,S. (1990) Mini review: positive control. J. Biol. Chem., 265, 3844–3850. [PubMed] [Google Scholar]
- 41.Opel M.L. and Hatfield,G.W. (2001) DNA supercoiling-dependent transcriptional coupling between the divergently transcribed promoters of the ilvYC operon of Escherichia coli is proportional to promoter strengths and transcript lengths. Mol. Microbiol., 39, 191–198. [DOI] [PubMed] [Google Scholar]
- 42.Ansari A.Z., Bradner,J.E. and O’Halloran,T.V. (1995) DNA-bend modulation in a repressor-to-activator switching mechanism. Nature, 374, 371–375. [DOI] [PubMed] [Google Scholar]
- 43.Ansari A.Z., Chael,M.L. and O’Halloran,T.V. (1992) Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature, 355, 87–89. [DOI] [PubMed] [Google Scholar]
- 44.Lamb J.W., Hombrecher,G. and Johnston,A.W.B. (1982) Plasmid-determined nodulation and nitrogen-fixation abilities in Rhizobium phaseoli. Mol. Gen. Genet., 186, 449–452. [Google Scholar]
- 45.Bagdasarian M., Lurz,R., Ruckert,B., Franklin,F.C.H., Bagdasarian,M.M., Frey,J. and Timmis,K.N. (1981) Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors and a host-vector system for gene cloning in Pseudomonas. Gene, 16, 237–247. [DOI] [PubMed] [Google Scholar]