Abstract
Maf1 is an essential and specific mediator of transcriptional repression in the RNA polymerase (pol) III system. Maf1-dependent repression occurs in response to a wide range of conditions, suggesting that the protein itself is targeted by the major nutritional and stress-signaling pathways. We show that Maf1 is a substrate for cAMP-dependent PKA in vitro and is differentially phosphorylated on PKA sites in vivo under normal versus repressing conditions. PKA activity negatively regulates Maf1 function because strains with unregulated high PKA activity block repression of pol III transcription in vivo, and strains lacking all PKA activity are hyperrepressible. Nuclear accumulation of Maf1 is required for transcriptional repression and is regulated by two nuclear localization sequences in the protein. An analysis of PKA phosphosite mutants shows that the localization of Maf1 is affected via the N-terminal nuclear localization sequence. In particular, mutations that prevent phosphorylation at PKA consensus sites promote nuclear accumulation of Maf1 without inducing repression. These results indicate that negative regulation of Maf1 by PKA is achieved by inhibiting its nuclear import and suggest that a PKA-independent activation step is required for nuclear Maf1 to function in the repression of pol III transcription. Finally, we report a previously undescribed phenotype for Maf1 in tRNA gene-mediated silencing of nearby RNA pol II transcription.
Keywords: nuclear import, phosphorylation, tRNA biosynthesis
The action of all three nuclear RNA polymerases (pols) in the synthesis of rRNAs, ribosomal protein mRNAs, and tRNAs is coordinately regulated to control ribosome biogenesis and cell growth in response to nutrients and many other conditions (1). In Saccharomyces cerevisiae, Maf1 has been identified as an absolute and specific effector of repression in the pol III system (2). The diversity of conditions that signal repression, combined with the essential role of Maf1 in this process, suggests that the Maf1 protein is targeted by multiple signaling pathways.
Genomewide localization of the pol III transcription apparatus has shown that nutrient deprivation and entry into stationary phase causes a significant decrease in polymerase occupancy on pol III genes (3, 4). This change is Maf1-dependent and is presumably a consequence of the direct interaction of Maf1 with the polymerase (5, 6). Consistent with this view, an in vitro system that recapitulates Maf1-dependent repression identified two steps that are inhibited as follows: polymerase recruitment to existing TFIIIB–DNA complexes and de novo assembly of the initiation factor TFIIIB onto DNA (5). In the latter step, Maf1 is thought to target the activity of TFIIIB via a direct interaction with one of its subunits, Brf1 (2, 5). However, the mechanism by which Maf1 inhibits TFIIIB–DNA assembly and transcription is not yet known.
Maf1 is a phylogenetically conserved and structurally novel protein that lacks homology to any motifs of known function (6). However, three conserved domains (A, B, and C) have been identified that contain predominantly charged residues and the previously undescribed signature sequences PDXDFS/T and WSXXYFFYNkkxKR, respectively (6). In this work, we establish the biological significance of conserved residues and motifs in Maf1 and report how signaling via the RAS/cAMP and TOR pathways negatively affects Maf1 localization and function in the repression of pol III transcription.
Results
Effects of MAF1 Mutations in Phenotypic Assays and in the Repression of Pol III Transcription.
Conserved charged residues in each domain of Maf1 were mutagenized individually or in pairs (Fig. 1A) and assessed for effects on Maf1 function in four assays: (i) repression of pol III transcription, (ii) growth on glycerol at 37°C, (iii) tRNA gene-mediated (tgm) silencing and (iv) tRNA-mediated nonsense suppression. The results for all mutations are summarized in Table 2, which is published as supporting information on the PNAS web site.
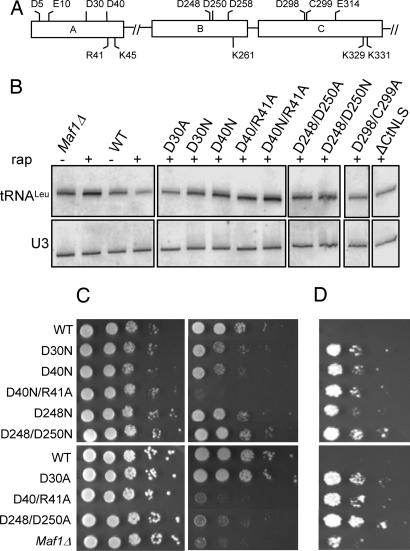
Fig. 1.
Analysis of charge substitutions in MAF1. (A) Mutation sites are shown schematically in conserved domains A, B, and C of Maf1. (B) Precursor tRNA- and U3 small nuclear RNA-specific probes were hybridized to a Northern blot of total RNA from rapamycin-treated or control strains. All images are from a single hybridization (Fig. 6). Lanes were cropped to remove mutants that are indistinguishable from WT. (C) Growth on glucose or glycerol at 37°C was compared by spotting 10-fold serial dilutions of the indicated WT or mutant MAF1 strains on SC-Trp (Left) and SGly-Trp (Right) media. (D) Analysis of tgm silencing. The strains indicated in C were transformed with a plasmid containing the SUP4-o tRNATyr gene and an adjacent GAL1 promoter-driven HIS3 gene (8). Equal cell numbers from 10-fold serial dilutions were spotted on SGal-Ura-Trp (shown in Fig. 6) and SGal-Ura-Trp-His media to monitor silencing of the HIS3 gene.
As an indicator of pol III synthesis, the level of short-lived tRNA precursors was quantified in control and rapamycin-treated cells and expressed as a percentage of the untreated WT control. Rapamycin significantly represses pol III transcription in the WT strain (to 35 ± 10% of the starting level), and this effect is quantitatively blocked in the maf1Δ strain (Fig. 1B and Table 2). Several mutations, including a conservative asparagine substitution at D40 in domain A and double mutations at positions D248 and D250 in the PDXDFS/T motif in domain B, were severely defective in their response to rapamycin. Other mutations, such as the K329/K331A (ΔCtNLS) substitution, which disrupts a putative nuclear localization sequence (NLS) in domain C (6), were partially defective.
Deletion of MAF1 causes temperature-sensitive growth on media containing glycerol (7). Only certain mutations with strong defects in transcriptional repression exhibited this phenotype (Fig. 1C). MAF1 alleles with partial defects in repression (between 47% and 58% residual transcription, e.g., D30A and D250N; Table 2) and the severely defective double asparagine substitution at D248/D250 had a WT glycerol growth phenotype (Fig. 1C).
The location of a tRNA gene in the 5′ upstream region of a pol II gene can suppress its transcription. This phenomenon, termed tgm silencing, does not involve steric occlusion of pol II factor binding but requires a transcriptionally competent tRNA gene and active pol III transcription and is associated with the clustering of tRNA genes in or near the nucleolus (8, 9). tgm silencing by the SUP4 tRNA gene was assayed from an adjacent GAL1 promoter-driven HIS3 gene. The WT MAF1 strain is phenotypically His− on galactose media (Fig. 1D). However, deletion of MAF1 generates His+ colonies, indicating a previously undescribed phenotype of Maf1 in tgm silencing. The MAF1 mutants generated either a WT silencing phenotype or a maf1Δ phenotype (Fig. 1D; see Fig. 6, which is published as supporting information on the PNAS web site). Thus, the silencing assay provides a sensitive, but all or none, measure of Maf1 function that correlates with the ability of MAF1 mutants to function in transcriptional repression (Table 2).
The W303 yeast strain contains an ochre-suppressible nonsense mutation (ade2-1) that causes accumulation of a red biosynthetic intermediate. In the presence of an efficient ochre suppressor, such as SUP4-o, readthrough of the ade2-1 mutation leads to a cream colony color (suppression). Deletion of MAF1 produces red colonies due to a decrease in readthrough efficiency (antisuppression) and is thought to result from hypomodification of the suppressor tRNA (10, 11). As expected for a phenotype based on efficient suppressor tRNA expression and function, MAF1 alleles exhibited the same rank order with respect to defects in transcriptional repression and antisuppressor activity (Fig. 6 and Table 2).
Despite some growth differences between the insensitive glycerol phenotype and the other three assays, the overall concordance in the behavior of the mutants suggests that each reports a common biochemical function of Maf1.
Maf1 Is Phosphorylated by PKA and Hypophosphorylation Correlates with Maf1 Function in Repression.
The ability of different signaling pathways and diverse conditions to effect repression through Maf1 suggested that the protein itself may be a target of one or more signaling pathways (10). To address this question, we first determined whether Maf1 is phosphorylated in vivo. Specific immunoprecipitation of a Maf1myc fusion protein from in vivo-labeled cell extracts identified a 32P-labeled band of the appropriate size (Fig. 2A). We next examined Maf1myc in lysates of cells treated with different repressing agents or grown to stationary phase by Western blotting of high resolving SDS/polyacrylamide gels (Fig. 2B). Slow- and fast-migrating forms of Maf1 were detected in control extracts and the loss of the slow-migrating form correlated with repression of pol III transcription. Because the slow-migrating form also was lost after phosphatase treatment of the extract, the data show that repression of pol III transcription correlates with a decrease in Maf1 phosphorylation.
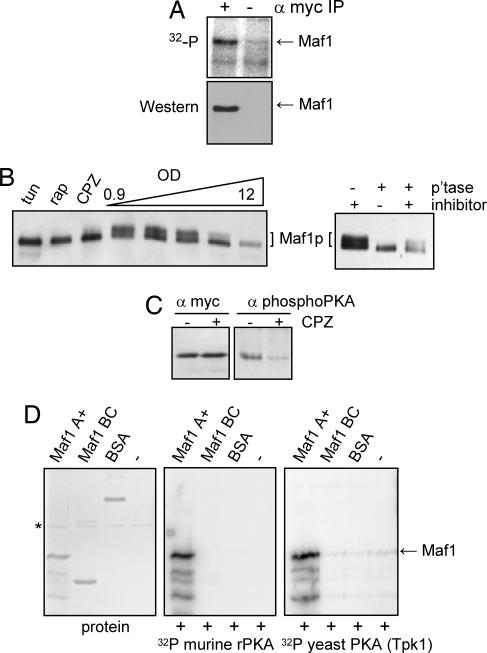
Fig. 2.
Hypophosphorylation of Maf1 on consensus PKA sites correlates with repression of pol III transcription. (A) In vivo 32P-labeling of Maf1myc. Immunoprecipitates obtained in the presence or absence of myc antibody were detected by autoradiography (Upper) and by Western blot (Lower). (B) Maf1 phosphorylation causes slow migration in PAGE. Cell extracts were processed to preserve phosphorylated Maf1 and separated by high-resolving PAGE, and Maf1 forms were detected with α-myc antibody. Samples are from cells treated with tunicamycin (tun), rapamycin (rap), or CPZ or taken from early log to stationary phase cultures (Left). Early log phase cell extracts were treated with alkaline phosphatase in the presence and absence of a phosphatase inhibitor (Right). (C) Maf1 is recognized by a PKA phosphosubstrate-specific antibody. Immunoprecipitated Maf1 from CPZ-treated and control extracts was detected by Western blot with α-myc antibody (Left) and with a PKA-phosphospecific antibody (Right). (D) Maf1 is a substrate for yeast PKA in vitro. Fragments of Maf1 that contain the A domain and adjacent sequences (A+) or the B+C domain were labeled in vitro by murine PKA (Gel-code blue stain, Left; and autoradiograph, Center) and yeast-purified GST-TPK1 (Right). Murine PKA is marked with an asterisk.
An evolutionary proteomics approach recently identified Maf1 as a substrate for cAMP-dependent PKA (12). Analysis of Maf1 reveals six sites that conform to the yeast PKA consensus R-3 R/K-2 X-1 S/T (13). To determine whether Maf1 is differentially phosphorylated on these sites under normal and repressing conditions, the protein was immunoprecipitated from control and repressed [chlorpromazine (CPZ)-treated; ref. 10] cell extracts and then detected by blotting with a PKA phosphosubstrate-specific antibody (see Methods and Fig. 7, which is published as supporting information on the PNAS web site). Phosphorylated Maf1 was readily detected in control extracts and the level of phospho-Maf1 detected in this manner decreased significantly under repressing conditions despite the presence of comparable levels of Maf1myc in both immunoprecipitates (Fig. 2C Left).
To determine whether Maf1 is a substrate for yeast PKA, recombinant fragments that split Maf1 at the beginning of domain B were incubated with [γ-32P]ATP and either murine or yeast (Tpk1) PKA. Consistent with the location of the consensus PKA sites between domains A and B, both enzymes phosphorylated the Maf1A+ fragment that contained these sequences but not the Maf1 BC fragment where these sequences are missing (Fig. 2D). Together these data demonstrate that Maf1 is phosphorylated on PKA sites under normal growth conditions and is a substrate for PKA in vitro. Moreover, PKA may function as a negative regulator of Maf1's activity in repression of pol III transcription because repression correlates with Maf1 hypophosphorylation at consensus PKA sites.
High PKA Activity Blocks Repression of Pol III Transcription.
The differential phosphorylation of Maf1 on consensus PKA sites (Fig. 2C) predicts that the catalytic activity of PKA should inversely correlate with Maf1 function: High PKA activity will limit the function of Maf1 in repression, whereas low PKA activity will enable repression by Maf1. PKA activity in yeast is provided by three catalytic subunits, Tpks1–3, that are genetically redundant for growth but have distinct substrate specificities (13). In preliminary experiments, we found that TPK1 alone (strain S7-7A) or TPK2 and TPK3 together (strain TF4-1) supports normal levels of pol III transcription in log phase and a normal transcriptional response to rapamycin (Fig. 3A and data not shown; see Table 3, which is published as supporting information on the PNAS web site). Deletion of BCY1, which encodes the regulatory subunit of PKA, causes high unregulated PKA activity (14). As predicted, bcy1Δ strains containing one, two, or all three catalytic PKA subunits were unable to signal repression efficiently (Fig. 3 A and B and data not shown). Also in agreement with the above predictions, a tpk1wi“wimpy” strain (14), which contains a debilitated kinase subunit as the sole source of PKA activity, allows repression of pol III transcription even in the absence of BCY1 (Fig. 3A).
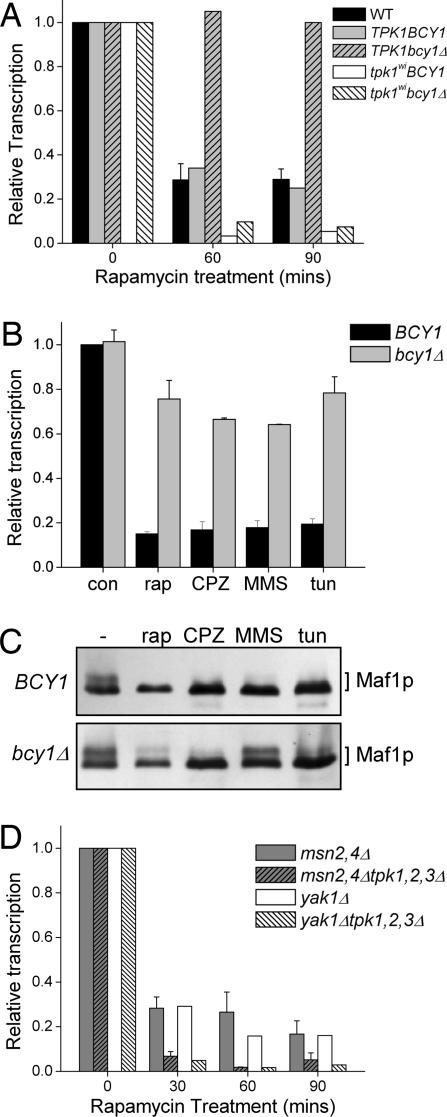
Fig. 3.
PKA activity affects repression of pol III transcription. (A) Repression of pol III transcription by rapamycin was detected by Northern blot analysis, quantified, and plotted relative to each untreated control sample. WT (TPK1,2,3, black bars), TPK1BCY1 (gray bars), TPK1bcy1Δ (gray hatched bars), tpk1wiBCY1 (open bars), and tpk1wibcy1Δ (white hatched bars) strains were treated with rapamycin for 60 and 90 min. (B) Repression by multiple conditions is blocked in the TPK1,2,3 bcy1Δ strain. WT (BCY1, black bars) and bcy1Δ strains (gray bars) were treated with rapamycin (rap), CPZ, methyl methanesulfonate (MMS), and tunicamycin (tun) for 60 min and repression of pol III transcription analyzed and expressed as in A. (C) Extracts from WT (BCY1; Upper) and bcy1Δ (Lower) strains were processed and detected for Maf1 forms as described in Fig. 2B. Hyperphosphorylated Maf1 accumulates in the bcy1Δ strain under the repressing conditions described in B. (D) PKA activity is not required to signal repression of pol III transcription. Repression of pol III transcription by rapamycin in PKA triple delete strains tpk1,2,3Δ,msn2,4Δ (gray hatched bars) and tpk1,2,3Δ,yak1Δ (white hatched bars) and their relevant control strains, TPK1,2,3,msn2,4Δ (gray bars) and TPK1,2,3,yak1Δ (open bars). Strains were treated with rapamycin for 30, 60, and 90 min.
Hyperactivation of PKA also can be caused by a mutant allele of RAS2 (RAS2Val-19; ref. 15). In RAS2Val-19 strains, the constitutive production of cAMP by adenylate cyclase dissociates the Bcy1 regulatory subunit, resulting in high PKA activity. In agreement with the effects seen in the bcy1Δ strains, the RAS2Val-19 mutation blocked the repression of pol III transcription in response to rapamycin (Fig. 8, which is published as supporting information on the PNAS web site).
In addition to rapamycin, repression induced by CPZ, methyl methane sulfonate, and tunicamycin treatments, which together involve at least three distinct signaling pathways (10), was also largely blocked by the high PKA activity in a bcy1Δ strain (Fig. 3B). Notably, the slow-migrating, phosphorylated form of Maf1 was enriched under repressing conditions in the bcy1Δ strain relative to WT, consistent with a negative role for PKA activity in Maf1 function (Fig. 3C). The variation in the absolute amount of phospho-Maf1 in this experiment may reflect a difficulty in maintaining the modification despite the presence of phosphatase inhibitors.
Strains deleted for all PKA catalytic subunits are inviable but can be rescued by deleting the glucose-sensing and stress-response kinase Yak1 or the general stress response transcription factors Msn2 and Msn4 (16, 17). These strains allowed us to assess whether the absence of PKA causes constitutive activation of Maf1 and repression of pol III transcription. Contrary to this possibility, equivalent absolute levels of pol III transcription were found in all of the strains during early log phase growth (data not shown). However, upon rapamycin treatment, the strains lacking Tpks1–3 were much more potently repressed than their corresponding WT counterparts (Fig. 3D). This result supports the finding that PKA activity opposes the function of Maf1 in repression and suggests that a PKA-independent step is required to achieve transcriptional repression by Maf1.
PKA Sites Overlap and Are Functionally Linked to an N-Terminal NLS (NtNLS) Sequence in Maf1.
The significance of PKA phosphorylation on Maf1 function was examined directly by generating alanine and glutamate substitutions at all six PKA consensus sites (residues 90, 101, 177, 178, 209, and 210; Fig. 4A) and testing their growth phenotypes and activity in transcriptional repression (Fig. 4 B–D and Table 1). The nonphosphorylatable 6SA mutant and the 6SE phosphorylation mimic exhibited normal levels of pol III transcription (86 ± 5% and 99 ± 7% of WT levels, respectively; data not shown) and the same extent of repression as WT Maf1. Both mutants were WT for the glycerol, antisuppression, and tgm-silencing phenotypes.
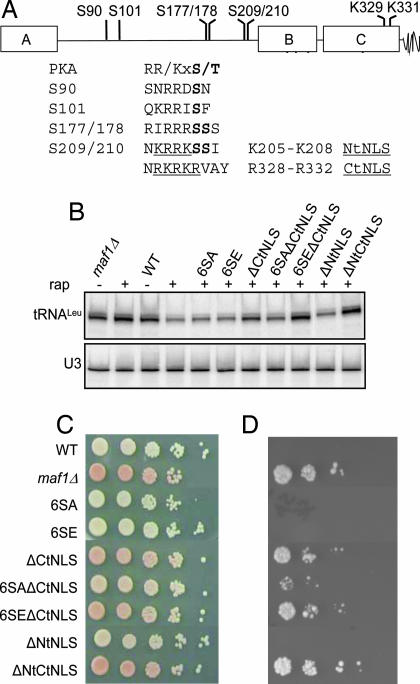
Fig. 4.
Mutations in Maf1 PKA recognition sites and NLS sequences affect Maf1 function. (A) Maf1 contains six PKA recognition sites (annotated in bold) and two potential NLS sequences (NtNLS and CtNLS, underlined). NtNLS is adjacent to the S209/210 pair of PKA sites. (B) Precursor tRNA- and U3 small nuclear RNA-specific probes were hybridized to a Northern blot of total RNA from PKA site and NLS mutant strains treated with rapamycin. Maf1 mutations are as follows: 6SA, S90/101/177/178/209/210A; 6SE, S90/101/177/178/209/210E; ΔCtNLS, K329/331A and ΔNtNLS, K205/R206A. (C and D) The WT, maf1Δ, and mutant strains indicated in B were transformed with a plasmid containing the SUP4-o tRNATyr gene and an adjacent GAL1 promoter-driven HIS3 gene (8). Equal cell numbers from 10-fold serial dilutions were spotted on SGal-Ura-Trp and SGal-Ura-Trp-His media to monitor the ade2-1 red color phenotype (C) and tgm silencing of the HIS3 gene (D), respectively.
Table 1.
Effect of NLS and PKA mutations on Maf1 function
| Mutations | Pol III repression* | Glycerol 37°C† | Growth SGal-His‡ | ade2-1 color§ | Location¶ |
|
|---|---|---|---|---|---|---|
| −rap | +rap | |||||
| WT | 28 | ++++ | − | − | N + C | N |
| maf1Δ | 112 | − | ++ | +++ | − | − |
| 6SA | 26 | ++++ | − | − | N > C | N |
| 6SE | 28 | ++++ | − | − | C > N | N |
| 6SAΔCtNLS | 42 | ++++ | (+) | + | N > C | N |
| 6SEΔCtNLS | 91 | +++ | +++ | ++ | C | C > N |
| ΔNtNLS | 35 | ++++ | − | − | C | C > N |
| ΔNtCtNLS | 101 | +++ | +++ | ++ | C | C |
| ΔCtNLS | 64 | +++ | +++ | ++ | C | N |
*Residual transcription after 90 min of rapamycin treatment expressed as a percentage of the untreated WT level (from Fig. 4). Data is representative of multiple experiments that generate an SD of 3–8%. An examination of unstressed transcription for many Maf1 mutants found no significant changes compared with WT or the maf1Δ strain.
†Glycerol phenotype: + represents each 10-fold dilution at which cells grew on SGly-Trp at 37°C.
‡tgm-silencing phenotype: + represents each 10-fold dilution at which cells grew on SGal-Ura-Trp-His at 30°C.
§Antisuppression: −, cream color; +, light pink; ++, pink; +++, red on SGal-Ura-Trp media.
¶Localization: Cytoplasmic (C) and nuclear signal (N) after 60 mins with (+) or without (−) rapamycin treatment. Qualitative changes in distribution are expressed relative to WT.
Two of the PKA sites in Maf1 (residues 209 and 210) lie adjacent to a possible second NLS sequence in the protein (Fig. 4A; ref. 6). Phosphorylation adjacent to an NLS (and at distal sites) is known to decrease the binding affinity for import factors and underlies the regulated nucleo-cytoplasmic trafficking of many proteins (18). Mutations that disrupt the potential NLS at residues 205–208 but retain the PKA site at position 210 (K205/R206A and ΔNtNLS) did not cause an obvious defect in Maf1 function. However, in combination with a partially defective disruption of the C-terminal NLS sequence (K329/K331A and ΔCtNLS; Figs. 1A and 4), the resulting ΔNtCtNLS mutant was as defective as the maf1Δ strain in all assays (Fig. 4 and Table 1). These results indicate that each NLS motif contributes to the function of Maf1.
The effect of PKA phosphorylation on the NtNLS motif was examined directly by combining the 6SE and ΔCtNLS mutations. The 6SEΔCtNLS mutant was as defective as the double NLS mutant, ΔNtCtNLS, in all assays (Fig. 4 and Table 1). Thus, the 6SE mutation phenocopies a disruption of the NtNLS motif in this context. Conversely, the 6SAΔCtNLS mutant increased Maf1 function relative to the ΔCtNLS mutation (Fig. 4). The phenotypes of these mutants, along with their activity in repressing pol III transcription (Table 1), suggest that the phosphorylation state of consensus PKA sites in Maf1 together with the NtNLS motif regulate Maf1 function. In accordance with the negative role of PKA in regulating Maf1-dependent repression (Fig. 3), the results predict that hyperphosphorylation of Maf1 on consensus PKA sites (Fig. 2) will negatively affect its accumulation in the nucleus.
PKA Consensus Sites Regulate the Nuclear Localization of Maf1 via the NtNLS.
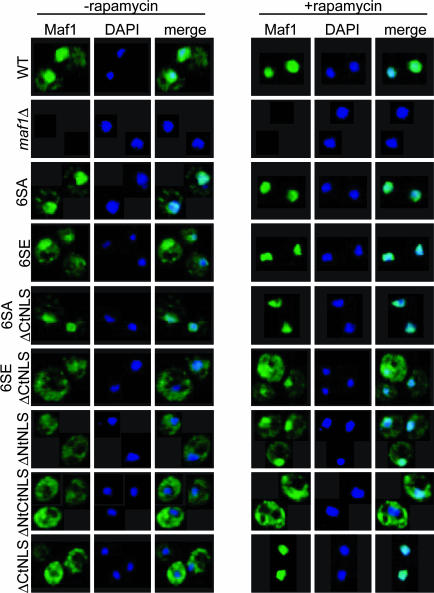
Recent studies have shown that Maf1 generates a largely cytoplasmic signal by standard fluorescence microscopy under normal growth conditions (19, 20). Although we also see this distribution at low resolution, higher resolution through optical sectioning clearly shows nuclear and cytoplasmic Maf1 signals in early log phase in synthetic media (Fig. 5). Analysis of the Maf1 mutants shows that each NLS motif influences the cellular distribution of Maf1: Both of the ΔNtNLS and ΔCtNLS mutants are predominantly cytoplasmic, and the ΔNtCtNLS mutant is excluded from the nucleus. Under the same conditions, the 6SE mutant is enriched in the cytoplasm and the 6SA mutant shows increased nuclear staining (Fig. 5 and Table 1). The effect of these mutations on the activity of the NtNLS is especially pronounced in the ΔCtNLS background. Notably, the nuclear localization of the 6SA mutant does not cause repression of pol III transcription (see above).
Fig. 5.
PKA recognition sites and NLS sequences regulate the nuclear localization of Maf1. Immunofluoresence of WT and mutant Maf1myc fusion proteins (Oregon Green 488) detected by fluorescence deconvolution microscopy. Maf1 nuclear localization is confirmed by the overlap with DAPI nuclear staining. Cells were untreated (Left) or rapamycin-treated (Right) for 60 min.
Treatment of cells with rapamycin, growth to stationary phase, and nutrient deprivation leads to the nuclear accumulation of Maf1 (refs. 19 and 20 and Fig. 5), consistent with its function in repressing pol III transcription. However, the ability of Maf1 to completely relocalize to the nucleus under repressing conditions is not a predictor of function. Although the nuclear accumulation of the inactive ΔNtCtNLS mutant was completely blocked under repressing conditions, the ΔCtNLS mutant that has intermediate activity efficiently relocalizes Maf1 to the nucleus, and the ΔNtNLS mutant that has WT activity is defective in this function (Fig. 5 and Table 1). The 6SEΔCtNLS mutation, which is predicted to decrease the affinity of the NtNLS sequence for the import machinery, significantly impairs the relocation of Maf1. These results show that both NLS motifs are independently able to direct Maf1 to the nucleus under normal conditions and support the conclusion that the activity of the NtNLS is required for efficient relocation under repressing conditions and is regulated by PKA.
Discussion
In this work, we provide evidence that the function of Maf1 is regulated at two levels: cellular localization (cytoplasm versus nucleus) and modulation of the activity of nuclear Maf1. Our experiments have identified two NLS sequences in Maf1 [(NtNLS) and C-terminal NLS (CtNLS)] that appear to play distinct roles in its localization and differentially affect Maf1 function. The data indicate that the NtNLS is negatively regulated by the action of PKA, and this regulation contributes to the cytoplasmic location of Maf1 under normal growth conditions. We note that our data does not exclude the possibility that other kinases may phosphorylate Maf1 at sites that overlap with PKA consensus sites. However, the phosphorylation of Maf1 by PKA in vitro (Fig. 2D), the conservation of PKA sites in Maf1 (12), the effects of PKA hyperactivation and deletion (Fig. 3), and the changes in the localization of PKA phospho-site and NLS mutants (Fig. 5) are explained most simply by PKA directly regulating Maf1 localization. PKA activity in yeast negatively regulates the general stress response and positively affects cell growth and proliferation in response to glucose and nutrients (21). Thus, the proposed negative regulation of Maf1 localization by PKA provides a mechanism to link glucose signaling via the RAS/cAMP pathway with pol III transcription.
The localization defects seen in the ΔNtNLS and ΔCtNLS mutants suggest that the NtNLS plays a dominant role in the redistribution of Maf1 under repressing conditions. However, the aberrant distribution in the ΔCtNLS mutant under normal growth conditions indicates that the CtNLS also has a biological role in Maf1 nuclear import. Unlike the NtNLS, the CtNLS is not adjacent to potential phosphorylation sites that might regulate its activity, and there is no evidence for Maf1-binding partners that could function in NLS-masking or cytoplasmic retention (22). These observations suggest that the CtNLS may be unregulated (i.e., constitutively active) and raise the possibility that the nuclear accumulation of the ΔNtNLS mutant is due to decreased nuclear export. It is plausible, therefore, that Maf1 may shuttle between the nucleus and cytoplasm, as described for a number of transcriptional regulators (23) and that changes in the relative rates of import and export lead to its nuclear accumulation. In this context, the nucleocytoplasmic shuttling of the stress response protein Msn2, controlled by protein kinases and phosphatases (24), provides an appealing paradigm for Maf1. In the case of Maf1 regulation, the activity of PKA appears to be opposed by protein phosphatase 2A (PP2A), because mutation of a PP2A scaffold subunit (Tpd3) affects pol III transcription (25) and reducing PP2A catalytic capacity affects Maf1 phosphorylation and localization (20).
The hypophosphorylated form of Maf1 that is observed under repressing conditions correlates with nuclear localization (Fig. 2). However, the requirements for repression of pol III transcription apparently are more complex than the simple dephosphorylation and redistribution of Maf1. The largely nuclear localization yet WT transcription of the 6SA mutant under normal growth conditions indicates that nuclear accumulation of Maf1 is not sufficient to cause repression of transcription per se (Fig. 5 and Table 1). This observation coupled with the normal but hyperrepressible pol III transcription in the complete absence of PKA (Fig. 3D) suggests that a PKA-independent activation step(s) is required for nuclear Maf1 to function in repression. The proposed activation step for nuclear Maf1 provides a mechanism for signaling pathways, other than those impinging on PKA, to regulate Maf1 function. Finally, we note that the extent of repression in the 6SA and 6SE mutants is not as great as in the tpk1wi and tpk1-3Δ strains. This difference suggests that Tpks1–3 affect other unknown cellular functions that directly or indirectly impact pol III transcriptional repression.
Methods
Yeast Strains and Assays.
Strains listed in Table 3 were grown in media containing a 2% carbon source. PCR-based deletions of YAK1 (26) and BCY1 (27) were confirmed by PCR with gene-specific primers. A pRS314 plasmid that contained Maf1 fused at the C terminus to a myc epitope and expressed from its own promoter was the template for site-directed mutagenesis (QuikChange II; Stratagene, La Jolla, CA). maf1Δ strains containing pRS314 with either WT MAF1myc or mutant derivatives were grown to OD 0.2 to 0.6 and treated as described in ref. 2. No mutation affected Maf1 protein stability as determined by Western blot analysis (data not shown), or caused a conditional phenotype on glucose-containing media. RNA extraction, Northern blot analysis, and quantitation were as described in ref. 28. Plate growth phenotypes (10-fold serial dilutions from equal cell numbers) were documented after 3–7 days. Antisuppression (decreased readthrough of ade2-1) was assessed on SC-Ura-Trp or SGal-Ura-Trp media after storage at 4°C for 4–7 days.
Analysis of Protein Phosphorylation.
For in vivo phosphorylation experiments, cells were grown to OD 0.5 in low-phosphate media, labeled with 5 mCi (1 Ci = 37 GBq) of carrier-free 32P-inorganic orthophosphate for 120 min (1), harvested with phosphatase inhibitors, and immunoprecipitated and detected as described in ref. 5. To preserve and detect modifications that change the mobility of the Maf1myc protein, cells were harvested directly into SDS sample buffer containing protease and phosphatase inhibitors, boiled before glass bead breakage (29), and separated by SDS/PAGE (acrylamide:bis-acrylamide 45:1). Phosphorylated Maf1 was detected in Western blots with a PKA phosphosubstrate-specific antibody that detects the sequence RRXpS/pT (catalog no. 9624; Cell Signaling Technology, Beverly, MA). In vitro phosphorylation used 1 μg of recombinant His-tagged Maf1 A+ (amino acids 1–200) or Maf1 BC (amino acids 231–395) fragments prepared under denaturing conditions and refolded (5) and either murine PKA catalytic subunit (NEB, Beverly, MA), or yeast Tpk1p (30) recovered from glutathione-agarose beads, in the presence of both protease and phosphatase inhibitors.
Fluorescence Microscopy.
Detailed fixation and detection methods are reported as Supporting Methods, which is published as supporting information on the PNAS web site. Anti-myc rabbit polyclonal antibody was detected with goat anti-rabbit antibody conjugated to Oregon Green 488 (Molecular Probes, Carlsbad, CA). Coverslips were mounted by using ProLong Antifade reagent, and images were captured in 0.1-μm optical slices on a Nikon (Tokyo, Japan) E800 microscope and deconvolved with ISEE software (Inovision, Raleigh, NC) as described in refs. 31 and 32.
Supplementary Material
Acknowledgments
We thank Gustav Ammerer (University of Vienna, Vienna, Austria) and Tamar Michaeli (Albert Einstein College of Medicine) for yeast strains and Jon Warner (Albert Einstein College of Medicine) for the RAS2Val-19-containing plasmid. This work was supported by National Institutes of Health Grants GM42728 (to I.M.W.) and GM063142 (to D.R.E.), Michigan Genetics Predoctoral Training Grant T32 GM07544 (to R.A.H.), and funds from the Albert Einstein College of Medicine.
Abbreviations
- CPZ
chlorpromazine
- NLS
nuclear localization signal
- CtNLS
C-terminal NLS
- NtNLS
N-terminal NLS
- pol
polymerase
- tgm
tRNA gene-mediated
Footnotes
The authors declare no conflict of interest.
References
- 1.Warner J. Trends Biochem Sci. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 2.Upadhya R, Lee J, Willis IM. Mol Cell. 2002;10:1489–1494. doi: 10.1016/s1097-2765(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 3.Roberts DN, Stewart AJ, Huff JT, Cairns BR. Proc Natl Acad Sci USA. 2003;100:14695–14700. doi: 10.1073/pnas.2435566100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harismendy O, Gendrel CG, Soularue P, Gidrol X, Sentenac A, Werner M, Lefebvre O. EMBO J. 2003;22:4738–4747. doi: 10.1093/emboj/cdg466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai N, Lee J, Upadhya R, Chu Y, Moir RD, Willis IM. J Biol Chem. 2005;280:6455–6462. doi: 10.1074/jbc.M412375200. [DOI] [PubMed] [Google Scholar]
- 6.Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, Hopper AK, Sentenac A, Boguta M. Mol Cell Biol. 2001;21:5031–5040. doi: 10.1128/MCB.21.15.5031-5040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boguta M, Czerska K, Zoladek T. Gene. 1997;185:291–296. doi: 10.1016/s0378-1119(96)00669-5. [DOI] [PubMed] [Google Scholar]
- 8.Hull MW, Erickson J, Johnston M, Engelke DR. Mol Cell Biol. 1994;14:1266–1277. doi: 10.1128/mcb.14.2.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Good PD, Haeusler RA, Thompson M, Engelke DR. J Biol Chem. 2005;280:8637–8639. doi: 10.1074/jbc.C500017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis IM, Desai NA, Upadhya R. Prog Nucleic Acid Res Mol Biol. 2004;77:323–353. doi: 10.1016/S0079-6603(04)77009-9. [DOI] [PubMed] [Google Scholar]
- 11.Kwapisz M, Smagowicz WJ, Oficjalska D, Hatin I, Rousset JP, Zoladek T, Boguta M. Curr Genet. 2002;42:147–152. doi: 10.1007/s00294-002-0342-7. [DOI] [PubMed] [Google Scholar]
- 12.Budovskaya YV, Stephan JS, Deminoff SJ, Herman PK. Proc Natl Acad Sci USA. 2005;102:13933–13938. doi: 10.1073/pnas.0501046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ptacek J, Devgan G, Michaud G, Zhu H, Zhu X, Fasolo J, Guo H, Jona G, Breitkreutz A, Sopko R, et al. Nature. 2005;438:679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
- 14.Cameron S, Levin L, Zoller M, Wigler M. Cell. 1988;53:555–566. doi: 10.1016/0092-8674(88)90572-7. [DOI] [PubMed] [Google Scholar]
- 15.Hlavata L, Aguilaniu H, Pichova A, Nystrom T. EMBO J. 2003;22:3337–3345. doi: 10.1093/emboj/cdg314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett S, Menold MM, Broach JR. Mol Cell Biol. 1991;11:4045–4052. doi: 10.1128/mcb.11.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith A, Ward MP, Garrett S. EMBO J. 1998;17:3556–3564. doi: 10.1093/emboj/17.13.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harreman MT, Kline TM, Milford HG, Harben MB, Hodel AE, Corbett AH. J Biol Chem. 2004;279:20613–20621. doi: 10.1074/jbc.M401720200. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DN, Wilson B, Huff JT, Stewart AJ, Cairns BR. Mol Cell. 2006;22:633–644. doi: 10.1016/j.molcel.2006.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oficjalska-Pham D, Harismendy O, Smagowicz WJ, Gonzalez de Peredo A, Boguta M, Sentenac A, Lefebvre O. Mol Cell. 2006;22:623–632. doi: 10.1016/j.molcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Thevelein JM, de Winde JH. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 22.Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, et al. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler EC, Ghosh S. Sci STKE. 2005;2005:re6. doi: 10.1126/stke.2842005re6. [DOI] [PubMed] [Google Scholar]
- 24.Jacquet M, Renault G, Lallet S, De Mey J, Goldbeter A. J Cell Biol. 2003;161:497–505. doi: 10.1083/jcb.200303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zyl W, Huang W, Sneddon AA, Stark M, Camier S, Werner M, Marck C, Sentenac A, Broach JR. Mol Cell Biol. 1992;12:4946–4959. doi: 10.1128/mcb.12.11.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, et al. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 27.Wach A, Brachat A, Pohlmann R, Philippsen P. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Moir RD, Sethy-Coraci IK, Warner JR, Willis IM. Mol Cell Biol. 2000;20:3843–3851. doi: 10.1128/mcb.20.11.3843-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shou W, Sakamoto KM, Keener J, Morimoto KW, Traverso EE, Azzam R, Hoppe GJ, Feldman RM, DeModena J, Moazed D, et al. Mol Cell. 2001;8:45–55. doi: 10.1016/s1097-2765(01)00291-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Klemic JF, Chang S, Bertone P, Casamayor A, Klemic KG, Smith D, Gerstein M, Reed MA, Snyder M. Nat Genet. 2000;26:283–289. doi: 10.1038/81576. [DOI] [PubMed] [Google Scholar]
- 31.Thompson M, Haeusler RA, Good PD, Engelke DR. Science. 2003;302:1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand E, Houser-Scott F, Kendall A, Singer RH, Engelke DR. Genes Dev. 1998;12:2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.