Abstract
Crystal (Cry) proteins produced by the soil bacterium Bacillus thuringiensis (Bt) are harmless to vertebrates, but they are highly toxic to insects and nematodes. Their value in controlling insects that destroy crops and transmit human diseases is well established. Although it has recently been demonstrated that a few individual Bt Cry proteins, such as Cry5B, are toxic to a wide range of free-living nematodes, the potential activity of purified Cry proteins against parasitic nematodes remains largely unknown. We report here studies aimed at characterizing in vitro and in vivo anthelminthic activities of purified recombinant Cry5B against the hookworm parasite Ancylostoma ceylanicum, a bloodfeeding gastrointestinal nematode for which humans are permissive hosts. By using in vitro larval development assays, Cry5B was found to be highly toxic to early stage hookworm larvae. Exposure of adult A. ceylanicum to Cry5B was also associated with significant toxicity, including a substantial reduction in egg excretion by adult female worms. To demonstrate therapeutic efficacy in vivo, hamsters infected with A. ceylanicum were treated with three daily oral doses of purified Cry5B, the benzimidazole anthelminthic mebendazole, or buffer. Compared with control (buffer-treated) animals, infected hamsters that received Cry5B showed statistically significant improvements in growth and blood hemoglobin levels as well as reduced worm burdens that were comparable to the mebendazole-treated animals. These data demonstrate that Cry5B is highly active in vitro and in vivo against a globally significant nematode parasite and that Cry5B warrants further clinical development for human and veterinary use.
Keywords: anthelminthic, Cry5B, nematode
Soil-transmitted nematode (STN) infections represent a major cause of morbidity in developing countries, with an estimated burden of human disease comparable with that of malaria or tuberculosis (1–3). In addition to human health, animal nematode infections are of major veterinary significance, resulting in millions of dollars of lost revenue for industries that provide products and food from livestock, including cattle and sheep (4–8). Although anti-nematode drugs (anthelminthics) exist, there are well founded concerns about the emergence of resistance to these compounds. Hence, there exists a pressing need to develop new, safe, and inexpensive agents for the treatment of human and veterinary nematode infections of global significance (8–12).
Bacillus thuringiensis (Bt) crystal (Cry) proteins are the most widely used, biologically produced insecticides in the world (13). Bt is a soil bacterium that produces large crystalline inclusions during sporulation. These crystals contain one or several Cry proteins that are highly toxic to invertebrates but nontoxic toward vertebrates (14, 15). For decades, Cry proteins have been applied in large quantities to kill both insects that eat plants and insects that vector viruses and helminth parasites (16). Transgenic corn and cotton expressing Bt Cry proteins for caterpillar protection have been planted for the past decade (17). In 2005, nearly 24% of all of the cotton grown in the world expressed a Cry protein (18). The tremendous success of this natural product is the result of multiple factors, including high efficacy, the absence of toxicity of Cry proteins toward mammals and other vertebrates, and the ability to produce Cry proteins inexpensively and in large quantities. Recently, three Bt Cry proteins, Cry5B, Cry14A, and Cry21A, were identified that are toxic to both free-living nematodes (roundworms) and the free-living stage of at least one parasitic nematode (15). These data raise the possibility that Cry proteins could provide a therapy for STN infections.
We report here that the purified recombinant Cry5B protein is highly active against the hookworm Ancylostoma ceylanicum, a parasitic nematode for which humans are permissive hosts (19–21). By using in vitro culture methods, we demonstrate that Cry5B targets multiple stages of hookworm development, including the intestinal bloodfeeding stage. We demonstrate that oral administration of purified Cry5B to hamsters infected with A. ceylanicum provides significant benefit in vivo, effecting a cure of the hookworm infection comparable with that achieved with mebendazole. Together, these data suggest that Bt Cry proteins have tremendous potential for treatment of parasitic nematode infections of humans and other mammals.
Results
Adult Hookworms Express Receptors for Cry5B.
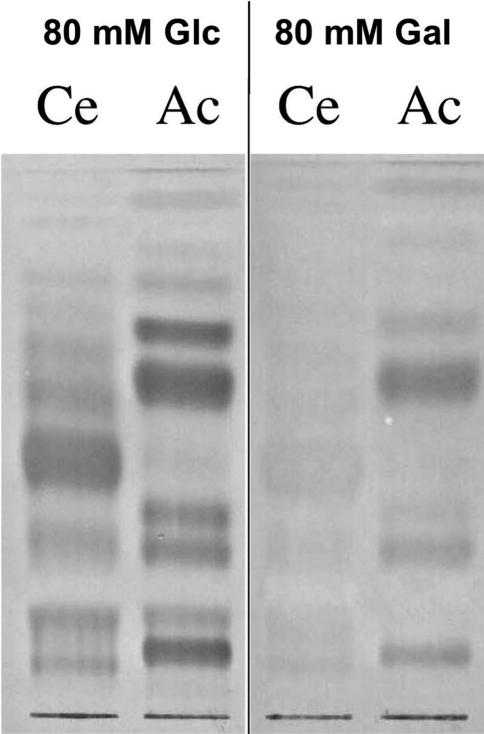
To determine whether the hookworm A. ceylanicum was susceptible to the nematicidal Bt Cry protein Cry5B, we investigated whether A. ceylanicum adults express a Cry5B receptor. As previously shown, the receptors for Cry5B in Caenorhabditis elegans are the carbohydrate moieties present on invertebrate-specific glycolipids (22). Because glycolipids are well conserved among nematodes (23), we hypothesized that Ancylostoma hookworms would similarly express Cry5B receptors. We extracted glycolipids from C. elegans and A. ceylanicum, resolved them by TLC, and performed an overlay/binding experiment with biotinylated Cry5B protein. As shown in Fig. 1, Cry5B binds multiple glycolipid species in both C. elegans and A. ceylanicum. Binding of Cry5B to C. elegans glycolipids was specifically inhibited in the presence of galactose but not glucose. Hookworm receptors demonstrated similar specificity because the addition of 80 mM galactose to the binding experiment reduced (but did not eliminate) binding of Cry5B to glycolipids from A. ceylanicum (Fig. 1). These data confirm that A. ceylanicum, like C. elegans, expresses Cry5B glycolipid receptors.
Fig. 1.
A. ceylanicum extracts contain Cry5B-binding glycolipids. Upper-phase glycolipids were extracted from mixed-stage C. elegans (Ce) and adult A. ceylanicum (Ac) and separated by TLC. The separated glycolipids were then subjected to a Cry5B overlay in the presence of 80 mM glucose (Glc) (left lanes) or 80 mM galactose (Gal) (right lanes). Cry5B bound to glycolipids from both nematodes in a galactose-dependent fashion.
Effect of Cry5B Toxin on Adult Hookworms in Vitro.
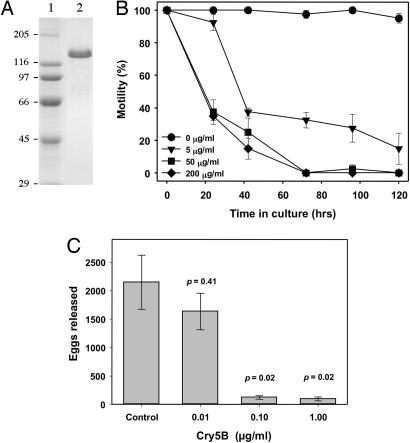
We evaluated the susceptibility of A. ceylanicum to Bt toxin by culturing adult worms in the presence of purified recombinant Cry5B (24) (Fig. 2A). The toxicity of Cry5B against adult worms as measured by motility was noted at all concentrations tested (Fig. 2B). In contrast, the motility of adult hookworms maintained in standard culture medium alone remained at 95% or greater throughout the observation period.
Fig. 2.
Effects of Cry5B on A. ceylanicum adults in vitro. (A) Six micrograms of purified Cry5B (lane 2) was subjected to SDS/PAGE and Coomassie blue staining. (B) Exposure to Cry5B impairs motility of adult hookworms. Groups of 10 adult A. ceylanicum worms were cultured in increasing concentrations of purified Cry5B toxin. Motility was monitored at the times indicated, and the data represent the mean values (±SE) of triplicate wells containing each concentration of toxin. Statistically significant (P < 0.001) differences in motility were observed between control worms (buffer only) and worms cultured in 50 or 200 μg/ml Cry5B toxin throughout the observation period (24–120 h). Statistically significant differences (P < 0.001) between control worms (0 μg/ml) and the 5 μg/ml toxin group were detected from 42–120 h in culture. (C) Cry5B toxin reduces A. ceylanicum egg excretion. Adult female hookworms were maintained for 24 h in the presence of increasing concentrations of purified Cry5B toxin. Experimental groups consisted of four replicate wells (three worms per well) at each concentration of toxin. Values represent the mean number of eggs (±SE) counted in each well. P values represent statistical comparisons between each group and the control worms (no toxin).
Cry5B toxin also reduced egg release by female hookworms (Fig. 2C). Control worms cultured in the absence of toxin released a mean of 2,150 ± 475 eggs over 24 h. Worms cultured in the lowest concentration of toxin (0.01 μg/ml) excreted fewer eggs (1,634 ± 320), although this difference was not statistically significant. In contrast, females exposed to 0.1 μg/ml or 1 μg/ml Cry5B demonstrated an ≈95% reduction in the number of eggs excreted (121 ± 31 and 97 ± 33, respectively) compared with control worms, differences that were statistically significant (P < 0.05). These concentrations of toxin did not impair the motility of adult females, and they are consistent with previous observations in free-living nematodes that progeny production is particularly sensitive to the effects of Cry proteins (15).
Effect of Cry5B Toxin on Hookworm Larval Development in Vitro.
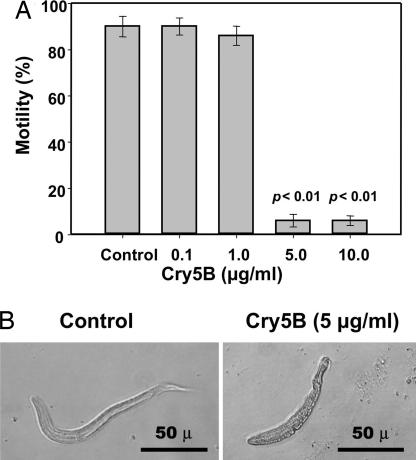
The susceptibility of early developmental stages of A. ceylanicum to Cry5B was evaluated by incubating eggs harvested from female hookworms in the presence of increasing concentrations of recombinant toxin. Purified Cry5B toxin had no effect on the percentage of eggs that hatched over 48 h (range, 29 ± 1 to 36 ± 4% in all treatment groups). However, there was a penetrant effect of Cry5B on the motility of larvae released from eggs at 48 h (Fig. 3A). In the absence of Cry5B, 90 ± 4% of cultured larvae demonstrated motility at the 48 h observation. There was no significant difference (at P < 0.05) in the motility of larvae hatched in the presence of 0.1 μg/ml (90 ± 3%) or 1.0 μg/ml (86 ± 4%) Cry5B (P > 0.6 for each group compared with control). At a concentration of 5 μg/ml Cry5B, however, larval motility was reduced to 6 ± 3%, a difference that was statistically significant (at P < 0.01). A similar effect was seen in larvae cultured in the presence of 10 μg/ml Cry5B, with 6 ± 2% motility (P < 0.01 compared with control) observed at 48 h. Our data thus indicate that early stage larvae are more sensitive to the effects of Cry5B than adult hookworms (compare Figs. 2B and 3A), consistent with results from studies on free-living nematodes (15). Incubation with Cry5B also resulted in significant morphological changes to A. ceylanicum larvae, as demonstrated by light microscopy (Fig. 3B). Hookworm larvae exposed to toxin exhibited stunted growth, and they showed obvious loss of integrity of most internal structures.
Fig. 3.
Effects of Cry5B on A. ceylanicum larvae. (A) Cry5B toxin impairs motility of early stage (L1/L2) hookworm larvae. A. ceylanicum eggs were allowed to hatch for 48 h in the presence of increasing concentrations of purified Cry5B toxin. Experimental groups consisted of four replicate wells containing larvae (mean of 20–46 per well) at each concentration of toxin. Values represent the mean percent of motile larvae (±SE) counted in each well. P values represent statistical comparisons between each group and the control worms (buffer only). (B) Cry5B toxicity in early larval (L1/L2) development. Representative photomicrographs show stunted growth and loss of integrity of the tegument and internal structures in A. ceylanicum larvae exposed to purified Cry5B toxin (5 μg/ml) for 48 h. (Magnification: ×200.)
The susceptibility of A. ceylanicum L3, which is the infectious stage of hookworm, to Cry5B was evaluated by incubating larvae cultured from the feces of infected hamsters in the presence of increasing concentrations of toxin. Worms were monitored daily for evidence of intoxication by determining their motility under light microscopy. These nonfeeding third-stage larvae, which possess a dense external sheath that covers the buccal capsule, were resistant to the effects of Cry5B up to concentrations of 10 μg/ml (data not shown).
Cry5B Treatment Reduces Pathology in Hookworm-Infected Hamsters.
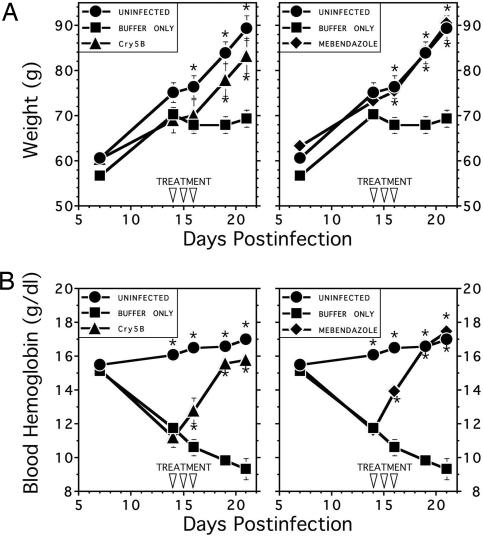
To evaluate the therapeutic potential of Cry5B, hamsters (n = 30) were infected with 150 A. ceylanicum larvae, and groups of 10 animals were orally dosed on days 14, 15, and 16 postinfection (PI) with buffer only, Cry5B, or the benzimidazole anthelminthic mebendazole. As expected, relative to uninfected animals, the infected hamsters in the buffer-treated group exhibited weight loss and significant reductions in blood hemoglobin levels temporally associated with the onset of hookworm bloodfeeding, which occurs at approximately day 10 PI (Fig. 4; see refs. 19 and 25). Persistent and statistically significant differences (P < 0.05) in weight were observed between the buffer-treated and uninfected control animals beginning at day 16 PI, whereas a difference in the blood hemoglobin level was detected by day 14 PI. In contrast, treatment with Cry5B was associated with significantly improved (P < 0.05) growth in infected animals (Fig. 4). By day 19 PI, the difference in weight between the Cry5B-treated animals (mean 77.9 ± 3.6 g) and buffer-treated controls (mean 67.9 ± 1.9 g) was highly significant (P = 0.008). This difference was even more pronounced by day 21 PI, with a mean weight of 83.2 ± 3.9 g in the Cry5B-treated group vs. 69.2 ± 1.9 g in the buffer-treated animals (P = 0.0008). Of note, by day 19 PI, the mean weights of the Cry5B-treated animals were found to be not statistically different at a cutoff of P < 0.05 from those of the uninfected hamsters (P = 0.1) or compared with those of infected hamsters treated with mebendazole(P = 0.08).
Fig. 4.
Cry5B treatment reduces clinical sequelae of hookworm infection as measured by weight gain (Upper) and blood hemoglobin (Lower). Hamsters were infected with 150 A. ceylanicum L3 on day 0 and treated with Cry5B (Left) or mebendazole (Right) on days 14, 15, and 16 PI as indicated by open arrowheads. All values are the means ± SE. Asterisks indicate statistical significance vs. the infected control group. For numerical values, see Results.
Cry5B treatment was also associated with rapid resolution of hookworm anemia as measured by blood hemoglobin levels (Fig. 4). Before treatment (day 14 PI), the mean blood hemoglobin levels in each of the three infected groups (buffer, Cry5B, mebendazole) ranged from 11.2 to 11.7 g/dl compared with a mean of 16.0 ± 0.2 g/dl in the uninfected control animals (P < 0.001 for each infected group vs. uninfected controls). However, a significant improvement in blood hemoglobin levels was noted in the Cry5B treatment group (mean 12.8 ± 0.8 g/dl) compared with the buffer-treated controls (mean 10.6 ± 0.5 g/dl) by day 16 PI (P = 0.003), which was the 3rd treatment day. The improvement in blood hemoglobin was sustained throughout the study period (P < 0.0001 vs. buffer-treated animals at days 19 and 21). As was observed for weight, the posttreatment blood hemoglobin levels (days 19 and 21) were statistically equivalent (based on a cutoff of P < 0.05 for statistical significance) in the uninfected and the Cry5B- and mebendazole-treated groups.
Cry5B Treatment Reduces Fecal Egg Excretion and Intestinal Worm Burden in Hookworm-Infected Hamsters.
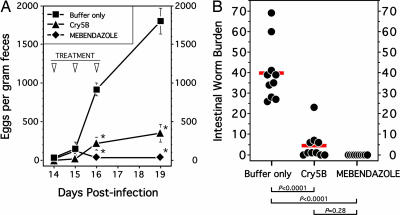
Hookworm eggs can be detected in the feces of hamsters infected with A. ceylanicum as early as day 14 PI, with egg counts increasing sharply over the next several days (26). In the buffer-treated animals, fecal egg counts increased from a mean of 917 ± 84 eggs per g (epg) of feces on day 16 PI to 1,800 ± 167 epg by day 19 PI (Fig. 5A). In contrast, hamsters treated with Cry5B excreted 217 ± 84 epg at day 16 and 350 ± 117 epg on day 19, reductions of 76% (P = 0.005 vs. the buffer-treated group) and 81% (P = 0.003), respectively. Although day 16 and day 19 epg in the Cry5B-treated group were higher than in the mebendazole-treated hamsters, this difference was not statistically significant at a cutoff of P < 0.05.
Fig. 5.
Cry5B treatment reduces fecal egg excretion (A) and intestinal hookworm burden (B) in infected hamsters. (A) Fecal samples from infected animals were collected at the times indicated, and hookworm eggs were quantified as described in Materials and Methods. All values are the means ± SE. Asterisks indicate statistical significance (P < 0.05) vs. the infected control group. (B) Individual worm burdens are indicated by closed circles, and the means of each group are shown by horizontal bars. Brackets indicate statistical comparisons between groups, with P values shown.
To determine whether the therapeutic effects of Cry5B also extend to a reduction in the intestinal parasite burden, animals were euthanized at day 22, and the number of adult worms was counted. As shown in Fig. 5B, the buffer-treated animals harbored a mean of 39.8 adult worms (range, 26–69). The animals in the Cry5B-treated group harbored significantly fewer adult worms (mean, 4.5; range, 0–23), which represented an 89% reduction in mean worm burden compared with the buffer-treated group (P < 0.0001). Of note, more than half of the 45 total worms in the Cry5B-treated group were recovered from a single hamster. Moreover, 3 of the 10 Cry5B-treated animals were cured of their infection, whereas only a single worm was found in 3 additional animals in this treatment group. There appear to be higher mean worm burdens in the Cry5B-treated group compared with the mebendazole-treated animals (4.5 vs. 0). However, further studies will be needed to confirm this finding because the observed difference was not statistically significant (P = 0.28).
Discussion
The data presented here demonstrate that Cry5B, a purified crystal protein from B. thuringiensis, is highly active against multiple life cycle stages of the human and animal hookworm parasite A. ceylanicum. Feeding Cry5B to adult and larval hookworms results in significantly impaired nematode motility, decreased egg release, and morphologic changes consistent with toxicity. Most importantly, our results demonstrate that oral administration of Cry5B to hookworm-infected hamsters results in significant therapeutic benefits comparable with those of a standard anthelminthic (mebendazole), including improved growth, increased blood hemoglobin levels, a >75% reduction in fecal egg excretion, and an ≈90% reduction in intestinal worm burden.
Previous data have pointed to the potential of Bt crystal proteins against animal parasitic nematodes. For example, Escherichia coli-expressing Cry5B are toxic to free-living stages of the rodent parasite Nippostrongylus brasiliensis (15), and spore–crystal lysates from two Bt strains were found to be toxic to three nematodes of veterinary importance in vitro (27). In the latter study, one of the strains also expressed Cry5B protein, although interpretation of these findings is complicated by the fact that each of the strains expressed multiple Cry proteins and that Bt spores were included in the assays. Our results demonstrate that a single purified crystal protein is toxic to a human parasitic nematode both in vitro and in vivo.
The data presented here suggest a similar mechanism for Cry5B action in Ancylostoma hookworms and in C. elegans. Both nematodes express glycolipid receptors, and the sensitivities to Cry5B of various stages (larval and adult) and phenotypes (e.g., reduction in egg production) are similar between the two organisms. Furthermore, our results with hookworm are consistent with the known mechanism of action of Cry proteins (28), i.e., that they must be ingested to intoxicate. Thus, it is not surprising that neither A. ceylanicum embryos nor infectious L3 stage larvae (both of which represent nonfeeding stages of hookworm) are susceptible to Cry5B toxicity.
Our most striking observation is that orally administered Cry5B is effective at reducing the clinical sequelae of hookworm infection, i.e., anemia and growth delay. These studies used the hamster model of A. ceylanicum, a well characterized system for investigations of hookworm pathogenesis and host–parasite interactions (19, 25). Animals treated with three orally administered doses of Cry5B showed statistically significant improvements in weight and blood hemoglobin levels, along with reductions in worm burdens (as measured by fecal egg excretion and intestinal worm recovery) compared with control hamsters receiving buffer alone (Figs. 4 and 5). The 3-day orally administered treatment regimen (Cry5B or mebendazole) is consistent with recommendations for treatment with benzimidazole anthelminthics of individuals with intestinal nematode infections, although community-based programs often use single-dose therapy (29, 30). By superficial observation or as detected by measurements of weight and hemoglobin levels, we observed no evidence of toxicity attributable to Cry5B in any of the treated animals.
Despite previously raised concerns that gastric acid would reduce the efficacy of orally administered Bt toxin (27), data from our study using oral administration show that the effect of Cry5B was comparable with that seen with mebendazole, as measured by improvements in weight and blood hemoglobin concentrations of treated animals compared with controls (Fig. 4). However, it is worth noting that Cry5B appears to be less effective at eliciting a complete cure of hookworm infection in the animal study (Fig. 5B). The degree to which this result reflects a fundamental difference in susceptibility of A. ceylanicum to Cry5B and mebendazole or in fact suggests a degree of inactivation of the toxin during intestinal transit needs to be investigated, as should defining the optimal dosing regimen for Bt against hookworm using the in vivo model.
Anthelminthic chemotherapy remains the cornerstone of current control measures for human and animal STN infections (30, 31). Periodic deworming of school-age children has been recommended to improve growth and nutritional status, and treatment of women infected with STNs during pregnancy may reduce the prevalence of anemia and improve birth outcomes (1). The advantages of currently available benzimidazole anthelminthics, e.g., mebendazole and albendazole, include their broad spectrum of activity against all major species of STN as well as their favorable safety profile and low cost (30, 32). However, evidence suggests that frequent deworming is necessary to achieve lasting benefit for school-age children, and in endemic communities this practice may have already led to the emergence of resistance in human isolates, particularly for hookworm (9, 10, 12, 31, 33–35). Repeated use of anthelminthics has previously been associated with widespread resistance in livestock, dramatically reducing the efficacy of these agents in many veterinary settings (4–8, 36, 37).
The development of chemotherapeutic agents that are safe and effective against a broad spectrum of human nematode parasites and inexpensive may prove essential to meet the World Health Organization goals for global control of morbidity caused by STN infections (38–40). Bt Cry proteins, including Cry5B, meet these criteria, and, as insecticides, they have an unprecedented track record of vertebrate safety (41). It is important to note that, whereas benzimidazole drugs target microtubules, Cry proteins are pore-forming toxins that target the intestinal tract of nematodes. Therefore, nematodes that become resistant to benzimidazole drugs are very unlikely to display cross-resistance to Cry proteins.
In summary, these studies define a potential class of anthelminthics for treatment of human parasitic nematode infections in billions of people at risk, and they provide a compelling rationale for further preclinical development of Bt Cry toxins for therapeutic use.
Materials and Methods
Parasites and Hosts.
The A. ceylanicum life cycle was maintained in 3–4-week-old male golden Syrian hamsters of the HsdHan:AURA outbred strain as described previously (19, 42). For the in vivo anthelminthic study, hamsters were orally infected with 150 third-stage A. ceylanicum larvae; the animals were then treated as described below and euthanized at day 22. The maintenance and care of experimental animals complied with the National Institutes of Health guidelines for the humane use of laboratory animals, and they were approved by the Yale University Animal Care and Use Committee.
In Vitro Assay of Cry5B Toxin-Binding Activity.
Upper-phase glycolipids from C. elegans and A. ceylanicum were extracted as previously described (22), except that A. ceylanicum adults were partially homogenized with a plastic pestle and extracted overnight. Equal amounts by mass (≈500 ng based on orcinol staining) of upper-phase glycolipids from C. elegans and A. ceylanicum were separated by TLC. Overlay experiments using biotinylated Cry5B were carried out as previously described (22) in the presence of either 80 mM glucose or 80 mM galactose.
Expression and Purification of Cry5B Protein.
Cry5B was purified from crystal toxin-deficient Bt strain HD1 transformed with a plasmid containing the Cry5B gene (43). Spore–crystal lysate from this strain was prepared by using standard methods (43). Cry5B protein was purified from spore–crystal lysate by using a sucrose gradient (44) with some modifications (details are given in Supporting Methods, which is published as supporting information on the PNAS web site). After purification, Cry5B was precipitated, suspended in double-distilled H2O, frozen in aliquots in liquid N2, and stored at −80°C. On the day of use, Cry5B aliquots were thawed and centrifuged at 15,000 × g for 5 min, and the supernatant was removed. The precipitated Cry5B was then resuspended to a final concentration of 5 mg/ml in 50 mM potassium citrate buffer (pH 3.0) immediately before use.
In Vitro Assays of Cry5B Toxicity Against Hookworm Life Cycle Stages.
To obtain adult hookworms for ex vivo study, hamsters were infected orally with 150–200 third-stage A. ceylanicum larvae (19, 25). At 20 days PI, the animals were euthanized, and live parasites were removed from the intestinal debris with forceps and washed in hookworm culture medium (HCM), consisting of RPMI 1640 (Invitrogen, Carlsbad, CA)/50% FCS (Sigma, St. Louis, MO)/2× penicillin/streptomycin (Invitrogen)/10 μg/ml Fungizone (Invitrogen). For studies of the effect of toxin on A. ceylanicum egg release, groups of 3 adult female hookworms were placed in wells of a microtiter plate containing HCM and increasing concentrations of Cry5B. After a 24-h incubation at 37°C, the adult worms were removed, and the eggs were suspended in 500 μl of RPMI 1640. The total number of eggs in the suspension was extrapolated from mean values obtained by counting the eggs in 6 aliquots (10 μl) from each well. Each treatment group consisted of three replicate wells. Eggs released from females over 24 h in the absence of toxin were placed in wells (100 eggs per well) of a plate containing HCM and increasing concentrations of Cry5B. The number of hatched larvae in each well was counted at 24-h intervals. The motility of hatched larvae in each treatment group was determined by observation under light microscopy. Worms were labeled as motile if they displayed typical undulating movement during 30 s of observation. For adult worms, the effect of Cry5B was measured by incubating groups of 10 worms (5 males, 5 females) in wells of a 24-well plate containing 1 ml of HCM and increasing concentrations of toxin. As above, motility was determined over 120 h by observation under light microscopy. For in vitro experiments, Cry5B was solubilized in citrate buffer (see above) before adding it to wells.
Effect of Anthelminthics on Hookworm Infection.
Weanling hamsters (n = 30) were orally infected with 150 A. ceylanicum L3 or left uninfected to serve as age-matched controls (n = 10). On days 14, 15, and 16 PI, infected animals (n = 10 in each group) were given 1 mg of Cry5B in citrate buffer or 1 mg of mebendazole (Sigma) in double-distilled H2O, each delivered orally in a volume of 0.2 ml. Infected control animals (n = 10) received 0.2 ml of citrate buffer only. Hamsters were monitored for weight, hemoglobin, and (when applicable) fecal eggs as described previously (19, 25). Blood hemoglobin was measured by using the total hemoglobin assay kit (Sigma). Fecal egg counts were performed by using a McMaster chamber (Hausser Scientific, Horsham, PA). At day 22, infected hamsters were euthanized, and adult hookworms were removed manually from the small and large intestines.
Statistical Analysis of Data.
Data are presented in the text and figures as the means ± SE. Significance testing was conducted by using the StatView 4.51 statistical analysis software package (Abacus Concepts, Piscataway, NJ). For multiple-group comparisons, ANOVA was performed followed by Fisher's protected least-significant difference as a posttest. P values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank the Burroughs Wellcome Fund for inspiring and supporting this work through Young Investigator Awards in Toxicology (to R.V.A.) and Molecular Parasitology (to M.C.). This work was supported by National Institutes of Health Grant 1 R01 AI056189 (to R.V.A. and M.C.).
Abbreviations
- Bt
Bacillus thuringiensis
- Cry
crystal
- epg
eggs per gram
- HCM
hookworm culture medium
- PI
postinfection
- STN
soil-transmitted nematode.
Footnotes
The authors declare no conflict of interest.
References
- 1.Crompton DW, Engels D, Montresor A, Neira MP, Savioli L. Acta Trop. 2003;86:121–124. doi: 10.1016/s0001-706x(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux DH, Hotez PJ, Fenwick A. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles GC. Res Vet Sci. 2005;78:99–108. doi: 10.1016/j.rvsc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Chandrawathani P, Waller PJ, Adnan M, Hoglund J. Trop Anim Health Prod. 2003;35:17–25. doi: 10.1023/a:1022023620599. [DOI] [PubMed] [Google Scholar]
- 6.von Samson-Himmelstjerna G, Blackhall W. Vet Parasitol. 2005;132:223–239. doi: 10.1016/j.vetpar.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Coles GC, Rhodes AC, Wolstenholme AJ. Vet Parasitol. 2005;129:345–347. doi: 10.1016/j.vetpar.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Albonico M. Acta Trop. 2003;86:233–242. doi: 10.1016/s0001-706x(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 10.Albonico M, Engels D, Savioli L. Int J Parasitol. 2004;34:1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Reynoldson JA, Behnke JM, Pallant LJ, Macnish MG, Gilbert F, Giles S, Spargo RJ, Thompson RC. Acta Trop. 1997;68:301–312. doi: 10.1016/s0001-706x(97)00106-x. [DOI] [PubMed] [Google Scholar]
- 12.De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J. Am J Trop Med Hyg. 1997;57:25–30. doi: 10.4269/ajtmh.1997.57.25. [DOI] [PubMed] [Google Scholar]
- 13.Whalon ME, Wingerd BA. Arch Insect Biochem Physiol. 2003;54:200–211. doi: 10.1002/arch.10117. [DOI] [PubMed] [Google Scholar]
- 14.Naimov S, Weemen-Hendriks M, Dukiandjiev S, de Maagd RA. Appl Environ Microbiol. 2001;67:5328–5330. doi: 10.1128/AEM.67.11.5328-5330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV. Proc Natl Acad Sci USA. 2003;100:2760–2765. doi: 10.1073/pnas.0538072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federici BA. J Invertebr Pathol. 2005;89:30–38. doi: 10.1016/j.jip.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay A, Bhatnagar NB, Bhatnagar R. Crit Rev Microbiol. 2004;30:33–54. doi: 10.1080/10408410490270712. [DOI] [PubMed] [Google Scholar]
- 18.James C. Global Status of Commercialized Biotech/GM Crops. Ithaca, NY: ISAAA; 2005. International Service for the Acquisition of Agri-Biotech Applications Briefs No 34. [Google Scholar]
- 19.Bungiro RD, Jr, Greene J, Kruglov E, Cappello M. J Infect Dis. 2001;183:1380–1387. doi: 10.1086/319867. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury AB, Schad GA. Am J Trop Med Hyg. 1972;21:300–301. doi: 10.4269/ajtmh.1972.21.300. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y, Okamoto K, Chiu JK. Am J Trop Med Hyg. 1968;17:378–381. doi: 10.4269/ajtmh.1968.17.378. [DOI] [PubMed] [Google Scholar]
- 22.Griffitts JS, Haslam SM, Yang T, Garczynski SF, Mulloy B, Morris H, Cremer PS, Dell A, Adang MJ, Aroian RV. Science. 2005;307:922–925. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- 23.Lochnit G, Dennis RD, Geyer R. Biol Chem. 2000;381:839–847. doi: 10.1515/BC.2000.106. [DOI] [PubMed] [Google Scholar]
- 24.Griffitts JS, Whitacre JL, Stevens DE, Aroian RV. Science. 2001;293:860–864. doi: 10.1126/science.1062441. [DOI] [PubMed] [Google Scholar]
- 25.Held MR, Bungiro RD, Harrison LM, Hamza I, Cappello M. Infect Immun. 2006;74:289–295. doi: 10.1128/IAI.74.1.289-295.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bungiro RD, Jr, Cappello M. Am J Trop Med Hyg. 2005;73:915–920. [PubMed] [Google Scholar]
- 27.Kotze AC, O'Grady J, Gough JM, Pearson R, Bagnall NH, Kemp DH, Akhurst RJ. Int J Parasitol. 2005;35:1013–1022. doi: 10.1016/j.ijpara.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 28.de Maagd RA, Bravo A, Crickmore N. Trends Genet. 2001;17:193–199. doi: 10.1016/s0168-9525(01)02237-5. [DOI] [PubMed] [Google Scholar]
- 29.Med Lett Drugs Ther. 2004;1189:1–12. [Google Scholar]
- 30.de Silva NR. Acta Trop. 2003;86:197–214. doi: 10.1016/s0001-706x(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 31.Horton J. Trends Parasitol. 2003;19:527–531. doi: 10.1016/j.pt.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Utzinger J, Keiser J. Expert Opin Pharmacother. 2004;5:263–285. doi: 10.1517/14656566.5.2.263. [DOI] [PubMed] [Google Scholar]
- 33.Saathoff E, Olsen A, Kvalsvig JD, Appleton CC. BMC Infect Dis. 2004;4:27. doi: 10.1186/1471-2334-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albonico M, Ramsan M, Wright V, Jape K, Haji HJ, Taylor M, Savioli L, Bickle Q. Ann Trop Med Parasitol. 2002;96:717–726. doi: 10.1179/000349802125001942. [DOI] [PubMed] [Google Scholar]
- 35.Albonico M, Wright V, Ramsan M, Haji HJ, Taylor M, Savioli L, Bickle Q. Int J Parasitol. 2005;35:803–811. doi: 10.1016/j.ijpara.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Mertz KJ, Hildreth MB, Epperson WB. J Am Vet Med Assoc. 2005;226:779–783. doi: 10.2460/javma.2005.226.779. [DOI] [PubMed] [Google Scholar]
- 37.McKellar QA, Jackson F. Trends Parasitol. 2004;20:456–461. doi: 10.1016/j.pt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Savioli L, Albonico M, Engels D, Montresor A. Parasitol Int. 2004;53:103–113. doi: 10.1016/j.parint.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Savioli L, Engels D, Endo H. Lancet. 2005;365:1520–1521. doi: 10.1016/S0140-6736(05)66433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Report by Secretariat. 2000 October 27, 2000. Division of Communicable Diseases, EB107/31. [Google Scholar]
- 41.Betz FS, Hammond BG, Fuchs RL. Regul Toxicol Pharmacol. 2000;32:156–173. doi: 10.1006/rtph.2000.1426. [DOI] [PubMed] [Google Scholar]
- 42.Garside P, Behnke JM. Parasitology. 1989;98:283–289. doi: 10.1017/s003118200006220x. [DOI] [PubMed] [Google Scholar]
- 43.Marroquin LD, Elyassnia D, Griffitts JS, Feitelson JS, Aroian RV. Genetics. 2000;155:1693–1699. doi: 10.1093/genetics/155.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debro L, Fitz-James PC, Aronson A. J Bacteriol. 1986;165:258–268. doi: 10.1128/jb.165.1.258-268.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.