Abstract
A high intake of the omega-3 fatty acid docosahexaenoate [docosahexaenoic acid (DHA)] has been associated with systemic antiinflammatory effects and cardiovascular protection. Cyclooxygenase (COX)-2 is responsible for the overproduction of prostaglandins (PG) at inflammatory sites, and its expression is increased in atheroma. We studied the effects of DHA on COX-2 expression and activity in human saphenous vein endothelial cells challenged with proinflammatory stimuli. A ≥24-h exposure to DHA reduced COX-2 expression and activity induced by IL-1, without affecting COX-1 expression. DHA effect depended on the NF-κB-binding site in the COX-2 promoter. EMSAs confirmed that DHA attenuated NF-κB activation. Because MAPK, PKC, and NAD(P)H oxidase all participate in IL-1-mediated COX-2 expression, we also tested whether these enzymes were involved in DHA effects. Western blots showed that DHA blocked nuclear p65 NF-κB subunit translocation by decreasing cytokine-stimulated reactive oxygen species and ERK1/2 activation by effects on both NAD(P)H oxidase and PKCε activities. Finally, to address the question whether DHA itself or DHA-derived products were responsible for these effects, we inhibited the most important enzymes involved in polyunsaturated fatty acid metabolism, showing that 15-lipoxygenase-1 products mediate part of DHA effects. These studies provide a mechanistic basis for antiinflammatory and possibly plaque-stabilizing effects of DHA
Keywords: docosahexaenoic acid, endothelium, inflammation, NF-κB
Vascular endothelial cells (EC) play a key role in the inflammatory aspects of atherosclerosis, by sustaining monocyte recruitment via leukocyte adhesion molecules and chemoattractants and cooperating with macrophages in the release of inflammatory cytokines and matrix-degrading enzymes (1). Inflammatory cytokines stimulate the production from arachidonic acid (AA) of lipid mediators, such as prostanoids, that contribute both to inflammation and vascular homeostasis (2). Rate-limiting enzymes in prostanoid production include cyclooxygenases (COX)-1 and −2. Although COX-1 is constitutively expressed, COX-2 is rapidly induced after inflammatory challenges (3). Enhanced COX-2 expression is found in inflammation (3) and also in atherosclerotic lesions, where a pathogenetic role has been suggested (4). COX-2 induced in macrophages and EC by oxidized low-density lipoprotein or IL-1 indeed catalyzes massive formation of eicosanoids that enhance vascular permeability and promote monocyte chemotaxis, proliferation, and cholesterol ester retention (5). Diets rich in marine-derived omega-3 fatty acids, mainly docosahexaenoic acid (DHA, 22:6 n-3) and eicosapentaenoic acid (EPA, 20:5 n-3), can attenuate chronic inflammatory diseases (6) and protect against atherosclerosis (7). By competing with AA, DHA and EPA inhibit enzymatic activity of both COX isoforms (8, 9), but their effects on COX-2 expression in vascular endothelium are poorly understood. We showed that incorporation of DHA into EC decreases cytokine-stimulated expression of leukocyte adhesion molecules (10). We have now examined whether DHA can directly affect IL-1-induced expression of COX-2 in EC.
Results
DHA Inhibits Endothelial COX Activity.
We previously showed that DHA (up to 25 μmol per liter) inhibited endothelial adhesion molecule expression without toxicity (10, 11). Because the effect of DHA was qualitatively similar to, but stronger than, the effect of EPA (11), all experiments were performed with DHA. We therefore first monitored the effects of prolonged (up to 48-h) exposure of human saphenous vein EC (HSVEC) to 25 μmol/liter DHA, by measuring production of 6-keto-PGF1α, the major metabolite of prostaglandin (PG) I2 that is, in turn, the main product of COX in EC. 6-keto-PGF1α increases in the presence of exogenous substrate, of stimuli for AA release and, more, of stimuli for EC activation, such as IL-1α or phorbol myristate acetate (PMA; Table 1, which is published as supporting information on the PNAS web site). Exposing HSVEC to DHA before stimulation with AA or thrombin significantly reduced 6-keto-PGF1α (Table 1). EC exposed to IL-1α required >12 h before 6-keto-PGF1α significantly rose (5-fold), and such production further increased after thrombin or AA addition (4- and 8-fold, respectively). DHA blocked thrombin or AA-stimulated PGI2 40% more in the presence of IL-1α than without IL-1α, and PMA effects were even more strongly inhibited (Table 1). Similar results were obtained with human umbilical vein EC (not shown). Stearate, not a substrate for COX, was used as control, and up to 50 μmol/liter did not inhibit PGI2 production.
Prolonged Exposure to DHA Is Necessary for Maximal Inhibition of COX Activity.
We tested the time dependence of DHA inhibitory effect in HSVEC over 0–48 h before addition of IL-1α (Fig. 1A). With coexposure to DHA and IL-1α, inhibition of COX-activity was minimal, but inhibition increased >50% when cells were preexposed to DHA for 48 h before adding IL-1α. Similar results were obtained by using PMA (Fig. 1B). This suggested that DHA inhibits PGI2 formation by two mechanisms: first as a competitive substrate and second by blocking COX-2 synthesis during EC activation.
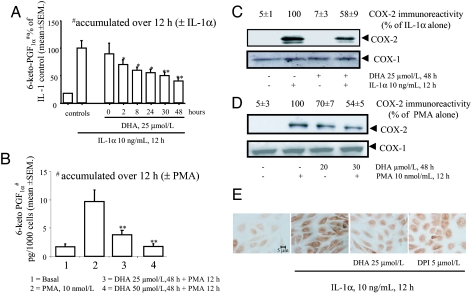
Fig. 1.
Inhibition of IL-1α- and PMA-mediated induction of COX-2 activity and protein by DHA. (A) HSVEC were preincubated in the absence (vehicle) or presence of 25 μmol/liter DHA for 0–48 h before stimulation with 10 ng/ml IL-1α for 12 h, then medium was collected and 6-ketoPGF1α measured by RIA. 6-ketoPGF1α is shown as percent of maximum response to IL-1α as a function of DHA preincubation time. Each bar represents the mean of eight determinations repeated in three separate experiments. ∗, P < 0.05; ∗∗, P < 0.01 vs. stimulated control. (B) HSVEC were preincubated with DHA, stimulated with 10 PMA for 12 h, then medium was collected and 6-ketoPGF1α measured and expressed as picogram per 1,000 cells for each of two DHA concentrations (25 and 50 μmol/liter). Each bar represents the mean of n = 8 determinations, repeated in three separate experiments. ∗∗, P < 0.01 vs. PMA alone. (C) HSVEC were preincubated with 25 μmol/liter DHA for 48 h, stimulated with 10 ng/ml IL-1α for 12 h, and then whole-cell lysates were analyzed by Western blot using antibodies specific for COX-1 and -2. Values of COX-2 are shown as percent of maximal control response (IL-1α alone). The blot depicted is representative of three similar ones. (D) HSVEC were preincubated with DHA for 48 h, stimulated with 10 nmol/liter PMA for 12 h, and cell lysates prepared and analyzed as in C. The blot depicted is representative of three similar ones. (E) HSVEC were preincubated with 25 μmol/liter for 48 h or 5 μmol/liter DPI, an inhibitor of NAD(P)H oxidase, for 30 min, then stimulated with 10 ng/ml IL-1α for 12 h, after which cells were fixed and immunostained as described in Materials and Methods. For C and D, densitometric values of COX-1 and -2 expression are reported as percent of maximal control response (IL-1α alone).
DHA Inhibits COX-2 Protein Expression.
To test for a dual inhibitory effect of DHA on EC, we studied DHA effects on COX-2 protein expression by Western blot and immunocytochemistry in HSVEC stimulated with IL-1α and PMA. As shown in Fig. 1C, basal COX-2 was minimal in unstimulated EC and rose after exposure to IL-1α. Incubation of HSVEC with DHA alone did not affect COX-2 expression, but in DHA-preincubated HSVEC, IL-1α induced only 50% as much COX-2 compared with EC preincubated with stearate, whereas COX-1 expression was unchanged (Fig. 1C Lower). Similar results were obtained using PMA for both HSVEC (Fig. 1D) and human umbilical vein EC (not shown). Immunocytochemistry confirmed these effects (Fig. 1E). Production of PGI2 was decreased in DHA-pretreated EC in a COX-2-dependent manner (Fig. 6, which is published as supporting information on the PNAS web site).
DHA Inhibits COX-2 Steady-State mRNA Levels Without Affecting mRNA Stability.
Consistent with reduced COX-2 protein expression, DHA also reduced COX-2 steady-state mRNA levels at Northern blot. There were, however, no changes in COX-2 mRNA stability (half-life) in experiments using the transcription blocker actinomycin-D, indicating a transcriptional effect (Fig. 7, which is published as supporting information on the PNAS web site).
DHA Reduces COX-2 Promoter Activity.
In transient transfection experiments using full-length or mutated human COX-2 promoter-luciferase constructs, we evaluated whether DHA regulated COX-2 promoter activity. We here used bovine aortic EC because of the difficulty in transfecting human EC, using PMA or LPS as stimuli. DHA pretreatment blocked COX-2 promoter activity after either stimulus (Fig. 8, which is published as supporting information on the PNAS web site). With a series of human COX-2 promoter/reporter constructs either deleted or site-mutated at specific transcriptionally active sites, we demonstrated that the proximal NF-κB-binding site is necessary for DHA inhibitory activity (Fig. 8).
DHA Reduces Activation of NF-κB and Nuclear Translocation of p65.
Having determined that NF-κB-binding sites are a crucial target in down-regulation by DHA of COX-2 protein expression, we next examined DHA effects on NF-κB activation by EMSA. At all concentrations tested, DHA pretreatment decreased by ≈60% the amount of shifted complex induced by IL-1α (Fig. 9A, which is published as supporting information on the PNAS web site). Because nuclear translocation of the p65 NF-κB subunit is key for NF-κB activity, we tested whether DHA affected expression and nuclear translocation of p65. Western blots of nuclear proteins from DHA-pretreated cells before IL-1α stimulation showed significantly less p65 nuclear translocation into nuclei (but not total cellular p65) from cells pretreated with DHA than from nontreated cells (Fig. 9 B and C), confirming and expanding EMSA results.
DHA-Mediated Reduction in Cytokine-Induced COX-2 Expression and p65 NF-κB Subunit Translocation Involves ERK1/2 but Not p38 MAPK.
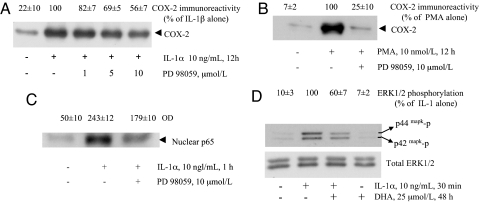
IL-1 activates the MAPK pathway, including ERK1/2 and p38 (12). ERK1/2 acts upstream to NF-κB activation in the IL-1 signaling pathway leading to COX-2 expression (13). To demonstrate that ERK1/2 and NF-κB activations and COX-2 expression are linked, we first blocked ERK1/2 activation by PD98059, inhibiting a kinase (MAPK/ERK kinase 1) immediately upstream to ERK (14). This strategy (i) significantly inhibited IL-1α- and PMA-induced COX-2 expression (Fig. 2A and B) and (ii) decreased translocation of p65 (Fig. 2C) and NF-κB activation (data not shown). We then evaluated whether DHA pretreatment affected IL-1α- and PMA-induced ERK1/2 phosphorylation (activation). In cells pretreated with DHA before IL-1α stimulation, ERK1/2 (but not p38 MAPK; data not shown) activation was significantly less than when no DHA was used (Fig. 2D Upper), suggesting that DHA blocks a molecular target upstream to ERK1/2. ERK total protein remained unchanged under all conditions tested (Fig. 2D Lower). Similar results (not shown) were obtained when ERK1/2 was activated by PMA.
Fig. 2.
Involvement of ERK1/2 in the stimulated expression of COX-2 and effect of DHA on ERK1/2 activation. (A) HSVEC were treated with MEK1 inhibitor PD 98059 at indicated concentrations for 30 min, then 10 ng/ml IL-1α was added for 12 h. Whole-cell lysates were analyzed by Western blot using an antibody specific for COX-2. Values of COX-2 are reported as percent of maximal control response (IL-1α alone). The blot shown is representative of three similar ones. (B) HSVEC were treated with 10 μmol/liter PD 98059 for 30 min, and then 10 nmol/liter of PMA was added for 12 h. Whole-cell lysates were used for Western blot as in A. The blot depicted is representative of three similar ones. (C) HSVEC were pretreated with 10 μmol/liter PD 98059 for 30 min and then stimulated with 10 ng/ml IL-1α. After 1 h, nuclear proteins were prepared and assayed by Western blot using a specific antibody against the p65 NF-κB subunit. Values of p65 expression are reported as units of OD. The blot shown is representative of three similar ones. (D) HSVEC were pretreated with 25 μmol/liter DHA for 48 h and then stimulated with 10 ng/ml IL-1α for 30 min. Whole-cell lysates were prepared and used for Western blot analysis using a specific antibody against phosphorylated ERK1/2. To ascertain that the total level of the ERK1/2 remained unchanged, the same blots were reprobed with an anti-ERK1/2 antibody that recognizes both phosphorylated and nonphosphorylated forms. Values of ERK1/2 phosphorylation are reported as percent of maximal control response (IL-1α alone). This blot is representative of three similar ones.
DHA Reduces the Production of Intracellular Reactive Oxygen Species (ROS) Induced by IL-1α.
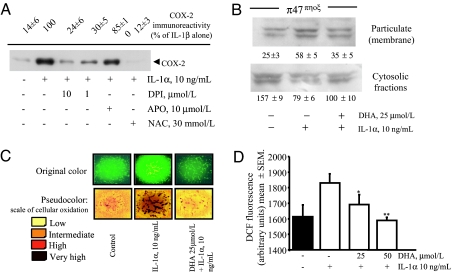
Redox events are involved in IL-1 signaling (15), and exogenous oxidants can activate the ERK1/2 pathway (16, 17). We therefore tested in HSVEC whether IL-1α induces the production of ROS, whether ROS mediate the expression of COX-2, which enzymes are involved in ROS generation, and finally whether DHA modulates this process. The thiol donor and antioxidant N-acetylcysteine dramatically attenuated IL-1α-induced expression of COX-2 (Fig. 3A). Similarly, diphenyl iodonium (DPI), an inhibitor of flavin-binding enzymes such as NAD(P)H oxidase, known to govern ROS generation in EC (18), concentration-dependently decreased IL-1α-stimulated COX-2 expression (Figs. 1E and 3A), without altering ERK1/2 activation (not shown). Correspondingly, we observed that IL-1α stimulates a strong production of ROS in HSVEC, as semiquantitatively assessed by dichlorofluorescein–fluorescence (Fig. 3 C and D). Here pretreatment with DHA before IL-1α decreased intracellular ROS (Fig. 3 C and D).
Fig. 3.
Effect of DHA on ROS production and p47phox translocation induced by IL-1α. (A) HSVEC were pretreated with DPI, apocynin, or N-acetylcysteine for 30 min before IL-1α stimulation for 12 h. Whole-cell lysates were subjected to Western blot for COX-2. Values of COX-2 are reported as percent of maximal control response (IL-1α alone). The blot shown is representative of three similar ones. (B) HSVEC were pretreated with 25 μmol/ liter DHA for 48 h and then stimulated with 10 ng/ml IL-1α for 20 min. Subcellular fractions (soluble and particulate) were isolated and Western blots performed with an antibody specific for p47phox. Values are in units of OD. The blot depicted is representative of two similar ones. (C) HSVEC were pretreated with 25 μmol/liter DHA for 48 h and then stimulated with 10 ng/ml IL-1α for 1 h. Monolayers were then washed and loaded with reduced dichlorofluorescein for 30 min and imaged as described in Materials and Methods. (Upper) Original microphotographs, where sides of each square are 300 μm long. (Lower) Corresponding pseudocolor transformation of digitalized images, where the yellow color indicates a low generation of ROS, and darker colors indicate increased ROS generation, proportional to color intensity. (D) Quantitative analysis of the effect of DHA on ROS production by IL-1α, as measured by dichlorofluorescein (DCF) fluorescence emission. Subconfluent HSVEC were treated with DHA at 25 and 50 μmol/liter for 48 h in 96-well plates, stimulated with 10 ng/ml IL-1α for 1 h, and finally loaded with reduced DCF. After 30 min, fluorescence was measured with a plate reader as described. More than eight replicates were used for each condition. Results are expressed as arbitrary fluorescence units ± SD. ∗, P < 0.05; ∗∗, P < 0.01 vs. IL-1α-stimulated control. This experiment is representative of a series of four, with similar results.
DHA Reduces Plasma Membrane Translocation of p47phox and PKCε.
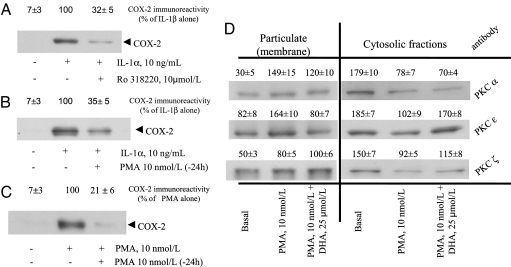
Our results highlighted a crucial role for NADPH oxidase in the production of ROS in cells stimulated to express COX-2. Because the p47phox component of NADPH oxidase is activated by its phosphorylation by PKC (19), which also activates ERK1/2 (20), we tested whether DHA, in altering NAD(P)H oxidase activity, alters membrane translocation of p47phox or of conventional, novel, and atypical PKCs (α, ε, and ζ isoforms). We studied these links by two different approaches: (i) treating cells with the nonspecific PKC inhibitor Ro 318220 and (ii) using PMA pretreatment to down-regulate PKC before IL-1α or PMA stimulation (20). As shown in Fig. 4A, cell treatment with Ro 318220 abrogated IL-1α-induced COX-2 expression. Similarly, PKC down-regulation by PMA pretreatment similarly blocked COX-2 protein induction during subsequent stimulation by IL-1α or PMA (Fig. 4 B and C, respectively), indicating that PKC is involved in IL-1α-induced COX-2 expression. Western blots of cytosolic and plasma membrane-enriched fractions obtained from DHA-treated cells also showed that DHA reduces the stimulated membrane translocation of both p47phox (Fig. 3B) and PKCε but not PKCα and -ζ (Fig. 4C).
Fig. 4.
Effects of DHA on PKC isoform translocations. (A) HSVEC were treated with Ro318220 for 30 min before IL-1α stimulation for 12 h. Whole-cell lysates were subjected to Western blot using an antibody specific for COX-2, and the value obtained at densitometric analysis is reported as percent of maximal control response (IL-1α alone). The blot is representative of three similar ones. (B) HSVEC were pretreated with PMA for 24 h before IL-1α stimulation for 12 h to down-regulate PKC activity. Whole-cell lysates were subjected to Western blot using an antibody specific for COX-2, as in A. The blot is representative of three similar ones. (C) HSVEC were pretreated as in B but then stimulated with PMA. The blot is representative of three similar ones. (D) HSVEC were pretreated with 25 μmol/liter DHA for 48 h and then stimulated with 10 nmol/liter PMA for 20 min. Subcellular fractions (soluble and particulate) were isolated, and Western blots were performed using anti-PKCα, -ε, or -ζ antibodies. Values of PKC translocations are reported as units of OD at densitometric analysis. The blot is representative of two similar ones.
Possible Role of Lipoxygenase (LO), P450 Epoxygenase, and COX Activities in DHA-Induced COX-2 Down-Regulation.
To address the question of whether DHA itself or some DHA-derived metabolite (21) is causally involved in DHA-induced COX-2 expression down-regulation, we treated EC with pharmacological and molecular (siRNA) inhibitors of the most important routes involved in the metabolism of polyunsaturated fatty acids. The inhibition of LO activities, and in particular of 15-LO-1, partially reverted the protective effect of DHA, indicating that, at least in part, DHA acts by these metabolites (Table 2, which is published as supporting information on the PNAS web site).
Discussion
Here we show that exposure of EC to DHA, under conditions that efficiently incorporate DHA into membrane phospholipids (11), decreases stimulated COX-2 mRNA transcription, COX-2 protein expression and PG production. DHA blocks NF-κB-mediated transcriptional regulation of COX-2 by decreased activation of ERK1/2, diminished intracellular production of ROS, and inhibition of p47phox activation and PKCε translocation.
Antiinflammatory properties of n-3 fatty acids have been mostly attributed to competition with AA as substrates for COX or 5-LO (22). Here we demonstrate that exposure of EC to DHA alters gene expression of COX-2 independently of acting as a competitive substrate. Here prolonged incubation of human EC with DHA decreased expression of COX-2 protein and PG production upon cytokine/PMA stimulation, a setting in which COX-2 is induced. These results both complement and expand prior demonstrations by Spector et al. (8) of decreased PGI2 production by human cultured EC briefly exposed to EPA or DHA (8). In Spector's experimental conditions, the decreased PGI2 resulted from the inability of EC COX to metabolize EPA and DHA instead of AA (8). We confirm that DHA acutely reduces (by 27% on average) PGI2 production when EC are stimulated by AA or thrombin, but we also demonstrate a stronger inhibition (40–71%) under conditions where COX-2 is induced. Our measurements likely underestimate DHA's inhibitory effect on PGI2 production by stimulated EC, because our RIA is not totally specific for 6-keto-PGF1α vs. Δ17–6-keto-PGF1α, the hydrolytic product of PGI3 (≈30% crossreactivity in our assay; data on file), and some retroconversion of DHA to EPA likely occurs.
The time course of these inhibitory effects of DHA is fully compatible with that of DHA incorporation into EC membranes, previously shown to plateau after 48 h (11), and with the kinetics of DHA inhibition of endothelial activation products, such as vascular cell adhesion molecule-1, E-selectin, IL-6, and IL-8 (11). Measurements of COX-2 protein confirmed that DHA decreases induced COX-2 by ≈50%, whereas expression of COX-1 was unaffected. Both aspirin and NS-398, nonselective and selective inhibitors of COX-2 activity, respectively, augment DHA effects on PG production; these data support the hypothesis that DHA has a different action from aspirin or NS-398.
Regulation of COX-2 is both transcriptional and, mostly, posttranscriptional (23). Our results on mRNA stability and promoter transfection experiments indicate that DHA mainly affects transcriptional regulation. As in many other inflammatory early response genes, 5′ promoter regulatory sites are important in COX-2 transcriptional control. Binding sites for transcription factors NF-IL-6, AP-2, CRE, and the proximal NF-κB-binding site (23), located between −327 and −220 bp 5′of the transcription start site, regulate COX-2 transcriptional activation by PMA and LPS. Of these promoter sites, only the NF-κB site is here shown to be essential for DHA modulation of COX-2 activity in EC, because only its deletion or mutation abolished DHA effects. The involvement of NF-κB in DHA regulation of COX-2 was further confirmed by EMSA and Western blot, showing reduced nuclear translocation of the p65 NF-κB subunit.
IL-1 receptor activation initiates a number of signaling pathways (15) involving ERK1/2, JNK, and p38 MAPK. ERK1/2, in particular, is clearly up-regulated in vivo in atherosclerosis (24) as well as in IL-1 (13)- and PMA (20)-induced COX-2 expression. Therefore, we examined whether DHA affected IL-1 and PMA-induced ERK1/2 activation. Having established a critical role for ERK1/2 in the expression of endothelial COX-2, because PD 98059 blocked p65 nuclear translocation and COX-2 protein induction, we then showed that DHA down-regulates IL-1α- and PMA-induced ERK1/2 activation. The effect of DHA on EC ERK1/2 confirms previous reports in non-EC (25, 26).
We next explored the possibility that DHA negatively affects one or more molecular target(s) upstream of ERK1/2. Because ROS production activates ERK-related pathways (27), as well as NF-κB (28), DHA might directly decrease IL-1-induced ROS by consuming superoxide anion by peroxidation of the double bonds in the polyunsaturated fatty acid chain (29). In addition, DHA might interfere with some ROS-producing enzyme system in the endothelium, because n-3 fatty acids can alter membrane lipid microdomains, such as lipid rafts and caveolae (30), involved in the compartmentalization, modulation, and integration of cell signaling components upstream of NF-κB, which are sensitive to hydrogen peroxide (27). NADPH oxidase is the principal source of ROS in the endothelium (18). Upon stimulation, its p47phox component becomes phosphorylated, promoting its translocation, together with p67phox, p40phox, and the small GTP-binding protein Rac to the plasma membrane to form the active enzyme complex (18). Although endothelial NADPH oxidase is constitutively active, with a low-level constant intracellular production of ROS, upon stimulation by agonists (such as PMA and cytokines), NADPH oxidase activity is augmented. This occurs through an increased amount of assembled complexes and/or changes in the phosphorylation status of p47phox. The phosphorylation of p47phox is mediated by PKC activation (19). We here demonstrate the involvement of NADPH oxidase activity in IL-1-mediated COX-2 expression by showing that DPI blocks IL-1-mediated COX-2 induction. Another NAD(P)H oxidase inhibitor, apocynin, had no effect on EC COX-2 expression, but it is known that apocynin inhibits NAD(P)H oxidase activity only in phagocytes, stimulating ROS production in nonphagocytic cells (31). We observed that DHA treatment of EC led to decreased p47phox membrane translocation, together with diminished NAD(P)H oxidase activity and intracellular ROS production. This effect is independent of the inhibition of ERK1/2 by DHA, because DPI alone does not alter ERK1/2 activation.
Because PKCε activity is also involved in the activation of NF-κB by ERK1/2 induction (32), and PKCs have been implicated in COX-2 expression (33), we explored the possibility that other molecular switches could be affected by DHA. We monitored membrane translocation of the main PKC isoforms in EC stimulated by PMA (34) in the presence of DHA. All such isoforms were activated by PMA, as demonstrated by their translocation to plasma membrane, but only the translocation of PKCε was reduced by DHA treatment. We conclude, therefore, that DHA inhibits at least two molecular switches, the activation of NAD(P)H oxidase and the activation of PKCε (which involves ERK1/2), both involved in COX-2 expression (Fig. 5).
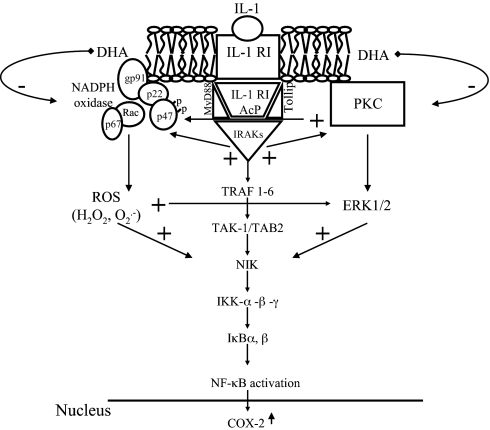
Fig. 5.
Proposed molecular model of dietary omega-3 fatty acid interference with IL-1 signaling pathways leading to COX-2 induction in EC. IL-1 binds to the IL-1 receptor type I (IL-1RI), which heterodimerizes with the IL-1 receptor accessory protein (IL-1RAcP). The IL-1R-associated kinases (IRAK) are then recruited and associated by the adapter proteins myeloid differentiation factor(MyD)88 and Toll-interacting protein (Tollip). The signaling pathway also includes the production of ROS (H2O2) through the activation of NAD(P)H oxidase by IRAK activation, as well as the activation of PKC, both contributing to NF-κB activation. DHA, by interfering with the production of ROS (through the inhibition of p47phox translocation and/or the scavenging of ROS by its multiple double bonds), would prevent the formation of H2O2, thus limiting all of the downstream cascade leading to COX-2 gene expression. Furthermore, DHA reduces PKCε activation, thus inhibiting ERK1/2 activation, also leading to NF-κB activation and COX-2 expression. TRAF, TNF receptor-associated factor; TAK-1, TGFβ-activated kinase 1; TAB-2, TAK1-binding protein 2; NIK, NF-κB-inducing kinase; IKK, IκB kinase.
We finally addressed the question whether DHA itself or some DHA-derived products are involved in this regulation of COX-2, because DHA can generate antiinflammatory lipid mediators through LO or COX pathways (21). We used pharmacological and molecular (siRNA) inhibitors of the main routes involved in polyunsaturated fatty acids metabilization. Here inhibition of LO activities, in particular 15-LO-1, partially reverted the DHA inhibitory effect on COX-2 expression. This suggests that DHA, after its incorporation in membrane lipids, acts at least in part through LO-derived structural rearrangements leading to antiinflammatory molecules, such as 10,17S-docosatriene, previously shown to inhibit oxidative stress in transformed human retinal pigment epithelium (35).
Overall, these findings provide insight into the mechanisms by which n-3 fatty acids may limit inflammation and atherogenesis. First, the effects shown here occur within a concentration range (≤25 μmol/liter) compatible with nutritional or pharmacological interventions in vivo (36). Second, inhibition of COX-2, which limits the amount of prostanoids released, may explain how n-3 fatty acids ameliorate rheumatoid arthritis, psoriasis, and inflammatory bowel disease (6). Third, in atherosclerotic vascular disease, COX-2 activation has been implicated in the growth of atherosclerotic plaques (37), as well as in plaque angiogenesis and the activation of matrix-metalloproteinases (38). Our results may therefore provide some explanation for the plaque-stabilizing effects recently ascribed to n-3 fatty acids on the basis of histological analyses (39). Although clinical results with selective COX-2 inhibitors in vascular disease have been recently found to be globally unfavorable (40), vascular effects of n-3 fatty acids on prostanoids are substantially different, because of milder effects on COX-2 and additional effects on thromboxane production. Thus, data presented here may explain part of the peculiar efficacy and safety profiles of these compounds in cardiovascular disease.
Materials and Methods
Materials.
DHA (22:6 n-3 all cis), AA, (20:4 n-6, all cis), and stearate (18:0) were obtained as >99% pure sodium salts from Nu-Chek (Elysian, MN). DHA from two other commercial sources [Calbiochem (La Jolla, CA) and Sigma-Aldrich (St. Louis, MO)] was also used as control. IL-1α was obtained from Hoffmann-La Roche (Basel, Switzerland). The COX-2 inhibitor NS-398, the MEK1inhibitor PD 98059 (inhibiting a kinase upstream of ERK1/2), the 5-LO inhibitor MK886, and the cytochrome P450 epoxygenase inhibitor SKF-525A were from Calbiochem. The 5- and 12-LO inhibitor 5,8,11-eicosatriynoic acid was from Alexis (Lausen, Switzerland). All other reagents were purchased from Sigma.
Cell Cultures.
We used two types of EC cultures: HSVEC and human umbilical vein EC (HUVEC), both harvested and maintained as described (41, 42). All experiments reported, when not otherwise specified, were performed in HSVEC, but control experiments were also done in HUVEC, with identical results. Cells were used at a time calibrated to reach confluence at the time of stimulation, and up to the fifth passage. For transfection assays, difficult in human EC, we used bovine aortic EC, as described (41).
Experimental Designs.
All cultured EC were preincubated with DHA or other fatty acids, as control, for 0–48 h, followed by stimulation with IL-1α, Escherichia coli LPS, or PMA for an additional 0–12 h, after which time cells and the supernatant medium were collected. In the experiments aimed at determining the production of 6-keto-PGF1α, some monolayers were also treated with aspirin or NS-398 for 30 min and then stimulated with AA or human thrombin for 5 min before medium collection.
Measurement of COX Activity.
We determined the concentration of 6-keto-PGF1α, the stable nonenzymatic product of PGI2, in the cell medium, by RIA (43).
Immunocytochemistry and Cell Lysis.
Immunocytochemistry and cell lysis were done with routine methods (see Supporting Text, which is published as supporting information on the PNAS web site).
Assessment of P47phox and PKC Translocation.
After exposure to IL-1α or PMA for 20 min, EC monolayers were washed three times in cold PBS and harvested by scraping. Cells were centrifuged, resuspended in extraction buffer, and disrupted by sonication on ice with three 15-s bursts. Sonicates were centrifuged at 500 × g for 10 min, the nuclei-rich pellet discarded, and the supernatant fluid recentrifuged to obtain the plasma membrane and the cytosolic fractions, which were used to detect specific subunit translocation after protein separation by SDS/PAGE and subsequent immunoblotting. For details, see Supporting Text.
Immunoblotting.
Equal amounts of proteins were separated by SDS/PAGE. The resolved proteins were transferred onto supported nitrocellulose sheets (Amersham Biosciences, Cardiff, U.K.) and, after saturation of nonspecific binding sites, incubated overnight with specific mono- and polyclonal antibodies against COX-1 and -2; various MAPK (also recognizing, in selected cases, specific activation epitopes); 15-LO-1; p47phox; PKCα, -ε, and -ζ; and the p65 NF-κB subunit. For details, see Supporting Text.
Northern Blot.
Northern blot was performed as published (41).
Transfections and Luciferase Assay.
We used the 5′-flanking sequence from −1432 to +59 of the human COX-2 gene and a series of deleted promoters progressively eliminating the entire sequence upstream of the proximal NF-κB-binding site (−327/+59 bp), the proximal NF-κB-binding site (−220/+59 bp), as well as all regulatory sequences upstream of the TATA box (−52/+59 bp). We also used a promoter site mutated at −224/−214 for the proximal NF-κB site (κBM), all kindly provided by Hiroyasu Inoue (National Cardiovascular Center Research Institute, Osaka, Japan), and all inserted into the promoterless luciferase expression pGL2 basic plasmid (Promega, Madison, WI). For details, see Supporting Text.
Preparation of Nuclear Extracts and EMSA.
After DHA treatment for 24–48 h and subsequent IL-1α stimulation for 1 h, nuclear proteins were purified as described (42). For the assay, we used the oligonucleotide 5′-AGTTGAGGGGACTTTCCCAGGC-3′ containing the consensus sequence for NF-κB (underlined; this is identical, with the only difference of a C→T substitution, to the proximal sequence of the NF-κB-binding site of the human COX-2 promoter (44), and a mutant oligonucleotide with a G→C substitution in the third nucleotide of the consensus sequence (5′-AGTTGAGGCGACTTTCCCAGGC-3′) (Santa Cruz Biotechnology, Santa Cruz, CA). For further details, see Supporting Text.
Measurement of Intracellular ROS.
After DHA treatment and IL-1α stimulation for 1 h, we measured the intracellular production of ROS in ECs, as described by us (ref. 29; see Supporting Text for further details).
Statistical Analysis.
Multiple comparisons were performed by one-way ANOVA, and individual differences then tested by the Fisher's protected least-significant difference test after demonstrating the existence of significant intergroup differences by ANOVA. Two-group comparisons were performed by unpaired Student's t test. Results are expressed as mean ± SEM, with a minimum of three separate experiments for each issue addressed.
Supplementary Material
Acknowledgments
This study was supported by a North Atlantic Treaty Organization collaborative grant (R.D.C. and B.B.W.), a grant from Sigma-Tau (Pomezia, Italy) (R.D.C.), the Center of Excellence on Aging Project (R.D.C.), and the Italian Ministry of the University and Scientific Research (R.D.C.). We thank Dr. Hiroyasu Inoue for supplying the COX-2 promoter constructs, Dr. Stephen Prescott (University of Utah, Salt Lake City, UT) for the gift of the COX-2 cDNA probe, and Dr. Kenneth K. Wu (University of Texas, Houston, TX) for advice on a critical revision of the experimental data on COX-2 promoter activity experiments.
Abbreviations
- DHA
docosahexaenoic acid
- AA
arachidonic acid
- EC
endothelial cell
- HSVEC
human saphenous vein EC
- COX
cyclooxygenase
- PMA
phorbol myristate acetate
- EPA
eicosapentaenoic acid
- PG
prostaglandin
- ROS
reactive oxygen species
- DPI
diphenyl iodonium
- LO
lipoxygenase.
Footnotes
Conflict of interest statement: R.D.C. has received research support and honoraria for lecturing on omega-3 fatty acids in cardiovascular disease through Pharmacia-Pfizer (Milan, Italy), Società Prodotti Antibiotici (SPA; Milan, Italy), Sigma-Tau (Pomezia, Italy), and Pronova (Lysaker, Norway).
References
- 1.Libby P. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Scholz H. Am J Physiol. 2003;285:R512–R514. doi: 10.1152/ajpregu.00298.2003. [DOI] [PubMed] [Google Scholar]
- 3.Pairet M, Engelhardt G. Fund Clin Pharmacol. 1996;10:1–15. doi: 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 4.Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Am J Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wohlfeil ER, Campbell WB. Arterioscler Thromb Vasc Biol. 1999;19:2901–2908. doi: 10.1161/01.atv.19.12.2901. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulos A. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 7.Kris-Etherton PM, Harris WS, Appel LJ. Arterioscler Thromb Vasc Biol. 2003;23:e20–e30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 8.Spector AA, Kaduce TL, Figard PH, Norton KC, Hoak JC, Czervionke RL. J Lipid Res. 1983;24:1595–1604. [PubMed] [Google Scholar]
- 9.Ringbom T, Huss U, Stenholm A, Flock S, Skattebol L, Perera P, Bohlin L. J Nat Prod. 2001;64:745–749. doi: 10.1021/np000620d. [DOI] [PubMed] [Google Scholar]
- 10.De Caterina R, Bernini W, Carluccio MA, Liao JK, Libby P. J Lipid Res. 1998;39:1062–1070. [PubMed] [Google Scholar]
- 11.De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Jr, Libby P. Arterioscler Thromb. 1994;14:1829–1836. doi: 10.1161/01.atv.14.11.1829. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 13.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 14.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brigelius-Flohe R, Banning A, Kny M, Bol GF. Arch Biochem Biophys. 2004;423:66–73. doi: 10.1016/j.abb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Milligan SA, Owens MW, Grisham MB. Arch Biochem Biophys. 1998;352:255–262. doi: 10.1006/abbi.1998.0603. [DOI] [PubMed] [Google Scholar]
- 17.Bae GU, Seo DW, Kwon HK, Lee HY, Hong S, Lee ZW, Ha KS, Lee HW, Han JW. J Biol Chem. 1999;274:32596–32602. doi: 10.1074/jbc.274.46.32596. [DOI] [PubMed] [Google Scholar]
- 18.Ray R, Shah AM. Clin Sci (London) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 19.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 20.Hirai K, Ezumi Y, Nishida E, Uchiyama T, Takayama H. Thromb Haemostasis. 1999;82:1545–1552. [PubMed] [Google Scholar]
- 21.Serhan CN, Arita M, Hong S, Gotlinger K. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 22.Dyerberg J, Bang HO. Lancet. 1978;1:152. doi: 10.1016/s0140-6736(78)90448-8. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe T, Tohnai N. Prostaglandins Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Dietrich H, Metzler B, Wick G, Xu Q. Arterioscler Thromb Vasc Biol. 2000;20:18–26. doi: 10.1161/01.atv.20.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Denys A, Hichami A, Khan NA. J Lipid Res. 2001;42:2015–2020. [PubMed] [Google Scholar]
- 26.Denys A, Hichami A, Maume B, Khan NA. Lipids. 2001;36:813–818. doi: 10.1007/s11745-001-0789-2. [DOI] [PubMed] [Google Scholar]
- 27.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Johnston G, Stebler B, Keller ET. Antioxid Redox Signal. 2001;3:493–504. doi: 10.1089/15230860152409121. [DOI] [PubMed] [Google Scholar]
- 29.Massaro M, Basta G, Lazzerini G, Carluccio M, Bosetti F, Solaini G, Visioli F, Paolicchi A, De Caterina R. Thromb Haemostasis. 2002;88:176–375. [PubMed] [Google Scholar]
- 30.Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. J Nutr Biochem. 2004;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Vejrazka M, Micek R, Stipek S. Biochim Biophys Acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. Am J Physiol. 2000;279:H1679–H1689. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- 33.Chang MS, Chen BC, Yu MT, Sheu JR, Chen TF, Lin CH. Cell Signal. 2005;17:299–310. doi: 10.1016/j.cellsig.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Ross D, Joyner WL. Endothelium. 1997;5:321–332. doi: 10.3109/10623329709052596. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burr M, Gilbert JF, Holliday RM, Elwood PC, Fehily AM, Rogers S, Sweetnam PM, Deadman NM. Lancet. 1989;334:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 37.Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, Marnett LJ, Morrow JD, Fazio S, Linton MF. Circulation. 2002;105:1816–1823. doi: 10.1161/01.cir.0000014927.74465.7f. [DOI] [PubMed] [Google Scholar]
- 38.Cipollone F, Prontera C, Pini B, Marini M, Fazia M, De Cesare D, Iezzi A, Ucchino S, Boccoli G, Saba V, Chiarelli F, Cuccurullo F, Mezzetti A. Circulation. 2001;104:921–927. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- 39.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald GA. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 41.De Caterina R, Libby P, Peng H-B, Thannickal VJ, Rajavashisth B, Gimbrone MAJ, Shin WS, Liao JK. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carluccio MA, Massaro M, Bonfrate C, Siculella L, Maffia M, Nicolardi G, Distante A, Storelli C, De Caterina R. Arterioscler Thromb Vasc Biol. 1999;19:220–228. doi: 10.1161/01.atv.19.2.220. [DOI] [PubMed] [Google Scholar]
- 43.De Caterina R, Giannessi D, Mazzone A, Bernini W, Lazzerini G, Maffei S, Cerri M, Salvatore L, Weksler B. Circulation. 1990;82:428–438. doi: 10.1161/01.cir.82.2.428. [DOI] [PubMed] [Google Scholar]
- 44.Inoue H, Tanabe T. Adv Exp Med Biol. 1997;407:139–144. doi: 10.1007/978-1-4899-1813-0_21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.