Abstract
The functional role of releasable Zn2+ in the central nervous system remains unknown. Here we show that zinc transporter 3 (ZnT-3), which maintains a high concentration of Zn2+ in synaptic vesicles and serves as a marker for zinc-containing neurons, is enriched in the lateral nucleus of the amygdala and in the temporal area 3 of the auditory cortex, an area that conveys information about the auditory conditioned stimulus to the lateral nucleus of the amygdala, but not in other conditioned stimulus areas located in the auditory thalamus. Using whole-cell recordings from amygdala slices, we demonstrated that activity-dependent release of chelatable Zn2+ is required for the induction of spike timing-dependent long-term potentiation in cortical input to the amygdala implicated in fear learning. Our data indicate that synaptically released Zn2+ enables long-term potentiation at the cortico-amygdala synapses by depressing feed-forward GABAergic inhibition of principal neurons. This regulatory mechanism, implicating pathway-dependent release of Zn2+, may serve an essential control function in assuring spatial specificity of long-lasting synaptic modifications in the neural circuit of a learned behavior.
Keywords: amygdala, synapse, synaptic plasticity, glutamate, GABA
Long-term potentiation (LTP) in afferent inputs to the amygdala is recruited during fear conditioning and used for retention of fear memory (1–5). A subset of glutamatergic neurons in fear conditioning pathways, including pyramidal neurons in the lateral nucleus of the amygdala (LA) where conditioned stimuli (CS) and unconditioned stimuli converge during fear learning (6–9), is enriched in zinc (Zn2+) (4). Zn2+ is found to be in a high concentration in synaptic vesicles at various synapses and to be colocalized with the neurotransmitters glutamate or GABA (10, 11). Previous studies have demonstrated that this vesicular Zn2+ can be released in a Ca2+-dependent manner in different regions of the brain, including the hippocampus, cortex, and amygdala, in response to synaptic stimulation at both glutamatergic and GABAergic synapses (11–19). This set of findings suggested to us the possibility that vesicular zinc may have a role in synaptic plasticity in the neural circuitry of fear learning.
Zn2+ has been implicated in a variety of neuronal functions in the mammalian brain both as a paracrine and as an autocrine mediator (20). However, despite intensive efforts the functional role of releasable zinc in synaptic transmission and plasticity remained elusive (10, 20). Here we report that chelatable zinc, released during synaptic activation, is critically involved in control of LTP in the cortico-amygdala pathway, which conveys auditory information about the CS to the amygdala during fear conditioning. We find that zinc acts by suppressing feed-forward inhibition of projection neurons by local circuit interneurons. The pathway-specific expression of zinc-containing cells suggests that this regulatory mechanism may define the spatial pattern of synaptic modifications in auditory inputs to the amygdala implicated in the acquisition of fear memory.
Results
Expression of ZnT-3 Gene and Distribution of Zn2+-Containing Cells in the LA and in the CS Pathways.
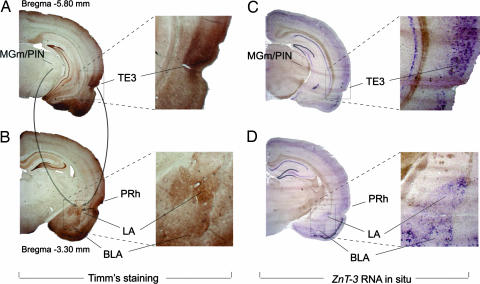
We earlier found that the principal cells in the lateral amygdala express the gene ZnT-3 (4), whose product, zinc transporter 3 (ZnT-3), controls Zn2+ concentration in synaptic vesicles (21). We have now analyzed the overlap between the distribution of Zn2+-containing cells and ZnT-3 expression in the fear conditioning pathways of the rat brain by performing Timm staining (Fig. 1A and B) and in situ hybridization with ZnT-3 RNA (Fig. 1 C and D), respectively. We found Zn2+ and ZnT-3 to be highly visible in the LA and in the temporal area 3 of the auditory cortex, the cortex that sends auditory information to the amygdala during fear learning. Surprisingly, neither Zn2+ nor ZnT-3 was found in the medial division of the medial geniculate nucleus/posterior intralaminar nucleus region of the auditory thalamus, another afferent area projecting to the LA and implicated in auditory fear conditioning (6, 7). These findings indicate that release of vesicular Zn2+ might be involved, in an input-specific fashion, in the regulation of synaptic function in the amygdala.
Fig. 1.
Vesicular Zn2+ and the ZnT-3 gene are colocalized in the cortico-amygdala pathway. (A and B) Timm staining for vesicular zinc. Solid gray arrows show the flow of the auditory information to the LA. (C and D) Digoxigenin-labeled RNA in situ hybridization for the ZnT-3 gene. Images in Right show higher-magnification views of the LA and the temporal area 3 of the auditory cortex (TE3). MGm, medial division of the medial geniculate nucleus; PIN, posterior intralaminar nucleus; PRh, perirhinal cortex.
Activity-Dependent Release of Zn2+ Is Required for the Induction of LTP in the Cortico-Amygdala Pathway.
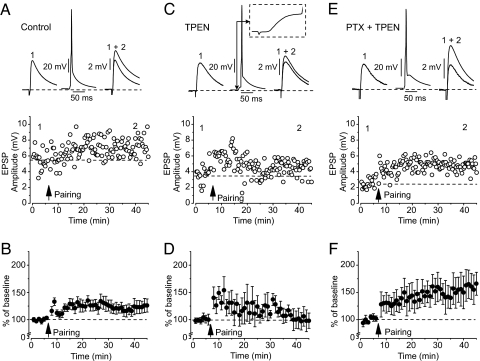
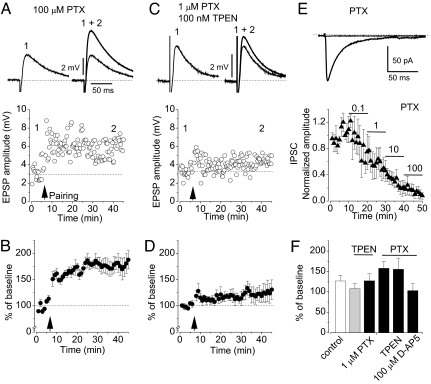
To explore the role of Zn2+ in synaptic plasticity in the lateral amygdala, we first examined whether Zn2+ released during presynaptic stimulation is necessary for the induction of a physiologically relevant form of LTP, spike timing-dependent LTP, at the cortico-amygdala pathway. This pathway contains zinc and expresses ZnT-3 (see Fig. 1). We obtained whole-cell recordings of excitatory postsynaptic potentials (EPSPs) in a current-clamp mode at resting membrane potential from visually identified pyramidal neurons in the dorsolateral division of the LA in brain slices in the presence of GABA-mediated inhibition. Pairing of EPSPs induced by low-frequency presynaptic stimulation (2 Hz) of the external capsule (EC) (cortical input) (3, 6) with action potentials (APs) evoked in a postsynaptic neuron with 4- to 6-ms delay from the onset of each EPSP led to LTP of the cortico-amygdala responses to 126.8 ± 13.7% (n = 9; significantly different from the baseline EPSP amplitude, paired t test, P < 0.05) (Fig. 2A and B). Inclusion of the selective Zn2+ chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (100 nM; KD = 0.26 fM) (11) in the bath solution abolished LTP in the cortical input to the LA (107.8 ± 12.1% of the baseline EPSP; n = 6; P > 0.1) (Fig. 2 C and D). TPEN, even in a significantly higher concentration than in our experiments, has no effect on glutamatergic EPSPs (22). When inhibition was eliminated by blocking the GABAA receptors (GABAAR) in the presence of TPEN with the selective antagonist picrotoxin (PTX) (100 μM), LTP was restored. Now the same induction paradigm resulted in significant potentiation of the cortico-amygdala EPSP (155.2 ± 27.6% of the baseline EPSP; n = 5; significantly different from the baseline EPSP amplitude, t test, P < 0.01) (Fig. 2 E and F). These findings indicate that Zn2+ released during the induction procedure promotes LTP at the cortico-amygdala synapses through inhibition of a GABA-dependent mechanism.
Fig. 2.
Chelatable Zn2+ is required for the induction of spike timing-dependent LTP in cortical input to the LA. (A Upper) Cortico-amygdala EPSPs recorded before (trace 1) and after (trace 2) LTP induction. (A Lower) AP-EPSP pairing-induced LTP (arrow) of the EPSP in the LA neuron in the same experiment as in Upper. (B) Summary graph of the LTP experiments as in A (n = 9). (C) Bath application of TPEN (100 nM) prevents LTP. Traces in Upper show EPSPs recorded before (trace 1) and after (trace 2) the pairing procedure. The trace in the middle demonstrates AP-EPSP pairing. (D) Summary graph of the LTP experiments as in C (n = 6). (E) Blockade of GABAARs (100 μM PTX) rescues LTP in the presence of TPEN. Traces are EPSPs recorded before (trace 1) and after (trace 2) LTP induction. (F) Summary graph of the LTP experiments as in E (n = 5). Error bars indicate SEM.
The effect on LTP was specifically linked to chelation of extracellular Zn2+. The membrane-impermeable Zn2+ chelator Ca-EDTA (1 mM) also abolished LTP fully in cortical input to the LA when GABAA inhibitory responses were not blocked (the EPSP remained at 101.8 ± 14.5% of the baseline amplitude 40 min after the induction; n = 5; P = 0.5). As is the case at the mossy fiber synapses in the hippocampus (14, 15), chelation of extracellular Zn2+ with Ca-EDTA had no effect on basal glutamatergic synaptic transmission in the LA with the EPSP remaining at 92 ± 4.7% of its initial value (n = 6; P = 0.6 vs. the baseline amplitude; 20 min after switching to Ca-EDTA-containing solution). This finding indicates that ambient Zn2+ does not modulate excitatory synaptic transmission in afferent inputs to the amygdala. The action of Ca-EDTA was specific to its ability to chelate Zn2+; it did not affect Ca2+-dependent glutamate release, as evidenced by unchanged paired-pulse facilitation recorded at a 50-ms interpulse interval (baseline paired-pulse facilitation value, 1.6 ± 0.1; paired-pulse facilitation value in the presence of Ca-EDTA, 1.5 ± 0.1; n = 6; P = 0.4).
Mimicking Zn2+ Release Suppresses GABAA Receptor-Mediated Inhibition of Principal Neurons in the LA.
As we have shown (4), the susceptibility of synapses in the lateral amygdala to LTP is under control of local circuit interneurons that provide feed-forward inhibition of principal cells. Similar to other central synapses, LTP in afferent inputs to the LA is enhanced when inhibitory influences are weakened either pharmacologically (23) or genetically (4). We therefore next asked whether mimicking Zn2+ release would decrease the level of GABA-mediated tonic inhibition of the principal neuron in the LA, thus potentially facilitating the induction of LTP. To this end we investigated the effects of exogenous Zn2+ on spontaneous inhibitory postsynaptic currents (sIPSCs) recorded under voltage-clamp conditions. We collected the sIPSCs at a holding potential of −70 mV using a chloride-based pipette solution in the presence of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors. Functional estimates indicate that activation of central synapses could lead to a peak extracellular Zn2+ concentration in brain slices of >100 μM and even up to 300 μM in response to strong stimulation (10, 14). In our experiments bath application of 100 μM Zn2+ decreased both the amplitude and frequency of sIPSCs recorded in the LA neurons. Mean sIPSC amplitude in the absence and presence of Zn2+ was 50.8 ± 9.4 pA and 29.5 ± 5.3 pA (n = 9; paired t test, P < 0.05) (Fig. 7 A, B, D, and F, which is published as supporting information on the PNAS web site), respectively, whereas the frequency of sIPSCs was decreased from 5.1 ± 1.0 Hz to 3.5 ± 0.9 Hz (n = 9; P < 0.05) (Fig. 7 C and E).
The observed changes in inhibition could be due to a decrease in the AP firing of local circuit interneurons and due to direct effects of Zn2+ on the function of GABAergic synapses. To explore this possibility we measured the size and the frequency of miniature IPSCs, which represent responses to the spontaneous release of single GABA-containing vesicles when the firing of AP is blocked with tetrodotoxin. We found that Zn2+ application led to a significant decrease in the miniature IPSCs amplitude from 42.8 ± 9.7 pA to 31.2 ± 9.8 pA (paired t test, P < 0.05) (Fig. 8 A, B, D, and F, which is published as supporting information on the PNAS web site). The frequency of miniature IPSCs was also decreased from 4.1 ± 1.0 to 2.2 ± 0.5 Hz (n = 6; P < 0.05) (Fig. 8 C and E). This finding indicates that Zn2+ was acting both presynaptically (decreasing GABA release) and postsynaptically (decreasing postsynaptic sensitivity to GABA).
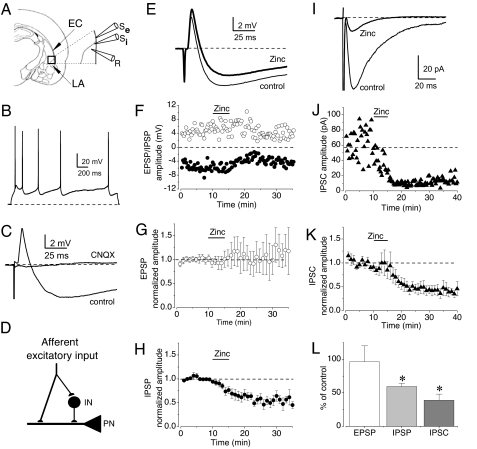
Only a fraction of all GABAergic inputs to a given projection neuron (assessed by recording the spontaneous GABAAR IPSCs) is activated during stimulation of a specific neural pathway. We therefore investigated the effects of Zn2+ on feed-forward inhibition generated by cortical afferent fibers that terminate in the LA. Stimulation of the EC (see Fig. 3A, stimulating electrode Se) led to the appearance of biphasic synaptic responses recorded in a current-clamp mode in cells with accommodating firing properties, which is indicative of pyramidal neurons (Fig. 3B). When the cell was depolarized by current injection from a normal resting membrane potential to −50 mV, a single presynaptic stimulus resulted in the CNQX-sensitive glutamatergic monosynaptic EPSP followed by the GABAAR inhibitory postsynaptic potential (IPSP) (Fig. 3C), as evidenced by its sensitivity to PTX (100 μM) (data not shown). The EPSP/IPSP sequences were completely abolished by CNQX (20 μM), revealing the disynaptic nature of the IPSP (Fig. 3 C and D). Zn2+ (100 μM) application now reduced the feed-forward IPSP to 59.5 ± 4.9% of its initial value (n = 10; P < 0.01) (Fig. 3 E, F, and H) whereas the size of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated EPSP was not significantly affected (P = 0.6) (Fig. 3 E–G). In agreement with earlier findings (23), the recruitment of feedback inhibition in the LA was unlikely in these experiments because we used a relatively low-intensity presynaptic stimulation that did not trigger AP in recorded projection neurons.
Fig. 3.
Zinc depresses feed-forward inhibition in the LA. (A) Schematic representation of a brain slice containing the LA that shows the position of the recording (R) and stimulation (Se and Si) pipettes. (B) Response of a neuron in the LA to prolonged depolarizing current injection showing significant spike frequency adaptation. (C) Stimulation of the EC evoked biphasic potentials in the LA at −50 mV consisting of the EPSP followed by the IPSP, sensitive to CNQX (20 μM). (D) Neural circuit for EPSP/IPSP sequences. IN, interneuron; PN, principal neuron. (E) Action of Zn2+ (100 μM) on EPSP/IPSP sequences. (F) The effect of Zn2+ on the amplitude of EPSPs (○) and IPSPs (●). (G and H) Summary graphs of the experiments as in F showing the effect of Zn2+ on the EPSP (G) and IPSP (H) (n = 10). (I) Zn2+ depresses monosynaptic GABA IPSC recorded at −70 mV. Stimulation pipette was placed within the LA. (J) The effects of Zn2+ (100 μM) on monosynaptic IPSCs. (K) Summary graph of the experiments as in J (n = 9). (L) Summary plot of the effects of Zn2+ on mean amplitude of evoked synaptic responses. Error bars indicate SEM.
We also studied the effects of Zn2+ on isolated monosynaptic IPSCs recorded under voltage-clamp conditions without contamination by glutamatergic synaptic responses that were blocked by CNQX (20 μM) and the NMDA receptor antagonist d-2-amino-5-phosphonopentanoic acid (50 μM). For this purpose, the stimulation electrode was placed in the LA to stimulate inhibitory interneurons directly (Fig. 3A, stimulating electrode Si). Similar to the experiments with disynaptic IPSPs, bath application of Zn2+ (100 μM) reduced the isolated monosynaptic IPSC to 38.9 ± 9.2% (n = 9; significantly different from the baseline responses, P < 0.01) (Fig. 3 I–L). Synaptic responses in the LA mediated by GABAARs exhibited a high sensitivity to actions of Zn2+ as they were depressed by zinc in concentrations as low as 10 μM. Moreover, we found that the amplitude of the GABAAR IPSP was not significantly affected by bath application of the benzodiazepine type 1-selective GABAAR agonist zolpidem (100 nM) (n = 5; P = 0.6). Low sensitivity of GABAAR IPSPs in the LA to zolpidem combined with a high sensitivity of these receptors to Zn2+ suggests that a relatively homogenous population of GABAAR subtypes exists at these synaptic inputs (24).
Synaptically Released Zn2+ Suppresses Feed-Forward Inhibition in the Cortico-Amygdala Pathway and Gates LTP.
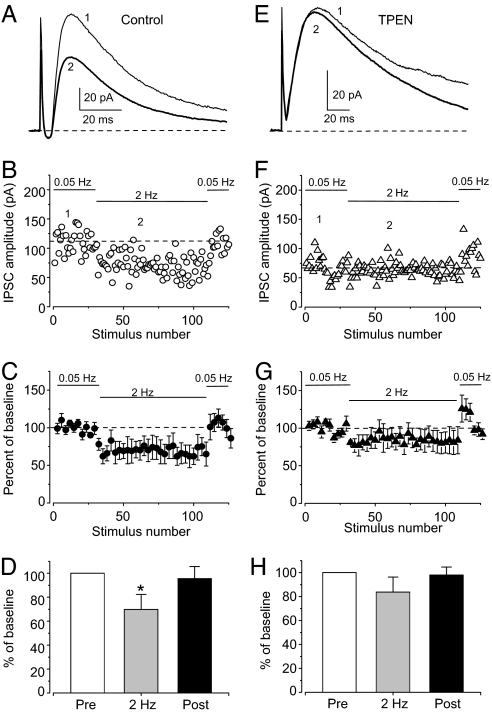
To obtain evidence that release of Zn2+ during delivery of the LTP protocol leads to depression of inhibitory inputs to principal cells in the LA, we recorded the feed-forward disynaptic inhibitory responses evoked by stimulation of the cortical input. We recorded GABAAR IPSCs at a holding potential of 0 mV, which is close to the reversal potential of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor EPSC. Under these conditions the contribution of monosynaptic glutamatergic component to the peak amplitude of the evoked synaptic response was negligible. To prevent synaptic plasticity in these experiments we included a high concentration of the Ca2+ chelator EGTA (10 mM) in the intracellular recording solution (25) and NMDA receptor antagonist d-2-amino-5-phosphonopentanoic acid (50 μM) in the bath solution. After recording baseline GABAAR IPSCs evoked by stimulation of once every 20 s, the cortical pathway was stimulated with a paradigm used to induce LTP at cortico-amygdala synapses (40 s at a frequency of 2 Hz; see Fig. 2). In the course of 2-Hz stimulation, the mean amplitude of GABAAR IPSC was reduced by 30.2 ± 12% (n = 8; significantly different from the baseline IPSC amplitude, paired t test, P < 0.04) (Fig. 4A–D). Consistent with the notion that the observed decreases in the magnitude of feed-forward IPSCs were due to action of synaptically released Zn2+, the zinc chelator TPEN abolished the effect of 2-Hz stimulation on the IPSC at a concentration that had no effect on baseline responses (100 nM). With TPEN in the bath solution, the IPSC recorded in the course of 2-Hz stimulation was decreased only by 16.2 ± 12.6% (n = 8; not significantly different from the baseline responses, P = 0.4) (Fig. 4 E–H).
Fig. 4.
Depression of feed-forward IPSCs by synaptically released Zn2+ depends on the frequency of presynaptic stimulation. (A) GABAAR IPSCs recorded at 0 mV in response to stimulation of the EC at 0.05-Hz (trace 1) and 2-Hz (trace 2) frequencies. (B) Data from an individual experiment. After recording baseline IPSCs, the cortical pathway was stimulated for 40 s at a frequency of 2 Hz. The same stimulation paradigm was used to induce LTP. Test stimulation with a frequency of 0.05 Hz was resumed immediately after 2-Hz stimulation. (C) Summary graph of the experiments as in B (n = 8). (D) Summary plot of the IPSC reduction during stimulation with a frequency of 2 Hz. (E) GABAAR IPSCs recorded in response to stimulation of the EC at 0.05-Hz (trace 1) and 2-Hz (trace 2) frequencies in the presence of TPEN (100 nM). (F) Data from an individual experiment with TPEN in the bath solution. (G) Summary graph of the experiments as in F (n = 8). (H) TPEN prevents the IPSC reduction during 2-Hz stimulation. Error bars indicate SEM.
Thus, we demonstrated that LTP in cortical input could not be induced when external Zn2+ is chelated, whereas blocking GABAAR-mediated inhibition with PTX allows LTP induction, even in the presence of Zn2+ chelator (see Fig. 2). However, the complete blockade of inhibition by a high concentration of PTX could unspecifically enhance LTP at the LA synapses. Indeed, in the presence of 100 μM PTX the EPSP was potentiated to 157.9 ± 16.8% (n = 6) of its initial amplitude (Fig. 5A and B; not significantly different from the amount of LTP observed in the presence of both PTX and TPEN) (see Fig. 2F; P > 0.1). To obtain evidence that the depression of GABAAR-mediated inhibition by zinc during the LTP-inducing stimulation might be responsible for the modulation of LTP, we tested the effect of an intermediate concentration of PTX that produces a block of inhibition that is similar to the block seen with synaptically released Zn2+. As shown in Fig. 5 C and D, 1 μM PTX, which reduced the amplitude of the isolated GABAAR IPSC by ≈30–40% (Fig. 5E; the IPSC was reduced to 57 ± 3% of its baseline amplitude; n = 4), which is nearly identical to the reduction in the size of the IPSC observed in the course of 2-Hz presynaptic stimulation (Fig. 4 C), also rescued LTP in the presence of TPEN. Under these conditions the EPSP was potentiated to 127.2 ± 17.4% (n = 6; paired t test, P < 0.05 vs. baseline). This finding is consistent with the view that zinc-mediated decreases in feed-forward inhibition of principal neurons observed during LTP-inducing presynaptic stimulation are sufficient to gate LTP at the cortico-amygdala synapses.
Fig. 5.
Partial block of feed-forward inhibition rescues LTP in the presence of Zn2+ chelator. (A) AP-EPSP pairing-induced LTP of the EPSP in the LA neuron in the presence of 100 μM PTX. Traces in Upper are the cortico-amygdala EPSPs recorded before (trace 1) and after (trace 2) LTP induction (arrow). (B) Summary graph of the LTP experiments as in A (n = 6). (C) LTP of the EPSPs in the presence of 1 μM PTX and TPEN (100 nM). Traces show EPSPs recorded before (trace 1) and after (trace 2) the pairing procedure. (D) Summary graph of the LTP experiments as in C (n = 6). (E) The effect of different concentrations of PTX on isolated monosynaptic GABAAR IPSC (n = 4). Traces in Upper show that the IPSC was completely blocked by 100 μM PTX. (F) Summary of LTP experiments. Error bars indicate SEM.
Discussion
Auditory fear conditioning results from the temporal association of conditioned and unconditioned stimuli in the course of behavioral training (6, 7, 9, 26). Information about the CS is mediated to the lateral nucleus via the thalamo-amygdala and cortico-amygdala pathways. Our present results provide direct evidence that Zn2+ released during synaptic activation enables LTP at amygdala synapses by decreasing feed-forward GABAergic inhibition of principal neurons. Reduced feed-forward inhibition could promote LTP by influencing back-propagation of AP (23). Because spike timing-dependent LTP at cortico-amygdala synapses is NMDA receptor-dependent and blocked by the NMDA receptor antagonist (see Fig. 5F), a weaker inhibitory drive would allow for the enhanced activation of NMDA receptors in cortical pathway and facilitate LTP induction.
Release of detectable quantities of zinc, even in response to individual presynaptic pulses, has been recently directly demonstrated in mouse hippocampal slices with imaging techniques (ref. 18, but see ref. 19). At mossy fiber synapses in the hippocampus, Zn2+ serves as a modulatory neurotransmitter that shapes the NMDA receptor-mediated synaptic currents by both voltage-dependent and voltage-independent mechanisms when coreleased with glutamate in response to synaptic activation (ref. 14; also see ref. 27). It has been shown earlier that the voltage-dependent Zn2+ inhibition of recombinant NMDA receptors could be observed with externally applied Zn2+ in the micromolar concentration range. This inhibition was found to be similar in NR2A and NR2B subunit-containing NMDA receptors (28). However, the voltage-independent inhibition occurs at significantly lower concentrations of Zn2+ in NR2A-containing receptors than in NR1-NR2B receptors (IC50 in the nanomolar and micromolar concentration range of Zn2+, respectively; see ref. 28). Because we recently found that the induction of spike timing-dependent LTP in the cortico-amygdala pathway depends entirely on activation of the NR2B-containing NMDA receptors (R. M. Shin and V.Y.B., unpublished data), it is likely that the amount of Zn2+ released during LTP-inducing stimulation in the amygdala, although sufficient for the high-affinity zinc inhibition of NR2A-containing receptors, does not reach levels required to block NMDA receptors containing the NR2B subunit and therefore does not directly affect the NMDA receptors implicated in the induction of LTP. Consistent with this notion, the detectable effects on the feed-forward IPSC and LTP in the cortico-amygdala pathway were observed in our study at a relatively low concentration of TPEN.
Zinc also blocks GABAAR with certain subunit composition (17, 24). Moreover, it has been shown that zinc can contribute to pathophysiology of epilepsy by inducing collapse of GABA-mediated inhibition in the dentate gyrus (29). A recent study demonstrated that zinc can be coreleased with GABA in the CA3 region of the guinea pig hippocampus (11). Consistent with our findings at the cortico-amygdala synapses, this activity-dependent release of zinc led to depression of monosynaptic GABAAR-mediated IPSCs evoked in CA3 principal neurons by stimulation in the dentate gyrus. However, the role of endogenous zinc released by synaptic activity in the induction of LTP was not demonstrated in this previous work. Although the effect on LTP observed in our study depended on chelation of extracellular Zn2+, a role of intracellular Zn2+ in modulation of synaptic function could not be ruled out, because zinc could act inside the neuron by entering it via voltage-dependent Ca2+ channels or through NMDA or Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors (15).
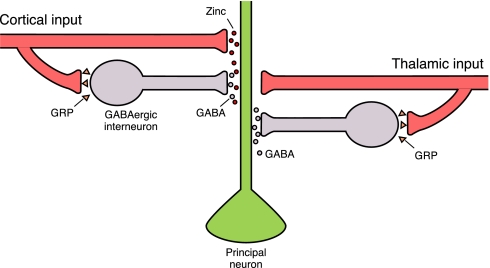
Our previous results provided evidence that gastrin-releasing peptide (GRP) is expressed by a fraction of glutamatergic neurons (also enriched in Zn2+) in the LA and in the auditory CS regions (4). Binding of GRP to its receptors on interneurons leads to release of GABA, thus decreasing susceptibility of synapses in the cortico-amygdala pathway to LTP (Fig. 6). Colocalization of GRP and zinc suggests an interesting possibility that different neuromodulators may be coreleased at the set of synapses conveying the CS information to the amygdala, providing a precise control of the inhibitory inputs to LA projection neurons, and therefore affecting the capability of neural network to undergo plastic changes needed for fear learning to occur.
Fig. 6.
A model for input-specific modulation of GABAergic inhibition by synaptically released zinc in the lateral amygdala. GRP is released by neurons projecting to the LA (see ref. 4). GRP binds to receptors on interneurons, leading to their enhanced firing and stronger inhibition of principal neurons. Glutamatergic cortico-amygdala afferents also contain Zn2+, whereas no Zn+-containing cells are detected in the thalamic CS areas also sending glutamatergic projections to the LA. Input-specific suppression of feed-forward GABAergic inhibition of principal neurons in the LA by synaptically released Zn2+ may control spatial specificity of LTP in fear conditioning pathways.
What is the potential functional significance of the observed effects of synaptically released Zn2+ in the lateral amygdala? Only a fraction of neurons in fear conditioning pathways expresses ZnT-3 transporter, a specific marker for zinc-containing neurons (4, 21, 30). Furthermore, in the present study both Zn2+ and ZnT-3 were detected in the LA and in the temporal area 3 of the auditory cortex, but not in the medial division of the medial geniculate nucleus/posterior intralaminar nucleus region of the auditory thalamus, afferent areas that deliver to the LA the auditory CS information during fear conditioning (see Fig. 1). Consistent with the lack of ZnT-3 expression in the auditory thalamus described by us, a previous study has demonstrated that the EPSP-AP pairing induction protocol does not result in LTP in thalamic input to the LA under conditions of intact GABAergic inhibition (23). Based on these findings, we propose a model according to which activation of Zn2+-containing/releasing cells could determine spatial specificity of synaptic modifications through highly localized release of Zn2+ in response to synaptic activation (Fig. 6). This specificity could lead to selective potentiation of synaptic inputs where vesicular Zn2+ is released during LTP-inducing activity, thus controlling spatial specificity of the information transfer in fear conditioning pathways and potentially contributing to the CS discrimination during recall of memory for fear.
Materials and Methods
In Situ Hybridization and Cytochemistry.
RNA in situ hybridization was performed as described previously (31). Timm staining was performed by using a modification of a previously published protocol (30, 32). Briefly, a 1-month-old rat (125 g body weight) was anesthetized by 3.5 ml of 1.25% Avertin solution i.p. The rat was transcardially perfused with 200 ml of buffered sulfate solution (9.002 g of Na2S·9H2O and 2.975 g of NaH2PO4·H2O in 500 ml of ddH2O) followed by 200 ml of a fixative [4% paraformaldehyde in 1× PBS (pH 7.3)]. The brain was removed, postfixed in the same fixative for 48 h, and then rinsed with 1× PBS (pH 7.3) for 30 min. Fifty-micrometer-thick sections were obtained by using Vibratome 1000 Plus, stored in 1× PBS (pH 7.3), and put on glass slides to air-dry overnight. The sections were developed in the dark for 2 h in a developing solution (30 ml of 50% gum arabic/5 ml of 2 M citrate buffer, pH 3.7/15 ml of 5.67% hydroquinone/0.25 ml of 17% AgNO3), washed in running tap water for 10 min, and rinsed in ddH2O. The sections were placed in 5% sodium thiosulfate solution for 15 min to stop the reaction and fix, rinsed again in ddH2O for 5 min three times, and mounted by using glycerol and a 1× PBS mixture (1:1).
Electrophysiology.
Amygdala slices (250–300 μm) were prepared from 3.5- to 5-week-old Sprague–Dawley rats with a vibratome. Slices were continuously superfused in solution containing 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 10 mM glucose and equilibrated with 95% O2 and 5% CO2 (pH 7.3–7.4) at room temperature. Whole-cell recordings were obtained from projection neurons in the LA under visual guidance (DIC/infrared optics) with an EPC-10 amplifier and Pulse v8.63 software (HEKA Elektronik, Lambrecht/Pfalz, Germany). In all current-clamp experiments the patch electrodes (3–5 MOm resistance) contained 120 mM K-gluconate, 5 mM NaCl, 1 mM MgCl2, 0.2 mM EGTA, 10 mM Hepes, 2 mM MgATP, and 0.2 mM NaGTP (adjusted to pH 7.2 with KOH). To record spontaneous and evoked IPSCs, 120 mM KCl was used instead of K-gluconate. To examine the effects of synaptically released Zn2+ on evoked GABAA IPSCs (the experiments shown in Fig. 4), cesium methane-sulfonate was substituted for potassium in the pipette solution. Series resistance was monitored throughout the experiment and was in the range of 10–20 MOm. Synaptic responses were evoked by low-intensity current pulses applied through a fine-tipped (≈5 μm) concentric stimulating electrode consisting of a patch pipette that was coated with silver paint. The stimulating pipette was positioned to activate the cortical input to the LA. To evoke monosynaptic GABAAR IPSCs, the stimulation electrode was placed within the lateral nucleus of the LA. Summary LTP graphs were obtained by normalizing data in 60-s epochs to the mean value of the baseline EPSP. The sIPSCs were analyzed with Mini Analysis v6 software (Synaptosoft, Decatur, GA). For the cumulative sIPSC or miniature IPSC graphs, responses were normalized by the median value of synaptic events collected under baseline conditions in each individual experiment.
Supplementary Material
Acknowledgments
We thank Keith Tully and Yan Li for helpful comments on the manuscript. This work was supported by National Institutes of Health Grants NS44185 and NS45625 (to V.Y.B.), the Ester A. and Joseph Klingenstein Fund (V.Y.B.), the National Alliance for Research on Schizophrenia and Depression (V.Y.B. and G.P.S.), The New Jersey Governor's Council on Autism (G.P.S.), and the Charles and Johanna Busch Memorial Fund (G.P.S.).
Abbreviations
- LA
lateral nucleus of the amygdala
- EPSP
excitatory postsynaptic potential
- IPSP
inhibitory postsynaptic potential
- IPSC
inhibitory postsynaptic current
- sIPSC
spontaneous IPSC
- CS
conditioned stimulus
- LTP
long-term potentiation
- AP
action potential
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- PTX
picrotoxin
- GRP
gastrin-releasing peptide
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- EC
external capsule
- GABAAR
GABAA receptor.
Footnotes
Conflict of interest statement: E.R.K. is one of four founders of Memory Pharmaceuticals and Chairman of its Scientific Advisory Board. In addition, E.R.K. is a member of the Scientific Advisory Board of Brain Cells.
References
- 1.Rogan MT, Staubli UV, LeDoux JE. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 2.McKernan MG, Shinnick-Gallagher P. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 3.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 4.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 5.Shumyatsky GP, Malleret G, Shin RM, Tully K, Takizawa S, Tsvetkov E, Joseph J, Vronskaya S, Yin DQ, Schubart UK, et al. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux JE. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 7.Maren S, Quirk GJ. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 8.Campeau S, Davis M. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis M, Whalen PJ. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 10.Huang EP. Proc Natl Acad Sci USA. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. J Neurophysiol. 2004;91:1091–1096. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- 12.Assaf SY, Chung SH. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 13.Howell GA, Welch MG, Frederickson CJ. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- 14.Vogt K, Mellor J, Tong G, Nicoll RA. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Hough CJ, Frederickson CJ, Sarvey JM. J Neurosci. 2001;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y. J Cell Biol. 2002;158:215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosie AM, Dunne EL, Harvey RJ, Smart TG. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- 18.Qian J, Noebels JL. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kay AR. J Neurosci. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baranano DE, Ferris CD, Snyder SH. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- 21.Palmiter RD, Cole TB, Quaife CJ, Findley SD. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinta-Ferreira ME, Matias CM. Brain Res. 2004;1004:52–60. doi: 10.1016/j.brainres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Bissiere S, Humeau Y, Luthi A. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 24.Defazio T, Hablitz JJ. J Neurophysiol. 1998;80:1670–1677. doi: 10.1152/jn.1998.80.4.1670. [DOI] [PubMed] [Google Scholar]
- 25.Tsvetkov E, Shin RM, Bolshakov VY. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- 26.Fanselow MS, Poulos AM. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 27.Westbrook GL, Mayer ML. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 28.Paoletti P, Ascher P, Neyton J. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buhl EH, Otis TS, Mody I. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 30.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Proc Natl Acad Sci USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeren-Wiemers N, Gerfin-Moser A. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.