Abstract
Alcoholism is a chronic relapsing disorder with substantial heritability. Uncovering gene–environment interactions underlying this disease process can aid identification of novel treatment targets. Here, we found a lowered threshold for stress-induced reinstatement of alcohol seeking in Marchigian–Sardinian Preferring (msP) rats genetically selected for high alcohol preference. In situ hybridization for a panel of 20 stress-related genes in 16 brain regions was used to screen for differential gene expression that may underlie this behavioral phenotype. An innate up-regulation of the Crhr1 transcript, encoding the corticotropin-releasing hormone receptor 1 (CRH-R1), was found in several limbic brain areas of msP rats genetically selected for high alcohol preference, was associated with genetic polymorphism of the Crhr1 promoter, and was accompanied by increased CRH-R1 density. A selective CRH-R1 antagonist (antalarmin, 10–20 mg/kg) was devoid of effects on operant alcohol self-administration in unselected Wistar rats but significantly suppressed this behavior in the msP line. Stress-induced reinstatement of alcohol seeking was not significantly affected by antalarmin in Wistar rats but was fully blocked in msP animals. These data demonstrate that Crhr1 genotype and expression interact with environmental stress to reinstate alcohol-seeking behavior.
Keywords: alcoholism, reinstatement, self-administration, corticotropin-releasing hormone
Alcohol use is the number three modifiable cause of death in the United States (1). Alcohol dependence, hereafter called alcoholism, is a complex behavioral disorder in which substantial heritable susceptibility factors interact with the environment to produce and maintain the disease state (2). Alcoholism is clinically characterized by a chronic relapsing course similar to other common medical conditions (3). Relapse, i.e., return to alcohol seeking and uncontrolled drinking after varying intervals of sobriety, is a key phenomenon in this process, making relapse prevention a primary therapeutic objective.
Gene–environment interactions are commonly implicated in alcoholism and propensity to relapse, but their exact nature is presently unknown. A behavioral analysis has long pointed to three broad categories of environmental stimuli with an ability to trigger relapse in susceptible individuals (4): consumption of small, “priming” doses of alcohol, presentation of conditioned cues associated with prior availability of alcohol, and stress. It is unclear whether, in alcohol-dependent individuals, these stimuli trigger relapse by interacting with preexisting genetic susceptibility factors, acquired CNS neuroadaptations secondary to a prolonged history of alcohol use, or both.
Models in experimental animals offer tools in the search for novel alcoholism treatments (5, 6) and may be helpful in addressing this question. Genetic selection for high alcohol preference in rats has resulted in several lines with pharmacologically relevant levels of voluntary intake of alcohol, as well as other alcohol-related phenotypes (7, 8), and an improved understanding of mechanisms mediating relapse-like behavior has been obtained through the use of models that use the ability of stimuli known to trigger relapse in humans to reinstate alcohol seeking in rats after extinction of operant responding for alcohol (9, 10). We reasoned that applying a reinstatement paradigm to animals genetically selected for high alcohol preference might help reveal genetic variation underlying altered sensitivity to relapse.
Clinically, alcoholism is commonly comorbid with anxiety disorders and depression, conditions characterized by maladaptive responses to stress (11). Here, we used an established selection-based model, the Marchigian–Sardinian Preferring (msP) rat, which has been bred for high alcohol preference at University of Camerino for >50 generations and is a subline of the previously established Sardinian Preferring (sP) line. These lines may offer a particularly useful model of genetic susceptibility to stress-mediated relapse, because anxiety and depression-like traits have cosegregated with high alcohol preference during the selection leading to their creation (12–14).
We first set out to confirm an elevated behavioral sensitivity to stress in the msP line and then demonstrated that it leads to a lowered threshold for stress-induced reinstatement of alcohol-seeking behavior. In search of genetic substrates of susceptibility to stress-induced relapse, we analyzed the expression of several families of stress-related genes across a range of brain areas thought to be involved in mediating behavioral effects of stress. Our analysis identified an up-regulated expression of the Crhr1 gene, encoding the corticotropin-releasing hormone receptor 1 (CRH-R1) as one of the most striking features of the msP line. Sequence data obtained pointed to genetic variation at this locus as a factor underlying the up-regulated expression and function of CRH-R1 receptors in msP rats. Finally, we used the selective CRH-R1 receptor antagonist antalarmin to demonstrate a causal role of up-regulated CRH-R1 expression and function for stress-induced relapse.
Results
Increased Behavioral Sensitivity to Stress in msP Rats.
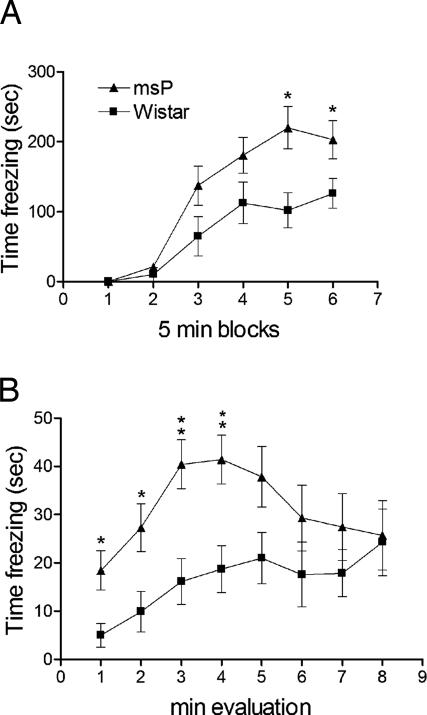
In the open field, under conditions of novelty, msP rats showed a markedly lower total distance traveled (3681.0 ± 184.6 vs. 4885.7 ± 271.4; mean ± SEM; F1,13 = 14.11, P < 0.001) and a markedly lower number of crossings in the center (50.5 ± 9.1 vs. 79.6 ± 8.3; F1,13 = 13.34, P < 0.001) compared with genetically heterogeneous Wistar rats, whereas immobility time was higher (341.8 ± 10.1 vs. 291.3 ± 11.1; F1,13 = 11.30, P < 0.01). After habituation, these three parameters were indistinguishable between the lines (data not shown). This pattern is characteristic of an anxiogenic-like phenotype. Accordingly, msP rats showed a markedly higher level of freezing during both acquisition (Fig. 1A; F1,1 = 132.9, P < 0.001) and recall (Fig. 1B; F1,12 = 6.10, P < 0.05) of fear conditioning. Pain thresholds, a potential confound, were determined by using a hot plate test but were identical in both lines (data not shown).
Fig. 1.
Increased freezing in msP rats during (A) acquisition and (B) recall sessions in a contextual fear-conditioning paradigm. Data are percentage time freezing (mean ± SEM, n = 7 per line) during six consecutive 5-min blocks of fear conditioning (A) or 8-min reexposure to context previously associated with foot shock (B).
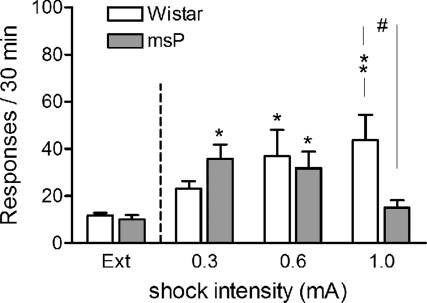
Decreased Threshold for Stress-Induced Reinstatement of Alcohol Seeking in msP Rats (Fig. 2).
Fig. 2.
Decreased threshold for stress-induced reinstatement of alcohol seeking in msP rats. Responding on a previously alcohol-paired lever after delivery of 15-min foot-shock stress at increasing current intensities (0.3, 0.6, and 1.0 mA; mean ± SEM; n = 8–10 for each line at each shock level) is shown. In msP rats, the highest level of relapse-like behavior was at 0.3 mA and in Wistar at 1.0 mA. In msP rats, behavior was disrupted by freezing at the highest shock intensity. Extinction responses (Ext) represent the mean number of lever presses during the last 3 days of extinction. Difference from extinction: ∗, P < 0.05 and ∗∗, P < 0.01. Difference between msP and Wistar: #, P < 0.05.
Foot shock robustly reinstated responding on the previously alcohol-associated lever (F1,1 = 132.9, P < 0.001). A reinstatement × strain × shock intensity interaction was found (F2,47 = 6.27, P < 0.01). Post hoc analysis demonstrated that msP animals reinstated after 0.3- and 0.6-mA foot shock. After 1.0 mA, freezing, a species-specific response to excessive stress, was instead observed in the msP line. In contrast, reinstatement in Wistar rats increased progressively with shock intensity, was not significant at 0.3 mA, reached significance at 0.6 mA, and was maximal at the highest current intensity (1.0 mA). There was a robust plasma corticosterone (CORT) response to foot-shock stress in both lines, but the lower threshold for stress-induced reinstatement in msP rats was not accompanied by a higher CORT response to shock (see Fig. 6, which is published as supporting information on the PNAS web site).
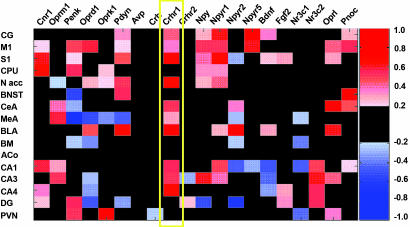
Screening for Differential Expression of Stress-Related Genes (Fig. 3).
Fig. 3.
Heat map representing the results of 20 in situ hybridizations comparing expression of candidate genes in forebrain regions of msP and Wistar rats. The color-coded SES (see Materials and Methods) is displayed in red or blue if expression levels where increased or decreased, respectively, in msP animals compared with Wistar rats. SES below 0.25 are not shown. On the x axis, genes are ordered according to their functional relationship as inferred from cocitation events between two genes in the PubMed database (http://www.ncbi.nlm.nih.gov/Entrez/). Gene symbols are shown according to the Entrez gene nomenclature: Cnr1, cannabinoid receptor 1; Oprm1, μ-opioid receptor; Penk, preproenkephalin; Oprd1, δ-opioid receptor; Oprk1, κ-opioid receptor; Pdyn, prodynorphin; Avp, arginine vasopressin; Crh, corticotrophin releasing hormone (CRH); Crhr1 and -2, CRH receptors 1 and 2, respectively; Npy, neuropeptide Y (NPY); Npyr1, -r2, and -r5, NPY receptors 1, 2, 5, respectively; Bdnf, brain-derived neurotrophic factor; Fgf2, basic fibroblast growth factor; Nr3c1, glucocorticoid receptor; Nr3c2, mineralocorticoid receptor; Oprl, nociceptin receptor; and Pnoc, prepronociceptin. The following brain regions are shown on the y axis: CG, cingulate cortex; M1, primary motor cortex; S1, primary somatosensory cortex; CPU, caudate putamen; N acc, nucleus accumbens; BNST, bed nucleus of stria terminalis; CeA, central amygdaloid nucleus; MeA, medial amygdaloid nucleus; BLA, basolateral amygdaloid nucleus; BM, basomedial amygdaloid nucleus; ACo, anterior cortical amygdaloid nucleus; CA1, -3, and -4, DG, dentate gyrus; Cornus Ammon areas of the hippocampal subregions 1, 3, and 4, respectively; DG, dentate gyrus; and PVN, hypothalamic paraventricular nucleus. The yellow frame highlights the up-regulated Crhr1 expression in msP vs. Wistar rats across numerous brain regions.
The most salient finding, shown by a consistent high standardized effect size (SES; see Materials and Methods) across most brain regions, was that of increased Crhr1 mRNA expression in the msP line.
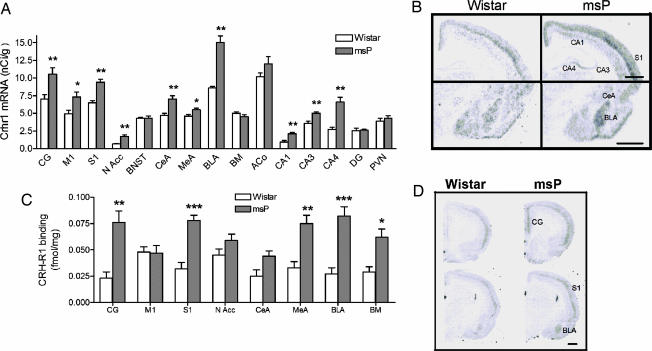
Directed Analysis of CRH-R1 Expression and Binding.
Focused analysis confirmed overall up-regulated Crhr1 expression in msP rats (Kruskal–Wallis nonparametric ANOVA, H1,231 = 12.5, P < 0.001], with post hoc tests demonstrating elevated expression in cortical, hippocampal, and amygdaloid areas as well as nucleus accumbens of msP rats (Fig. 4A and B). Receptor autoradiography showed that increased Crhr1 expression was paralleled by increased overall CRH-R1 binding-site density (ANOVA, main effect of line: F1,126 = 125.5, P ≪ 0.001). Post hoc analysis showed significant increased CRH-R1 densities in cingulate and somatosensory cortex, as well as medial, basolateral, and basomedial amygdala of msP rats (Fig. 4 C and D).
Fig. 4.
Quantitation of Crhr1 expression and CRH-R1 binding in nonselected Wistar and genetically selected, alcohol-preferring mSP rats. (A) Up-regulated Crhr1 expression in msP rats (black bars) compared with Wistar (white bars) rats (nCi/g ± SEM, n = 7–8; ∗∗, P < 0.01; ∗, P < 0.05). (B) Illustrative autoradiogram showing increased Crhr1 expression in msP. (Scale bars, 1 mm.) Bregma = −3.0. (C) Elevated Crhr1 expression in msP rats was accompanied by increased density of CRH-R1 sites in numerous brain regions, shown with [125I]sauvagine autoradiography and using stressin 1 to mask R2 sites (black bars, msP; white bars, Wistar; n = 8 per group; mean ± SEM; ∗∗∗, P < 0.001; ∗, P < 0.05, Newman–Keul test). (D) Illustrative autoradiogram showing increased total [125I]sauvagine binding (CRH-R1 + CRH-R2) in msP rats. (Scale bar, 1 mm.) Bregma = +2 and −3.0 mm. CG, cingulate cortex; M1, primary motor cortex; S1, primary somatosensory cortex; N Acc, nucleus accumbens; BNST, bed nucleus of stria terminalis; CeA, central amygdaloid nucleus; MeA, medial amygdaloid nucleus; BLA, basolateral amygdaloid nucleus; BM, basomedial amygdaloid nucleus; ACo, anterior cortical amygdaloid nucleus; CA1, -3, and -4, Cornu Ammon areas of the hippocampal subregions 1, 3, and 4; DG, dentate gyrus; PVN, hypothalamic paraventricular nucleus.
Sequence Variation in the Promoter Region of Crhr1.
Two SNPs in the Crhr1 promoter region segregated msP and Wistar lines (−1836 and −2097 vs. first start codon of mRNA sequence, PubMed database accession no. NM_030999.1). These markers were in allelic identity, constituting two distinct allelic variants. One [G-G; wild type (wt)] is identical at the corresponding positions to the Rattus norvegicus reference sequence (Ensembl database no. ENSRNOG00000004900). All 20 Wistar subjects analyzed were wt/wt. The other allele carries A-to-A substitutions at these positions and represents a previously unrecognized variant (var) exclusively encountered in msP rats. At least one var copy was found in 17/20 msP rats analyzed (3 var/var, 14 var/wt, 3 wt/wt), yielding a highly significant association of allele frequencies with line (χ2 = 29.53, P < 0.00001) and suggesting that these Crhr1 variants segregated during selection for alcohol preference in msP animals.
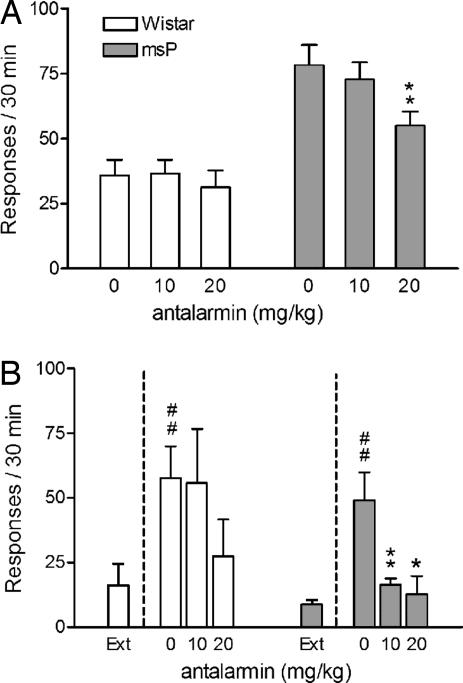
Suppressed Alcohol Self-Administration and Eliminated Reinstatement of Alcohol Seeking by the CRH-R1 Antagonist Antalarmin Selective to msP Rats.
Systemic antalarmin reduced alcohol responding in the msP line (Fig. 5A; F2,6 = 3.9, P < 0.05). Self-administration in Wistar rats was not affected by antalarmin (F2,7 = 0.2, not significant). Antalarmin eliminated reinstatement of alcohol seeking in msP rats evoked by 0.6-mA foot shock (Fig. 5B, F2,19 = 6.5, P < 0.001), a current intensity that evokes identical magnitude of reinstatement in msP and nonselected Wistar rats (see Fig. 2). On post hoc analysis both antalarmin doses eliminated stress-induced reinstatement in msP rats. In contrast, in Wistar rats, the 10 mg/kg dose was entirely ineffective, whereas a trend for suppression at 20 mg/kg failed to reach statistical significance.
Fig. 5.
Suppression of alcohol self-administration and blockade of stress-induced reinstatement of alcohol seeking in msP but not Wistar rats after treatment with the CRH-R1 antagonist antalarmin (10–20 mg/kg i.p.). (A) Ethanol self-administration rates (mean ± SEM responses on the active lever; Wistar, n = 8; and msP, n = 7). (B) Stress-induced reinstatement ethanol seeking after 15-min intermittent foot shock (0.6 mA; mean ± SEM of responses on the previously active lever). Treatment with antalarmin given 15 min before intermittent foot-shock stress blocked reinstatement of alcohol seeking in msP rats but not in Wistar rats. Extinction responses (Ext) represent the mean number of lever pressing during the last 3 days of extinction. Difference from extinction: #, P < 0.001. Difference between controls (0) and antalarmin-treated rats: ∗, P < 0.05 and ∗∗, P < 0.01.
Discussion
CRH is known to mediate behavioral stress responses through its central type 1 receptors (15). Alcohol is a potent antianxiety agent, and it has been shown that elevated anxiety predicts its use in rats (16), whereas involvement of CRH transmission in effects of stress on alcohol drinking has been demonstrated in nonfunctional Crhr1 mouse mutants (17). It has recently been proposed that long-term up-regulation of CRH transmission acquired through a prolonged history of alcohol use is central to developing and maintaining alcoholism (18). We show here that genetic variation associated with increased CRH-R1 expression can emulate this state even in the absence of a long drinking history. These data emerge just as human association findings suggest the Crhr1 locus to mediate genetic susceptibility for excessive drinking. Based on an analysis of 14 human Crhr1 polymorphisms, two haplotype tagging SNPs were recently identified and examined for association with drinking phenotypes. In an adolescent sample, association was observed with binge drinking, lifetime prevalence of alcohol intake, and lifetime prevalence of drunkenness; whereas in a sample of adult alcohol-dependent patients, an association was found with high amount of drinking (19). Together with these findings, our data suggest that CRH-R1 antagonists may be useful for treatment of alcoholism, and particularly so in carriers of susceptibility alleles at the Crhr1 locus, in analogy with data emerging for a functional variant of the μ-opioid receptor (20, 21). Of note, the CRH-mediated increase in behavioral stress sensitivity of msP rats was not accompanied by a corresponding up-regulation of hypothalamic-pituitary-adrenal-axis responsiveness to stress, in agreement with the notion that behavioral actions of CRH are mediated through separately controlled, extrahypothalamic CRH systems (15).
Our data highlight an important commonality that is emerging for the role of CRH in alcohol self-administration and relapse into alcohol seeking. Robust responding for alcohol is commonly achieved in nonselected Wistar rats without a history of dependence, but this basal level of self-administration is independent of the CRH system, as shown by our present antalarmin data as well as prior results (22). In contrast, excessive self-administration resulting from a prolonged history of dependence (23, 24) is fully reversed by CRH-receptor blockade (22). In the present study, a similar excessive self-administration is seen in msP rats in the absence of a history of dependence, due to genetic factors. It is also fully reversed by antalarmin and thus similarly driven by CRH signaling. The convergence of the acquired postdependent (22) and the genetically determined msP phenotype is further shown by increased levels of experimental anxiety in both models, also reversed by CRH antagonism in both cases.
Similarly, systemic antalarmin was ineffective in blocking reinstatement of alcohol seeking in Wistar rats without a history of dependence but fully blocked this behavior in the msP line, demonstrating an increased sensitivity of these animals to CRH-R1 blockade. Central administration of the CRH antagonist d-Phe-CRF12–41 has previously been reported to block shock-induced reinstatement in Wistar rats with a history of dependence (10). Together, these data indicate a common pattern for alcohol self-administration and stress-induced reinstatement of alcohol seeking. Both these behaviors appear to become sensitive to CRH-blockade only after a recruitment of the CRH system, either through preexisting genetic factors, as seen in the msP line, or through a history of dependence. An implication of this phenotypic convergence is that even when carefully characterized for stress- and anxiety-related intermediate phenotypes, clinical “alcoholism” may represent an aggregate of phenotypically similar but biologically distinct entities (phenocopies), only some of which reflect genetic susceptibility factors. A similar dilemma has been described for major depression (25).
The variant Crhr1 allele identified here was strongly enriched in the msP line and absent in Wistar rats. Additional studies will be required to determine whether the msP variant directly drives overexpression in this line or is merely in linkage disequilibrium with a functional locus. Furthermore, the msP–Wistar comparison may have some limitations for determining the extent to which behavioral traits of msP rats map to the Crhr1 locus, whereas an alcohol-avoiding line that is a direct counterpart to the msP line is not available. The choice of comparison line represents a challenge is several respects. An alcohol-avoiding phenotype after bidirectional selection may reflect enrichment of protective alleles at other loci rather than selection against a susceptibility allele that contributes to high preference. An attractive strategy to resolve this will be to selectively transfer the msP Crhr1 variant onto a Wistar genetic background.
The up-regulation of Crhr1 expression in msP rats lowered the threshold for reinstatement to subnormal levels. This effect of genetic variation at the Crhr1 locus represents a prototypical gene × environment interaction. The rate by which previously alcohol-reinforced responding is extinguished does not differ between msP rats carrying the variant allele and nonselected Wistar rats. Similarly, after extinction and in the absence of an environmental stimulus, no differences in alcohol seeking are seen between the two lines. It is only with the introduction of an environmental stressor that the functional role of the gain-of-function allele is revealed, as a lowered threshold and thus increased propensity for reinstatement.
In summary, genetically up-regulated activity of the CRH system confers susceptibility for excessive self-administration of alcohol and for relapse into alcohol seeking after abstinence. These data provide a functional validation for antagonism at CRH-R1 receptors as a mechanism for novel treatments aimed at relapse prevention in susceptible individuals.
Materials and Methods
Experimental Subjects.
Male msP rats (University of Camerino) were compared with outbred Wistar rats (Charles River, Sulzfeld, Germany), from which the msP line was derived. Subjects (350–400 g at the time of the experiments) were housed on a reverse 12-hour light/dark cycle (lights off at 0900 hours), at 20–22°C and 45–55% humidity, and with free access to tap water and food pellets. All procedures followed the EU Directive for Care and Use of Laboratory Animals.
Drugs.
Antalarmin was synthesized as described (26), dissolved in 20% Tween 80 and 80% distilled water, and injected i.p. 30 min before behavioral testing.
Open Field.
An open arena with a square white floor was enclosed by Plexiglass walls (MedAssociates, Georgia, VT). Interruptions of 10 equally spaced infrared light beams recorded total distance traveled, immobility, and number of entries in the central zone of the arena (a measure of anxiety). Animals (n = 7 per line) were tested for 10 min on day 1 (nonhabituated), and the same test was repeated on day 2 (habituated).
Fear Conditioning in msP and Wistar Rats.
Fear conditioning (n = 7 per line) was assessed as described (27) by using shock chambers with a camera for behavioral recording. Conditioning was for 30 min and 5 s and was performed by using five unsignalled 1-s foot shocks (1.0 mA) separated by 5-min blocks. After 24 h, rats were returned to the chamber and freezing to context was assessed for 8 min. In a separate experiment, pain thresholds were determined by using a hot plate test to control for possible bias from differential pain sensitivity between msP and Wistar rats. Animals (eight msP and seven Wistar) were placed on a metal plate kept at a constant temperature of 52.5 ± 0.6°C. Each animal was tested in five trials carried out every 20 min. Latency to first observable response to heat (paw licking or jumping) was measured, with a cutoff time of 40 s.
Ethanol Self-Administration and Stress-Induced Reinstatement of Alcohol Seeking.
Self-administration and reinstatement were studied as described (28). Briefly, for all these experiments, operant self-administration of 10% alcohol was first established by using a saccharin fading procedure and daily 30-min fixed ratio (FR) 1 sessions. After completion of saccharin fading, all animals were allowed daily sessions until a stable baseline of self-administration was achieved (15 days). At this point, either experiments to assess drug effect on self-administration were initiated or responding for alcohol reinforcement was extinguished over an additional 15 days, for subsequent assessment of shock-intensity or drug effects on reinstatement. Reinstatement tests began 1 day after the last extinction session. Foot shock was administered via the grid floor of the chamber under a variable-interval 40-s schedule (interval range: 10–70 s). After termination of foot shock, the levers were extended into the chambers and responses on the previously alcohol-associated lever were recorded for 30 min. Reinstatement sessions were conducted under conditions identical to those during extinction.
To assess effects of antalarmin on self-administration, a within-subject design was used. msP (n = 7) and Wistar (n = 8) each received 0 (vehicle), 10, and 20 mg/kg of antalarmin in a Latin square counterbalanced design, with experiments every 4 days.
To determine the relationship between shock intensity and reinstatement, a between-subjects design was used. Animals were divided into six groups (three msP and three Wistar groups; n = 8–10 per group) to receive 0.3, 0.6, or 1.0 mA of intermittent electric foot shock, respectively.
To evaluate the ability of antalarmin to block shock-induced reinstatement, a between-subjects design was also used. Three groups of msP (n = 7–8 per group) and three groups of Wistar (n = 7 per group) rats were treated with vehicle or antalarmin (10 or 20 mg/kg) and 30 min later, reinstatement of alcohol-seeking induced by foot-shock stress was studied. In this experiment, a 0.6-mA current intensity was used because the assessment of reinstatement as a function of shock intensity had showed that at this level, reinstatement in msP and Wistar rats does not differ.
Endocrine Response to Foot Shock.
CORT levels were measured by obtaining tail tip blood 30 min and 4 h after exposure to foot shock. Samples in triplicates were processed for CORT enzymatic immunoassay (EIA) according to the manufacturer's instructions (Cayman Chemicals, Ann Arbor, MI).
Screening for Differential Gene Expression.
In situ hybridization was performed by using riboprobes described in Table 1 (which is published as supporting information on the PNAS web site). Alcohol-naïve msP and Wistar rats were used (n = 8 per group). Procedures were as described in ref. 29. Rats were killed between 7 and 10 h into the light cycle, and brains were quickly removed, snap frozen in −40°C isopentane, and stored at −70°C until use. Brain sections (10 μm) were taken at Bregma levels (i) +2.5 to + 1.7 mm, (ii) −0.3 to −0.4 mm, (iii) −1.7 to −2.0 mm, and (iv) −2.3 to −3.3 mm according to the atlas of Paxinos and Watson (30). The hybridized sections were exposed to BAS-5000 PhosphorImager plates (Fuji, Tokyo, Japan). PhosphorImager-generated digital images were analyzed by using AIS Image Analysis Software (Imaging Research Inc., St. Catharines, Ontario, Canada). Regions of interest were defined by anatomical landmarks as described in the atlas (30). Based on the known radioactivity in the 14C standards, image values were converted to nCi/g. For detailed visualization, slides were subsequently exposed for 1 month to Kodak BioMax MR film (Eastman Kodak Company, Rochester, NY).
Analysis of Crhr1 Sequence Variation.
The promoter region of Crhr1 was sequenced in 20 msP and 20 nonselected Wistar rats. DNA was isolated from whole blood by using the DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Six primer pairs were designed to amplify ≈2 kb of the promoter and 400 base pairs of the 3′ UTR of the Crhr1 gene. Primer sequences and PCR parameters are given in Table 2 (which is published as supporting information on the PNAS web site).
CRH-R1 Receptor Binding.
Receptor autoradiography using [125I]sauvagine was carried out as described with minor modifications (31, 32).
Statistical Analysis.
Behavioral experiments and endocrine response were analyzed by ANOVA followed by Newman–Keuls test for post hoc analysis. For visualization of the in situ hybridization screen for differential gene expression, the SES was used. For each contrast, SES = t2df/t2df + (df), where df = N − 2 (n = total number of observations). In a multiple-factor ANOVA, SES for a factor represents the proportion of variance explained by that factor (33). SES was defined as positive in cases of increased expression and negative in cases of decreased expression in msP vs. Wistar rats. A SES of ±0.25 was used as threshold for visualization. Crhr1 gene expression data deviated from homogeneity of variances (Levene's test, F29,201 = 5.5, P < 0.001), and were analyzed by Kruskal–Wallis nonparametric ANOVA for difference between the two lines, followed by post hoc test. CRH-R1 binding data had homogenous variances, and standard two-way ANOVA with line and brain region as factors was carried out and followed by post hoc tests. To test for line differences in Crhr1 allele frequencies, a Yates corrected χ2 test was used.
Supplementary Material
Acknowledgments
This work was supported by funding from the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program and the European Community (TARGALC consortium Grant QLRT-2001-01048).
Abbreviations
- msP
Marchigian–Sardinian Preferring
- CRH-R1
corticotropin-releasing hormone receptor 1
- CORT
corticosterone
- SES
standardized effect size.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Goldman D, Oroszi G, Ducci F. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 3.Dackis CA, O'Brien CP. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- 4.Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Am Psychol. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- 5.Heilig M, Egli M. Drug Discovery Today: Disease Models. 2005;2:313–318. [Google Scholar]
- 6.Heilig M, Egli M. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Ciccocioppo R, Hyytia P. Addict Biol. 2006;11:193–194. doi: 10.1111/j.1369-1600.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- 9.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. Psychopharmacology (Berlin) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Weiss F. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 12.Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- 13.Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M. Psychopharmacology (Berlin) 1999;144:151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- 14.Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrichs SC, Koob GF. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 16.Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Psychopharmacology (Berlin) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 17.Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- 18.Valdez GR, Koob GF. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, et al. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 20.Bond C, LaForge KS, Tian MT, Melia D, Zhang SW, Borg L, Gong JH, Schluger J, Strong JA, Leal SM, et al. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 22.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 23.Rimondini R, Arlinde C, Sommer W, Heilig M. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 24.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS, Thornton LM, Gardner CO. Am J Psychiatry. 2001;158:582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- 26.Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 27.Bast T, Zhang WN, Feldon J. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- 28.Ciccocioppo R, Martin-Fardon R, Weiss F. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- 29.Hansson AC, Sommer W, Rimondini R, Andbjer B, Stromberg I, Fuxe K. J Neurosci. 2003;23:6013–6022. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 31.Primus RJ, Yevich E, Baltazar C, Gallager DW. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 32.Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. J Pharmacol Exp Ther. 1998;286:459–468. [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.