Abstract
The homeobox transcription factors Engrailed-1 and Engrailed-2 are required for the survival of mesencephalic dopaminergic neurons in a cell-autonomous and gene-dose-dependent manner. Because of this requirement, the cells die by apoptosis when all four alleles of the Engrailed genes are genetically ablated (En1−/−;En2−/−). In the present study, we show that viable and fertile mice, heterozygous null for Engrailed-1 and homozygous null for Engrailed-2 (En1+/−;En2−/−), have an adult phenotype that resembles key pathological features of Parkinson's disease. Specifically, postnatal mutant mice exhibit a progressive degeneration of dopaminergic neurons in the substantia nigra during the first 3 mo of their lives, leading to diminished storage and release of dopamine in the caudate putamen, motor deficits similar to akinesia and bradykinesia, and a lower body weight. This genetic model may provide access to the molecular etiology for Parkinson's disease and could assist in the development of novel treatments for this neurodegenerative disorder.
Keywords: dopamine, midbrain, mouse mutant, neurodegenerative disease, substantia nigra
Mesencephalic dopaminergic (mesDA) neurons are the main source of dopamine in the mammalian central nervous system (1). They are located in three distinct nuclei, the substantia nigra pars compacta (SN), the ventral tegmentum (VTA), and the retrorubral field (RRF). Their main innervation targets are the basal ganglia, where they play a major role in controlling emotion, motivation, and motor behavior (2, 3), recognized by their involvement in schizophrenia, addiction, and most prominently in Parkinson's disease (PD; ref. 4). PD is a multisystemic degenerative disorder of the central and peripheral nervous systems. Its hallmark is the progressive degeneration of DA neurons in the SN. The clinical symptoms are resting tremor; muscular rigidity; postural instability; hyposomia; a positive response to the application of l-3,4-dihydroxyphenylalanine (l-DOPA); and the presence of cytoplasmatic inclusions (Lewy bodies) in postmortem brains (5). Additionally, PD patients are often characterized by weight loss starting before diagnosis and continuing with disease progression (6, 7).
The homeodomain transcription factors Engrailed-1 and -2 (En1 and En2) are expressed by all mesDA neurons soon after differentiation and then continuously into adulthood. They are cell-autonomously and in a gene-dose-dependent manner required for the survival of this neuronal population, leading to the complete loss of the cells in mutant mice homozygous null for both genes (En1−/−;En2−/−; refs. 8 and 9). In their function as survival factors for mesDA neurons, the two genes are redundant and can compensate for each other (9, 10). The single-null mutants for either En1 (En1−/−) or En2 (En2−/−) show no significant alterations in the organization of the mesDA system at birth, but the mice with genotypes intermediate to the single and double mutants are distinctively different from each other. Whereas En1−/−;En2+/− mice die at postnatal day 0 (P0) and exhibit a large loss of mesDA neurons, En1+/−;En2−/− (EnHT hereafter) mice are viable and fertile and show a wild-type mesDA phenotype at birth.
Results
Specific Loss of Nigral Dopaminergic Neurons in Engrailed Mutant Mice.
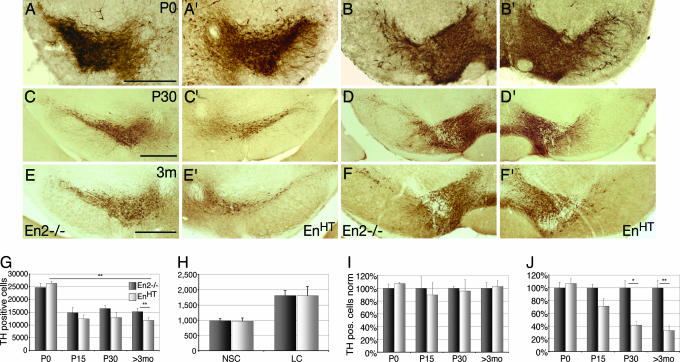
To determine the role of the Engrailed genes in mature animals, we examined the mesDA system in mice with different Engrailed genotypes at various postnatal ages. In accord with our previous reports, En1−/−, En1−/−;En2+/−, and En1−/−;En2−/− mice die at birth (8, 9) and show a gene-dose-dependent reduction of mesDA neurons (9). Mice of other Engrailed genotypes are viable and fertile. Among these, En1+/−, En1+/−;En2+/−, En2+/−, and En2−/− mice displayed a wild-type-like distribution of the neurons at all ages examined (data not shown; for En2−/−; see Fig. 1E and F). In contrast, EnHT mice showed a specific loss of DA neurons in the SN (Fig. 1E′), whereas the VTA and RRF were unaffected (Fig. 1F′). Because the distribution of mesDA neurons was normal in En2−/− mice, we continued our analysis using En2−/− mice as control littermates. By using stereological methods (11), we counted the tyrosine hydroxylase (TH)-positive cell bodies in the adult SN, VTA, and RRF separately (12). These counts showed that En2−/− mice were equivalent to wild-type C57/Bl6 (data not shown), whereas the number of nigral DA neurons in EnHT mutants was, on average, 67.2% lower (Fig. 1I). In contrast, no significant difference between genotypes was found in VTA and RRF (Fig. 1J).
Fig. 1.
Gradual postnatal loss of DA neuron in substantia nigra. (A–F and A′–F′) TH immunostaining on coronal brain section at P0 (A, A′, B, and B′), P30 (C, C′, D, and D′), and 3 mo (E, E′, F, and F′) of En2−/− and En1+/tLZ;En2−/− (EnHT) mouse mutants on the level of the substantia nigra (A, A′, C, C′, E, and E′) and the VTA (B, B′, D, D′, F, and F′). (G–J) Cell count of DA neurons in ventral midbrain (G, I, and J) and noradrenergic neurons in locus coeruleus (LC) and nucleus subcoeruleus (NSC) (H) (n ≥ 4 for ages <3 mo; n = 12 for 3 mo and older). (I) VTA and RRF. (J) SN. (Error bars, standard error; ∗, P < 0.01; ∗∗, P < 0.001.) (Scale bars: 500 μm.)
To determine the age at which neuronal degeneration sets in, we extended our analysis to mice of different postnatal ages starting at birth. At postnatal day 0 (P0), the mesDA system of EnHT mice was unaltered, neither the distribution of the cells (Fig. 1 A and A′) nor their numbers (Fig. 1G) were significantly different from their En2−/− littermate controls. In total, we counted 26,349 ± 805 and 24,715 ± 1,734 TH-positive neurons in the ventral midbrain of EnHT and En2−/− mice, respectively; separated into individual nuclei, we found 5,582 ± 384 and 5,223 ± 464 in the SN, 17,302 ± 405 and 15,733 ± 1,163 in the VTA, and 3,465 ± 225 and 3,759 ± 525 in the RRF, respectively. As in wild-type mice, both genotypes showed a decline in the numbers of mesDA neurons in the next 14 days (13); however, in the EnHT mice, this normal cell death overlaps with an additional genotype-associated loss of TH-positive cells reaching in total 16% at P15 (12,463 ± 1,496 in EnHT mice and 14,819 ± 2,109 in En2−/− control littermates; P = 0.05). In En2−/− mice, like in wild type, the numbers of mesDA neurons stabilized at this age. In the EnHT mice, however, the numbers continued to decrease until ≈3 mo after birth. From this age onward, the mutants had on average 32.6% fewer mesDA neurons than their En2−/− littermate controls (11,226 ± 935 for EnHT vs. 16,645 ± 1,786 for En2−/− mice; P = 0.006; Fig. 1G). Separate counts of SN, VTA, and RRF revealed that, at all ages (the oldest animal counted was 22 mo), cell loss was restricted to the SN (Fig. 1 I and J). In particular, the average number of DA neurons was 1,882 ± 708 and 6,115 ± 1,008 (−69.2 ± 11.6%, P = 0.002) in the SN and 9,766 ± 1,471 and 10,530 ± 1,234 in VTA and RRF of EnHT and En2−/− mice, respectively. (For a two-factorial ANOVA considering genotype and age, see Supporting Text, which is published as supporting information on the PNAS web site.) During the phase of cell loss, the mutants showed a high variance between individuals of the same age and sometimes even an asymmetric cell loss between the left and right hemispheres.
Data from our previous prenatal in vivo and in vitro studies had demonstrated that loss of mesDA neurons in Engrailed double- mutant embryos is a cell-autonomous function of the two transcription factors and not a result of deficiencies in the surrounding tissue (8, 14). To provide evidence that the EnHT phenotype also reflects a cell-autonomous function of En1 and En2, we examined the noradrenergic neurons of the locus coeruleus in the mutants. These neurons do not express the Engrailed genes but depend on them for their development during embryogenesis (14), suggesting they are sensitive to morphological alterations in mid- and hindbrain. We found that neither the locus coeruleus nor the neighboring nucleus subcoeruleus was affected in EnHT mutants. In particular, we counted 1,809 ± 302 and 1,813 ± 156 noradrenergic neurons in the locus coeruleus and 969 ± 101 and 984 ± 65 in the nucleus subcoeruleus of EnHT and En2−/− animals, respectively (Fig. 1H).
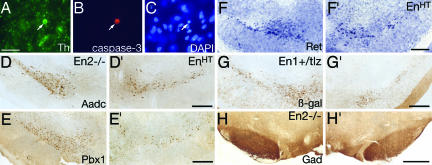
It was possible that nigral DA neurons were not detectable in EnHT mice by immunolabeling against TH due to a change of their neurotransmitter phenotype. To elaborate on this possibility, we first investigated whether there were signs of cell death in nigral DA neurons; during the postnatal stages, they were most rapidly disappearing but were beyond the age of naturally occurring developmental cell death due to a glial cell-derived neurotrophic factor requirement (15). In the SN of P20 EnHT mutants, we typically detected ≈12–15 very small rounded TH-positive cell bodies, which were labeled against activated caspase-3 and showed pyknotic nuclei (Fig. 2A–C). In the littermate control, the amount of TH-positive apoptotic figures was never more than three. Despite the presence of these apoptotic cells, we could not detect signs of activated astrocytes when using a glial fibrillary acidic protein antibody (data not shown). Second, we examined the expression pattern of seven additional genes expressed by mesDA neurons: Pitx3, Pbx1, Aadc, Ahd2, Ret, α-Synuclein, and γ-Synuclein (8, 16–18). Third, we stained against the En1 reporter β-Gal (19) and compared EnHT with En1+/tLZ;En2+/+ mice. Fourth, we performed a Nissl staining, which is often used in model systems for PD. The expression analysis and histological stain revealed a normal distribution of neurons in the VTA and RRF and a loss of cell bodies in the SN comparable with that seen after TH immunohistochemistry (for a selection of these markers, see Fig. 2 D–G). Furthermore, to investigate whether the loss of nigral DA neurons is a result of a general morphological alteration, which we could not detect with Nissl staining, we analyzed the GABAergic population in the SN, which is located just ventral to nigral DA neurons and does not express En1 or En2 (20, 21). The GABAergic cell bodies and axons were normal in EnHT mice (Fig. 2H). Together, the immunohistochemical analysis and the histological stain show that nigral DA neurons of EnHT mice die by apoptosis and do not convert their neurotransmitter phenotype. Furthermore, the En1taulacZ expression pattern in an En2 wild-type background (En1+/tLZ;En2+/+; Fig. 2G) revealed that the functional deletion of one En1 allele is necessary but not sufficient to cause the loss of nigral DA neurons, and that En2 also plays an essential role.
Fig. 2.
Cell loss by apoptosis and no change in neurotransmitter phenotype. (A, B, and C) Coronal section at level of SN of P20 EnHT mouse stained against TH (green), activated caspase-3 (red), and DAPI (blue). The nigral DA neuron shows signs of apoptosis, a small rounded cell body, activated caspase-3, and a pyknotic nucleus (arrows). (D–H) Immunostaining against aromatic amino acid decarboxylase (Aadc) (D), Pbx1 (E), the En1-reporter β-Gal (G), and glutamate decarboxylase (Gad; H), and in situ hybridization against Ret (F), all on adult coronal brain sections of En2−/− (D–F, H), En1+/tLZ;En2−/− (EnHT) (D′–H′) and En1+/tLZ mice (G). Aadc, Pbx1, Ret, and β-Gal expression reveal all loss of nigral DA neurons in EnHT mice, whereas the Gad staining is the same in both genotypes (H). [Scale bars (A–C): 20 μm; (D–H): 500 μm.]
Molecular Changes in Basal Ganglia of EnHT Mice.
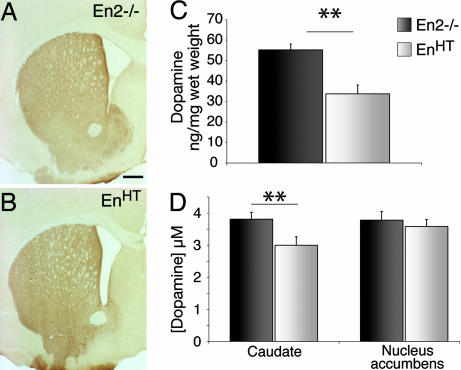
Because deficiencies in the nigral DA system are associated with defects in their main innervation target, the caudate putamen (CPu), we investigated adult EnHT mice (>3 mo) for anatomical and molecular changes in the CPu. Despite the loss of almost 70% of nigral DA neurons, we did not see marked differences in the density of TH-positive terminals by immunohistochemistry (Fig. 3A and B). To obtain a more accurate picture, we measured dopamine levels by HPLC. We found that the amount of dopamine in the striatum was significantly lower in EnHT mice (−38.9 ± 5.1%, P = 0.001) than in their En2−/− littermate controls (Fig. 3C). Furthermore, when we measured dopamine release in striatal slices by fast-scan cyclic voltametry (22), the amount of dopamine discharged upon a defined stimulus in the CPu was 21% lower in slices from EnHT animals (3.0 ± 0.28 and 3.8 ± 0.23 μM; P = 0.001 for EnHT and En2−/− mice, respectively). As expected from the cell counts, the dopamine release in the nucleus accumbens, which is innervated by VTA neurons, was unaltered (Fig. 3D).
Fig. 3.
Molecular changes in dorsal striatum. (A and B) TH immunostaining of basal ganglia showing no significant difference between genotypes. (C) Dopamine content in the dorsal striatum measured by HPLC (n = 6). (D) Dopamine release measured by cyclic voltametry (n = 6). (Scale bars: 500 μm; error bars, standard error; ∗∗, P < 0.001.)
A loss of DA innervation to the CPu had been shown to result in molecular changes in GABAergic output circuits (23). We therefore measured the expression levels of genes specific to the direct (substance P and dynorphin) and indirect pathways (dopamine receptor 2), All three genes were expressed significantly lower in EnHT than in En2−/− mice (53.1 ± 11.8%, P = 0.011; 55.2 ± 8.1%, P = 0.003, and 54.3 ± 6.3%, P = 0.001, respectively; n ≥ 8). Taken together, these data demonstrate that the loss of DA neurons in the SN results in diminished storage and release of dopamine in the CPu and in concomitant molecular changes in GABAergic neurons deprived of that input.
Motor Performance in EnHT Mice.
To determine the behavioral consequences in EnHT mice, we conducted a battery of tests that evaluate motor coordination, strength, balance, and locomotion. Some of these tests had previously been used to assess motor performance in rodent models for PD. Specifically, the mice were assessed in the following tests: open field, hang and grid performances, swimming, horizontal ladder, rotorod, stride, and cylinder (24–27). In addition, we examined the mice on two tasks that have cognitive components associated with them: the Morris water task and the elevated plus maze. It had previously been shown that the loss of En2 causes alterations in the cerebellar organization (28) and an impairment of motor learning performance (29). Therefore, to limit our analysis to behavioral changes that may be associated with the loss of nigral DA neurons, we compared EnHT mice to their En2−/− littermates and not to wild type. Furthermore, because we did not observe any change in the numbers of nigral DA neurons after 3 mo of age (Fig. 1i), we chose to conduct the tests in animals of 8 mo and again at 18 mo of age to evaluate whether they exhibited an age-related decline in performance. The swimming performance and horizontal ladder tests were adopted only after the tests at 8 mo had been completed.
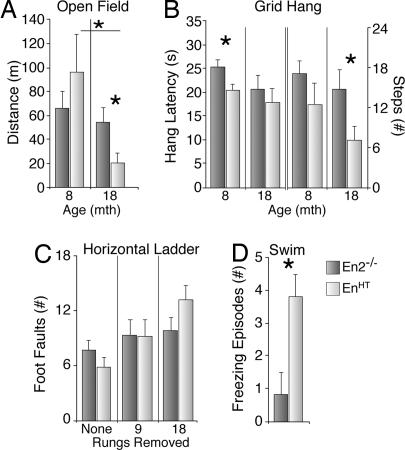
EnHT mice were impaired, relative to their En2−/− littermates, in four of the 10 tests that we used (Fig. 4). In the open field test, a general assessment of locomotor and exploratory behavior, EnHT mice were not impaired at 8 mo; however, by 18 mo of age, they showed a significant reduction in forward locomotion compared with En2−/− littermate controls (−63%; P < 0.05) and to their own performance at 8 mo (−79%; P < 0.05), whereas in En2−/− mice, locomotion was not significantly altered (−18%; Fig. 4A). In the grid/hang performance tests, which provides an index of motor strength in combination with locomotor ability, EnHT mice were able to hang from the inverted grid for a significantly shorter duration than their En2−/− littermate controls at 8 mo of age (P < 0.05) and, although there was no significant difference in hang time at 18 mo of age, the number of steps they took while hanging was significantly reduced (−52%; P < 0.05) relative to En2−/− mice (Fig. 4B).
Fig. 4.
Behavioral phenotype. (A) Open field test. En1+/−;En2−/− (EnHT) mice exhibited reduced locomotor activity at 18 mo of age, but not at 8 mo. (B) Grid hang test. Eight-month-old EnHT mice were not able to hang onto the mesh grid as long as their En2−/− counterparts (Left). Although this effect was less pronounced at 18 mo, at this age, the locomotor activity of EnHT mice was reduced by more than half (Right). (C) Horizontal ladder test. Although there were performance decrements as more rungs were removed, the performance of EnHT mice was not significantly impaired relative to En2−/− littermates. (D) Swimming test. EnHT mice exhibited four times more bouts of freezing than the controls (n ≥ 8 for all tests). Error bars, standard deviation; ∗, P < 0.05.
We did not observe significant differences between groups in the four remaining behavioral tests of motor coordination. The EnHT mice performed increasingly worse in the horizontal ladder test as more rungs were removed; however, their performance was not statistically different from that of En2−/− mice (Fig. 4C). Furthermore, although both groups showed a significant age-related decline in their ability to stay on the rotating spindle during the rotorod task, there were no between-group differences (data not shown). Finally, we found no differences between groups when we assessed rearing behavior in the cylinder task or measured forelimb and hindlimb stride lengths at either 8 or 18 mo of age (data not shown).
The performance of EnHT and En2−/− mice was also compared in two tests that provide indices of nonmotor behavior. We found no difference between or within groups across time in performance on the elevated plus maze, a measure of anxiety-related behavior. In the Morris water task, a test of spatial learning, 8 mo-old EnHT mice required significantly more time to find the hidden platform than their En2−/− littermates. However, we observed that the EnHT mice exhibited long periods of time when they would freeze in the water and just float. When the animals were startled with an auditory stimulus each time they stopped swimming, EnHT and En2−/− littermate controls performed equally well (data not shown). To more specifically investigate this behavioral deficit, we examined the animal's swimming performance in a water-filled alley. Although we observed no asymmetries in paw use or differences in forepaw inhibition, EnHT mice exhibited four times as many episodes of freezing compared with En2−/− littermates (Fig. 4D).
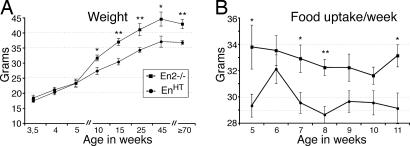
Weight Reduction During Nigral Degeneration.
Weight loss is a common feature among PD patients (6, 7). Although the cause is still debated, gastrointestinal dysfunction had been suggested due to the often-occurring degeneration of the enteric nervous system during disease progression (30). It is plausible, however, that the diminished DA innervation to the CPu is the cause for the weight loss, because patients with subthalamic deep-brain stimulation gain weight after onset of treatment (31, 32). To determine whether the degeneration of the nigrostriatal system in EnHT mice is accompanied by lower weight, we recorded the body weight of the mutants and their En2−/− littermate controls over their entire lifespan (Fig. 5). For the first 5–6 wk after birth, the weight was the same for both genotypes; however, thereafter the rate of weight gain slowed down in EnHT mice, and weight differences became apparent. From 15 weeks onward, EnHT mice weighed ≈16–20% less than their En2−/− littermate controls (80.2 ± 1.1%; P = 0.001 for 15-wk-old animals; Fig. 5A). To examine whether the weight disparity is caused by lower food uptake, we measured food consumption biweekly between 5 and 11 wk after birth. During this period, EnHT mice consumed significantly less food per day than the En2−/− controls (29.1 g ± 0.81 and 32.2 g ± 0.83, respectively; P = 0.0031; Fig. 5B). Because the lower weight uptake of EnHT animals is correlated to the loss of nigral DA neurons, and the Engrailed genes are not expressed by the enteric nervous system (20), it is likely that the lower weight of EnHT mutants is the result of the DA depletion in the CPu, but other causes cannot be excluded.
Fig. 5.
Body weight and food uptake. Charts representing weight and food uptake in EnHT and En2−/− littermates over time. (A) EnHT mice are not subject to the same weight gains as their En2−/− littermate controls. The x axis is skewed toward the first postnatal weeks to emphasize difference between early postnatal and adult phases. (B) Weekly food uptake per mouse. EnHT mice eat significantly less per week than their littermate control. Each data point reflects the average from measurements of at least eight mice, sometimes up to 44. Error bars, standard error; ∗, P < 0.01; ∗∗, P < 0.001.
Discussion
We provide evidence that partial deletion of the Engrailed genes in mice leads to a selective and progressive degeneration of nigral DA neurons over the first 3 mo of their lives. This loss, which does not affect other DA groups in the mesencephalon, results in reduced storage and release of dopamine in the CPu, molecular changes in the GABAergic output neurons, motor deficits, and lower body weight. The phenotype of these mutant mice resembles key pathological features of PD.
Because EnHT mice are not the result of a genetic deletion targeted exclusively to mesDA neurons, it is possible that the deficiencies in other neuronal populations and not the reduced dopamine signaling in the CPu causes the altered motor behavior and the weight loss. However, the options are limited. Because all results were obtained by a comparison between EnHT and En2−/− mice, it is unlikely that neurons expressing only En2, like the cerebellar granule cells, contribute to the phenotype (20). En1 is expressed in the postnatal brain by a subset of Bergmann glia in the cerebellum, by radial glia in the ventral midbrain, and by undefined cell types in superior and inferior colliculus (9). Furthermore, it is transiently expressed by a subset of interneurons in the spinal cord (33), which control the speed of the vertebrate locomotor outputs. The latter could be a cause for the altered motor behavior; however, an En1-heterozygous phenotype was not been reported for these interneurons (19). Alternatively, the phenotype of EnHT mice could be linked to the early embryonic function of En1. It participates in the regionalization of the neural tube in a gene-dose-dependent manner during early embryogenesis, leading in Engrailed double mutant mice to different degrees of deletion in mid-/hindbrain tissue correlated to the allelic composition (34). The brain morphology of EnHT and En2−/− were not apparently different, and the number of noradrenergic neurons in the locus coeruleus was the same, suggesting that the phenotype of EnHT mice is not related to the early embryonic function of En1. However, this needs further investigation.
The specific loss of nigral DA neurons in EnHT mice during the first 3 postnatal mo is a unique feature not found in other genetic models for PD. In Tgfα-null mutants (35) and Aphakia (Pitx3−/−) mice (17), the loss is also specific to nigral DA neurons but occurs in the embryo and is already completed before birth. In Weaver mutant mice, a gain-of-function mutation of the inward-rectifying potassium channel Girk2 (36) results in an unspecific opening of the channel and influx of sodium ions. In these animals, degeneration is a postnatal event but affects also VTA and RRF (37). In strong contrast to EnHT mutant mice, cell loss is also observed in other neuronal populations that express Girk2. For example, the cerebellar granule cells fail to differentiate and die before they migrate into deeper layers of the cerebellum (38). Despite strong expression of En2 in granule cells, this cell population appears normal in EnHT mutants, with the exception of a small alteration in the organization of the cerebellar folia (28).
Adult EnHT mice exhibit a larger reduction in nigral DA neurons than idiopathic PD patients (39); however, their motor deficits are less severe. It is possible that significant differences in the neural circuits exist between rodents and humans, but alternatively, young mice may be able to activate compensatory mechanisms that are lost with age. This is supported by previous findings that young adult mice recover from 1-methyl-4-phenyl-1,3,6-tetrahydropyridine (MPTP) intoxication, whereas older animals do not (40). How the EnHT mice compensate for the loss of nigral DA neurons still needs to be addressed; however, the proportionally smaller reduction in dopamine content and release suggests that the remaining nigral DA neurons counteract the disappearance of their neighbors. On the other hand, the unexpected down-regulation of the dopamine receptor 2 in the CPu, which is in contrast to the results obtained after acute experimental dopamine depletion (23), suggests that the adaptation could happen through changes in the GABAergic output neurons.
The loss of DA neurons in the SN but not in the VTA and the RRF, even though they all express the two Engrailed genes, indicates that the lowered En1 expression in EnHT mice exposes a distinct vulnerability of nigral DA neurons. This vulnerability is likely an innate Engrailed-independent property of this cell population, because PD pathogenesis in the mesencephalon (41) and toxin-based experimental models also shows a preferential susceptibility to degeneration of nigral DA neurons (42). These similarities suggest that detailed examination of the underlying molecular mechanism that leads to the cell death in the Engrailed mutants could improve our understanding of the molecular etiology of PD.
Materials and Methods
Animals.
The generation of the En2-null mutant and the En1tau-LacZ knockin mice were previously described (19, 43). In all experiments, the En1-null allele is En1tau-LacZ. The En2 mutants with an original mixed genetic background of 129 and Swiss–Webster were crossed three times into a C57/BL6 background. The line was bred as En1+/tlZ;En2−/− at the central animal facility, University of Heidelberg.
Immunohistochemistry and in Situ Hybridization.
All immunostainings and in situ hybridizations were performed as described (8, 9). The following additional antibodies were used: rabbit antiglutamate decarboxylase 65 and 67 (Gad; AB1511) and rabbit antiglial fibrillary acidic protein (MAB360), both from Chemicon (Hampshire, U.K.).
Estimation of Cell Number in the Mesencephalon.
To count TH-positive cells, a computer-assisted image system was used. The counting area was depicted at low magnification (×5), and SN, VTA, and RFF were separated by using exact anatomical criteria (12). These criteria can be imagined in a simplified but almost accurate manner by laying a line from the most lateral tip of the SN to the most ventral point of the midline of the brain section. Cells ventral to this line are counted as SN and in dorsal position as VTA. SN and RRF were distinguished by a morphological gap in rostral to caudal direction separating them. The number of TH-positive cells was estimated with an optical fractionator probe (Stereoinvestigator 5.04 MicroBrightField, Williston, VT). The settings were determined empirically; the grid size was set at 100 μm, and the sampling size was 30 × 30 μm. Sections were sampled with a frequency of 2. Coefficient of error (<0.1) was taken into account to validate each estimate. In the case of the noradrenergic neurons in the locus coeruleus and nucleus subcoeruleus, we did not estimate the numbers with the fractionator probe; instead, we blindly counted each cell.
HPLC.
To measure the dopamine content, we dissected the dorsal striatum from the nucleus accumbens and olfactory tubercle and analyzed the tissue for dopamine content by reverse-phase HPLC, as described (44).
Quantitative RT-PCR.
Quantitative RT-PCRs were performed with a 7000 Sequence Detection System from Applied Biosystems (Foster City, CA) by using preformulated assays on-demand and calculating the results with the comparative computed tomography method. The assays had the following identification tags: Mm00436880_m1 for substance P, Mm00438541_1020;m1 for dopamine receptor 2, and Mm00457572_1020;m1 for dynorphin; as standard control, Mm00507222_1020;s1 ribosomal protein S18, Mm00435617_1020;m1 for phosphoglycerate kinase 1 (Pgk1), and Mm00446973_1020;m1 for TATA box-binding protein (Tbp). The dissected striata were homogenized and the RNA isolated and reverse-transcribed by using random hexamers to initiate transcription. Each individual PCR was done in three biological replicates, and at least two of three standard controls were run in parallel each time.
Fast-Scan Cyclic Voltametry in Brain Slices.
DA overflow was measured in 300-μm-thick coronal striatal slices from EnHT mice and En2−/− controls (4–6 mo old). Animals were anesthetized with isoflurane and decapitated and the brains rapidly removed and kept in ice-cold high-magnesium artificial cerebrospinal fluid (aCSF) containing 124 mM NaCl, 3.3 mM KCL, 1.2 mM KH2PO4, 2.6 mM MgSO4, 2.5 mM CaCl2, 20 mM NaHCO3, and 10 mM glucose, saturated with 95% O2− 5% CO2. Slices were cut with a vibratome (Leica VT1000S, Nussloch, Germany) and kept after preparation at room temperature in normal magnesium aCSF (1.3 mM MgSO4). Slices were then transferred to a submerged recording chamber, where they were perfused at 2–3 ml/min aCSF at room temperature. DA release was evoked by a single pulse (0–500 μA, 300 μsec) applied through a bipolar stimulation electrode (bipolar stainless steel, 100 μm, insulated except at the tip) every 10 sec. DA was detected with 5-μm carbon-fiber electrodes (ALA Scientific Instruments, Westbury, New York) by using fast-scan cyclic voltametry (22). Cyclic voltamograms (ramps from −500 to +1,000 mV and back to −500 mV at 300 V per sec) were repeated every 100 ms by using an EPC9 amplifier (HEKA Electronic, Lambrecht, Germany). The background current obtained before release was subtracted from the current measured after release. The oxidation current of dopamine (between 500 and 700 mV) was converted to DA concentration by electrode calibration at the end of the experiment by using 0–10 μM DA as standards. Group averages were compared by using Student's t test.
Behavioral Studies.
Except for the open-field and the water maze tests, behavioral activity was videotaped and analyzed by using video replay. Behavior in the open field and water maze tests was recorded and quantified by using an automated video tracking system. With the exception of the limb-use asymmetry and stride tests, behavioral testing was performed once per day for 5 days consecutively. All tests were performed during the animal's light cycle.
Open field test.
A circular area (1.55-m diameter, 0.46-m high) constructed of white fiberglass served as the open field arena. The mice permitted to freely explore for 10 min while an image tracking system (HVS Image, Hampton, U.K.) recorded total distance traveled, average walking speed, and percent time spent in the central, intermediate, and peripheral regions. The arena was cleaned between trials with 70% ethanol and distilled water.
Swim task.
Swimming behavior was assessed in a glass-fronted alley (1.2 m × 8.5 cm) filled with 21°C water to a depth of 43 cm. The mice were placed in one end of the alley, and their behavior was video-recorded as they swam to a wire-mesh exit ramp at the other end. Swim latency, limb-use asymmetry, episodes of forepaw disinhibition, and freezing behavior were quantified. Freezing was defined as motionless floating for a minimum duration of 3 sec.
Rotorod test.
An automated rotorod (Rotomex Rotorod 950, Columbus Instruments, Columbus, OH) was used to assess motor coordination and balance. Mice were individually placed on the spindle, whereupon it began rotating at 6 rpm and accelerated linearly to 30 rpm over 2 min. The latency to fall off the rod was recorded.
The hang test, grid performance test, Morris water task, horizontal ladder test, stride length test, elevated plus maze, and cylinder task are described elsewhere (24–27, 45, 46).
Body Weight and Food Consumption.
Male mice, which were provided with food ad libitum, were weighed at different ages after weaning until senescent ages (17 mo). The average and standard error were calculated for all groups of ages. For measuring food consumption, the animals were individually housed and the food pellets biweekly weighed.
Statistical Analysis.
Values are expressed as mean ± standard error. Differences between means were analyzed by using a paired two-tailed Student's t test. For weight analysis, we performed Student's t test with two-sample equal variance (homeoscedastic) and one-tailed distribution. For motor performance tests, the null hypothesis was rejected at the 0.05 level and for all others, at the 0.01 level. All P values shown are rounded up at the third or fourth digit.
Supplementary Material
Acknowledgments
We thank Martyn Goulding (Salk Institute, La Jolla, CA) for the En1/tauLacZ and Alex Joyner (New York University School of Medicine, New York, NY) for the En2 mutant mice and Marco Chiarandini and Tommaso Schiavinotto for help with the statistical analysis. We are particularly grateful to Andrew Lumsden and Peter Burbach for discussions on the manuscript. We also thank Gabriele Döderlein, Richard Hertel, Kendra Laustsen, and Rae Kokotailo for technical assistance. This work was supported by grants from the Federal Secretary for Education and Research (BMBF) Biofuture 98; the Michael J. Fox Foundation; the National Parkinson Foundation (to H.H.S.); the Canadian Institutes of Health Research; and the Natural Sciences and the Engineering Research Council of Canada (to R.H.D.).
Abbreviations
- mesDA
mesencephalic dopaminergic
- SN
substantia nigra pars compacta
- VTA
ventral tegmentum
- RRF
retrorubral field
- PD
Parkinson's disease
- TH
tyrosine hydroxylase
- CPu
caudate putamen
- Pn
postnatal dayn.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fallon JH, Loughlin SE. In: The Rat Nervous System. Paxinos G, editor. London: Academic; 1994. pp. 215–237. [Google Scholar]
- 2.Schultz W. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 3.Girault JA, Greengard P. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 4.Eells JB. Curr Med Chem. 2003;10:857–870. doi: 10.2174/0929867033457700. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW, Tatton WG. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 6.Beyer PL, Palarino MY, Michalek D, Busenbark K, Koller WC. J Am Diet Assoc. 1995;95:979–983. doi: 10.1016/S0002-8223(95)00269-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Ann Neurol. 2003;53:676–679. doi: 10.1002/ana.10577. [DOI] [PubMed] [Google Scholar]
- 8.Alberi L, Sgadò P, Simon HH. Development (Cambridge, UK) 2004;131:3229–3236. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- 9.Simon HH, Saueressig H, Wurst W, Goulding MD, O'Leary DD. J Neurosci. 2001;21:3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanks M, Wurst W, Anson-Cartwright L, Auerbach AB, Joyner AL. Science. 1995;269:679–682. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- 11.West MJ. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 12.German DC, Manaye KF. J Comp Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- 13.Oo TF, Kholodilov N, Burke RE. J Neurosci. 2003;23:5141–5148. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon HH, Thuret S, Alberi L. Cell Tissue Res. 2004;318:53–61. doi: 10.1007/s00441-004-0973-8. [DOI] [PubMed] [Google Scholar]
- 15.Burke RE, Antonelli M, Sulzer D. J Neurochem. 1998;71:517–525. doi: 10.1046/j.1471-4159.1998.71020517.x. [DOI] [PubMed] [Google Scholar]
- 16.Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP. Nat Neurosci. 2000;3:337–341. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- 17.Smidt MP, Smits SM, Bouwmeester H, Hamers FP, Van Der Linden AJ, Hellemons AJ, Graw J, Burbach JP. Development (Cambridge, UK) 2004;131:1145–1155. doi: 10.1242/dev.01022. [DOI] [PubMed] [Google Scholar]
- 18.McCaffery P, Drager UC. Proc Natl Acad Sci USA. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saueressig H, Burrill J, Goulding M. Development (Cambridge, UK) 1999;126:4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- 20.Davis CA, Joyner AL. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- 21.Davis CA, Holmyard DP, Millen KJ, Joyner AL. Development (Cambridge, UK) 1991;111:287–298. doi: 10.1242/dev.111.2.287. [DOI] [PubMed] [Google Scholar]
- 22.Kawagoe KT, Zimmerman JB, Wightman RM. J Neurosci Methods. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- 23.Gerfen CR. Trends Neurosci. 2000;23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- 24.Tillerson JL, Miller GW. J Neurosci Methods. 2003;123:189–200. doi: 10.1016/s0165-0270(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 25.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 26.Metz GA, Whishaw IQ. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 27.Fernagut PO, Diguet E, Labattu B, Tison F. J Neurosci Methods. 2002;113:123–130. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- 28.Millen KJ, Wurst W, Herrup K, Joyner AL. Development (Cambridge, UK) 1994;120:695–706. doi: 10.1242/dev.120.3.695. [DOI] [PubMed] [Google Scholar]
- 29.Gerlai R, Millen KJ, Herrup K, Fabien K, Joyner AL, Roder J. Behav Neurosci. 1996;110:126–133. doi: 10.1037//0735-7044.110.1.126. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer RF. Lancet Neurol. 2003;2:107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 31.Macia F, Perlemoine C, Coman I, Guehl D, Burbaud P, Cuny E, Gin H, Rigalleau V, Tison F. Mov Disord. 2004;19:206–212. doi: 10.1002/mds.10630. [DOI] [PubMed] [Google Scholar]
- 32.Barichella M, Marczewska AM, Mariani C, Landi A, Vairo A, Pezzoli G. Mov Disord. 2003;18:1337–1340. doi: 10.1002/mds.10543. [DOI] [PubMed] [Google Scholar]
- 33.Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 34.Simon HH, Scholz C, O'Leary DD. Mol Cell Neurosci. 2005;28:96–105. doi: 10.1016/j.mcn.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Blum M. Nat Neurosci. 1998;1:374–377. doi: 10.1038/1584. [DOI] [PubMed] [Google Scholar]
- 36.Roffler-Tarlov S, Martin B, Graybiel AM, Kauer JS. J Neurosci. 1996;16:1819–1826. doi: 10.1523/JNEUROSCI.16-05-01819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaspar P, Ben Jelloun N, Febvret A. Neuroscience. 1994;61:293–305. doi: 10.1016/0306-4522(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 38.Harkins AB, Fox AP. Cerebellum. 2002;1:201–206. doi: 10.1080/14734220260418420. [DOI] [PubMed] [Google Scholar]
- 39.Dunnett SB, Bjorklund A. Nature. 1999;399:A32–9. doi: 10.1038/399a032. [DOI] [PubMed] [Google Scholar]
- 40.Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Neuropharmacology. 1987;26:97–99. doi: 10.1016/0028-3908(87)90051-7. [DOI] [PubMed] [Google Scholar]
- 41.Damier P, Hirsch EC, Agid Y, Graybiel AM. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 42.Dauer W, Przedborski S. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 43.Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Science. 1991;251:1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- 44.Wachtel SR, Bencsics C, Kang UJ. J Neurochem. 1997;69:2055–2063. doi: 10.1046/j.1471-4159.1997.69052055.x. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland RJ, Dyck RH. Can J Psychol. 1984;38:322. [Google Scholar]
- 46.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.