Abstract
The concept of initiators for continuous activator regeneration (ICAR) in atom transfer radical polymerization (ATRP) is introduced, whereby a constant source of organic free radicals works to regenerate the CuI activator, which is otherwise consumed in termination reactions when used at very low concentrations. With this technique, controlled synthesis of polystyrene and poly(methyl methacrylate) (Mw/Mn < 1.2) can be implemented with catalyst concentrations between 10 and 50 ppm, where its removal or recycling would be unwarranted for many applications. Additionally, various organic reducing agents (derivatives of hydrazine and phenol) are used to continuously regenerate the CuI activator in activators regenerated by electron transfer (ARGET) ATRP. Controlled polymer synthesis of acrylates (Mw/Mn < 1.2) is realized with catalyst concentrations as low as 50 ppm. The rational selection of suitable Cu complexing ligands {tris[2-(dimethylamino)ethyl]amine (Me6TREN) and tris[(2-pyridyl)methyl]amine (TPMA)} is discussed in regards to specific side reactions in each technique (i.e., complex dissociation, acid evolution, and reducing agent complexation). Additionally, mechanistic studies and kinetic modeling are used to optimize each system. The performance of the selected catalysts/reducing agents in homo and block (co)polymerizations is evaluated.
Keywords: controlled radical polymerization, electron transfer, catalysis, green chemistry, block copolymer
The widespread industrial application of chemical synthetic techniques is often contingent upon the efficiency with which these processes can be implemented. This dependency is particularly true in the field of controlled radical polymerization (CRP). The vast array of polymeric materials that have been produced in the last decade by atom transfer radical polymerization (ATRP) (1, 2), an especially powerful CRP technique, is striking. The extraordinary control over topologies, compositions, microstructures, and functionalities (3–6) that ATRP can provide in polymeric synthesis has led to an explosive development in nanocomposites, thermoplastic elastomers, bioconjugates, drug delivery systems, etc. (7–10).
While such polymers are finding industrial applications, (11), the large-scale production of these materials has been rather limited. This fact can be attributed mostly to the high catalyst concentrations required by ATRP, often approaching 0.1 M in bulk monomer. Added expense is therefore associated with purifying any polymers generated in these homogenous reactions (12). An additional problem of industrial relevance involves the use of highly active (i.e., very reducing) ATRP catalysts. Special handling procedures are often required to remove all oxygen and oxidants from these systems. Previous research intending to streamline the process and products of ATRP has focused on maximizing the efficiency of catalyst removal or recycling through the use of ion-exchange resins (13), biphasic systems (14), immobilized/solid-supported catalysts (12), and immobilized/soluble hybrid catalyst systems (15). By contrast, the work presented hereafter demonstrates how several recently developed ATRP initiation systems can be implemented to both scavenge oxidants and decrease the amount of catalyst needed (to ppm levels) where its removal or recycling would be unwarranted for many industrial applications.
Controlled radical polymerization processes (16) function by establishing an equilibrium between propagating radicals and dormant chains. Radical termination is diminished in “living” polymerizations obeying the persistent radical effect (17) as the equilibrium becomes strongly shifted toward the dormant species. The ATRP equilibrium (characterized by KATRP = kact/kdeact) involves homolytic cleavage of an alkyl halide bond R–X by a transition metal complex activator Mtn/L that reversibly generates an alkyl radical R• and the corresponding higher oxidation state metal halide deactivator Mtn+1X/L (Scheme 1a). R• can then propagate with a vinyl monomer (M), be deactivated in this equilibrium by Mtn+1X/L, or terminate by either coupling or disproportionation with another R•, at which point two equivalents of deactivator accumulate as persistent radicals. With consideration of the complexing ligand of the ATRP catalyst (and consequently, the reducing power of the complex), the ATRP equilibrium in Scheme 1 can be easily and appropriately adjusted for more or less reactive monomers.
Scheme 1.
Proposed mechanism for ATRP processes.
In principle, the absolute amount of metal catalyst in Cu-based ATRP can be decreased under normal ATRP conditions without affecting the rate of polymerization, which is ultimately governed by a ratio of the concentrations of CuI to CuII species according to Eq. 1.
 |
In reality, radical termination occurs, and CuII deactivator accumulates as a persistent radical. As much as 1–10% of polymeric chains terminate under typical ATRP conditions (18). This observation suggests that in a system with a targeted degree of polymerization 100, if 10% of the chains were to terminate, polymerization would not reach full conversion unless the total catalyst concentration exceeded one part per thousand vs. monomer (≈1,000 ppm). However, a special situation occurs in the polymerization of styrene, in which thermal initiation generates radicals that can reduce accumulated CuII deactivator. We demonstrate herein that exceptionally small concentrations of catalyst can mediate controlled polymerization of styrene in this manner. For monomers that do not undergo thermal initiation, very small amounts of free radical initiators can be used to simulate slow thermal initiation in a process dubbed initiators for continuous activator regeneration (ICAR) ATRP (Scheme 1b).
The limitation of ICAR ATRP is evident in the production of block copolymers, because the free radical initiator will also generate new homopolymer chains that may alter the properties of the copolymer. Following early reports that zero-valent metals could reduce the CuII deactivator (19), and later observations that monosaccharides could increase rates of polymerization in ATRP by reducing the deactivator concentration (20), AGET (activators generated by electron transfer) ATRP used a stoichiometric amount of tin(II) 2-ethylhexanoate (Sn(EH)2) (21) or ascorbic acid (22) to reduce CuII to CuI, which could then catalyze ATRP under appropriate polymerization conditions. The concentration of catalyst relative to initiator can be significantly decreased when the reducing agent in this system (which cannot initiate new chains) is present in excess relative to the catalyst. CuII that accumulates as a persistent radical is then continuously reduced to CuI as the activator is regenerated by electron transfer (ARGET) (Scheme 1b) (23, 24).
The catalyst concentration cannot be decreased indefinitely in any ATRP system, because control over molecular weight distribution in Cu-based ATRP depends on absolute deactivator concentration according to the following relationship:
 |
in which PDI is the polydispersity index, Mw is the weight-average molecular weight, Mn is the number-average molecular weight, DPn is the degree of polymerization, []0 refers to the concentration at time 0, and Conv. is conversion expressed as a dimensionless fraction. However, good control over styrene polymerization was established with ARGET using only 10 ppm Cu catalyst (23). Additionally, the catalyst and excess reducing agent can effectively work to scavenge and remove dissolved oxygen from the polymerization system.
Herein, the rational selection of suitable catalysts for these systems is discussed in regards to specific side reactions that occur in ICAR and ARGET; mechanistic studies and kinetic modeling are used to optimize various parameters in each system; the performance of the selected catalysts/reducing agents in several different polymerization systems is evaluated; and the advantages and limitations of each method are discussed.
Results and Discussion
ICAR ATRP.
Several factors should be considered when attempting to optimize and select the appropriate conditions for ATRP processes in very dilute solutions of catalyst. First, control over molecular weight distributions in ATRP depends on absolute deactivator concentration and the rate constant of deactivation of a given catalyst (see Eq. 2). Because the Cu catalyst is present in such small quantities in this work, only complexes with high values of KATRP (resulting in sufficiently high concentrations of CuII in solution) and relatively fast deactivation rate constants should efficiently control molecular weight and polydispersity. Second, the catalyst should not dissociate appreciably at the low concentrations used in either ARGET or ICAR. This problem will be compounded by competitive monomer complexation with the ligand to the metal. Third, at very low concentrations of Cu, it is not immediately clear whether radical concentration and consequently rate of polymerization will be governed by KATRP (as in normal ATRP) or by the rate of new radical generation. The latter concern will largely be addressed with kinetic modeling. The results of several experiments designed to study different variables in the ICAR process are presented in Table 1 and discussed hereafter.
Table 1.
ICAR ATRP of styrene (St), methyl methacrylate (MMA), and n-butyl acrylate (BA)
| Entry | Temp, °C | Monomer/initiator/Cu | [Cu], ppm | Ligand/ratio to Cu | AIBN/Initiator | Time, min | Conv., % |
Mn |
Mw/Mn | |
|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical | GPC | |||||||||
| 1 | 60 | 200 St/1/0.01 | 50 | Me6TREN/1 | 0.1 | 2,760 | 44 | 8,700 | 7,900 | 1.12 |
| 2 | 60 | 200 St/1/0.01 | 50 | TPMA/1 | 0.1 | 2,880 | 39 | 7,800 | 6,800 | 1.09 |
| 3 | 60 | 200 St/1/0.01 | 50 | PMDETA/1 | 0.1 | 2,880 | 29 | 5,600 | 4,500 | 1.62 |
| 4 | 60 | 200 St/1/0.01 | 50 | dNbpy/2 | 0.1 | 2,940 | 36 | 7,200 | 5,600 | 1.68 |
| 5 | 70 | 200 St/1/0.01 | 50 | Me6TREN/1 | 0.1 | 2,400 | 47 | 9,500 | 7,600 | 1.11 |
| 6 | 70 | 200 St/1/0.01 | 50 | Me6TREN/1 | 0.2 | 2,500 | 60 | 11,900 | 10,000 | 1.15 |
| 7 | 70 | 200 St/1/0.01 | 50 | Me6TREN/1 | 0.4 | 1,140 | 66 | 13,200 | 10,100 | 1.22 |
| 8 | 110 | 200 St/1/0.01 | 50 | Me6TREN/10 | — | 1,775 | 65 | 12,900 | 11,000 | 1.25 |
| 9 | 110 | 200 St/1/0.01 | 50 | TPMA/10 | — | 1,930 | 49 | 9,800 | 9,600 | 1.13 |
| 10 | 110 | 200 St/1/0.002 | 10 | TPMA/50 | — | 1,720 | 42 | 8,400 | 7,600 | 1.38 |
| 11 | 110 | 200 St/1/0.0002 | 1 | TPMA/500 | — | 1,700 | 55 | 11,000 | 8,400 | 1.72 |
| 12 | 60 | 200 MMA/1/0.01 | 50 | TPMA/1 | 0.1 | 1,120 | 50 | 10,100 | 11,100 | 1.23 |
| 13 | 65 | 200 BA/1.25/0.01 | 50 | TPMA/1 | 0.01 | 1,910 | 35 | 7,100 | 8,900 | 1.46 |
Boldface numbers emphasize the variable of interest in a series of experiments. Ratios are molar. AIBN, azobisisobutyronitrile; GPC, gel-permeation chromatography; EtBrPA, ethyl α-bromophenylacetate. [St]0/[EtBrIB]0 = 200; [St]0 = 5.82 M; 50% anisole by volume; [BA]0/[EtBrIB]0 = 160; [BA]0 = 5.88 M; 20% anisole by volume; [MMA]0/[EtBrPA]0 = 200; [MMA]0 = 6.23 M; 50% anisole by volume.
Rational selection of the ligand.
Four ATRP catalysts with a broad range of KATRP values (25) were selected for this study. These included the CuCl2 complexes of tris[2-(dimethylamino)ethyl]amine (Me6TREN) (26), tris[(2-pyridyl)methyl]amine (TPMA) (27), N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA) (28), and 4,4′-di-(5-nonyl)-2,2′-dipyridyl (dNbpy) (29). ICAR ATRP of styrene (St) was first conducted at low temperature (60°C), where organic radicals were produced by the slow decomposition of azobisisobutyronitrile (AIBN) (0.1 eq vs. ethyl 2-bromoisobutyrate (EtBrIB) initiator) in the presence of 50 ppm CuCl2/L complexes (entries 1–4 in Table 1).
Interestingly, rates of polymerization differ by less than a factor of 2 among reactions catalyzed by these four CuCl2/L complexes of Me6TREN, TPMA, PMDETA, and dNbpy. This finding was initially surprising, given that values of KATRP, which govern radical concentration and the rate of polymerization under normal ATRP conditions, differ by more than four orders of magnitude among these four complexes (25). Additional experiments and kinetic simulations (see below) explore the possibility that (i) rates of polymerization and radical concentration under ICAR ATRP conditions are actually controlled by the rate of free radical initiator decomposition and (ii) relative CuI and CuII concentrations conform accordingly as dictated by the KATRP value.
While polymerizations mediated by CuCl2/Me6TREN and CuCl2/TPMA were very well controlled in terms of molecular weight and Mw/Mn (entries 1 and 2 in Table 1; Fig. 1), control over Mw/Mn was significantly worse in the polymerization mediated by CuCl2/PMDETA and CuCl2/dNbpy. This behavior is consistent with the fact that the latter two catalysts have the lowest KATRP values of the four complexes. Such observations concerning attainable control can also be rationalized on the basis of the stability of these complexes toward dissociation at high dilution and high temperature. The temperature dependence of the stability of the CuII complexes of PMDETA (30), Me6TREN (30), and TPMA (31) in aqueous media is discussed in the Supporting Text, which is published as supporting information on the PNAS web site, and can be used as a general guide for ligand selection in these systems. These constants suggest that CuCl2 complexes of TPMA and Me6TREN are quite stable. However, significant dissociation of the CuCl and CuCl2/PMDETA complexes would ultimately result in a lower absolute value of deactivator concentration, helping explain the observed poor control over Mw/Mn in this system (entry 3, Table 1).
Fig. 1.
Kinetic plot (Left) and molecular weight (Lower Right) and Mw/Mn (Upper Right) as a function of conversion in the ICAR ATRP of St with 50 ppm and 1 ppm Cu. St/EtBrIB/CuCl2/Me6TREN/AIBN = 200/1/0.01/0.01/0.1; St/EtBrIB/CuCl2/TPMA/AIBN = 200/1/0.0002/0.1/0.1; [St]0 = 5.82 M; 60°C; 50% anisole by volume (entries 1 and 11 in Table 1).
Variable free radical initiator concentration, Cu concentration, and monomer.
Entries 5–7 of Table 1 illustrate that varying the amount of AIBN versus alkyl halide initiator clearly affects the rate of polymerization, as hypothesized above when it was discovered that the value of KATRP for a given catalyst did not appear to govern the rate of polymerization. All three of those reactions mediated by CuCl2/Me6TREN were well controlled in terms of molecular weight and Mw/Mn (≈1.1 to 1.2).
At higher temperatures (110°C), where the complexes are more prone to dissociate, a 10-fold excess of ligand to Cu was used to suppress dissociation. The radical reducing agents were generated from the thermal initiation of St. Polymerization mediated by CuCl2/TPMA was better controlled than that mediated by CuCl2/Me6TREN (in terms of Mw/Mn) under these conditions. When the Cu concentration was decreased from 50 ppm to 10 and then just 1 ppm (entries 10 and 11, Table 1), Mw/Mn was broader (≈1.4 and ≈1.7 for 10 and 1 ppm of Cu, respectively) and molecular weights were slightly higher than theoretical values. However, quite impressively, just 1 ppm of Cu in the presence of excess TPMA was ultimately sufficient to control molecular weights in this ATRP system (Fig. 1).
The ICAR ATRP of methyl methacrylate (MMA) proved very efficient when mediated by 50 ppm CuCl2/TPMA and initiated by ethyl α-bromophenylacetate (EtBrPA) (entry 12, Table 1). Linear first-order kinetics were observed, molecular weights agreed well with theoretical values, and polydispersities were low (Mw/Mn ≈ 1.2). Good control over molecular weights and acceptable Mw/Mn in the polymerization of n-butyl acrylate (BA) initiated by EtBrIB was also attained with 50 ppm CuCl2/TPMA (entry 13, Table 1), illustrating the broad application of ICAR ATRP.
Kinetic modeling.
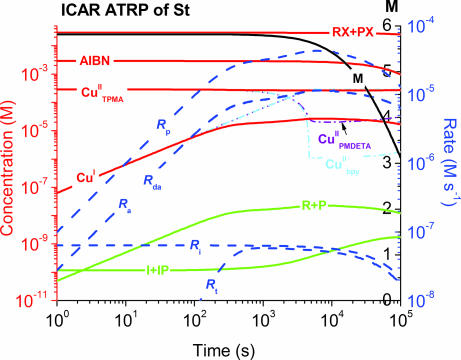
Polymerizations were simulated (predici version 6.3.1) in an effort to obtain a clear picture of the kinetics of ICAR ATRP and demonstrate that (i) rate of polymerization in ICAR is governed by the rate of AIBN decomposition and (ii) attainable control is dictated by the value of KATRP and rate of deactivation for a given catalyst. The multitude of parameters necessary for these simulations, and typical rate constants for three CuBr2/L complexes [with bipyridine (bpy), PMDETA, and TPMA], are shown in Table 3, which is published as supporting information on the PNAS web site. The three chosen catalysts represent a broad range of KATRP values (spanning over three orders of magnitude) (25). Fig. 2 and, which are published as supporting information on the PNAS web site, illustrate the results and intricacies of these ICAR simulations with St.
Fig. 2.
predici simulation of kinetic plots for ICAR ATRP of St employing TPMA, PMDETA, or bpy with 50 ppm Cu and AIBN as the free radical initiator.
According to these simulations, the polymerization rates for all three complexes are essentially the same (Fig. 5). All rates and species concentrations in a simulated polymerization mediated by CuBr2/TPMA are illustrated on a double-logarithmic scale in Fig. 2. It can be seen that the concentration of dormant species (ATRP initiator RX and polymeric dormant species) remains constant throughout the reaction, which gives rise to a linear increase in molecular weight with monomer conversion, and further indicates that most of the chain end functionality survives throughout the reaction. The ATRP quasi-equilibrium (activation rate Ra ≈ deactivation rate Rda during the entire time span) was reached almost immediately because of the initial presence of CuII species. Once this state is reached, the concentrations of radicals, CuI, and CuII remain essentially constant, and the termination rate (Rt) approaches the decomposition rate of AIBN (Ri) with a rate constant kdc. The steady-state radical concentration ([R]s) can be estimated by setting Ri = Rt, i.e.,
and
Eq. 4 shows how the radical concentration (and hence, the polymerization rate) under steady-state conditions is dependent only on the AIBN decomposition rate constant, its concentration, and the radical termination rate constant. Radical concentration should therefore not be governed by the choice of ATRP catalyst, KATRP, or the initial concentration of CuII species. This dependance further suggests that polymerization rates can be adjusted with the choice of an appropriate free radical initiator. These predictions are in relatively good agreement with experimental observations, where apparent rates of polymerization mediated by CuCl2/Me6TREN, TPMA PMDETA, and dNbpy (entries 1–4, Table 1) are very similar.
However, control over molecular weight and Mw/Mn is very catalyst dependent. As shown in Fig. 6, when TPMA is used as the ligand (i.e., appropriate values of activation and deactivation rate constants are used in the simulations), Mw/Mn is low (<1.5) throughout the reaction and approaches 1 at high conversion. Molecular weights increase linearly with conversion and are equal to theoretical values. Similar results are predicted with PMDETA, although Mw/Mn is higher than in the reaction with TPMA. However, neither Mw/Mn nor molecular weights are well controlled in a reaction where CuBr2/(bpy)2 is modeled (Fig. 6).
The ratio of polymerization rate to the deactivation rate, i.e., (kp[M])/(kdeact[CuII]), represents the number of monomer units that will add to an actively propagating radical chain before it is deactivated to the dormant state. This ratio can be used in a qualitative estimation of how well a given catalyst can control a polymerization. Because such a small amount of Cu catalyst is used in ICAR ATRP, catalysts with large values of KATRP (high concentration of CuII) and fast deactivation rate constants will minimize this ratio, allowing for more even polymer chain growth and ultimately better control. Cu complexes with TPMA have a large value of KATRP with the model polystyrene chain end 1-phenylethyl bromide (estimated as ≈7 × 10−6 at 60°C). Whereas the analogous KATRP value of the Cu/PMDETA complex is much lower (≈5 × 10−8), kdeact for Cu/PMDETA is larger than that of TPMA, which can compensate for the product of kda[CuII]. The Cu catalyst formed with bpy is the least active among the three complexes in discussion, with KATRP ≈ 1 × 10−9 but a relatively large kdeact (3.0 × 107 M−1·s−1). The concentration of CuII species at quasi-steady state can be estimated from the ATRP equilibrium
Therefore, where [R]s is estimated from Eq. 4,
As illustrated in Fig. 2 and calculated from Eq. 6 with their respective values of KATRP, 90% of the total concentration of Cu in the quasi-steady state exists in the CuII oxidation state for complexes with TPMA under those conditions. This can be compared with just 7% for PMDETA and only 0.3% for bpy. The ratios of (kp[M])/(kdeact[CuII]) at the quasi-steady state can be calculated with Eqs. 4 and 6. Four, nine, and 230 monomer units will initially add to a propagating chain when it is activated by Cu/L complexes with TPMA, PMDETA, and bpy, respectively. These values are qualitatively consistent with the attainable control illustrated in Fig. 7, which is published as supporting information on the PNAS web site, for each system. However, in ICAR systems employing PMDETA as the ligand, control may be overestimated in Fig. 6 because the complex stability at dilution and elevated temperature is not taken into account in these simulations.
ARGET ATRP.
One advantage of ARGET over ICAR ATRP is the use of reducing agents that do not generate new chains. The following paragraphs predominantly focus on the use of hydrazine (NH2NH2) and phenylhydrazine (PhNHNH2) to regenerate the activating CuI complex by a redox process, where the products of oxidation are either nitrogen gas or organic in nature (for further discussion of the mechanism of the reduction process, see Supporting Text). Similar rules for catalyst selection exist in ARGET as in ICAR (i.e., concerning complex dissociation). Additionally, the release of acid during the oxidation of these reducing agents can destabilize copper-based ATRP catalysts derived from amines. Excess base (or excess ligand or reducing agent acting as a base) will likely be required to trap the acid. Several variables examined in this technique are now discussed and presented in Table 2.
Table 2.
PBA prepared by ARGET ATRP under various conditions
| Entry | Monomer/initiator/Cu | CuCl2, ppm | Ligand/ratio to Cu | Reducing agent/ratio to Cu | Time, min | Conv., % |
Mn |
Mw/Mn | |
|---|---|---|---|---|---|---|---|---|---|
| Theoretical | GPC | ||||||||
| 1 | 200/1/0.01 | 50 | Me6TREN/10 | PhNHNH2/10 | 1,098 | 78 | 19,994 | 26,100 | 1.23 |
| 2 | 200/1/0.01 | 50 | Me6TREN/3 | PhNHNH2/10 | 1,098 | 33* | 8,500 | 20,200 | 2.3 |
| 3 | 200/1/0.01 | 50 | TPMA/10 | PhNHNH2/10 | 3,780 | 59 | 15,124 | 16,700 | 1.27 |
| 4 | 200/1/0.01 | 50 | TPMA/3 | PhNHNH2/10 | 1,300 | 32* | 8,202 | 8100 | 1.57 |
| 5 | 200/1/0.01 | 50 | PMDETA/10 | PhNHNH2/10 | No reaction | ||||
| 6 | 200/1/0.1 | 500 | PMDETA/10 | PhNHNH2/10 | 1,230 | 64 | 16,405 | 25,481 | 1.70 |
| 7 | 200/1.28/0.01 | 50 | Me6TREN/10 | (Me6TREN) | 1,240 | 86 | 17,100 | 21,600 | 1.83 |
| 8 | 200/1.28/0.01 | 50 | TPMA/10 | NH2NH2/5 | 2,520 | 41 | 8,270 | 8,690 | 1.32 |
| 9 | 200/1.28/0.01 | 50 | TPMA/10 | NH2NH2/10 | 2,520 | 60 | 11,840 | 12,490 | 1.25 |
| 10 | 200/1.28/0.01 | 50 | TPMA/3 | NH2NH2/5 | 1,200 | 28* | 5,650 | 5540 | 1.37 |
| 11 | 200/1.28/0.01 | 50 | TPMA/3 | NH2NH2/10 | 1,200 | 21* | 4,320 | 4730 | 1.40 |
| 12† | 200/1/0.01 | 50 | TPMA/3 | MPO/200 | 1,920 | 16* | 3,200 | 4300 | 1.33 |
Boldface numbers emphasize the variable of interest in a series of experiments. MPO, 4-methoxyphenol. [BA]0 = 5.88 M; 60°C; ≈20% anisole by volume.
*Polymerization did not occur past this limited conversion.
†90°C.
Effect of the ligand and ligand concentration.
A dramatic difference was observed in the level of attainable control over molecular weight distribution of poly(n-butyl acrylate) (PBA), depending on the ligand used (PMDETA, Me6TREN, or TPMA) and the concentration of the ligand relative to Cu (entries 1–6 in Table 2). First, when a 10-fold excess of ligand to 50 ppm Cu was used, polymerization could reach high conversion, as opposed to when just a 3-fold excess was used. Second, considering together polymer molecular weights and Mw/Mn, reactions were best controlled when TPMA was used, followed by Me6TREN and then PMDETA, the latter of which required much higher Cu concentrations (500 ppm) to mediate any polymerization.
The behavior of the three complexes can in part be rationalized on the basis of their stability toward dissociation, as discussed previously with ICAR ATRP, compounded by the fact that these reducing agents can complex with the catalyst. However, 50 ppm of CuCl2/PMDETA can mediate polymerization in ICAR ATRP, whereas it cannot in ARGET. This difference is attributed to the instability of the complex toward protonation. The pH dependence of the stability of the CuII complexes of PMDETA (32), Me6TREN (33), and TPMA (34) can be calculated knowing the protonation constants in water, which are available in literature (see Eq. 7, Fig. 7, and Discussion in Supporting Text). On the basis of those trends observed in aqueous media, it is clear why PMDETA is not a good choice of ligand for ARGET ATRP. Complexes of PMDETA and Me6TREN are markedly more destabilized in acidic media than are those of the less basic ligand TPMA. From the point of view of temperature and pH stability, TPMA appears the superior choice among these ligands for ARGET reactions.
Variable reducing agent and Cu concentrations.
The appropriate reducing agent and concentration of reducing agent is not immediately obvious. The reagent will quickly be consumed if too little is used, and too much might lead to fast and uncontrolled polymerizations or unwanted side reactions with the catalyst. The picture becomes more clouded by the fact that amine-based ligands can also act as mild reducing agents (35).
In a control experiment, a 10-fold excess of Me6TREN (with four tertiary amine groups capable of reducing CuII) was used in the absence of any other reducing agent (entry 7 in Table 2). Polymerization occurs under these conditions in the presence of alkyl halide. However, control over Mw/Mn is very poor (>1.8 at 86% conversion) but is much better when PHNHNH2 is used as the reducing agent (entry 1 in Table 2). BA polymerization is also well controlled in terms of molecular weight and Mw/Mn in the presence of NH2NH2. First-order linear kinetics are observed when a 10-fold excess of TPMA to Cu is used in the presence of both a 5- and 10-fold excess of NH2NH2. The rate of polymerization increases with increasing concentration of NH2NH2, and first-order kinetics remain linear (Fig. 3). When just a 3-fold excess of TPMA to Cu is used (entries 10 and 11 in Table 2), polymerization reaches only limited conversion (again, attributed to the consumption of ligand with the evolution of acid).
Fig. 3.
Kinetic plot (Left) and molecular weight (Lower Right) and Mw/Mn (Upper Right) as a function of conversion in the CuCl2/TPMA-mediated ARGET ATRP of BA, with variable hydrazine (N2H4) reducing agent. [BA]0/[EtBrIB]0/[CuCl2]0/[TPMA]0/[N2H4]0 = 200/1.28/0.01/0.1/0.05 or 0.1; [BA]0 = 5.88 M; 60°C; 20% anisole by volume (entries 8 and 9 in Table 2).
In addition to hydrazines, another class of organic reducing agent was investigated with 4-methoxyphenol (MPO). When 10 eq of this reducing agent were used with CuCl2/TPMA in the ARGET ATRP of BA at 90°C, no polymerization was observed. With the use of 200 eq of MPO, only 16% conversion was reached in 32 h (although Mw/Mn was relatively low; entry 12 in Table 2). The inefficiency of MPO as a reducing agent (in terms of polymerization rate) compared with NH2NH2 and PhNHNH2 is fully consistent with the voltammetric data for these organic complexes; literature values for the oxidation waves of phenols are typically one full volt more positive than the oxidation waves of hydrazine derivatives in acetonitrile (36).
Synthesis of PSt-b-PBA prepared by ICAR and ARGET ATRP.
To demonstrate the utility of these ATRP methods in the production of block copolymers, and to confirm that chain-end functionality remains high in ICAR ATRP, PSt macroinitiator was prepared by ICAR and then extended with BA by using ARGET ATRP (to minimize the production of new chains). Both polymerizations were carried out with 50 ppm copper catalyst. Chain extension of the PSt macroinitiator with BA by using ARGET ATRP with 50 ppm copper proved very efficient (Mn by GPC = 61,200, theoretical Mn = 52,900, Mw/Mn = 1.29). Fig. 4 shows the GPC traces recorded after each synthetic step and illustrates the utility of these techniques in the production of block copolymers.
Fig. 4.
GPC traces after each step in synthesis of block copolymer PSt-b-PBA. Experimental conditions for ICAR ATRP of St with 50 ppm Cu catalyst (solid line): St/EtBrIB/CuII/TPMA = 200/1/0.01/0.1; [St]0 = 5.82 M; 110°C; 50% anisole by volume. Experimental conditions for ARGET ATRP of BA with 50 ppm Cu catalyst (dashed line): BA/PSt/CuII/Me6TREN/PhNHNH2 = 400/1/0.02/0.1/0.1; [BA]0 = 4.90 M; 60°C; 50% dimethylformamide by volume.
Conclusions
The concept of ICAR in ATRP was introduced. Only 50 ppm Cu catalyst was needed to mediate well controlled polymerizations of St and methyl methacrylate (Mw/Mn < 1.2) with this technique. The rational selection of suitable Cu-complexing ligands was discussed in detail, primarily in regards to the value of KATRP for a given catalyst but also with respect to complex stability at high dilution and at elevated temperatures. For these reasons, it was determined that Me6TREN and TPMA were more suitable ligands than PMDETA and dNbpy in ICAR ATRP at low Cu catalyst concentrations. Experimental data as well as simulations confirmed that the rate of polymerization in ICAR is governed by the rate of free radical initiator decomposition, whereas control is ultimately determined by KATRP and the rate of deactivation.
Additionally, derivatives of NH2NH2 and MPO were used as reducing agents in ARGET ATRP. Controlled polymer synthesis (Mw/Mn < 1.2) of acrylate was implemented with catalyst concentrations as low as 50 ppm. The rational selection of the catalyst-complexing ligand was discussed in regards to complex stability and protonation in the presence of acid. From the point of view of temperature and pH stability, TPMA was reasoned to be the superior choice over PMDETA and Me6TREN for ARGET reactions. The utility of these processes was further illustrated with the production of a well defined block copolymer by employing only 50 ppm Cu catalyst.
Methods
Materials and Analyses.
Me6TREN (26, 37) and TPMA (27, 38) were synthesized according to literature procedures. All other complexes, reagents, and solvents used in this study were obtained from commercial sources and were purified and deoxygenated as described in refs. 23 and 24. Monomer conversions and polymer molecular weights and Mw/Mn were determined by using a gas chromatograph (GC) and a GPC system, respectively (details in Supporting Text).
Representative Polymerization.
A deoxygenated solution of CuCl2 and ligand was prepared with the appropriate monomer and solvent in a Schlenk flask and was placed in a thermostated oil bath. After an initial sample was taken, the organic reducing agent or AIBN and alkyl halide initiator were added. Samples were withdrawn at regular intervals and analyzed by GC and GPC to follow the progress of the reaction (details in Supporting Text).
Kinetic Modeling.
The predici program (version 6.3.1) was used for all kinetic modeling (39); it employs an adaptive Rothe method as a numerical strategy for time discretization. The concentrations of all species can be followed with time.
Supplementary Material
Acknowledgments
This work was financially supported by National Science Foundation Grants DMR-05-49353 and CHE-04-05627 (to K. Matyjaszewski) and by the members of the Controlled Radical Polymerization Consortium at Carnegie Mellon University.
Abbreviations
- AIBN
azobisisobutyronitrile
- ARGET
activators regenerated by electron transfer
- ATRP
atom transfer radical polymerization
- BA
n-butyl acrylate
- bpy
bipyridine
- dNbpy
4,4′-di-(5-nonyl)-2,2′-dipyridyl
- EtBrIB
ethyl 2-bromoisobutyrate
- GPC
gel-permeation chromatography
- ICAR
initiators for continuous activator regeneration
- Me6TREN
tris[2-(dimethylamino)ethyl]amine
- MPO
4-methoxyphenol
- PBA
poly(n-butyl acrylate)
- PhNHNH2
phenylhydrazine
- PMDETA
N,N,N′,N″,N″-pentamethyldiethylenetriamine
- PSt
polystyrene
- St
styrene
- TPMA
tris[(2-pyridyl)methyl]amine.
Footnotes
Conflict of interest statement: No conflicts declared.
This article is a PNAS direct submission.
References
- 1.Wang J-S, Matyjaszewski K. J Am Chem Soc. 1995;117:5614–5615. [Google Scholar]
- 2.Matyjaszewski K, Xia J. Chem Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 3.Coessens V, Pintauer T, Matyjaszewski K. Prog Polym Sci. 2001;26:337–377. [Google Scholar]
- 4.Pyun J, Matyjaszewski K. Chem Mater. 2001;13:3436–3448. [Google Scholar]
- 5.Davis KA, Matyjaszewski K. Adv Polym Sci. 2002;159:2–166. [Google Scholar]
- 6.Matyjaszewski K. Prog Polym Sci. 2005;30:858–875. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bontempo D, Maynard H D. J Am Chem Soc. 2005;127:6508–6509. doi: 10.1021/ja042230+. [DOI] [PubMed] [Google Scholar]
- 8.Tang C, Qi K, Wooley KL, Matyjaszewski K, Kowalewski T. Angew Chem Int Ed. 2004;43:2783–2787. doi: 10.1002/anie.200353401. [DOI] [PubMed] [Google Scholar]
- 9.Koh K, Ohno K, Tsujii Y, Fukuda T. Angew Chem. 2003;42:4194–4197. doi: 10.1002/anie.200351398. [DOI] [PubMed] [Google Scholar]
- 10.Lele BS, Murata H, Matyjaszewski K, Russell AJ. Biomacromolecules. 2005;6:3380–3387. doi: 10.1021/bm050428w. [DOI] [PubMed] [Google Scholar]
- 11.Matyjaszewski K, Spanswick J. Mat Today. 2005;8(3):26–33. [Google Scholar]
- 12.Shen Y, Tang H, Ding S. Prog Polym Sci. 2004;29:1053–1078. [Google Scholar]
- 13.Matyjaszewski K, Pintauer T, Gaynor S. Macromolecules. 2000;33:1476–1478. [Google Scholar]
- 14.Haddleton DM, Jackson SG, Bon SAF. J Am Chem Soc. 2000;122:1542–1543. [Google Scholar]
- 15.Hong SC, Paik H-J, Matyjaszewski K. Macromolecules. 2001;34:5099–5102. [Google Scholar]
- 16.Matyjaszewski K, Davis TP. Handbook of Radical Polymerization. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 17.Fischer H. Chem Rev. 2001;101:3581–3610. doi: 10.1021/cr990124y. [DOI] [PubMed] [Google Scholar]
- 18.Matyjaszewski K, Kajiwara A. Macromolecules. 1998;31:548–550. [Google Scholar]
- 19.Matyjaszewski K, Coca S, Gaynor SG, Wei M, Woodworth BE. Macromolecules. 1997;30:7348–7350. [Google Scholar]
- 20.de Vries A, Klumperman B, de Wet-Roos D, Sanderson RD. Macromol Chem Phys. 2001;202:1645–1648. [Google Scholar]
- 21.Jakubowski W, Matyjaszewski K. Macromolecules. 2005;38:4139–4146. [Google Scholar]
- 22.Min K, Gao H, Matyjaszewski K. J Am Chem Soc. 2005;127:3825–3830. doi: 10.1021/ja0429364. [DOI] [PubMed] [Google Scholar]
- 23.Jakubowski W, Min K, Matyjaszewski K. Macromolecules. 2006;39:39–45. [Google Scholar]
- 24.Jakubowski W, Matyjaszewski K. Angew Chem. 2006;45:4482–4486. doi: 10.1002/anie.200600272. [DOI] [PubMed] [Google Scholar]
- 25.Tang W, Tsarevsky NV, Matyjaszewski K. J Am Chem Soc. 2006;128:1598–1604. doi: 10.1021/ja0558591. [DOI] [PubMed] [Google Scholar]
- 26.Xia J, Gaynor SG, Matyjaszewski K. Macromolecules. 1998;31:5958–5959. [Google Scholar]
- 27.Xia J, Matyjaszewski K. Macromolecules. 1999;32:2434–2437. [Google Scholar]
- 28.Xia J, Matyjaszewski K. Macromolecules. 1997;30:7697–7700. [Google Scholar]
- 29.Patten TE, Xia J, Abernathy T, Matyjaszewski K. Science. 1996;272:866–868. doi: 10.1126/science.272.5263.866. [DOI] [PubMed] [Google Scholar]
- 30.Paoletti P, Ciampolini M. Inorg Chem. 1967;6:64–68. [Google Scholar]
- 31.Anderegg G, Hubmann E, Podder NG, Wenk F. Helv Chim Acta. 1977;60:123–140. [Google Scholar]
- 32.Navon N, Golub G, Cohen H, Paoletti P, Valtancoli B, Bencini A, Meyerstein D. Inorg Chem. 1999;38:3484–3488. doi: 10.1021/ic9812048. [DOI] [PubMed] [Google Scholar]
- 33.Golub G, Lashaz A, Cohen H, Paoletti P, Bencini A, Valtancoli B, Meyerstein D. Inorg Chim Acta. 1997;255:111–115. [Google Scholar]
- 34.Ambundo EA, Deydier M-V, Grall AJ, Aguera-Vega N, Dressel LT, Cooper TH, Heeg MJ, Ochrymowycz LA, Rorabacher DB. Inorg Chem. 1999;38:4233–4242. [Google Scholar]
- 35.Wang F, Sayre LM. J Am Chem Soc. 1992;114:248–255. [Google Scholar]
- 36.Sawyer DT, Sobkowiak A, Roberts JJL. Electrochemistry for Chemists. 2nd Ed. New York: Wiley; 1995. [Google Scholar]
- 37.Ciampolini M, Nardi N. Inorg Chem. 1966;5:41–44. [Google Scholar]
- 38.Tyeklar Z, Jacobson RR, Wei N, Murthy NN, Zubieta J, Karlin KD. J Am Chem Soc. 1993;115:2677–2689. [Google Scholar]
- 39.Wulkow M. Macromol Theor Simul. 1996;5:393–416. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.