Abstract
The reaction between BunLi in benzene and the solid polystyrene support PS-C6H4CH2NH2 leads to a lithiated species that can be represented as PS-C6H4CH2NHLi(LiBu)x, where x ∼ 4, which is active in the ring-opening of the cyclic esters L-lactide, rac-lactide, and 2,5-morpholinediones. With ≈10 eq of these monomeric six-membered rings and with heating, cyclic esters (MeCHC(O)O)n and [MeCHC(O)OCHRC(NH)O]n are reversibly released to the solution. These have been characterized by electrospray ionization MS, and some small rings have been separated by gel-permeation chromatography. Addition of NaBPh4 to a heated benzene solution containing these rings preferentially removes the 18-membered rings from solution. For lactide this is shown to form the basis for chemical amplification from a dynamic combinatorial library and lactide can be converted to (MeCHC(O)O)6 in >80% yield. Metallated supports derived from Me2Mg and Et2Zn are less reactive but do show some ability for lactide ring-enlarging. The 18-membered ring (R,R,R,S,S,S)- and meso-(R,S,R,S,R,S)-(MeCHC(O)O)6 and the 24-membered ring (MeCHC(O)OCHPriC(NH)O)4 have been characterized by single-crystal x-ray diffraction studies, together with the complex Na[η3-S,S,S,S,S,S-(MeCHC(O)O)6]2BPh4.
Keywords: dynamic combinatorial library, ring enlarging
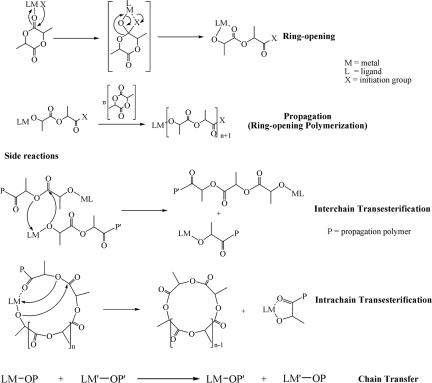
The ring-opening polymerization of cyclic esters such as lactides (LA) is a well established procedure for the production of polyesters (1). This is commonly brought about by the use of metal catalyst precursors, although organic catalysis is possible by employing a nucleophilic base such as 4-dimethyl aminopyridine or an N-heterocyclic carbene with alcohol initiators (2–5). It is now generally accepted that metal-alkoxide species are the key reactive intermediates in the propagation steps and, furthermore, that these, too, play a role in the competing side reactions of transesterification and chain transfer (6). These reactions are collectively shown in Scheme 1.
Scheme 1.
Reactions involved in ring-opening polymerization employing a coordinate catalyst.
Transesterification is normally a deleterious side reaction because it leads to a loss of control of molecular mass in a living polymerization and, because in a stereoselective ring-opening polymerization, transesterification scrambles the stereocenters along a chain. As shown in Scheme 1, transesterification can occur by a bimolecular mechanism or a reaction involving a single chain. The latter reaction, intrachain transesterification leads to cycles. Previously, we found that the polymerization of lactide by Ph2SnX2 initiators, where X = OPri or NMe2, gave different ratios of chains to cycles with the cycles being significantly more prominent for X = NMe2. This apparently arises because of the favorable chelation of the SnOCHMeC(O)NMe2 groups (7).
Prompted by these observations, we set out to explore a synthetic route for cyclic oligoesters derived from LA and we describe herein our findings. It is worthy of mention that others have synthesized cyclic esters and carbonates principally as a route for the formation of polyesters and polycarbonates (8).
Brunelle and coworkers (9) at GE Corporate Research and Development specifically developed high-yielding syntheses of cyclic esters derived from 1,4-aromatic acyl chlorides and diols. These ringed compounds melt to yield low viscous media, which by a thermo-neutral ring-opening polymerization give polyesters with no elimination products. This chemistry forms the technology platform of Cyclics Corporation.
Kricheldorf and coworkers (10, 11) have used principally tin(IV) diolates as initiators for the ring-opening polymerization and the reaction with dicarboxylic acid chlorides to cleave the cyclic esters.
Hodge and coworkers (12) used a solid support and step growth polymerization to produce a polyester chain followed by treatment with a metal catalyst, most often Bun2SnO, to affect intrachain transesterification and release of the cyclic esters to the solution phase.
Watanabe et al. (13) have reported in the patent literature that various lithium reagents, specifically alkoxides, thiolates, and amides, react with rac-LA in THF at approximately −80°C to produce 75∼95% cyclic oligolactides. Aside from a condensation procedure, this is the only previous report of the synthesis of cyclic oligolactides, and although significant quantities of cyclic oligomers are formed they are present along with chains.
Finally, we note that Bachmann and Seebach (14) have reported the preparation and characterization of cyclic lactones (MeCHCH2C(O)O)n, where n = 4 and 8.
In all of the above, the formation of the cyclic oligoesters involves a stoichiometric and stepwise procedure. In this work, we describe a method that provides for a “living” or “immortal” catalytic procedure for the ring enlarging of lactide and 2,5-morpholinediones that, as shown below, may be viewed as symmetric A2 and asymmetric AB rings. The immortal nature of the catalyst arises from the fact that it is recoverable and reusable.
Results and Discussion
Cyclic Oligolactides.
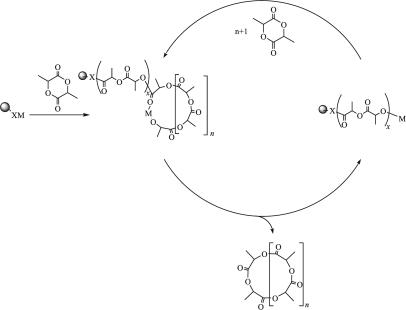
To suppress bimolecular reactions, we have used a commercially available polystyrene resin having benzylamine functionality, represented hereafter as polystyrene-supported (PS)-C6H4CH2NH2. The strategy that was followed was to metallate the support with species known to facilitate ring-opening polymerization of LA and to rely on intrachain transesterification to release cycles to the solution. This reaction sequence is depicted in Scheme 2, and, depending on the relative rates of ring-opening polymerization, krop, and transesterification, ktrans, two limiting scenarios can be envisaged: (i) If krop ≫ ktrans, then a two-step process could be operated where polymerization was run at a temperature T1 and cyclization at T2 (T2 > T1). (ii) Alternatively, if krop ∼ ktrans, then the reaction could be run at one temperature. In the latter case the reaction could be run to reach equilibrium, whereas in the former case the rings could be released from the support under kinetic conditions.
Scheme 2.
Releasing of cyclic oligolactides from solid supported catalyst. As shown the rings are released containing whole numbers of lactide, but back-biting may occur anywhere along the chains to release rings (MeCHC(O)O)n, where n is even or odd.
The PS-C6H4CH2NH2 was allowed to react with LiBun (excess) in benzene solution for 72 h, and then the support was collected by filtration, washed with benzene, and dried under vacuum. A similar procedure was followed for the preparation of Mg and Zn metallated supports by employing Me2Mg and Et2Zn, respectively.
Analysis of the lithiated support revealed that LiBun was still present, and we formulate the active species as a lithium aggregate of the type PS-C6H4CH2NHLi(LiBu)x, where x ∼ 4 based on Gilman titration (15). The aggregation of lithium is perhaps not surprising since LiBun is known to be a hexamer in hexane and an amido group could easily be incorporated within this type of aggregate (16). This procedure can be viewed as the preparation of the “catalyst precursor.”
The lithiated support was then allowed to react with L-LA (10 eq per N) in benzene at room temperature for 10 h. The support was collected by filtration and resuspended in benzene and heated to 60–70°C. The support was again collected by filtration and washed with benzene and dried under a dynamic vacuum yielding “the catalyst.” By elemental analysis the N-to-Li ratio is ≈1:5, which supports the view that the resting state of the catalyst can be viewed as an alkoxide lithium aggregate attached to the support. Note that a lithium amide will ring-open lactide to generate LiOCHMeC(O)OCHMeC(O)NHR and that the butyllithium will react to give the lithium enolate of lactide. Collectively, these will represent the species initially present in the lithium aggregate and each may ring-open further lactide molecules.
The catalyst system so prepared reacts with LA in benzene at room temperature to take up LA (chain growth), but only upon heating to ≈70°C are cycles released to the solution in significant quantities. The combined reactions of ring-opening polymerization and transesterification can thus be run together at ≈70°C with conversion of LA to cyclic oligoesters.
Under the same conditions the magnesium- and zinc-supported catalysts were notably less reactive and only at 70°C in benzene did significant reactions occur such that within 4 h 47% conversion to cycles was observed for M = Mg and 14% conversion for M = Zn. The reactivity order Li > Mg > Zn is consistent with what is known for homogeneous reactions in solvents such as benzene.
In the above reactions, the cyclic oligoesters present in solution are isolated by filtration and removal of the solvent from the filtrate. They are waxy solids soluble in common organic solvents such as toluene, benzene, chloroform, and acetone.
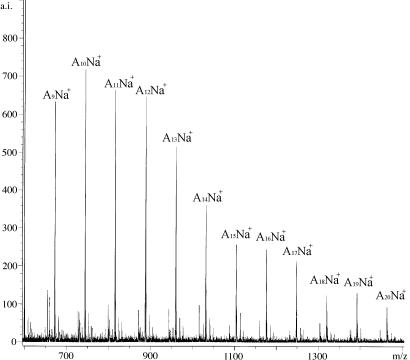
When ≈10 eq of L-LA was used in the reaction with the lithiated support a range of cyclic species, An, where A = MeCHC(O)O, was observed in the mass spectrum (electrospray ionization MS and MALDI-TOF) as shown in Fig. 1. By gel-permeation chromatography (GPC), we observe a fairly broad molecular-mass distribution, polydispersity index ∼ 2.0 and Mn ∼ 500–1,000 Da.
Fig. 1.
MALDI-TOF of cyclic oligolactide derived on PS-C6H4CH2NHLi·(BuLi)x (x = 3.7). A = C3H4O2.
By 1H NMR spectroscopy the cycles show many resonances in contrast to poly(L-LA). This may be attributable in part to the presence of many different cycles An, but it is also because epimerization has occurred. Indeed, examination of the products formed from the lithiated support indicates essentially complete loss of optical activity when L-LA is used as the monomer source, whereas those An rings formed from Mg and Zn supports retain some optical activity.
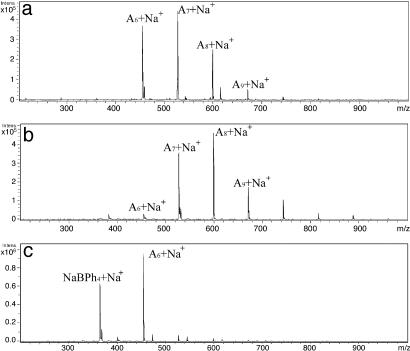
The release of cycles to the solution could occur in a reversible manner and thus at some time, t∞, the system would be described as a dynamic combinatorial library of rings (17–19). If a guest ion was present that selectively bound to one of the rings then the system could be moved to favor the presence of that host–guest complex ion. To test for this hypothesis, we introduced an isolated group of An molecules in benzene solution to a suspension of NaBPh4. Upon heating to 60°C the A6 rings were selectively removed from the solution as is evident from the mass spectra shown in Fig. 2. Based on the known structure of a glycolide complex Na(CH2C(O)O)6·BPh4·(CH3CN)2, we propose that the A6 molecules form a polymer chain in the solid state where three ketonic oxygen atoms donate to one Na+ ion and three to the other (unpublished results). This mode of bonding is suggested by the structure of (MeCHC(O)O)6 molecules (see below) and is akin to that seen in the solid state of K(eniatin-B)NCS, where the K+ ions form a polymeric chain bridged by the cyclodepsipeptide (20).
Fig. 2.
Electrospray ionization MS of results of cyclic oligolactide reacted with NaBPh4. (a) Cyclic oligolactide before reaction. (b) Cyclic oligolactide after reaction. (c) NaBPh4 after reaction.
Chemical Amplification for the Formation of A6 Rings.
The ability of NaBPh4 to sequester the (MeCHC(O)O)6 rings suggested that we could achieve conversion of LA monomers to this specific ring provided that the rings present in solution behaved as a dynamic combinatorial library. This principle has been elegantly demonstrated by the Sanders group (21) for cyclic peptide-hydrazones. We have found that when the polymer support (1 eq based on N atom content) is allowed to react with L-LA (24 eq) in the presence of a suspension of NaBPh4 (10 eq) in benzene at 80°C for 20 h, the A6 rings are removed from solution with the formation of NaBPh4(MeCHC(O)O)6. The latter is soluble in acetonitrile, and based on the integration of the proton signals arising from BPh4− and A6 in the 1H NMR spectrum recorded in CD3CN the conversion of LA to A6 is >80% based on NaBPh4.
By 1H NMR spectroscopy, we also see that the A6 rings sequestered in this manner are enriched to the order of ≈50% in one stereo isomer (see Separation and Isolation of Rings). Thus, we can conclude that the rate of epimerization is less than the rate of ring-opening of L-LA and that complete thermodynamic control is not operative when NaBPh4 preferentially removes the A6 rings.
In the formation of An rings the lithiated support can be collected and repeatedly used in the production of cyclic oligolactides, and in our hands this has been done nine times with little loss of activity. In this regard, we consider the catalyst to be living or immortal, and thus the present procedure is distinct from earlier procedures that produce cyclic esters by stoichiometric reactions.
Ring Enlarging of 2,5-Morpholinediones.
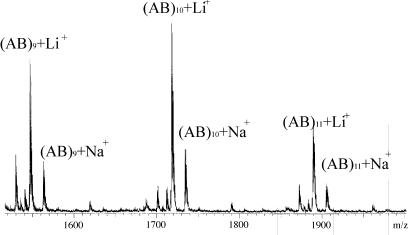
When the morpholinediones where R = Pri, CH2Pri are allowed to react with the lithiated support in benzene solution at room temperature (10–20 h) and the filtrate is collected, it is found to contain some unreacted morpholinedione and a series of rings (AB)n as determined by mass spectrometry. For R = Me and CH2Ph the procedure to obtain (AB)n rings was the same as for lactide. As is shown in Fig. 3, only ring sizes in multiples of six are released to the solution, which implies a regioselective transesterification process.
Fig. 3.
MALDI-TOF of cyclic 3-isopropyl-6-methyl-2,5-morpholinedione oligomers derived on PS-C6H4CH2NHLi(BuLi)x. AB = C8H13O3N.
Separation and Isolation of Rings.
The lower-molecular-mass rings An or (AB)n can be isolated by careful and repeated GPC separation, and as noted before in the case of A = MeCHC(O)O, the A6 rings can be sequestered by complexation with NaBPh4.
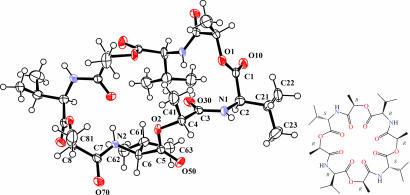
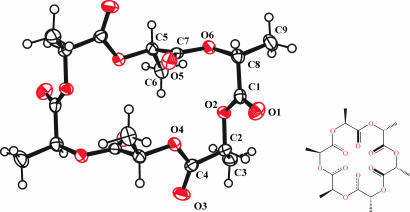
For a reaction involving the 2,5-morpholinedione where R = iPr, we isolated a crystalline component from a set of (AB)4 rings and determined its structure by single-crystal x-ray crystallography. An Oak Ridge Thermal Ellipsoid Plot (ORTEP) drawing of this 24-membered cyclodepsipeptide is shown in Fig. 4. The four stereocenters of the CHMe carbons are all R, whereas for the CHPri carbons two are R and two are S; in the starting six-membered ring all of the methine carbons have S stereochemistry. The molecule has crystallographically imposed C2 symmetry.
Fig. 4.
ORTEP drawing of the molecular structure of cyclic 3-isopropyl-6-methyl-2,5-morphylinedione tetramer found in the solid state. The molecule contains a twofold rotation axis. The ellipsoids are drawn at the 50% probability level. The hydrogen atoms are drawn with an arbitrary radius.
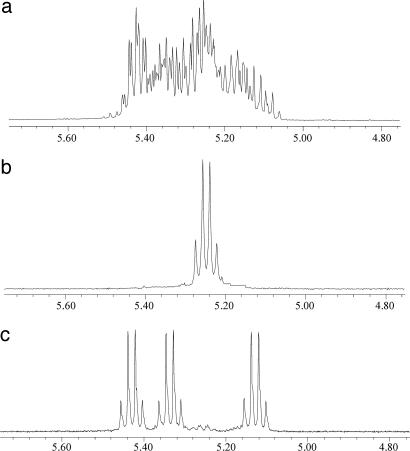
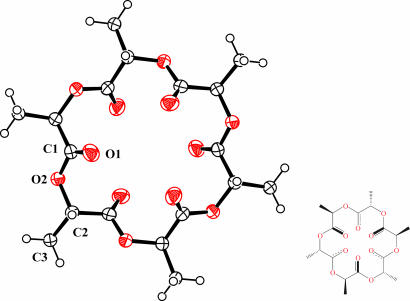
As noted earlier the lithiated support causes epimerization of the lactide methine carbons, and when the reactions are run under complete thermodynamic control the An rings are collectively optically inactive. The 1H NMR spectrum of a GPC-separated A6 sample is shown in Fig. 5a. Only the methine 1H resonance is shown, and this is seen to be a complex pattern of overlapping quartets due to 1H-1H coupling with the methyl groups. However, the meso-A6 ring R,S,R,S,R,S-(MeCHC(O)O)6 can be isolated from this mixture of 14 isomers, which include six enantiomeric pairs, by crystallization from chloroform solutions. This isomer is notably less soluble and readily crystallizable. The 1H NMR spectrum of this meso isomer is shown in Fig. 5b and consists of just one methine quartet. The molecular structure of this isomer was also determined by crystallography, and an ORTEP drawing is shown in Fig. 6. In the solid state the molecule has crystallographically imposed S6 symmetry. All six ketonic oxygens are directed inward, but three are directed above and three below the plane of the 18-membered ring.
Fig. 5.
1H NMR spectroscopy of (CH3CHC(O)O)6, A6 at the methine region. (a) A6 prepared with PSC6H4CH2NHLi + L-LA and separated by GPC. (b) (S,R,S,R,S,R)-(CH3CHC(O)O)6. (c) (S,S,S,R,R,R)-(CH3CHC(O)O)6.
Fig. 6.
ORTEP drawing of the molecular structure of (S,R,S,R,S,R)-(CH3CHC(O)O)6 found in the solid state. The molecule contains a crystallographic 3 axis. The ellipsoids are drawn at the 50% probability level. The hydrogen atoms are drawn with an arbitrary radius.
When the A6 rings are formed and sequestered by NaBPh4, they have to be removed by chemical and chromatographic procedures (see Experimental Procedures). When reactions are carried out with L-LA, the A6 rings are enriched in the enantiomers 6S and 5S,1R. Reactions employing rac-LA, conversely, yield enrichment of the 3R,3S and 2R,4S/4R,2S isomers. By chromatography several of these isomers have now been separated and characterized by NMR spectroscopy and single-crystal x-ray crystallography. At this time we note the structure of the meso isomer R,R,R,S,S,S-(MeCHC(O)O)6 shown in Fig. 7. The molecule has a crystallographically imposed center of inversion, and it can be seen that only two of the six ketonic oxygens are directed inward. The 1H NMR spectrum of the R,R,R,S,S,S-A6 isomer is shown in Fig. 5c. There are three methine quartets because each methine carbon is flanked in an asymmetric environment by either a ketonic group or an ester oxygen.
Fig. 7.
ORTEP drawing of the molecular structure of (S,S,S,R,R,R)-(CH3CHC(O)O)6 found in the solid state. The molecule contains a crystallographic inversion center. The ellipsoids are drawn at the 50% probability level. The hydrogen atoms are drawn with an arbitrary radius.
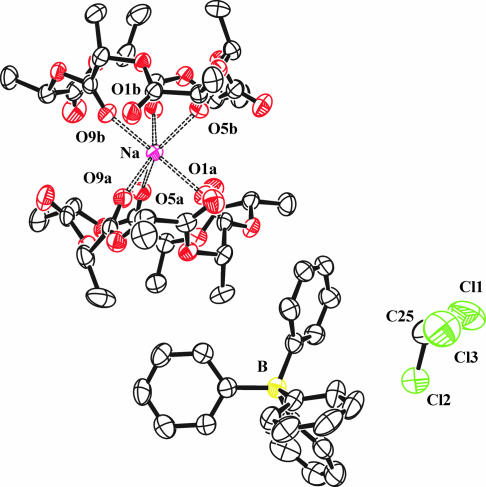
The 6S-(MeCHC(O)O)6 isomer has also been isolated as a complex with sodium tetraphenylborate by crystallization from chloroform, namely as the salt Na(A6)2BPh4·CHCl3. As shown in Fig. 8, the two A6 rings bind to the Na+ cation to generate a six-coordinate pseudo octahedral NaO6 geometry. Each A6 ring forms a facial η3-O unit. Three ketonic oxygens are directed inward toward the Na+ ion, and three are directed out of the plane of the 18-membered ring. This complexation of the A6 ring also causes the methyl groups to be directed in a similar manner with three above the ring and three directed outward.
Fig. 8.
ORTEP drawing of the molecular structure of Na[(S,S,S,S,S,S)-(CH3CHC(O)O)6]2BPh4·CHCl3 found in the solid state. The ellipsoids are drawn at the 50% probability level. The hydrogen atoms are not shown. Selected bond distances: Na-O(1a), 2.289(2) Å; Na-O(5a), 2.297(2) Å; Na-O(9a), 2.326(2) Å; Na-O(1b), 2.323(2) Å; Na-O(5b), 2.291(2) Å; Na-O(9b), 2.294(2) Å.
Concluding Remarks
The procedures described above allow for the conversion of lactide and 2,5-morpholinediones to their respective cyclic oligoesters and cyclodepsipeptides. The ring enlargement occurs by the combined operations of ring opening and monomer enchainment followed by back-biting and ring expulsion from the supported catalyst to the solution. In contrast to the conventional preparation of cycles, high-dilution techniques are not required and no linear chains are present in the solution. The rings present in solution can back-react with support so that in time the rings present will be in equilibrium and as such form a dynamic combinatorial library. That for lactide the 18-membered rings are removed selectively by NaBPh4 in >80% yield based on the reaction 3 A2 = A6 testifies to the process of chemical amplification from a dynamic equilibrium of An rings. Although we have not examined the kinetics of these reactions, we can state that k(ring opening) > k(transesterification) > k(epimerization) for the lithiated support and moreover that the reactivities of the metallated support is Li > Mg > Zn. We believe it should be possible to find active supported metal catalysts that will be capable of ring opening and transesterification without epimerization and thus yield rings where the stereocenters of the monomer are retained. We also propose that other substrates may be used to effect chemical amplification of rings of different sizes.
Given the utility of crown ethers in cation binding and the synthesis of a wide variety of macromolecular assemblies (22–24), these cyclic esters may find some use in the transport and release (by hydrolysis) of substrates to which they have a high affinity. Similar considerations pertain for the preparation of cyclodepsipeptides, which are a class of nature's cation binding and transport agents.
Experiment Procedures
General.
Standard vacuum line, Schlenk flasks, and N2-atmosphere dry box techniques were used. Benzene was distilled from a potassium suspension. Acetonitrile was distilled from a CaH2 suspension. 1.0 M Et2Zn solution in hexane, 3.0 M MeMgCl solution in hexane, 2.5 M n-BuLi solution in hexane, sodium tetraphenylborate, and N,N,N′,N′-tetramethylethylenediamine (TMEDA) were purchased from Aldrich and used as received. Polystyrene-supported amine (PS-C6H4CH2NH2) was purchased from Matrix Innovation and used as received. L- and (D,L)-LA were purchased from Aldrich and sublimed twice at 60°C under 10−2 mbar of pressure (1 bar = 100 kPa). 2,5-Morpholinedione complexes were prepared according to the procedures described in ref. 25.
Preparation of PS amino lithium catalyst [PS-C6H4CH2NHLi·(n-BuLi)x].
PS amine (PS-C6H4CH2NH2, product of Matrix Innovation; 3.00 g, 4.71 mmol) was suspended in benzene (20 ml) under a nitrogen atmosphere. n-BuLi (2.5 M, 11 ml, 28 mmol) was added, and the suspension was stirred for 72 h. The beads were then filtered off, washed with benzene (two times, 10 ml) and hexane (three times, 10 ml), and dried under vacuum. The product (3.8 g) was purple-colored powder with reduced absorptions at 3,060 cm−1 (N-H) compared with the PS-C6H4CH2NH2 starting material. From Gilman titration the N-to-Li ratio was determined to be 1:4.7. Subsequent to treatment with L-LA and the synthesis of cyclic oligolactides, elemental analysis gave an N-to-Li ratio of 1:4.9.
Preparation of PS amino magnesium and zinc catalyst.
PS amino magnesium and zinc catalyst are given in the Supporting Text, which is published as supporting information on the PNAS web site.
Preparation of cyclic oligolactide using PS amino lithium catalyst [PS-C6H4CH2NHLi·(BuLi)x].
(i) PS amino lithium [PS-C6H4CH2NHLi·(BuLi)x; 0.37 g, 0.59 mmol] and L-LA (0.88 g, 6.1 mmol) were mixed together. Benzene (20 ml) was added, and the mixture was stirred at room temperature for 18 h. The beads were then filtered off and washed with benzene (two times, 10 ml). The combined filtrate and washings were evaporated to give a yellow, wax-like solid (0.34 g, 38%). The recovered resin was resuspended in benzene (20 ml) and heated at 60°C for 4 h. The beads were then filtered off and washed with benzene (two times, 5 ml). The combined filtrate and washings were evaporated to give a yellow, waxy solid (0.19 g, 22%).
(ii) The resin from experiment i was recovered, L-LA (0.84 g, 5.8 mmol) and benzene (20 ml) were added, and this was stirred at room temperature for 10 h. The beads were then filtered off, washed with benzene (two times, 10 ml), resuspended in benzene (20 ml), and heated at 60°C for 6 h. The resin was then filtered off again and washed with benzene (two times, 10 ml). The combined filtrate and washings were evaporated to give a colorless, wax-like solid (0.37 g, 44%). 1H NMR spectrum showed that it was 98% oligomers (2% LA). The solid was dissolved in methanol and applied in electrospray ionization MS, showing that 100% cyclic oligolactide were present.
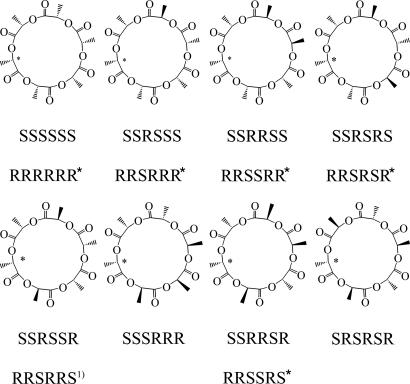
(iii) Repeating step ii gave recycling reactions. Preparative GPC was applied over the 2.0 g of the cyclic oligolactide prepared above to give 0.035 g of A6, which crystallized out the (S,R,S,R,S,R)-(CH3CHC(O)O)6 (Fig. 9).
Fig. 9.
Stereo isomers of (CH3CHC(O)O)6 (A6). Asterisk indicates mirror enantiomer.
Chemical amplification for the formation of A6 rings.
PS amino lithium catalyst (0.42 g) prepared with PS amine (0.22 g, 0.35 mmol) according to the procedure of step i above was mixed with L-LA (1.20 g, 8.3 mmol) and NaBPh4 (1.16 g, 3.4 mmol). Benzene (30 ml) was added, and the mixture was stirred at 80°C for 20 h. The beads were then filtered off, washed with hot benzene (70°C, 20 ml), and dried under vacuum. To the resulting beads was added 40 ml of CH3CN, and the suspension was stirred for 1 h before filtration and washing. The filtrate and washings were combined and evaporated to give a white solid (2.26 g). 1H NMR and electrospray ionization MS show the molecular formula of [(CH3CHC(O)O)6]0.83NaBPh4 (yield 95% based on NaBPh4).
See Supporting Textand Data Sets 1–4, which are published as supporting information on the PNAS web site, for detailed experimental information, crystallographic data sets, and preparation of (S,S,S,R,R,R)-(CH3CHC(O)O)6, Na[(S,S,S,S,S,S)-(CH3CHC(O)O)6]2BPh4·CHCl3, cyclic oligolactide using PS amino magnesium and zinc catalysts (PS-C6H4CH2NHMgCH3), and cyclodepsipeptides using PS amino lithium catalyst [PS-C6H4CH2NHLi·(BuLi)x] and monomer (3S,6S)-3-isopropyl-6-methyl-2,5-morpholinedione, (3S,6S)-3-isobutyl-6-methyl-2,5-morpholinedione, (3S,6S)-3,6-dimethyl-2,5-morpholinedione, and (3S,6S)-3-benzyl-6-methyl-2,5-morpholinedione.
Summary of Crystal Data.
(S,R,S,R,S,R)-(CH3CHC(O)O)6: C18H24O12, Cambridge Crystallographic Data Centre (CCDC) reference no. 271312, M = 432.37, trigonal, space group R3, a = 12.128(3) Å, c = 11.821(4) Å, V = 1,505.7(7) Å3, T = 150(2) K, Z = 3, μ = 0.122 mm−1, 9,752 reflections collected, 771 independent (Rint = 0.031), R1 = 0.045 for 584 reflections with I > 2σ(I); (AB)4: C32H52N4O12, CCDC reference no. 271309, M = 684.78, orthorhombic, space group C2221, a = 14.837(1) Å, b = 14.951(1) Å, c = 17.066(1) Å, V = 3,785.8(4) Å3, T = 95(2) K, Z = 4, μ = 0.022 mm−1, 7,626 reflections collected, 1,927 independent (Rint = 0.031), R1 = 0.057 for 1,724 reflections with I > 2σ(I); (S,S,S,R,R,R)-(CH3CHC(O)O)6: C18H24O12, CCDC reference no. 602674, M = 432.37, triclinic, space group P1, a = 6.927(2) Å, b = 8.341(2) Å, c = 10.428(4) Å, α = 71.04(1)°, β = 74.21(1)°, γ = 66.98(1)°, V = 517.1(3) Å3, T = 150(2) K, Z = 1, μ = 0.118 mm−1, 12,235 reflections collected, 2,340 independent (Rint = 0.031), R1 = 0.034 for 1,766 reflections with I > 2σ(I); Na[(S,S,S,S,S,S)-(CH3CHC(O)O)6]2BPh4·CHCl3: [(C18H24O12)2Na]+[B(C6H5)4]−·CHCl3, CCDC reference no. 602675, M = 1,326.31, triclinic, space group P1, a = 10.975(1) Å, b = 10.979(1) Å, c = 15.835(2) Å, α = 85.878(3)°, β = 71.854(4)°, γ = 67.253(5)°, V = 1,669.6(3) Å3, T = 220(2) K, Z = 1, μ = 0.220 mm−1, 35,501 reflections collected, 11,322 independent (Rint = 0.027), R1 = 0.038 for 9,643 reflections with I > 2σ(I).
Supplementary Material
Acknowledgments
We thank Dr. Maren Pink (Indiana University, Bloomington, IN) for the synchrotron data collection of C32H52N4O12 at the Advanced Photon Source, Argonne National Laboratory and the Department of Energy, Office of Basic Sciences, Chemical Division, for support of this work. The Advanced Photon Source is supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract W-31-109-Eng-38. ChemMatCARS Sector 15 is principally supported by the National Science Foundation/Department of Energy under Grant CHE0087817 and the Illinois Board of Higher Education.
Abbreviations
- GPC
gel-permeation chromatography
- LA
lactide(s)
- ORTEP
Oak Ridge Thermal Ellipsoid Plot
- PS
polystyrene-supported.
Footnotes
Conflict of interest statement: No conflicts declared.
This article is a PNAS direct submission.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference nos. 271309, 271312, 602674, and 602675).
References
- 1.Dechy-Cabaret O, Martin-Vaca B, Bourissou D. Chem Rev. 2004;104:6147–6176. doi: 10.1021/cr040002s. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe BJ, Hillmyer MA, Tolman WB. J Chem Soc Dalton Trans. 2001:2215–2224. [Google Scholar]
- 3.Nederberg F, Connor EF, Glausser T, Hedrick JL. Chem Commun. 2001:2066–2067. doi: 10.1039/b106125a. [DOI] [PubMed] [Google Scholar]
- 4.Nederberg F, Connor EF, Moeller M, Glauser T, Hedrick JL. Angew Chem Int Ed. 2001;40:2712–2715. [PubMed] [Google Scholar]
- 5.Nyce GW, Glauser T, Connor EF, Möck A, Waymouth RM, Hedrick JL. J Am Chem Soc. 2003;125:3046–3056. doi: 10.1021/ja021084+. [DOI] [PubMed] [Google Scholar]
- 6.Chisholm MH, Delbridge EE. New J Chem. 2003;27:1177–1183. [Google Scholar]
- 7.Chisholm MH, Delbridge EE, Gallucci JC. New J Chem. 2004;28:145–152. [Google Scholar]
- 8.Semlyen JA. Cyclic Polymers. 2nd Ed. Boston: Kluwer Academic; 2000. [Google Scholar]
- 9.Brunelle DJ, Bradt JE, Serth-Guzzo J, Takekoshi T, Evans T, Pearce EJ, Wilson PR. Macromolecules. 1998;31:4782–4790. doi: 10.1021/ma971491j. [DOI] [PubMed] [Google Scholar]
- 10.Kricheldorf HR, Chatti S, Schwarz G. Macromol Symp. 2004;210:93–99. [Google Scholar]
- 11.Kricheldorf HR. J Polym Sci Polym Chem Ed. 2004;42:4723–4742. [Google Scholar]
- 12.Ruddick CL, Hodge P, Cook A, McRiner A. J Chem Soc Perkin Trans. 2002;1:629–637. [Google Scholar]
- 13.Watanabe M, Takano J, Ishihara Y, Murakami M. WO 2001021612 A1 20010329. PCT International patent application. 2001
- 14.Bachmann BM, Seebach D. Helv Chim Acta. 1998;81:2430–2461. [Google Scholar]
- 15.Gilman H, Carledge FK, Sim SY. J Organomet Chem. 1963;1:8–14. [Google Scholar]
- 16.Verstraete P, Deffieux A, Fritsch A, Rayez JC, Rayez MT. THEOCHEM. 2003;631:53–66. [Google Scholar]
- 17.Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, Stoddart JF. Angew Chem Int Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Lehn J-M, Eliseev AV. Science. 2001;291:2331–2332. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]
- 19.Furlna RLE, Otto S, Sanders JKM. Proc Natl Acad Sci USA. 2002;99:4801–4804. doi: 10.1073/pnas.022643699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhukhlistova NE. Crystallogr Rep. 2002;47:433–442. [Google Scholar]
- 21.Roberts SL, Furlan RL, Otto S, Sanders JKM. Org Biomol Chem. 2003;1:1625–1633. doi: 10.1039/b300956d. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen CJ. J Am Chem Soc. 1967;89:7017–7036. [Google Scholar]
- 23.Pedersen CJ. Angew Chem Int Ed Engl. 1988;27:1021–1027. [Google Scholar]
- 24.Collin J-P, Dietrich-Buchecker C, Gaviña P, Jimenez-Molero MC, Sauvage J-P. Acc Chem Res. 2001;34:477–487. doi: 10.1021/ar0001766. [DOI] [PubMed] [Google Scholar]
- 25.Jörres V, Keul H, Höcker H. Macromol Chem Phys. 1998;199:825–833. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.