Abstract
Coiled-coil proteins contain a characteristic seven-residue sequence repeat whose positions are designated a to g. The interacting surface between α-helices in a classical coiled coil is formed by interspersing nonpolar side chains at the a and d positions with hydrophilic residues at the flanking e and g positions. To explore how the chemical nature of these core amino acids dictates the overall coiled-coil architecture, we replaced all eight e and g residues in the GCN4 leucine zipper with nonpolar alanine side chains. Surprisingly, the alanine-containing mutant forms a stable α-helical heptamer in aqueous solution. The 1.25-Å resolution crystal structure of the heptamer reveals a parallel seven-stranded coiled coil enclosing a large tubular channel with an unusual heptad register shift between adjacent staggered helices. The overall geometry comprises two interleaved hydrophobic helical screws of interacting cross-sectional a and d layers that have not been seen before. Moreover, asparagines at the a positions play an essential role in heptamer formation by participating in a set of buried interhelix hydrogen bonds. These results demonstrate that heptad repeats containing four hydrophobic positions can direct assembly of complex, higher-order coiled-coil structures with rich diversity for close packing of α-helices.
Keywords: protein design, protein structure, helix–helix interfaces, buried polar interactions, cavity
Helix–helix interactions are ubiquitous in the native structure of proteins and in associations among proteins, including supramolecular assemblies and transmembrane receptors that mediate cellular signaling and transport. Coiled coils afford a unique model system for elucidating principles of molecular recognition between helices (1–4). The conformation of coiled coils is prescribed by a characteristic 7-aa repeat, the 3–4 heptad repeat denoted as a-b-c-d-e-f-g (5). Positions a and d are typically occupied by hydrophobic amino acids such as Leu, Ile, Val, and Ala, whereas residues at positions e and g are frequently polar or charged (5–8). Crick's (9) classical analysis proposed that the nonpolar a and d side chains associate by means of complementary “knobs-into-holes” packing to form supercoiled α-helical ribbons. Beyond the role of hydrophobic side chains at the core a and d positions, coiled coils can use intra- and interhelical electrostatic interactions to tune their stability, especially those between the flanking e and g positions of neighboring chains (10–18). Despite their seeming regularity in sequence and packing patterns, coiled coils exhibit remarkable diversity in the number and arrangement of the associating helices: two- to five-stranded structures have been identified with parallel or antiparallel helix orientation (2). The structural parameters of dimers have been described by Crick (9), and general rules governing the close packing of α-helices in hexamers and dodecamers have been deduced (19, 20).
Analysis of coiled coils provides insight into the general problem of protein folding by simplifying the geometrical and topological context. Although the hydrophobic cores of globular proteins can be repacked quite freely, formation of an apolar core in coiled coils is spatially constrained, allowing dissection of their structure and folding in unprecedented detail (21, 22). The canonical knobs-into-holes packing of coiled coils confers exquisite sensitivity to the stereochemical properties of the core a and d residues. Interior packing of the side chains at the a and d positions, in fact, has been shown to dominate the global architecture of coiled coils (23). Polar side chains at the a and d positions also can destabilize coiled-coil structure yet impose a high degree of conformational selectivity (4, 24). Moreover, ionic interactions between the e and g residues have been shown to influence the specificity of coiled-coil assembly (10–18). For example, repulsive or attractive interactions between the e and g side chains can control the extent of homo- versus heterodimerization in model coiled coils (10). This current level of understanding of the determinants of coiled-coil structure has allowed formulation of robust rules for coiled-coil design, embodied in algorithms that are available for this purpose. Thus, it seems remarkable that fundamental elements of coiled-coil formation still remain incompletely understood. Here, we report a striking example resulting from an unanticipated role of nonpolar side chains at the normally charged e and g positions.
Recent experiments show that the presence of apolar amino acids at either the e or g position of the heptad repeat can specify formation of antiparallel four-helix structures (see refs. 17 and 18). In these tetramers, the buried a, d, e and a, d, g side chains interlock in nonclassical combinations of knobs-against-knobs and knobs-into-holes packing to form the hydrophobic core. The lac repressor type of core packing, for example, is of the a, d, e class, with the helices offset by 0.25 heptad (25, 26); on the other hand, in the severe acute respiratory syndrome coronavirus S2 protein, the packing involves the a, d, g side chains with the helix register shifted by 0.5 heptad (27). Evidently, patterning of hydrophobic and polar residues beyond the canonical 3–4 coiled-coil sequence can lead to distinctive three-dimensional structures with characteristic supercoil shapes and exceptional stability. We have previously investigated the basis of a, d, g core packing by characterizing variants of the GCN4 leucine zipper that adopt antiparallel tetrameric structures in response to hydrophobic substitutions at the g positions (17). Here, we report the engineering of a GCN4 leucine-zipper variant containing exclusively alanine residues at the e and g positions (Fig. 1). Surprisingly this mutant peptide folds into a hitherto unreported seven-stranded coiled coil consisting of two interleaved parallel helical screws in which the helices are staggered in phase with the heptad repeat. This heptamer highlights the need to extend current rules for coiled-coil folding and assembly to include nonpolar side chains at the e and g positions. In addition, it provides a soluble model for analyzing intrahelical interactions in more complex protein interfaces and makes available a unique nanoscale model cavity to explore by using different solutes.
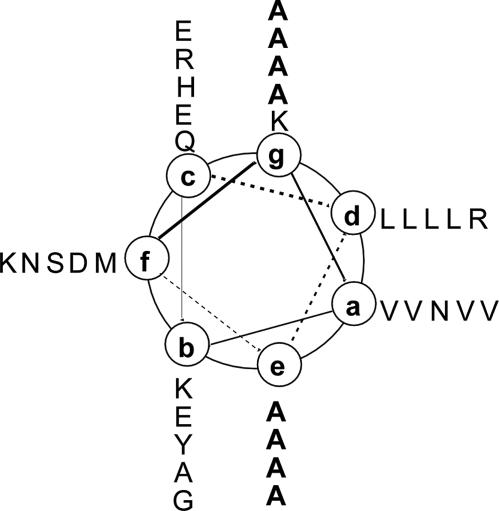
Fig. 1.
Helical wheel projection of residues Met-1 to Arg-34 of the GCN4-pAA sequence. The view is from the N terminus. Heptad repeat positions are labeled a through g. GCN4-pAA differs from the recombinant dimeric leucine-zipper peptide GCN4-pR by alanine substitutions at four e and four g positions (bold). The sequences of GCN4-pR and GCN4-pAA (with the eight mutated e and g positions set in italics) are as follows: GCN4-pR, MK VKQLEDK VEELLSK NYHLENE VARLKKL VGER; GCN4-pAA, MK VKQLADA VEELASA NYHLANA VARLAKA VGER. The GCN4-pR sequence contains an additional Met-Lys-Val and no Arg-Met at its N terminus but is otherwise identical to GCN4-p1.
Results and Discussion
Design of GCN4-pAA.
We chose the dimeric GCN4 leucine zipper as our engineering scaffold because it represents the prototypical and best-studied model with which to decipher the structural determinants of coiled-coil assembly and geometry (23). The hydrophobic interface between the two α-helical chains of the leucine zipper is formed by interlocking of residues at positions a, d, e, and g (28). Interhelical ionic interactions between charged side chains at the e and g positions also can contribute to dimerization specificity (28). Our previous studies have demonstrated that replacing g residues with nonpolar amino acids in the recombinant leucine-zipper peptide GCN4-pR can switch both stoichiometry and helix orientation to produce a stable antiparallel tetramer structure (17). This finding suggests that the selection of hydrophobic residues at both the e and g positions of the 3–4 heptad repeat might offer another mechanism of mediating association of α-helices. To investigate this possibility, we engineered a GCN4-pR variant called GCN4-pAA with alanine substitutions at four e and four g positions (Fig. 1). Alanine was selected for its minimal apolar side chain and high helix-formation propensity (29). Moreover, because alanine has relatively low hydrophobicity, we surmised that bulky apolar side chains at the a and d positions of the parental sequence might still act as the dominant factor in the folding reaction of GCN4-pAA.
Solution Properties of GCN4-pAA.
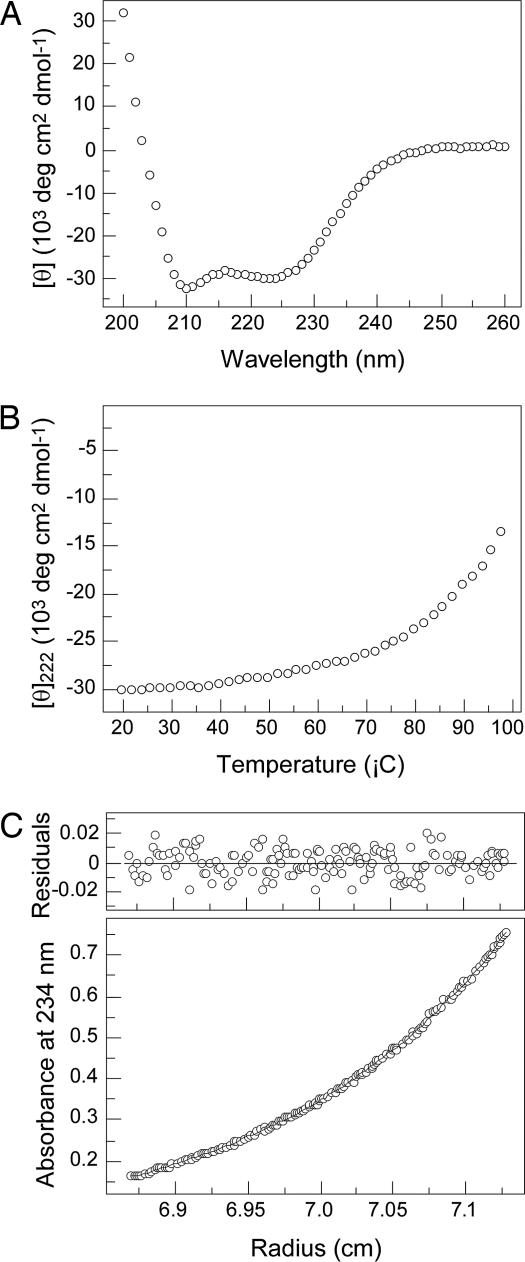
The GCN4-pAA peptide is only marginally soluble in aqueous solutions in the pH range of 6.5–8.5 (<10 μM) but attains solubility of >1 mM in acetate-buffered saline (ABS) (50 mM sodium acetate, pH 5.2/150 mM NaCl), which might be attributed to the high hydrophobic side-chain content and high isoelectric point (pI = 8.21) of the engineered peptide. CD measurements at 50 μM peptide concentration in ABS show that GCN4-pAA is ≈90% helical at 20°C (Fig. 2A) and displays a cooperative thermal unfolding transition with a melting temperature of ≈95°C (Fig. 2B). Sedimentation equilibrium experiments indicate that GCN4-pAA forms a clean heptamer and exhibits no systematic dependence of molecular mass on peptide concentration between 30 and 300 μM (Fig. 2C). Thus, alanine residues at the e and g positions of the dimeric leucine zipper serve to direct the formation of a stable, well ordered seven-helix bundle in solution.
Fig. 2.
The GCN4-pAA peptide forms a seven-stranded helical bundle. (A) CD spectrum at 20°C in ABS (pH 5.2) and 50 μM peptide concentration. (B) Thermal melt monitored by CD at 222 nm. (C) Representative sedimentation equilibrium data of a 100 μM sample at 20°C and 18,000 rpm in ABS (pH 5.2). The data fit closely to a heptameric complex. (Upper) The deviation in the data from the linear fit for a heptameric model is plotted.
Structure of the Heptamer.
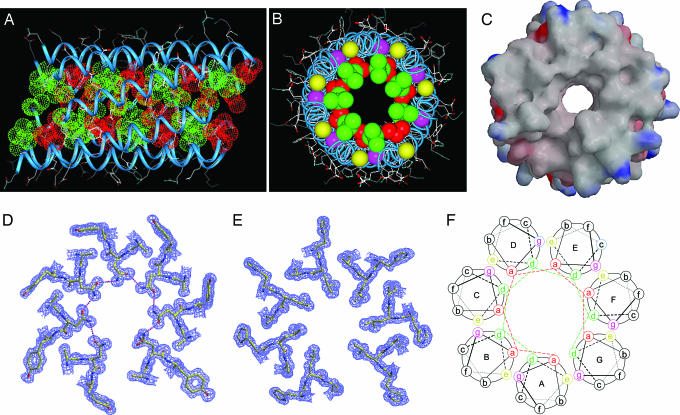
To investigate the basis for the switch between dimer and heptamer conformations, the x-ray crystal structure of the GCN4-pAA peptide was determined at 1.25-Å resolution (Table 1). The GCN4-pAA heptamer consists of seven parallel α-helical peptide monomers wrapped in a gradual left-handed superhelix with several unique features (Fig. 3). The superhelix includes ≈30 residues (3–32) from each chain (the two N- and C-terminal-most residues are not well defined in the electron density maps). This seven-helix bundle has a straight supercoil axis and forms a cylinder that is ≈48 Å in length and ≈31 Å in diameter. The individual helices in the heptamer can be superimposed on each other with an rmsd for Cα atoms of 0.14–0.40 Å.
Table 1.
Crystallographic data and refinement statistics
| Data collection | |
| Resolution, Å | 47.5–1.25 |
| No. of unique reflections | 51,370 |
| Redundancy | 2.0 |
| Completeness, % | 92.2 (91.4) |
| Rmerge,* % | 4.6 |
| I/σ(I) | 12.2 (2.3) |
| Space group | P1 |
| Unit-cell parameters | a = 31.5 Å, b = 35.3 Å, c = 49.0 Å |
| α = 86.5°, β = 104.3°, γ = 99.9° | |
| No. of molecules, AU | 7 |
| Solvent content, % | 36.7 |
| Refinement | |
| Resolution, Å | 47.5–1.25 |
| No. of reflections | 51,370 |
| No. of protein atoms | 1,612 |
| No. of water molecules | 179 |
| No. of hexane-1,6-diols | 8 |
| Rcryst/Rfree,† % | 18.1/20.9 |
| rmsd bond lengths, Å | 0.009 |
| rmsd bond angles, ° | 1.1 |
| Average B factors, Å2 | 23.3 |
| rmsd B values, Å2 | 1.7 |
Values in parentheses refer to the highest-resolution shell, 1.27–1.25 Å. AU, asymmetric units.
*Rmerge = Σ |I − 〈I〉|/Σ I, where I is the integrated intensity of a given reflection.
†Rcryst = Σ |Fo − Fc|/Σ Fobs, Rfree = Rcryst calculated by using 5% of the reflection data chosen randomly and omitted from the start of refinement.
Fig. 3.
Crystal structure of GCN4-pAA. (A) Lateral view of the heptamer (residues 5–31). Red van der Waals surfaces identify residues at the a positions, and green surfaces identify residues at the d positions. (B) Axial view of the heptamer. The view is from the N terminus looking down the superhelical axis. Red spheres identify residues at the a positions, green spheres identify residues at the d positions, yellow spheres identify residues at the e positions, and pink spheres identify residues at the g positions. (C) Molecular surface representation of the heptamer. The solvent-accessible surface is colored according to the local electrostatic potential, ranging from +32 V in dark blue (most positive) to −30 V in deep red (most negative). (D) Cross-section of the superhelix in the Asn-17(a) layer. The 1.25-Å 2Fo − Fc electron density map at 1.5σ contour is shown with the refined molecular model. Hydrogen bonds are denoted by pink dotted lines. (E) Omit map showing the Leu-20(d) layer in a 2Fo − Fc difference Fourier synthesis (contoured at 1.5σ). (F) Helical wheel projection of the heptamer showing interhelical packing interactions. The view is from the N terminus. Heptad positions are labeled a through g.
Serendipitously, the seven parallel helices in the GCN4-pAA structure are offset from each other by one amino acid residue, causing a heptad shift in register between the A and G helices (Fig. 3 A and B). Consequently, residues at positions a and d make unique side-to-side contacts with side chains of neighboring helices throughout the core of the heptamer, leading to the formation of two distinctive screws of interacting cross-sectional layers (Fig. 3A). One of these helical screws is formed by the 21 valine and 7 asparagine side chains at the a positions (Fig. 3D; the Val-3 side chains at the N terminus lack core packing), and the second is formed by the 28 leucine side chains at the d positions (Fig. 3E). All of the a and d side chains except one (Leu-27 of the C helix) adopt the most preferred rotamer conformations in α-helices (30). Alanine residues at positions e and g along the neighboring helices flank the a and d side chains to efficiently sequester the hydrophobic interface from the solvent (Fig. 3B).
Hydrophobic Channel.
The GCN4-pAA heptamer contains a 7-Å-wide central channel that is formed by spaces in the middle of each a and d layer and lined by hydrophobic side chains; the channel spans the superhelix and is open at both ends (Fig. 3 B and C). Within this channel, the experimentally phased map shows a strong string of electron density on the coiled-coil heptamer axis between residues 6 and 22, separate from the protein portion of the map. For the purpose of crystallographic model building and refinement, we have modeled this string as five hexanediol molecules (a precipitant that is present in the crystallization buffer). Given the fact that large internal cavities in natural proteins are unstable in the aqueous milieu, the heptameric channel formed from the parallel GCN4-pAA α-helices may offer a valuable model for investigating how cavities alter protein folding and stability.
Helix Interactions.
Although the superhelical radius differs significantly in the parallel GCN4-pLI tetramer (with leucine at each a position and isoleucine at each d) (23) and GCN4-pAA heptamer, these coiled-coil structures can still maintain similar pitch and residues per supercoil turn (Table 2). Moreover, the surface area of each helix is less buried in the heptamer (1,453 Å2 per helix) than in the tetramer (1,640 Å2 per helix) because of the smaller size of the e and g alanine side chains and the lower burial values of the a and d side chains in GCN4-pAA. Relative to the side chains of isolated helices, residues at the a, d, e, and g positions of the heptamer are substantially buried (>75%); residues at the b and c positions are partly buried (≈25%), whereas the f position remains completely exposed. The distance between the axes of neighboring helices (except for the A and G chains) is 7.6 Å, whereas the distance between the axis of helix A and the axis of helix D is 17.1 Å, and the distance between the axis of helix A and the axis of helix E is 17.7 Å (Fig. 3F). The A and G helices are 0.9 Å farther apart; this gap is due to a heptad phase shift.
Table 2.
Structural parameters of GCN4 leucine-zipper variants
| Parameter | GCN4-pIL | GCN4-pA | GCN4-pV | GCN4-pAA |
|---|---|---|---|---|
| Superhelix | ||||
| Supercoil radius, r0, Å | 7.1 | 6.9 | 7.4 | 11.4 |
| Residues per supercoil turn, n | 133.1 | 110.9 | 107.3 | 141.3 |
| Supercoil pitch, P, Å | 198 | 161 | 156 | 202 |
| Buried surface area, Å2 per helix | 1,640 | 1,368 | 1,368 | 1,453 |
| α-Helix | ||||
| α-Helix radius, r1, Å | 2.25 | 2.29 | 2.29 | 2.29 |
| Residues per α-helical turn, n | 3.58 | 3.61 | 3.63 | 3.61 |
| Rise per residue, h, Å | 1.53 | 1.51 | 1.51 | 1.50 |
| Angular frequency, ω1, ° per residue | −100.8 | −99.7 | −99.3 | −99.7 |
A Buried Hydrogen-Bonding Network.
To achieve efficient side-chain packing, Asn-17 residues at an a position form a network of hydrogen bonds within the hydrophobic channel of the GCN4-pAA heptamer (Fig. 3D). Substitution of Asn-17 with valine, serine, or threonine causes the resulting molecule to unfold in solution, whereas introducing glutamine into this position creates a variant with hydrodynamic properties identical to those of the Asn-17-containing peptide. In addition, our initial crystallographic analysis indicates that the Gln-17 side chain makes good interhelical packing interactions and is well accommodated without significantly altering the seven-helix structure. Thus, interhelix hydrogen bonds between the Asn side chains are essential for the folding and assembly of GCN4-pAA and likely act to nucleate and maintain the heptad phase register of the seven parallel helices in the heptamer. Our results reinforce the notion that buried polar interactions of appropriate geometry can impart structural specificity in coiled coils even if they do so at the expense of thermodynamic stability (23).
Core Packing.
The heptamer interface shows nonclassical meshing of interfacial side chains in knobs-into-holes packing (Fig. 4E and J). Looking down the superhelical axis from the N terminus, knobs formed by a residues of one helix fit into holes formed by the a and g side chains and two adjacent d side chains of the counterclockwise-related monomer. Similarly, knobs at d positions pack into holes formed by the d and e side chains and two adjacent a side chains of the clockwise-related monomer. By contrast, the alanine side chains at e positions pack into triangular spaces between the c, d, and g residues of the neighboring helix; those at g positions pack into triangular spaces between the a, b, and e residues. Thus, the a, b, c, d, e, and g residues of the heptad repeat segregate into two geometrically distinct helix–helix interfaces so as to create two continuous hydrophobic seams between the seven α-helical chains. As far as we know, these interleaved parallel helical screws have not been seen before in natural proteins.
Fig. 4.
Comparison of helix–helix interfaces in parallel coiled coils. (A) Parallel packing at position a (180°) in the GCN4-p1 dimer (23). The view is from the N terminus looking down the superhelical axis. The Cα–Cβ bond of each knob (blue) is oriented parallel to the Cα–Cα vector (red) at the base of the recipient hole on the neighboring helix. The Cα–Cβ bonds of the e and g residues are shown in pink and cyan, respectively. (B) Acute packing at position a (120°) in the GCN4-pII trimer (52). (C) Perpendicular packing at position a (90°) in the GCN4-pLI tetramer (23). (D) Packing at position a (72°) in the Trp-14 pentamer (31). (E) Packing at position a (51.4°) in the GCN4-pAA heptamer. (F) Perpendicular packing at position d (90°) in the dimer. (G) Acute packing at position d (150°) in the trimer. (H) Parallel packing at position d (180°) in the tetramer. (I) Packing at position d (198°) in the pentamer. (J) Packing at position d (218.6°) in the heptamer.
The seven parallel α-helical peptide monomers in the heptamer structure have crossing angles of ≈10° for both neighboring helices and relative to the superhelical axis of the heptamer. Thus, the heptamer conforms to a coiled-coil model proposed by Crick (9) to explain α-helix packing in α-keratin: an interhelix packing angle near 20°C with knobs-into-holes packing of side chains between adjacent helices. In addition, the Cα–Cα and Cα–Cβ vectors at the a and d layers of the heptamer are 51.4° and 218.6°, respectively (Fig. 4 E and J). The local packing geometry in the hydrophobic interface of the heptamer thus follows a general trend observed in the structures of parallel coiled coils containing two to five helices (31). This trend shows how residues at the b, c, e, and g positions of the heptad repeat become increasingly buried as the number of strands grows (Fig. 4). The results suggest that the amino acid sequence at these core positions will play a crucial role in determining the higher-order structures of coiled coils.
Protein Structure.
Accurate prediction of side-chain packing and its influence on tertiary conformation is an important objective in modern protein structure and design efforts. De novo protein design and engineering of coiled-coil interfaces have been used to test and enhance our growing ability to predict folded structures (23, 32). Previous studies have analyzed the potential of van der Waals interactions beyond the a and d side chains to generate higher-order coiled-coil structures (20, 33–36). The dimeric GCN4 leucine zipper represents the simplest protein–protein interface with which to study the detailed relationship between local side-chain interactions and global three-dimensional architecture. In the heptamer conformation, the core a and d residues from the parent dimer sequence form two continuous helical screws because of local packing of these nonpolar side chains. Conserved asparagines at a positions direct parallel helix orientation by forming a network of buried hydrogen bonds. The lateral displacement of adjacent helices in a sevenfold screw symmetry observed in the heptamer structure is unique among known coiled-coil structures.
Although the mechanism by which the geometric details of packing among side chains at the a, d, e, and g positions confer specificity toward the native state cannot be predicted at present, the availability of the seven-helix coiled coil provides a soluble model for detailed exploration of individual side-chain–side-chain interactions within a large-scale structure. The properties of the nanoscaled central cavity in this structure merit further investigation as well: How do various solutes influence the cavity and the stability of the heptamer? We anticipate that additional fundamental rules prescribing helix-to-helix packing in coiled coils remain to be discovered and will provide insight into the subtle relationship between amino acid sequence and the higher levels of structure adopted by this diverse class of proteins.
Materials and Methods
Protein Expression and Purification.
The recombinant peptide GCN4-pAA and its variants were expressed in Escherichia coli BL21(DE3)/pLysS by using a modified pET3a vector (Novagen, San Diego, CA). Substitutions were introduced into the pGCN4-pA plasmid (17) by using the method of Kunkel et al. (37) and were verified by DNA sequencing. Cells were lysed by glacial acetic acid and centrifuged to separate the soluble fraction from inclusion bodies. The soluble fraction containing peptide was subsequently dialyzed into 5% acetic acid overnight at 4°C. The GCN4-pAA(V) construct was appended to the TrpLE′ leader sequence (38) and cloned into the pET24a vector (Novagen). GCN4-pAA(V) was purified from inclusion bodies and cleaved from the TrpLE′ leader sequence with cyanogen bromide as described in ref. 39. Final purification of all peptides was performed by reverse-phase HPLC on a C18 preparative column using a water-acetonitrile gradient in the presence of 0.1% trifluoroacetic acid. Peptide identities were confirmed by electrospray mass spectrometry (Voyager Elite; PerSeptive Biosystems, Framingham, MA). Peptide concentrations were determined by using the method of Edelhoch (40).
Biophysical Analysis.
CD spectra were measured on a 62A/DS CD spectrometer (Aviv, Lakewood, NJ) at 4°C in ABS (50 mM sodium acetate, pH 5.2/150 mM NaCl) and 50 μM peptide. Thermal stability was assessed by monitoring [θ]222 as a function of temperature under the same conditions. A [θ]222 value of −35,000° cm2 dmol−1 was taken to correspond to 100% helix (41). Sedimentation equilibrium measurements were carried out on an XL-A analytical ultracentrifuge (Beckman Coulter, Fullerton, CA) equipped with an An-60 Ti rotor at 20°C. Peptide samples were dialyzed overnight against ABS (pH 5.2), loaded at initial concentrations of 30, 100, and 300 μM, and analyzed at rotor speeds of 18,000 and 21,000 rpm. Data sets were fitted to a single-species model. Random residuals were observed in all cases.
Crystallization and Structure Determination.
GCN4-pAA was crystallized at room temperature by using the hanging drop vapor-diffusion method by equilibrating against reservoir buffer (0.1 M sodium citrate, pH 5.4/0.1 M KH2PO4/1.4 M hexanediol), a solution containing 1 μl of 6 mg·ml−1 peptide in water, and 1 μl of reservoir buffer. Crystals belonged to space group P1 (a = 31.5 Å, b = 35.3 Å, c = 49.0 Å, α = 86.5°, β = 104.3°, and γ = 99.9°) and contained seven monomers in the asymmetric unit. The crystals were harvested in 0.1 M sodium citrate, pH 5.4/0.1 M KH2PO4/1.7 M hexanediol/15% glycerol and frozen in liquid nitrogen. Diffraction data were collected on beamline X4C at the National Synchrotron Light Source (Brookhaven, NY). Reflection intensities were integrated and scaled with DENZO and SCALEPACK (42). Initial phases were determined by molecular replacement with Phaser (43), using the structure of the GCN4-pA monomer (17) as a search model. Seven GCN4-pA molecules were oriented and placed in the asymmetric unit with a Z score of 7.8 and final refined log-likelihood gain of 431.7, corresponding to the seven GCN4-pAA molecules. This model and the data set for GCN4-pAA were directly fed to Arp/Warp (44), which provided a largely complete asymmetric unit of the seven chains and allowed ≈93% of the final model to be interpreted. The resulting experimental electron density map was of excellent quality and showed the location of most of the side chains. Crystallographic refinement of the GCN4-pAA structure was carried out by using Refmac (45). Density interpretation and manual model building were performed with O (46). The final model (Rcryst = 18.1% and Rfree = 20.9% for the resolution range 47.5–1.25 Å) consists of residues 3–33 (monomer A), 3–33 (monomer B), 3–33 (monomer C), 3–34 (monomer D), 3–33 (monomer E), 1–32 (monomer F), and 3–32 (monomer G) in the asymmetric unit, eight hexane-1,6-diols, and 179 water molecules. Bond lengths and bond angles of the model have rmsds from ideality of 0.009 Å and 1.1°, respectively. All protein residues are in the most favored regions of the Ramachandran plot.
Structure Analysis.
Coiled-coil parameters were calculated by using TWISTER (47). The rmsds were calculated with LSQKAB in the CCP4i program suite (48). Buried surface areas were calculated from the differences of the accessible side-chain surface areas of the seven-helix complex and of the individual helical monomers by using CNS 1.0 (49). Residues 3–32 of GCN4-pAA were used in the calculations. To calculate an omit map, target residues were removed, and the remaining atoms were shaken randomly by 0.3 Å to minimize model bias and then refined. Figures were generated by using SETOR (50), Insight II (Accelrys, San Diego, CA), and GRASP (51).
Acknowledgments
We thank John Schwanof at the National Synchrotron Light Source (beamline X4C) for support, Sergei Strelkov for help with the superhelical parameter calculations, and David Eliezer for comments on the manuscript. This work was supported by National Institutes of Health Grant AI42382, the Irma T. Hirschl Trust, and a Fellowship from the National Science Council of Taiwan (to C.-S.C.).
Abbreviation
- ABS
acetate-buffered saline.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2HY6).
References
- 1.Cohen C, Parry DA. Science. 1994;263:488–489. doi: 10.1126/science.8290957. [DOI] [PubMed] [Google Scholar]
- 2.Lupas AN, Gruber M. Adv Protein Chem. 2005;70:37–78. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 3.Kohn WD, Mant CT, Hodges RS. J Biol Chem. 1997;272:2583–2586. doi: 10.1074/jbc.272.5.2583. [DOI] [PubMed] [Google Scholar]
- 4.Betz SF, Bryson JW, DeGrado WF. Curr Opin Struct Biol. 1995;5:457–463. doi: 10.1016/0959-440x(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 5.McLachlan AD, Stewart M. J Mol Biol. 1975;98:293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- 6.Parry DA. Biosci Rep. 1982;2:1017–1024. doi: 10.1007/BF01122170. [DOI] [PubMed] [Google Scholar]
- 7.Lupas A, Van Dyke M, Stock J. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 8.Hodges RS, Sodek J, Smillie LB, Jurasek L. Cold Spring Harbor Symp Quant Biol. 1972;37:299–310. [Google Scholar]
- 9.Crick FHC. Acta Crystallogr. 1953;6:689–697. [Google Scholar]
- 10.O'Shea EK, Lumb KJ, Kim PS. Curr Biol. 1993;3:658–667. doi: 10.1016/0960-9822(93)90063-t. [DOI] [PubMed] [Google Scholar]
- 11.Kohn WD, Kay CM, Hodges RS. J Mol Biol. 1998;283:993–1012. doi: 10.1006/jmbi.1998.2125. [DOI] [PubMed] [Google Scholar]
- 12.Krylov D, Mikhailenko I, Vinson C. EMBO J. 1994;13:2849–2861. doi: 10.1002/j.1460-2075.1994.tb06579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monera OD, Kay CM, Hodges RS. Biochemistry. 1994;33:3862–3871. doi: 10.1021/bi00179a010. [DOI] [PubMed] [Google Scholar]
- 14.McClain DL, Binfet JP, Oakley MG. J Mol Biol. 2001;313:371–383. doi: 10.1006/jmbi.2001.5044. [DOI] [PubMed] [Google Scholar]
- 15.Burkhard P, Ivaninskii S, Lustig A. J Mol Biol. 2002;318:901–910. doi: 10.1016/S0022-2836(02)00114-6. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X, Zhu H, Lashuel HA, Hu JC. Protein Sci. 1997;6:2218–2226. doi: 10.1002/pro.5560061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y, Liu J, Zheng Q, Eliezer D, Kallenbach NR, Lu M. Structure (London) 2006;14:247–255. doi: 10.1016/j.str.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav MK, Leman LJ, Price DJ, Brooks CL III, Stout CD, Ghadiri MR. Biochemistry. 2006;45:4463–4473. doi: 10.1021/bi060092q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North B, Summa CM, Ghirlanda G, DeGrado WF. J Mol Biol. 2001;311:1081–1090. doi: 10.1006/jmbi.2001.4900. [DOI] [PubMed] [Google Scholar]
- 20.Calladine CR, Sharff A, Luisi B. J Mol Biol. 2001;305:603–618. doi: 10.1006/jmbi.2000.4320. [DOI] [PubMed] [Google Scholar]
- 21.Harbury PB, Plecs JJ, Tidor B, Alber T, Kim PS. Science. 1998;282:1462–1467. doi: 10.1126/science.282.5393.1462. [DOI] [PubMed] [Google Scholar]
- 22.Bryson JW, Betz SF, Lu HS, Suich DJ, Zhou HX, O'Neil KT, DeGrado WF. Science. 1995;270:935–941. doi: 10.1126/science.270.5238.935. [DOI] [PubMed] [Google Scholar]
- 23.Harbury PB, Zhang T, Kim PS, Alber T. Science. 1993;262:1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez L, Jr, Woolfson DN, Alber T. Nat Struct Biol. 1996;3:1011–1018. doi: 10.1038/nsb1296-1011. [DOI] [PubMed] [Google Scholar]
- 25.Fairman R, Chao HG, Mueller L, Lavoie TB, Shen L, Novotny J, Matsueda GR. Protein Sci. 1995;4:1457–1469. doi: 10.1002/pro.5560040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman AM, Fischmann TO, Steitz TA. Science. 1995;268:1721–1727. doi: 10.1126/science.7792597. [DOI] [PubMed] [Google Scholar]
- 27.Deng Y, Liu J, Zheng Q, Yong W, Lu M. Structure (London) 2006;14:889–899. doi: 10.1016/j.str.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Shea EK, Klemm JD, Kim PS, Alber T. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 29.O'Neil KT, DeGrado WF. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 30.Lovell SC, Word JM, Richardson JS, Richardson DC. Proteins. 2000;40:389–408. [PubMed] [Google Scholar]
- 31.Liu J, Yong W, Deng Y, Kallenbach NR, Lu M. Proc Natl Acad Sci USA. 2004;101:16156–16161. doi: 10.1073/pnas.0405319101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovejoy B, Choe S, Cascio D, McRorie DK, DeGrado WF, Eisenberg D. Science. 1993;259:1288–1293. doi: 10.1126/science.8446897. [DOI] [PubMed] [Google Scholar]
- 33.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 34.Walshaw J, Woolfson DN. J Mol Biol. 2001;307:1427–1450. doi: 10.1006/jmbi.2001.4545. [DOI] [PubMed] [Google Scholar]
- 35.Walshaw J, Woolfson DN. Protein Sci. 2001;10:668–673. doi: 10.1110/ps.36901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walshaw J, Woolfson DN. J Struct Biol. 2003;144:349–361. doi: 10.1016/j.jsb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Kunkel TA, Roberts JD, Zakour RA. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 38.Kleid DG, Yansura D, Small B, Dowbenko D, Moore DM, Grubman MJ, McKercher PD, Morgan DO, Robertson BH, Bachrach HL. Science. 1981;214:1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- 39.Shu W, Ji H, Lu M. Biochemistry. 1999;38:5378–5385. doi: 10.1021/bi990199w. [DOI] [PubMed] [Google Scholar]
- 40.Edelhoch H. Biochemistry. 1967;6:1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- 41.Chen YH, Yang JT, Chau KH. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 42.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 43.Storoni LC, McCoy AJ, Read RJ. Acta Crystallogr D. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 44.Lamzin VS, Wilson KS. Acta Crystallogr D. 1993;49:129–149. doi: 10.1107/S0907444992008886. [DOI] [PubMed] [Google Scholar]
- 45.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 46.Jones TA, Zou JY, Cowan SW, Kjeldgaard Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 47.Strelkov SV, Burkhard P. J Struct Biol. 2002;137:54–64. doi: 10.1006/jsbi.2002.4454. [DOI] [PubMed] [Google Scholar]
- 48.Potterton E, Briggs P, Turkenburg M, Dodson E. Acta Crystallogr D. 2003;59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 49.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 50.Evans SV. J Mol Graphics. 1993;11:134–138. doi: 10.1016/0263-7855(93)87009-t. 127–128. [DOI] [PubMed] [Google Scholar]
- 51.Nicholls A, Sharp KA, Honig B. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 52.Harbury PB, Kim PS, Alber T. Nature. 1994;371:80–83. doi: 10.1038/371080a0. [DOI] [PubMed] [Google Scholar]