Abstract
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality in allogeneic stem cell transplantation (alloSCT). Donor T cells that accompany stem cell grafts cause GVHD by attacking recipient tissues; therefore, all patients receive GVHD prophylaxis by depletion of T cells from the allograft or through immunosuppressant drugs. In addition to providing a graft-versus-leukemia effect, donor T cells are critical for reconstituting T cell–mediated immunity. Ideally, immunity to infectious agents would be transferred from donor to host without GVHD. Most donors have been exposed to common pathogens and have an increased precursor frequency of memory T cells against pathogenic antigens. We therefore asked whether memory CD62L–CD44+ CD4+ T cells would induce less GVHD than unfractionated or naive CD4+ T cells. Strikingly, we found that memory CD4 cells induced neither clinical nor histologic GVHD. This effect was not due to the increased number of CD4+CD25+ regulatory T cells found in the CD62L–CD44+ fraction because memory T cells depletion of these cells did not cause GVHD. Memory CD4 cells engrafted and responded to antigen both in vivo and in vitro. If these murine results are applicable to human alloSCT, selective administration of memory T cells could greatly improve post-transplant immune reconstitution.
Introduction
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality in allogeneic stem cell transplantation (alloSCT). In GVHD, mature donor T cells that accompany the stem cell graft attack recipient tissues, especially the skin, liver, and gastrointestinal tract. All patients, therefore, receive some type of GVHD prophylaxis either by depletion of T cells from the allograft or through pharmacologic treatment with agents that impair T cell function. This GVHD prophylaxis has adverse effects because mature donor T cells play a critical role in mediating reconstitution of the adaptive immune system, especially in adults with diminished thymic function (1–3). Thus, stem cell transplant recipients are at great risk for infections, particularly when prolonged immunosuppression is required for treatment of acute and chronic GVHD. The difficulty in balancing immune reconstitution versus GVHD prophylaxis has prevented the more widespread application of allogeneic stem cell transplantation to treat common inherited disorders of hematopoiesis, such as sickle cell anemia and thalassemia (4, 5). Donor T cells also play an important role in eliminating neoplastic cells. This antitumor effect has also proven difficult to deliver without GVHD.
In an effort to permit the safe engraftment of donor T cells, much effort has focused on testing the GVHD-inducing capacity of different T cell subsets. In particular, investigators have examined T1 or T2 T cell subsets (6–10) and T cells lacking molecules for T cell effector functions such as FasL, perforin, or TNF-α (11–14). In general, impairment of individual pathways have resulted in moderation of GVHD, but these results have not yet translated into widely applicable clinical protocols.
Peripheral T cells can also be broadly divided into those that have never been activated by antigen (naive T cells) and antigen-experienced T cells, which include effector, central memory, and effector memory cells (15–18). To date, these subpopulations have not been tested in murine models of alloSCT. It is attractive to consider the selective transfer of memory T cells. Most donors have been exposed to common pathogens and already have an increased precursor frequency of memory T cells that can respond quickly when rechallenged with antigen. There are reasons to think that memory T cells would be more potent at inducing GVHD than naive T cells, however. When challenged by antigen, memory T cells enter the cell cycle and produce cytokines more rapidly than do naive T cells (15, 16, 19). Memory cells may also have less stringent APC requirements, and thus allospecific memory T cells that do not contact professional APCs might also expand and participate in GVHD reactions (20). Memory T cells also undergo more rapid homeostatic proliferation in irradiated recipients than do naive T cells (21). Memory T cells might have a skewed T cell receptor repertoire, which could either increase or decrease GVHD. On the other hand, naive cells could be more potent at GVHD induction because they uniformly express CD62L and CCR7, which promotes T cell trafficking into secondary lymphoid tissues where they can encounter APCs presenting alloantigens. A potentially more diverse repertoire in naive cells may also enhance the ability of this subset to cause GVHD. If either naive or memory cells were less potent in GVHD induction, then this could open an avenue for mediating T cell–immune reconstitution and graft-versus-leukemia (GVL) with less toxicity from GVHD.
While there is no unequivocal definition of naive and memory T cells, investigators have correlated immunophenotype with naive or memory cell properties. Naive and memory T cells are thought to be enriched in the CD44–CD62L+ and CD44+CD62L– fractions, respectively. For convenience, throughout this paper we will refer to these cells as “naive” and “memory,” recognizing that these populations are not homogeneous. Nonetheless, the fact that these phenotypic markers segregate naive and memory cells made it feasible to test whether CD44–CD62L+ and CD44+CD62L– T cells have different capacities to cause GVHD.
We found that in a CD4-dependent, MHC-identical, multiple minor histocompatibility antigen–incompatible (miHA-incompatible) model of chronic GVHD, memory CD4 cells do not cause GVHD. In contrast, naive T cells are potent inducers of GVHD. We also show that this difference is not due to the increased number of CD4+CD25+ cells in the memory fraction, which have been implicated in regulation of various immunopathologic situations, including GVHD (22–26). We believe these results could have important clinical implications because memory cells could be capable of mediating immune reconstitution and possibly GVL with less GVHD.
Methods
Mice.
Recipient 8– to 12-week-old male BALB/c (H-2d) and donor male 8- to 12-week-old B10.D2 (H-2d) mice were purchased from NCI Laboratories (Frederick, Maryland, USA) and The Jackson Laboratory (Bar Harbor, Maine, USA), respectively. Adult-thymectomized male BALB/c recipients were purchased from Taconic Farms (Germantown, New York, USA) and rested 3 weeks prior to transplant. Mice were housed in microisolator cages and fed nonautoclaved food and acidified water containing sulfatrim for 2 weeks after transplant.
GVHD model.
Recipient BALB/c mice received 700–850 cGy from a cesium irradiator (see legends for individual experiments) and were reconstituted with 8 × 106 B10.D2 T cell–depleted bone marrow with spleen cells or purified naive or memory CD4+ T cells as described below.
Bone marrow T cell depletion.
Bone marrow (BM) cells were collected by flushing femurs and tibias from B10.D2 donor mice into MACS buffer (1× PBS, 5 mM EDTA, 3% calf serum). After red blood cell lysis, BM cells were incubated with biotinylated anti-Thy1.2 (30H12) mAb for 20 minutes on ice, washed once, then incubated with streptavidin-conjugated microbeads (SA-beads; Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) for 20 minutes at 4°C. Cells were depleted of Thy1.2-positive cells using an AutoMACS (Miltenyi Biotech GmbH). Remaining Thy1.2-positive cells were routinely less than 0.5% of BM cells. Cells were resuspended in injection buffer (1× PBS, 10 mM HEPES, 2.5% acid citrate dextrose anticoagulant, 0.5% penicillin-streptomycin) prior to transplant.
T cell purifications.
B10.D2 spleens were crushed through 70-μm screens in MACS buffer. After red blood cell lysis, spleen cells were resuspended in injection buffer prior to transplant or underwent further cell purifications.
CD4 cells were enriched from total spleen cells using BioMag (QIAGEN, Hilden, Germany) cell separation as follows. Cells were incubated with the following Ab supernatants for 30 minutes on ice, followed by two washes in MACS buffer: anti-CD8-α (TIB105), anti-B220 (RA3-6B2), anti–Mac-1 (M1/70), and anti-FcR (24G.2). Cells were incubated with prepared BioMag goat anti-rat magnetic beads for 30 minutes on ice in a T75 or T125 flask, which was then placed next to a strong magnet. Cells not bound to magnetic particles were collected and were typically 70–80% CD4+ without contaminating CD8+, B220+, CD11b+, or FcR+ cells.
For initial separations of naive and memory cells, enriched CD4+ T cells were incubated with mAb’s anti–CD4-APC/Cy7 (GK1.5; Caltag Laboratories Inc., Burlingame, California, USA), anti–CD62L-FITC (Mel-14; lab prepared), anti–CD44-APC (Pgp-1; BD PharMingen, San Diego, California, USA) for 20 minutes on ice, washed once in MACS buffer, and resuspended in sort buffer (1× PBS, 5 mM EDTA, 0.5% calf serum, and 1× gentamicin). Cells were separated into CD62L+CD44– naive and CD62L–CD44+ memory populations using a FACSVantage cell sorter (Becton Dickinson Immunocytometry Systems, San Jose, California, USA).
Two procedures were used for the purification of CD25– naive and memory cells. In the first, spleen cells enriched for CD4+ T cells were stained with anti–CD4– APC-Cy7, anti–CD62L-FITC, anti–CD44-APC, and biotin anti–CD25 (7D4; BD PharMingen) for 20 minutes on ice, washed once in MACS buffer, and incubated with streptavidin-phycoerythrin (streptavidin-PE) (Intergen Co., Purchase, New York, USA) for 15 minutes on ice, washed again, and resuspended in sort buffer. Gated CD4+CD25– cells were separated into CD62L+CD44– naive and CD62L–CD44+ memory subsets using a FACSVantage.
In the second approach, separation of CD25+ and CD25– naive and memory CD4+ T cells was achieved by first incubating BioMag-enriched CD4+ T cells with biotinylated anti-CD62L and biotinylated anti-CD25 cells for 30 minutes on ice. Cells were washed once in MACS buffer and then incubated with SA-beads for 25 minutes at 4°C. Cells were separated on the AutoMACs into CD62L+CD25+ and CD62L–CD25– fractions. The negative fraction (CD62L–CD25–) was used as memory cells. The positive fraction, containing CD62L+CD25+ cells, was incubated with anti–CD4-APC-Cy7, anti–CD62L-FITC, anti–CD44-APC, and anti–CD25-PE for 20 minutes on ice, washed once in MACS buffer, and resuspended in sort buffer. Naive CD4+CD62L+CD25– cells were then isolated on a FACStar cell sorter (Becton Dickinson Immunocytometry Systems).
Immunizations.
Donor B10.D2 mice were immunized by intraperitoneal injection with 50 μg chicken γ globulin (CGG; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) in CFA (Sigma-Aldrich, St. Louis, Missouri, USA) 3 weeks prior to transplantation. Adult thymectomized (ATX) BALB/c mice underwent a GVHD-inducing transplant as described above, using CGG-immunized B10.D2 mice as BM and CD4+ T cell donors. Mice received (a) no CD4 cells, (b) CD25– memory CD4 cells, or (c) unfractionated CD4 cells. Thirty-seven days after transplant, recipients were immunized with either 50 μg CGG or pigeon cytochrome c (PCC; an irrelevant control antigen) in CFA by foot-pad and tail-base subcutaneous injections. Unmanipulated ATX mice were also immunized as a control.
Lymphocyte proliferation assay.
Draining LN cells were collected from transplanted and unmanipulated ATX BALB/c mice 14 days after immunization. Prior to the LN collection, ATX recipient mice were inspected for the absence of a thymus to ensure complete thymectomy. Cells from each experimental group were pooled, and residual recipient cells from transplanted groups were depleted using MACS depletion with recipient-specific biotinylated anti-Ly9.1 (clone 30C7; BD PharMingen). Residual contaminating Ly9.1+ cells were less than 1% of total. Cells were resuspended in complete Click’s media (Irvine Scientific, Irvine, California, USA) with 5% FCS (Gemini Bio-Products, Woodland, California, USA), 100 U/ml penicillin G, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 2 mM L-glutamine, and 0.0568 mM 2-mercaptoethanol. Conventional T lymphocyte proliferation assays were performed (106 cells/well); all assays were set up with triplicate samples and incubated with media only (no antigen), CGG titrations (12.5 μg, 25 μg, or 50 μg), or 15 μg PPD (Mycobacterium tuberculosis H37 RA; Difco Laboratories, Detroit, Michigan, USA) for 72 hours. Lymphocyte proliferation was assessed by [3H]-thymidine incorporation (1 μCi/well; ICN Radiochemicals Inc., Irvine, California, USA) during the last 16 hours of culture. Sample wells were harvested onto filters, and incorporated radioactivity was counted in a Betaplate liquid scintillation counter (LKB/Wallac, Gaithersburg, Maryland, USA).
GVHD clinical-scoring system.
Animals were weighed every 3 days following BM transplantation and scored for skin manifestations of GVHD beginning on day 18. The scoring system was as follows: skin ulcers with alopecia less than 1 cm2 in area = 1; skin ulcers with alopecia 1–2 cm2 in area = 2; skin ulcers with alopecia greater than 2 cm2 in area = 3; mice lacking skin ulcers and alopecia received a score of 0. Additionally, animals were assigned 0.3 point each for skin disease (ulcers or scaling) on ears, tails, and paws. Thus, the minimum score was 0, and the maximum score was 3.9. Incidence is graphed as percentage of mice that have never achieved a clinical score of 0.6 or greater. Once a mouse has had a score of at least 0.6, it is always considered affected, even if GVHD subsides to a score of less than 0.6. We also plotted disease severity of an experimental group, which is the mean clinical score of all mice in that group that were affected by GVHD (i.e., have had a score of 0.6 or greater). When mice either died or were euthanized for humane reasons, their disease severity scores at time of death remained included in subsequent mean scores.
Pathologic scoring.
Shaved skin from the interscapular region (approximately 2 cm2) was fixed in 10% formalin, embedded in paraffin, sectioned, slide mounted, and stained with hematoxylin and eosin. Slides were scored by a dermatopathologist (J. McNiff; blinded to experimental groups) on the basis of dermal fibrosis, fat loss, inflammation, epidermal interface changes, and follicular drop-out (0–2 for each category).
Statistical methods.
The significance of differences in GVHD incidence was calculated as a χ2 at the last observation day as no events occurred in the memory T cell recipients. The significance of differences between clinical scores and pathology scores were calculated by Mann-Whitney. Significance of differences in proliferation were calculated by an unpaired t test.
Results
Memory CD4 cells do not cause GVHD.
To compare the GVHD-inducing potency of naive and memory T cells, we used the B10.D2 (H-2d) → BALB/c (H-2d) model of chronic GVHD. In this model CD4 cells are both required and sufficient for GVHD, whereas, in contrast, CD8 cells alone are incapable of causing GVHD (27, 28). The syndrome induced with purified CD4 cells is indistinguishable from that induced by unfractionated splenocytes. In this model, GVHD is primarily cutaneous, manifested by alopecia, erythema, ulceration, and fibrosis.
Naive and memory phenotype CD4 cells were purified by magnetically depleting B cells and granulocytes from spleens, followed by FACS sorting. Naive CD4 cells were defined as CD62L+CD44–, whereas memory cells were CD62L–CD44+ (Figure 1). BALB/c recipients were irradiated and reconstituted with B10.D2 T cell–depleted BM along with no T cells, 107 unfractionated spleen cells containing 1.2 × 106 CD4 cells, 106 memory CD4 cells, or 106 naive CD4 cells.
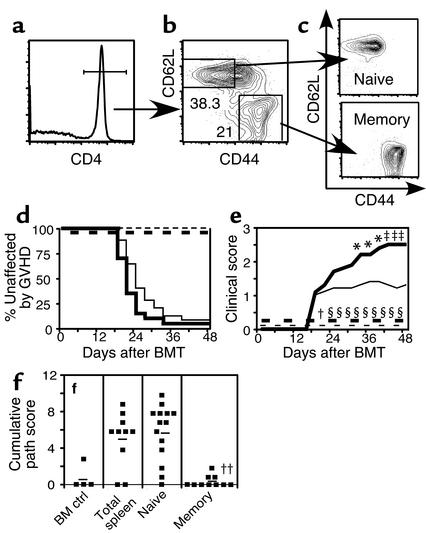
Figure 1.
Memory CD4+ T cells do not cause GVHD. Naive and memory T cells were purified as described in Methods. After gating on CD4+ T cells (a), cells were sorted into CD62L+CD44– naive and CD62L–CD44+ memory fractions (b). Reanalyses of sorted populations are shown in (c). BALB/c mice were lethally irradiated and reconstituted with 8 × 106 B10.D2 T cell–depleted BM alone (thin dashed line, n = 9) or with 107 B10.D2 total spleen cells (thin solid line, n = 25), 106 naive T cells (thick solid line, n = 20), or 106 memory T cells (thick dashed line, n = 10). Data are combined from two independent experiments. GVHD incidence and mean clinical score are shown in d and e. Statistical comparisons are as follows: (d). P < 0.0001 for GVHD incidence in recipients of memory CD4 versus spleen cells or naive CD4 cells. (e) For clinical score, *P < 0.05 (time points 1–3) and ‡P < 0.01 (time points 4–6) for recipients of naive versus total spleen cells; †P < 0.05. §P < 0.001 (time points 2–10) for recipients of memory versus total spleen cells. P < 0.0001 for recipients of memory versus naive cells at all time points. BM control mice and BM plus memory cell groups did not get GVHD, but the clinical score lines were offset for clarity. Pathology scores from representative mice are shown in (f). Mean scores are indicated by horizontal bars. ††P < 0.005 and P < 0.0004 for recipients of memory versus total (unfractionated) spleen cells and memory versus naive CD4 cells, respectively.
Strikingly, in two independent experiments, memory CD4 cells did not induce clinical or histologic GVHD, while naive CD4 cells caused more severe GVHD than unfractionated splenocytes containing a greater number of CD4 cells (Figure 1, d–f). Between the two experiments, a total of ten mice received memory cells, and none developed GVHD. In contrast, 19 of 20 recipients of naive cells and 23 of 25 spleen cell recipients developed GVHD (P < 0.0001 for both memory versus spleen and memory versus naive). Affected naive CD4 recipients had a mean clinical score of 2.5 compared with 1.5 in spleen cell recipients (P < 0.05 for time points 1–3; P < 0.01 for time points 4–6). Memory cell recipients had a score of 0 (P < 0.05 for time point 1 and P < 0.001 for time points 2–10, memory versus spleen; P < 0.0001 for all time points, memory versus naive). Histologically, memory cell recipients had scores indistinguishable from hosts that received no T cells. In contrast, recipients of spleen cells and naive T cells had mean scores of 5.2 and 5.9, respectively (P < 0.005, memory versus spleen; P < 0.0004, memory versus naive cells). Thus, GVHD in this model is induced only by naive and not memory CD4 cells. The differences in clinical scores between naive and spleen cell recipients were not reflected by histologic analysis of affected skin as the clinical score measures the extent of skin involvement. The nature of the lesions in affected skin was quantitatively and qualitatively similar in the total spleen cell and naive groups.
Memory CD4 cells depleted of regulatory CD4+CD25+ cells do not cause GVHD.
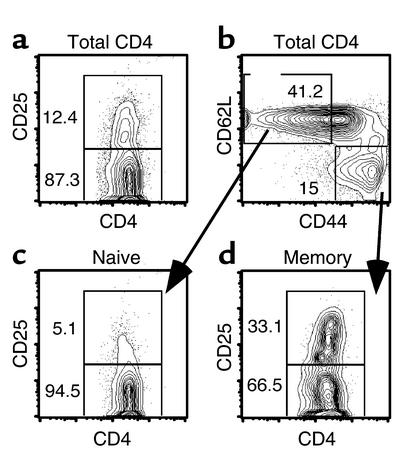
Differential GVHD-inducing capacity could be an intrinsic property of naive and memory T cells. Alternatively, the CD44+CD62L– population that contains memory T cells might also contain a regulatory cell that suppresses GVHD that is not present among the naive cell population. Potential candidate regulatory cells include CD4+CD25+ T regulatory cells (Treg), which have been shown to suppress GVHD in MHC-incompatible GVHD models (22–24). We therefore enumerated the percentage of CD4+CD25+ cells present in B10.D2 naive and memory CD4 cells. We found that 5.5% of naive cells and 33% of memory CD4 cells were CD25+ (Figure 2). Thus, memory cells could have been less efficacious at inducing GVHD due to the large number of putative Treg cells present.
Figure 2.
CD25 expression on naive and memory CD4 subsets. B10.D2 spleen cells enriched for CD4 cells as described in Methods were stained with mAb’s against CD4, CD25, CD62L, and CD44. We found that 12.4% of CD4 cells were CD25+ (a). We gated on CD62L+CD44– naive and CD62L–CD44+ memory CD4 cells (b) and analyzed their expression of CD25 (c and d). Note that 33.1% of cells with a memory phenotype express CD25 versus 5.1% of cells with a naive phenotype.
To evaluate this possibility, we performed GVHD experiments with naive and memory CD4+ T cells that were depleted of CD4+CD25+ cells (see Methods). In two independent experiments (Figures 3 and 4), 106 memory CD4 cells depleted of CD4+CD25+ cells did not cause GVHD, whereas an equal number (Figure 3) or only 250,000 naive CD4 cells (Figure 4) caused severe GVHD.
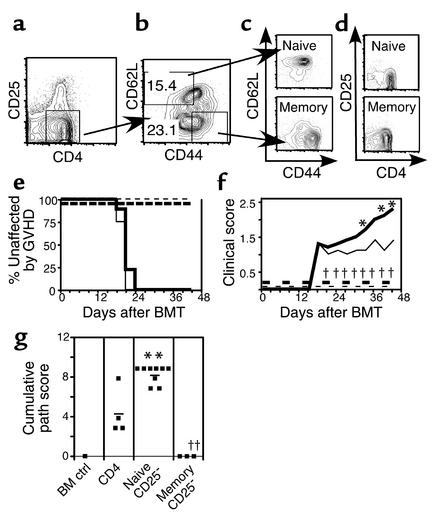
Figure 3.
FACS-sorted memory CD25– T cells do not cause GVHD. Donor B10.D2 spleen cells were enriched for CD4+ T cells using BioMag separation, then stained with mAb’s for CD4, CD25, CD62L, and CD44. After gating on CD4+CD25– cells (a), T cells were sorted on the basis of CD62L and CD44 expression (b). Reanalyses of sorted populations are shown in (c) (CD44 versus CD62L) and (d) (CD4 versus CD25). BALB/c mice were lethally irradiated and reconstituted with 8 × 106 B10.D2 T cell–depleted BM alone (thin dashed line, n = 1) or with 2 × 106 B10.D2 unfractionated CD4+ T cells (thin solid line, n = 4), 106 purified naive CD4+CD25– T cells (thick solid line, n = 9), or 106 memory CD4+CD25– T cells (thick dashed line, n = 3). Incidence of GVHD is shown in (e). P < 0.0082 and P < 0.0005 comparing GVHD incidence in recipients of CD25– memory CD4 versus unfractionated and CD25– naive CD4 cells, respectively. Average clinical disease score for affected mice (f). *P < 0.05 (time points 1–3) and for recipients of CD25– naive cells versus unfractionated CD4 cells. P < 0.02 for all comparisons between recipients of CD25– memory and naive cells. Pathology scoring from representative mice (g). ††P < 0.0034 and P < 0.017 for recipients of memory versus total and naive CD4+ T cells, respectively. **P < 0.016 for recipients of naive versus total CD4+ T cells.
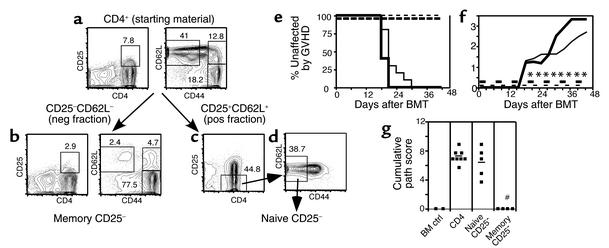
Figure 4.
AutoMACS- and FACS-sorted CD25-depleted memory T cells do not cause GVHD. Donor B10.D2 spleen cells enriched for CD4+ T cells using BioMag beads were stained with biotinylated anti-CD62L and anti-CD25 mAb’s, followed by staining with SA-beads. Cells were separated into CD25–CD62L– (negative [neg] fraction) and CD25+CD62L+ (positive [pos] fraction) cells using an AutoMACS. Phenotype of presort CD4+ T cells is shown in (a). Phenotype of CD25–CD62L– negative fraction (memory cells) is shown in (b). CD25+CD62L+ cells (positive fraction) were sorted on a FACStar cell sorter to purify CD25– (c) and CD62L+CD44– cells (d). Reanalysis of the sorted population is not available. BALB/c mice were lethally irradiated and reconstituted with 8 × 106 B10.D2 T cell–depleted BM alone (thin dashed line, n = 5) or with 1.5 × 106 unfractionated B10.D2 CD4+ T cells (thin solid line, n = 10), 2.5 × 105 CD4+CD25– naive T cells (thick solid line, n = 5), or 106 CD4+CD25– memory T cells (thick dashed line, n = 4). Incidence of GVHD (e). P < 0.0002 and P < 0.003 for difference between recipients of CD25– memory and total CD4 and CD25– naive CD4 cells, respectively. Average clinical disease score for mice affected with GVHD (f). *P < 0.02 (all time points) for CD25– memory versus total CD4. P < 0.01 on days 19–43 after transplant for recipients of CD25– memory versus naive CD4 cells. Pathology scoring from representative mice (g). #P < 0.007 and P < 0.014 for recipients of CD25– memory versus total and CD25– naive CD4 cells, respectively.
In the first experiment, naive and memory CD25– cells were isolated by FACS sorting (Figure 3, a–d). Irradiated BALB/c hosts received 8 × 106 B10.D2 T cell–depleted BM with no T cells, 2 × 106 unfractionated CD4 cells, and 106 naive or 106 memory CD4 cells. As in our experiments with CD25-replete naive and memory cells, CD25– memory CD4 cells did not cause GVHD, whereas naive CD25– cells caused more severe GVHD than did an equal number of unfractionated CD4 cells (Figure 3, e–g). Differences in GVHD incidence between recipients of memory versus naive (P < 0.0005) and memory versus total CD4 cells (P < 0.008) were highly significant.
In the second experiment, naive and memory CD25– cells were isolated by MACS depletion to obtain memory cells, followed by FACS sorting to obtain naive cells (see Methods and Figure 4, a–d). Again, memory CD4 cells caused no clinical GVHD, whereas only 250,000 naive CD25– memory CD4 cells induced GVHD similar to that induced by 1.5 × 106 unfractionated CD4 cells (Figure 4, e–g). As in the first experiment, differences in GVHD incidence between recipients of memory cells versus naive cells (P < 0.0027) and memory versus total CD4 cells (P < 0.0002) were highly significant. Thus, between the two experiments, none of the seven mice that received CD25-depleted memory cells developed GVHD, whereas 14 of 14 recipients of CD25-depleted naive cells got GVHD.
The clinical differences we observed were also borne out histologically. Mice were sacrificed on day 42, and skin histology was scored as described in Methods (Figure 3g and Figure 4g). Memory CD25– T cell recipients had no evidence of GVHD, whereas recipients of total unfractionated CD4 cells (including CD4+CD25+ cells) and naive CD25– CD4 cells developed severe histologic GVHD. Representative histology is shown in Figure 5.
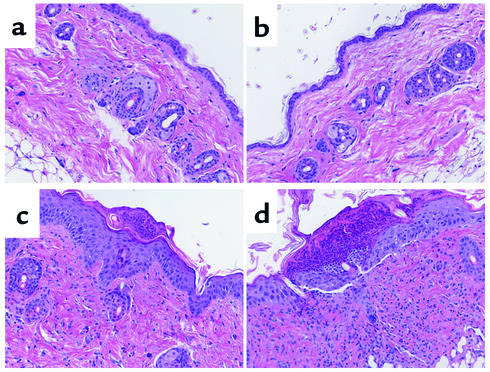
Figure 5.
Representative histology. Representative skin histology from BALB/c recipients of B10.D2 T cell–depleted BM alone (a), with memory cells (b), unfractionated CD4 cells (c), or naive CD4 cells (d). Note thickening of keratinocyte layer, interface dermatitis, and ulcerations (c and d) not present in a and b.
Engrafted memory CD4 cells respond to antigen in vivo.
For donor memory T cell infusions to be effective in immune reconstituting alloSCT recipients, they must engraft and be able to respond to antigen. We could not ask this in the experiments described above because memory CD4 cell recipients also had donor BM-derived T cells. To evaluate memory donor CD4 cell engraftment and function, we therefore used ATX BALB/c mice as recipients. Because donor BM-derived cells cannot differentiate into T cells due to the absence of a thymus, the only donor-derived T cells in these mice are derived from those infused at the time of transplant. Donor- and host-derived cells can be distinguished by expression of the recipient-specific Ly9.1 isoform.
To mimic the situation of a recipient responding to an antigen against which the donor has already been exposed, we used B10.D2 mice that were immunized 21 days prior to transplant with CGG as CD4+ T cell and BM donors. ATX BALB/c mice were irradiated and reconstituted with T cell–depleted B10.D2 BM, with (a) no CD4 cells, (b) unfractionated CD4 cells, or (c) CD25– memory CD4+ T cells. To test functional in vivo memory of donor T cells, recipients and unmanipulated ATX mice were immunized on day 37 after transplant with CGG or the irrelevant control antigen PCC. Fourteen days later, mice were sacrificed and draining lymph node cells were harvested. Residual recipient host cells were depleted (less than 1% contaminating host cells), and the remaining cells were restimulated in vitro with CGG. Proliferation was measured by [3H]-thymidine incorporation.
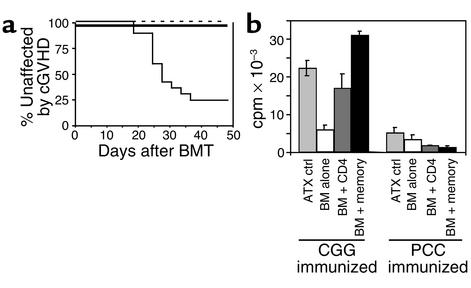
As in previous experiments, memory T cell recipients did not develop clinical GVHD (Figure 6a), whereas most recipients of unfractionated CD4 cells did. Donor memory T cells engrafted, with 75% of LN CD4 cells being Ly9.1– and therefore derived from the mature donor T cells given at the time of transplant. These memory cells made strong proliferative recall responses to CGG, even greater than those made by recipients of unfractionated CD4 cells. This is particularly striking because the fraction of CD4+ T cells in the memory group was only one-third of that in the unfractionated CD4 cell group (not shown). Memory recipients immunized with PCC in vivo failed to respond to CGG in vitro, which confirms that proliferation depended on in vivo priming. Importantly, CGG-immunized recipients of T cell-depleted BM had poor responses, demonstrating that residual host CD4 cells could not have made a significant contribution. Thus, donor memory CD4+ T cells both engrafted and responded vigorously to antigen.
Figure 6.
Donor memory cells engraft and respond to antigenic challenge. B10.D2 mice were immunized intraperitoneally with CGG in CFA and used 3 weeks later as CD4+ cell and BM donors. ATX BALB/c mice were irradiated and reconstituted with 8 × 106 T cell–depleted BM cells with no CD4 cells (thin dashed line, n = 9), 1.5 × 106 unfractionated CD4 cells (thin solid line, n = 17), or 106 CD4+CD25– memory cells (thick line, n = 14). Incidence of GVHD (a). P < 0.001 for GVHD incidence in recipients of CD25– memory cells versus total CD4 cells. Transplanted memory cells respond to CGG (b). Thirty-seven days after the transplant, recipients and unmanipulated ATX BALB/c mice were immunized with CGG or PCC in CFA. Two weeks later, draining LN cells were collected, depleted of residual recipient cells, and rechallenged with 50 μg CGG in vitro in a standard proliferation assay. Cells were pooled from all animals (n = 3–7) of an experimental group: untransplanted ATX control, BM alone, BM plus unfractionated CD4 cells, BM plus CD25–CD4+ memory cells. Background counts (no antigen) were subtracted from plotted data. P = 0.0002 for proliferation to CGG for BM plus memory cells versus BM plus unfractionated CD4 cells. P < 0.0001 for BM plus memory cells versus BM alone. Error bars indicate standard deviation of samples run in triplicate.
Discussion
These experiments show that in an MHC-compatible, multiple miHA-incompatible murine model of CD4-dependent chronic GVHD, donor memory cells alone are ineffective in mediating GVHD. In contrast, an equal or even a lower number of naive donor CD4 cells induced severe GVHD. This difference was not due to an effect of CD4+CD25+ Treg cells because we observed similar results when we depleted these cells. Memory CD4 cells engrafted and were able to mount strong proliferative recall responses when challenged in vivo. If translatable to humans, these results could have an important clinical impact. AlloSCT recipients could potentially receive substantial numbers of donor memory T cells, leading to immune reconstitution with a lower risk of GVHD. These cells might also be capable of mediating antitumor effects, an issue that will have to be addressed in future studies.
There are several potential explanations for why memory T cells failed to induce GVHD. In general, the differences we observed could be due to differences in T cell receptor (TCR) repertoire, intrinsic properties of memory T cells, or both. It is presumed that T cells with a memory phenotype have responded to environmental antigens. Thus, the TCR repertoire could be altered such that the immunodominant miHAs targeted by naive T cells are not recognized by memory cells. It is also possible that the primary stimulation that created these memory cells polarized them toward a T1 or T2 response, and if these phenotypes were preserved on restimulation (29), this could account for the decreased GVHD. Using a number of approaches, however, we have not seen evidence that either T1 or T2 cells are exclusively required for GVHD in this model (our unpublished observations). In addition, memory T cells might not traffic efficiently to secondary lymphoid organs. At least some memory T cells did traffic to lymph nodes, however, because memory CD4 cell recipients mounted proliferative responses to CGG. This is consistent with data on the responsiveness of memory CD4 cells transferred in nontransplant settings (30). Finally, the population that includes memory T cells could contain a regulatory T cell population other than CD4+CD25+ cells. These possibilities will need to be addressed in a series of future experiments that will require unique genetics to distinguish donor-engrafted mature T cells from donor marrow–derived newly generated T cells.
While memory cells are clearly less potent at inducing GVHD, they may still be capable of doing so under certain conditions. In other GVHD models, immunizing T cell donors with recipient antigens reduces the number of T cells required for GVHD induction (28). In these cases, however, the precursor frequency of alloreactive cells would be greatly increased. Thus, even though memory T cells from immune mice mediate GVHD, they may be less effective at inducing GVHD than naive T cells bearing the same TCRs on a per cell basis. Furthermore, GVHD in these experiments may have been caused by persistent activated effectors rather than memory cells, since donor cell purifications were not performed.
Naive T cells, with or without CD25+ cells, were more potent than unfractionated cells and caused more severe GVHD. Approximately 10% of unfractionated CD4 cells are CD25+ as compared with 5.5% of naive CD4 cells. It is therefore possible that a reduction in CD25+ cells from 10% to 5.5% that accompanies naive cell purification accounts for this difference. Alternatively, differences in regulatory activity among subsets of CD4+CD25+ cells could account for this. CD4+CD25+CD62L+CD44– cells could be less efficacious in suppressing GVHD than are CD4+CD25+CD62L–CD44+ cells. Against this idea, CD62L+ and CD62L– CD4+CD25+ cells were equally efficacious in suppressing T cell proliferation in vitro (31), though to our knowledge they have not yet been compared in vivo. Finally, as mentioned above, a regulatory cell that is CD25– may also be among cells with a CD62L–CD44+ phenotype, and the exclusion of this cell from the naive population could account for its greater potency compared with unfractionated cells. We think it is unlikely that naive cells are resistant to the activity of CD4+CD25+ regulatory cells because it appears that most, if not all, of the GVHD-inducing activity in this model resides in the naive population, and depletion of CD25+ cells from CD4 cells results in more severe GVHD (our unpublished observations).
If memory CD4 cells cause less GVHD than unfractionated CD4 cells in human alloSCT recipients, selective administration of memory T cells could greatly improve immune reconstitution, as was the case in our murine model. The importance of donor immunity in reconstituting host immunity in human alloSCT is dramatically demonstrated by the increased incidence of EBV-related post-transplant lymphoproliferative disorders (PTLDs) in recipients of T cell–depleted allografts as compared with recipients of T cell–replete grafts. Infusions of donor T cells from EBV-immune donors induce regressions of PTLDs (32). Adaptive immunotherapy with ex vivo–generated donor T cells specific for CMV or EBV have been effectively used to reconstitute anti-CMV immunity and treat PTLD (29, 33–36). Such a practice is not likely to become widespread due to the cost and technical difficulties in generating these cells. Transfer of a smaller number of naturally occurring donor memory cells might be a more widely applicable approach. Not only would the precursor frequency of cells that can respond to infectious pathogens be increased, immunosuppressive therapy that restricts their function might also be reduced, if not eliminated. Such an approach would be particularly important for adult patients, in which the majority of T cell reconstitution is mediated by donor T cells rather than by thymically derived T cells (1), especially in those patients with GVHD (37).
Acknowledgments
This work was supported by NIH grants R01 HL66279 (to M.J. Shlomchik, W.D. Shlomchik, and B.E. Anderson), K08 HL03979-02 (to W.D. Shlomchik), and NIH T32 AI 071019-23-25 (to B.E. Anderson).
Footnotes
See the related Commentary beginning on page 25.
Mark J. Shlomchik and Warren D. Shlomchik contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: graft-versus-host disease (GVHD); allogeneic stem cell transplantation (alloSCT); graft-versus-leukemia (GVL); minor histocompatibility antigen (miHA); bone marrow (BM); streptavidin-conjugated microbeads (SA-beads); phycoerythrin (PE); chicken γ globulin (CGG); adult thymectomized (ATX); pigeon cytochrome c (PCC); T regulatory cells (Treg); T cell receptor (TCR); post-transplant lymphoproliferative disorders (PTLD).
References
- 1.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol. Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 2.Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2989–2994. doi: 10.1073/pnas.052714099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 4.Walters MC, et al. Bone marrow transplantation for sickle cell disease. N. Engl. J. Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 5.Lucarelli G, et al. Bone marrow transplantation in adult thalassemic patients. Blood. 1999;93:1164–1167. [PubMed] [Google Scholar]
- 6.Teshima T, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J. Clin. Invest. 1999;104:317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YG, Sykes M. The role of interleukin-12 in preserving the graft-versus-leukemia effect of allogeneic CD8 T cells independently of GVHD. Leuk. Lymphoma. 1999;33:409–420. doi: 10.3109/10428199909058446. [DOI] [PubMed] [Google Scholar]
- 8.Fowler DH, Breglio J, Nagel G, Hirose C, Gress RE. Allospecific CD4+, Th1/Th2 and CD8+, Tc1/Tc2 populations in murine GVL: type I cells generate GVL and type II cells abrogate GVL. Biol. Blood Marrow Transplant. 1996;2:118–125. [PubMed] [Google Scholar]
- 9.Fowler DH, Gress RE. CD8+ T cells of Tc2 phenotype mediate a GVL effect and prevent marrow rejection. Vox Sang. 1998;74(Suppl. 2):331–340. doi: 10.1111/j.1423-0410.1998.tb05439.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, et al. Selective T-cell subset ablation demonstrates a role for T1 and T2 cells in ongoing acute graft-versus-host disease: a model system for the reversal of disease. Blood. 2001;98:3367–3375. doi: 10.1182/blood.v98.12.3367. [DOI] [PubMed] [Google Scholar]
- 11.Hill GR, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J. Clin. Invest. 1999;104:459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh MH, Korngold R. Differential use of FasL- and perforin-mediated cytolytic mechanisms by T-cell subsets involved in graft-versus-myeloid leukemia responses. Blood. 2000;96:1047–1055. [PubMed] [Google Scholar]
- 13.Schmaltz C, et al. Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect. Blood. 2001;97:2886–2895. doi: 10.1182/blood.v97.9.2886. [DOI] [PubMed] [Google Scholar]
- 14.Via CS, Nguyen P, Shustov A, Drappa J, Elkon KB. A major role for the Fas pathway in acute graft-versus-host disease. J. Immunol. 1996;157:5387–5393. [PubMed] [Google Scholar]
- 15.Sprent J, Surh CD. Generation and maintenance of memory T cells. Curr. Opin. Immunol. 2001;13:248–254. doi: 10.1016/s0952-7915(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 16.Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology. 2002;106:127–138. doi: 10.1046/j.1365-2567.2002.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 19.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 20.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 21.Dummer W, Ernst B, LeRoy E, Lee D, Surh C. Autologous regulation of naive T cell homeostasis within the T cell compartment. J. Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 25.Read S, Powrie F. CD4(+) regulatory T cells. Curr. Opin Immunol. 2001;13:644–649. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 27.Hamilton BL. L3T4-positive T cells participate in the induction of graft-vs-host disease in response to minor histocompatibility antigens. J. Immunol. 1987;139:2511–2515. [PubMed] [Google Scholar]
- 28.Korngold R, Sprent J. Variable capacity of L3T4+ T cells to cause lethal graft-versus-host disease across minor histocompatibility barriers in mice. J. Exp. Med. 1987;165:1552–1564. doi: 10.1084/jem.165.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heslop HE, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 31.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J. Immunol. 2000;164:4551–4557. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 32.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulos E, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 34.Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 35.Rooney CM, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 36.Rooney CM, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 37.Weinberg K, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]