Abstract
Calcium ion is a universal signaling intermediate, which is known to control various biological processes. In excitable cells, voltage-gated calcium channels (Cav) are the major route of calcium entry and regulate multiple functions such as contraction, neurotransmitter release, and gene transcription. Here we show that T lymphocytes, which are nonexcitable cells, express both regulatory β and pore-forming Cav1 α1 subunits of Cav channels, and we provide genetic evidence for a critical role of the Cav β3 and Cav β4 regulatory subunits in T lymphocyte function. Cav β-deficient T lymphocytes fail to acquire normal functions, and they display impairment in the T cell receptor-mediated calcium response, nuclear factor of activated T cells activation, and cytokine production. In addition, unlike in excitable cells, our data suggest a minimal physiological role for depolarization in Cav channel opening in T cells. T cell receptor stimulation induces only a small depolarization of T cells, and artificial depolarization of T cells using KCl does not lead to calcium entry. These observations suggest that the Cav channels expressed by T cells have adopted novel regulation/gating mechanisms.
Keywords: calcium, Cav β4, Cav β3
Calcium ion plays critical and specific roles in various T cell functions, including activation, differentiation, proliferation, and cytokine production (1, 2). In T lymphocytes, ligation of the T cell receptor (TCR) by antigen leads to the release of calcium from intracellular stores, triggering the calcium release-activated calcium current (3), and a potential candidate for calcium release-activated calcium current channel was recently reported (4–6). But the complexity of the calcium response in T cells suggests the expression of more than one plasma membrane calcium channel. Although it is established that, in excitable cells, Cav channels constitute the major route of calcium entry (7), the functional presence of the Cav1 channels in T lymphocytes has been suggested (8–13) but has remained controversial because of the lack of a reliable and specific loss-of-function approach.
Cav β subunits are cytoplasmic proteins that strongly regulate Cav channels through direct interaction with pore-forming α1 subunits (14–17). The β subunits are also critical for assembly of the channel complex (18), correct plasma membrane targeting (19, 20), and stimulation of channel activity (21). A number of potential α1–β combinations are likely to form a Cav channel complex (22), and, among these, the β4 and β3 subunits are key subunits that associate with Cav1 channels (23–25).
A spontaneous mutation named lethargic, which arose in the mouse inbred strain BALB/cGn in 1962, is recognizable in homozygous mice at 2 weeks of age by the onset of ataxia, seizures, and lethargic behavior (26, 27). These mice also exhibit a generalized immunological disorder including defective cell-mediated immune responses (28). It has been reported, using a positional cloning approach, that this syndrome was the result of a mutation of the Cav β4 subunit gene (29). This mutation is characterized by a 4-nt insertion into a splice donor site, which results in exon skipping and translational frameshift with loss of the α1 subunit-binding site and a severe reduction of β4 expression (29).
In this study we first show that members of the Cav1 family of Cav channels are expressed along with Cav β regulatory subunits by WT T cells. Cav1.1, Cav1.2, and Cav1.4 exhibit a differential expression as T cells transit from the naïve stage to the effector stage (day 4 after stimulation). Second, using two independent Cav β subunit-deficient mice, Cav β4 mutant and Cav β3 knockout (KO) (30), we show that functional Cav β regulatory subunits are necessary for a normal TCR-mediated calcium response, nuclear factor of activated T cells (NFAT) nuclear translocation, and cytokine production but are unnecessary for proliferation. Finally, we show that TCR stimulation of T cells generates only a small depolarization and that even strong depolarization of T cells using KCl does not lead to calcium entry. These observations suggest a minor physiological role for depolarization in Cav channel opening in T cells and suggest that Cav channels have developed distinct gating mechanisms in T cells compared with excitable cells.
Results and Discussion
CD4 T Cells Express both Pore-Forming and Regulatory Subunits of Cav1 Channels.
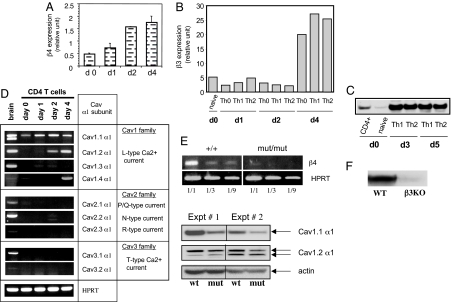
Real-time quantitative PCR (Fig. 1A) and semiquantitative RT-PCR (Fig. 5A, which is published as supporting information on the PNAS web site) revealed expression of the Cav β4 regulatory subunit in unstimulated naïve T cells of wild-type mice and up-regulation after TCR stimulation. Real-time quantitative PCR (Fig. 1B) and Western blots (Fig. 1C) revealed a similar expression profile for the Cav β3 regulatory subunit in T cells (expression in unstimulated naïve T cells and up-regulation after TCR stimulation). Because Cav β subunits can potentially interact with all Cav channels through an α1–β interaction involving highly conserved residues in both subunits (31, 32), we also assessed which Cav channel subtypes are expressed by T cells before and after TCR stimulation (Fig. 1D). Three Cav channels belonging to the Cav1 family were detected, Cav1.1, Cav1.2, and Cav1.4, displaying differential expression as T lymphocytes transit from the naïve stage toward an effector stage (day 4) (Fig. 1D). A dramatic reduction of Cav β4 subunit mRNA expression (Fig. 1E Upper) and a substantial reduction of Cav1.1 but not Cav1.2 α1 subunit proteins (Fig. 1E Lower) was also detected in Cav β4 mutant CD4 T cells compared with WT. The absence of β3 subunit expression in β3-deficient T cells is shown in Fig. 1F.
Fig. 1.
Expression of Cav channel subunits by CD4 T cells. Purified total CD4 T cells (CD4+) or naïve T cells (CD62L-high and CD44-low) were left unstimulated (day 0) or were stimulated with plate-bound anti-CD3 plus anti-CD28 Abs for the indicated period. (A and B) Analysis of β4 and β3 subunit mRNA expression by CD4 T cells using real-time quantitative PCR. (C) Western blot analysis of β3 subunit expression in CD4 T cells. (D) Expression of the Cav channel α1 subunits by CD4 T cells at different stages of differentiation. (E) Expression of Cav channel subunits by β4 mutant T cells assessed by using RT-PCR and Western blot. (F) Western blot analysis of the expression of β3 subunit in effector WT or β3 KO T cells. Results in A and D are representative of at least three independent experiments. For B, C, E, and F, two experiments were performed.
Impairment of TCR-Mediated Calcium/NFAT Pathway Activation in β4- and β3-Deficient CD4 T Cells.
The role of Cav β4 and β3 regulatory subunits in TCR-mediated calcium response was then tested by using T cells from β subunit-deficient mice and control littermates. The analysis of the thymus of Cav β4 mutant mice showed normal intrinsic T cell development. In fact, when mice were analyzed before the onset of the neurological syndrome (<2 weeks old), T cell development was normal (Fig. 6A, which is published as supporting information on the PNAS web site). In contrast, mice exhibiting the neuropathy (>2 weeks old) showed a reduction in the CD4/CD8 double-positive population (data not shown). Together, the data suggest that this reduction is secondary to the neuropathy including the corticosterone hypersecretion previously described in the mutant mice (33). This finding was confirmed by using RAG1−/− bone marrow chimera mice to allow stem cells from both WT and β4 mutant to develop in similar physiological environment. In these conditions, thymic development was indeed normal (Fig. 6B). In addition, no difference in the percentage of CD4 and CD8 cells or in the expression of different activation markers (CD25, CD69, CD62L, and CD44) was observed in the mutant relative to WT littermate mice (Fig. 6C), suggesting that the overall development of T cells is normal in these Cav β4 mutant mice. The analysis of the thymus of Cav β3 KO mice also revealed normal T cell development (Fig. 6D).
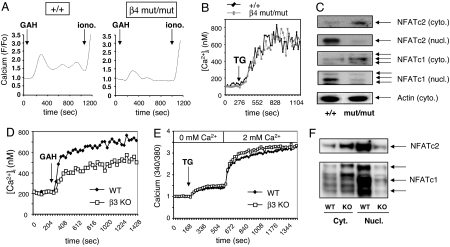
Although typical calcium responses were obtained with WT cells, both the initial peak and the plateau calcium responses were attenuated in both β4 and β3 subunit-deficient CD4 T lymphocytes (Fig. 2 A and D and Fig. 7A, which is published as supporting information on the PNAS web site). Furthermore, no defect in the calcium response was observed when WT and both β3 or β4 subunit-deficient CD4 T lymphocytes were stimulated by using thapsigargin, which mediates a passive release of calcium from intracellular stores (2, 34), indicating that store-operated calcium channels were not affected. No defect was also observed in the release of calcium from intracellular stores as assessed by using TCR and thapsigargin stimulations in a calcium-free medium (Figs. 2E and 7C). Finally, the ability of both groups of cells to take up the calcium probe fluo-3 was similar between T cells from WT and β4 mutant mice (Fig. 7B), which rules out the possibility that the difference observed in the calcium response is secondary to a difference in the ability of the two groups of cells to take up fluo-3. Altogether, these data demonstrate that the Cav β regulatory subunits are necessary for a normal TCR-mediated calcium response in CD4 T cells.
Fig. 2.
Calcium/NFAT pathway is impaired in both β4- and β3-deficient CD4 T cells. (A and D) The calcium response of CD4 T cells from β4-deficient (mut/mut) or β3-deficient (β3 KO) mice and WT littermate was evaluated by using 2C11 anti-CD3 Ab and goat anti-hamster (GAH) in a cross-linking system. (B and E) Calcium response of WT and β4- or β3-deficient CD4 T lymphocytes upon stimulation with thapsigargin (1 μM) under physiological Ca2+ concentrations (B) or in Ca2+ add-back experiments (E). (C) Western blot assay was performed on cytoplasmic (cyto.) and nuclear (nucl.) extracts prepared from β4 mutant CD4 T cells and control littermate stimulated for 48 h in the presence of human IL-2. Actin was used as internal control. (F) Analysis of β3-deficient CD4 T cells for NFAT nuclear translocation, using Western blot, after 18 h of TCR stimulation. Results are representative of five (A and D), three (B, C, and E), and two (F) independent experiments.
Increases in [Ca2+]i in T cells lead to nuclear translocation of the calcium-dependent transcription factor NFAT after its dephosphorylation by the phosphatase calcineurin (35, 36). This calcium/NFAT pathway is crucial for multiple T cell functions, including cytokine production (37). Nuclear translocation of both NFAT subtypes, NFATc2 and NFATc1, was inhibited in both β4- and β3-deficient T cells relative to WT (Fig. 2 C and F), demonstrating that the complete calcium/NFAT pathway is significantly impaired upon the inactivation of β regulatory subunits. Interestingly, a similar link between Cav1 channels and NFATc4 was reported in the hippocampal neurons (38).
Defective Cytokine Production in β Subunit-Deficient CD4 T Cells.
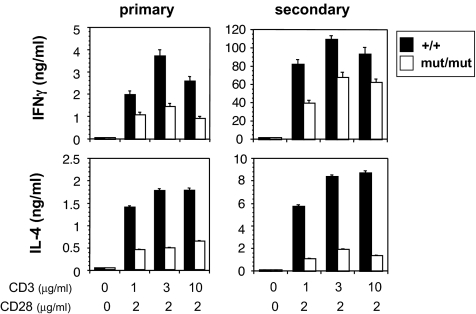
Although β4 mutant CD4 T lymphocytes retained their ability to proliferate when stimulated via the TCR (Fig. 8A, which is published as supporting information on the PNAS web site), TCR-mediated IL-2 production was partially inhibited in the β4 mutant T cells (Fig. 8B). In addition, the production of effector T cell cytokines, IFNγ and IL-4, was also reduced in both β4- and β3-deficient CD4 T cells under both primary and secondary conditions (Fig. 3 and data not shown). Finally, to exclude the possibility that the immune cell phenotype observed in β4 mutant T cells was secondary to the neuronal/endocrine deficiency, we generated bone marrow chimeras using WT or β4 mutant bone marrow transfer into irradiated RAG1-deficient mice. The analysis of CD4 T cells purified from the chimeras showed a similar defect in IFNγ and IL-4 production (Fig. 9, which is published as supporting information on the PNAS web site), corroborating the findings observed with T cells from the β4 mutant mice (Fig. 3). Thus, the T cell deficiency observed in β4 mutant mice was intrinsic to T lymphocytes.
Fig. 3.
Defective CD4 T cell differentiation in the absence of functional β4 subunit. CD4 T cells were stimulated by plate-bound anti-CD3 plus anti-CD28. Three days later, IFNγ and IL-4 production was measured by ELISA (primary). In some experiments cells were stimulated for 4 days, then washed and restimulated (secondary) by plate-bound anti-CD3 Ab. Twenty-four hours later, IFNγ and IL-4 production was measured by ELISA. Results are representative of at least three independent experiments.
Our data show that the TCR-mediated, β subunit-dependent, calcium/NFATc1/NFATc2 pathway activation is critical for cytokine production but is dispensable for proliferation of T lymphocytes. A selective defect in cytokine production but not T cell proliferation was also reported in NFATc2/NFATc1 double KO T cells (39).
Depolarization Is Unlikely to Be the Main Physiological Switch for T Cell Cavs.
To assess the physiological role of depolarization in Cav1 channel opening in T lymphocytes, we monitored changes in the resting potential of T cells after TCR stimulation using the voltage-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4) (3). A small depolarization was detected in stimulated T cells relative to unstimulated T cells (Fig. 4A Upper). This depolarization peaked at 120 s. A gradual repolarization was then observed after 300 and 600 s after TCR stimulation (Fig. 4A Upper). The peak depolarization observed was similar to the depolarization obtained with 10–15 mM KCl (Fig. 4A Lower) and was nifedipine-sensitive (Fig. 10, which is published as supporting information on the PNAS web site), suggesting that Cav channels contribute to the generation/maintenance of this small depolarization.
Fig. 4.
TCR stimulation induces only a small depolarization, and depolarization by KCl does not trigger T cell Cav opening. (A Upper) A modest depolarization of T lymphocytes after TCR stimulation (in black). Depolarization was assessed with the voltage-sensitive dye DiBAC4 (3) by using flow cytometric analysis. (A Lower) Depolarization using different concentrations of KCl (10–40 mM). Results shown in A are representative of four independent experiments. (B) CD4 T cells were purified from C57BL/6 mice and then differentiated under Th1 (IL-12 plus anti-IL-4), Th2 (IL-4 plus anti-IFNγ), or Th0 (no cytokine) conditions using Abs to CD3 and CD28. The calcium response of fura-2-loaded T or C2C12 cells was evaluated after the addition of KCl (40 mM). Results shown in B are representative of three independent experiments.
Subsequently, we tested the susceptibility of T cell Cavs to depolarization induced by KCl. Artificial depolarization of CD4 T cells that have been differentiated under Th1 (IL-12 plus anti-IL-4), Th2 (IL-4 plus anti-IFNγ), or Th0 (no cytokine) conditions with KCl did not lead to calcium influx (Fig. 4B). KCl was used at 40 mM, a dose that induces a significant depolarization of T cells (Fig. 4A Lower). However, under the same conditions and as expected, KCl triggered a transient calcium response in the C2C12 skeletal muscle excitable cell line as previously reported (40). In addition, all four groups of cells, Th0, Th1, Th2, and C2C12, were able to mount a calcium response after stimulation with the calcium ionophore ionomycin (data not shown). Altogether, these observations suggest that depolarization is unlikely to play a major role in opening T cell Cav channels. Alternatively, Cav1 channels could be regulated in a voltage-independent manner as previously suggested for human Jurkat cells (12), where other TCR-triggered signaling events such as phosphorylation would induce opening. This hypothesis is strongly supported by the earlier surprising findings showing that Cav1.3 channels can be activated at voltages of approximately −60 mV under physiological calcium concentrations (41, 42). Further studies are needed to clarify the contribution of regulatory events other than voltage in Cav1 channel physiological gating in T lymphocytes. T cells should respond only to antigen stimulation through cognate TCR. A voltage-dependent opening in the absence of TCR dependence would lead to a random opening of Cav channels and subsequent activation of T cells, which could lead to inflammation in the absence of antigen. Unlike excitable cells, T cells migrate and roam the body through variable extracellular environments and tissues with various ion concentrations. It is therefore conceivable that Cav channels expressed by T cells have developed a more specific control of their opening than mere voltage sensing.
Finally, the present study provides an answer to a long-standing question pertaining to the role of Cav channels in T lymphocyte function. The T cell functional defect described in the present work in both β4- and β3-deficient T cells reflects the contribution of these subunits to Cav1 channel-dependent calcium response in T lymphocytes. It is not yet clear which Cav1 channel(s) is controlled by each one of these subunits. It now remains to determine the specific role of each one of these individual Cav1 channels.‖
Materials and Methods
Preparation and Differentiation of CD4 T Cells in Vitro.
CD4 T cells from spleens were isolated from 6- to 8-week-old C57BL/6 mice purchased from the National Cancer Institute (Frederick, MD). CD4 cells were isolated by immunomagnetic negative selection using Abs against CD8, NK1.1, and MHC class II followed by incubation with anti-mouse and anti-rat Ig-coated magnetic beads (PerSeptive Biosystems, Framingham, MA). For experiments involving β4 mutant mice, 3- to 4-week-old B6EiC3H-a/A-Cacnb4lh/+ mice (purchased from The Jackson Laboratory, Bar Harbor, ME) were used. Cells were cultured in Bruff's medium containing 10% FCS and were stimulated for various time periods in the presence of plate-bound anti-CD3 and anti-CD28 Abs. In some experiments, 20 units/ml recombinant human IL-2 (a gift from Biogen, Cambridge, MA) was added to the culture.
Semiquantitative RT-PCR and Real-Time Quantitative PCR.
Total RNA was extracted and reverse-transcribed as previously described (8). Subsequently, each sample was subjected to PCR with sense and antisense primers: hypoxanthine phosphoribosyltransferase, 5′-GTTGGATACAGGCCAGACTTTGTTG (sense) and 5′-TCGGATATCCGGTCGGATGGGAG (antisense); Cav1.1 α1, 5′-TGTGGTATGTCGTCACTTCCTCC (sense) and 5′-CGTCAATGATGCTGCCGATG (antisense); Cav1.2 α1, 5′-CAAGCCCTCACAAAGGAATGC (sense) and 5′-AAAGTTGCCCCTGCTGTCACTC; Cav1.3 α1, 5′-ATCTCACACACCGCCAGGACTATG (sense) and 5′-CATCACCTTTGACCTCTCTCGTG (antisense); Cav1.4 α1, 5′-AAGATTTACCTATCCCAGGCACCTAC (sense) and 5′-CATCAAAGCGGGAAAGAATAGACTC (antisense); Cav2.1 α1, 5′-AGTACCTCACCCGAGATTCTTCCATC (sense) and 5- GCTGCGTAGATCTTACCCACTGTCA (antisense); Cav2.2 α1, 5′-CTTCCATCCTAGGGCCTCACCACTTA (sense) and 5′-CTCCGGGAGCTTGGTGAGTCTGAT (antisense); Cav2.3 α1, 5′-CGAAAGCACCTCCTCTCTCCAGATGT (sense) and 5′-GTGCAAGGAGTTGGAAGACTCGGTC (antisense); Cav3.1 α1, 5′-CACTCTCTGCCCAATGACAGCTACAT (sense) and 5′-CTGCTTCTGGTCTCTTGAGGGTCCTTGGC (antisense); Cav3.2 α1, 5′-CAGGATGCTCTCGCTACCCAATGACA (sense) and 5′-CTCCGTGTAGTCTGGGATGCCGTCTTC (antisense); β4 subunit, 5′-TGGCTTCATCCCAAGTCCACTGCG (sense) and 5′-CAGTGATGGCCCTACTAACACCAC (antisense); genotyping β4 mutant mice, 5′-AAATGGTATCAGGAACATTCCGAGC (sense) and 5′-TTTCCAAACCAGTGAAAGCGTTAGC (antisense). For real-time PCR, a fluorogenic probe was synthesized by Biosearch Technologies (Novato, CA): probe sequence, 5′ 6-FAM d(CACCCACACACGAGCAGTAGCACCCCT) BHQ-1 3′; primers, 5′-CTGGAGGCATACTGGCGTG-3′ and 5′-ACGTTCCGCCCCAGTAATG-3′. Real-time PCR analysis of β3 was performed by using commercially available primers and probe from Applied Biosystems (Foster City, CA). Cycling conditions were 2 min at 94°C followed by 25–35 repeats of 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min. To ensure that amplified products were not from genomic DNA, a sample of purified RNA that was not subjected to reverse transcription was introduced in PCRs.
Analysis of Intracellular Calcium Concentration.
The intracellular free Ca2+ concentration was measured by using either fluo-3/AM (Molecular Probes, Eugene, OR) as previously described (45) or Fura-2/AM (Molecular Probes). For experiments where fluo-3 was used, cells were loaded with 5 μM fluo-3/AM (Molecular Probes) for 30 min at 37°C. Cells were scanned by using the ACAS 570 video laser cytometer (Meridian Instruments, Lansing, MI). Fluo-3/AM-loaded T cells were stimulated with anti-CD3 and anti-hamster IgG in a cross-linking system. For experiments where fura-2 was used as calcium probe, fluorescence was monitored in ratio mode by using a fluorometer (Polarstar Galaxy; BMG Labtechnologies, Offenburg, Germany). Collected data were analyzed by using Fluostar Galaxy Software (BMG Labtechnologies). At the end of each experiment, cells were treated with 5 μM ionomycin in calcium-containing medium, then with calcium-free medium supplemented with 5 mM EGTA. Experimental 340/380 ratios were converted to [Ca2+]i according to the equation described by Tsien and colleagues (46).
Flow Cytometry.
Fluo-3-loaded or Ab-stained T cells were analyzed by using a FACScan flow cytometer and CellQuest 3.1 software (Becton Dickinson, Franklin Lakes, NJ).
Western Blotting.
Protein extracts (10 μg) were subjected to 8% SDS/PAGE, transferred to PVDF membranes (Millipore, Bedford, MA), and immunoblotted with appropriate Abs. The following Abs were used: mouse monoclonal Ab against NFATc2 (4G6-G5; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal Ab to NFATc1 (Affinity Bioreagents, Golden, CO), and anti-actin goat polyclonal Ab (Santa Cruz Biotechnology). The Ab to Cav β3 (Ab 828) was generated in the laboratory of V.F.
5-(and -6)-Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Labeling.
CFSE (Molecular Probes) was added to the cells at a final concentration of 0.5 μM, and cells were incubated for 10 min at 37°C. At the end of the incubation period, the cells were immediately washed three times in PBS containing 10% FCS. Cells were then stimulated for the indicated period, and CFSE staining was measured by flow cytometry.
ELISA.
Cytokine levels in cell culture supernatants were evaluated by using two-site sandwich ELISA with paired Abs purchased from Pharmingen (San Diego, CA).
Evaluation of CD4 T Cell Depolarization.
DiBAC4 (3) is a negatively charged lipophilic dye that partitions across cell membrane depending on membrane potential. Increases in fluorescence correspond to depolarization. CD4 T cells were pretreated with 300 nM DiBAC4 (3) and then incubated in the absence or presence of precoated anti-CD3 plus anti-CD28 Abs or different concentrations of KCl (10–40 mM) for the indicated period. Subsequently, cells were washed twice, and fluorescence level was determined by using flow cytometry.
Statistics.
Student's t test was used to calculate the statistical significance of differences between two sets of averaged data.
Supplementary Material
Acknowledgments
We thank F. Manzo for assistance with manuscript preparation. A.B. was supported by the Fondation pour la Recherche Médicale and by the Arthritis National Research Foundation, D.M. was supported by a Cancer Research Institute postdoctoral fellowship, and M.K.J. is supported by an Arthritis Foundation postdoctoral fellowship. A.B. and M.K.J. were Associates and R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- TCR
T cell receptor
- DiBAC4
bis-(1,3-dibutylbarbituric acid) trimethine oxonol
- NFAT
nuclear factor of activated T cells
- KO
knockout.
Footnotes
The authors declare no conflict of interest.
Some of the data presented in this article were initially published in Science (43) and were subsequently retracted (44) because of the erroneous nature of figure 4B, which was prepared by Srisaila Basavappa. In this article, in addition to the analysis of the β4 mutant mice, we present new evidence corroborating the role of the Cav channels in T lymphocyte functions by using Cav β3 KO mice. Therefore, this previously unpublished genetic evidence is compatible with the genetic evidence previously published in the Science article (i.e., β4 mutant mice) and emphasizes a more generalized role for the Cav β subunits in T cell functions.
References
- 1.Cantrell D. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 3.Zweifach A, Lewis RS. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 5.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catterall WA. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 8.Badou A, Savignac M, Moreau M, Leclerc C, Pasquier R, Druet P, Pelletier L. J Biol Chem. 1997;272:32411–32418. doi: 10.1074/jbc.272.51.32411. [DOI] [PubMed] [Google Scholar]
- 9.Savignac M, Badou A, Moreau M, Leclerc C, Guery JC, Paulet P, Druet P, Ragab-Thomas J, Pelletier L. FASEB J. 2001;15:1577–1579. doi: 10.1096/fj.00-0733fje. [DOI] [PubMed] [Google Scholar]
- 10.Kotturi MF, Carlow DA, Lee JC, Ziltener HJ, Jefferies WA. J Biol Chem. 2003;278:46949–46960. doi: 10.1074/jbc.M309268200. [DOI] [PubMed] [Google Scholar]
- 11.Savignac M, Gomes B, Gallard A, Narbonnet S, Moreau M, Leclerc C, Paulet P, Mariame B, Druet P, Saoudi A, et al. J Immunol. 2004;172:5206–5212. doi: 10.4049/jimmunol.172.9.5206. [DOI] [PubMed] [Google Scholar]
- 12.Stokes L, Gordon J, Grafton G. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 13.Kotturi MF, Jefferies WA. Mol Immunol. 2005;42:1461–1647. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Richards MW, Butcher AJ, Dolphin AC. Trends Pharmacol Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 16.Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 18.Tareilus E, Roux M, Qin N, Olcese R, Zou J, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien AJ, Gao T, Perez-Reyes E, Hosey MM. J Biol Chem. 1998;273:23590–23597. doi: 10.1074/jbc.273.36.23590. [DOI] [PubMed] [Google Scholar]
- 20.Gao T, Chien AJ, Hosey MM. J Biol Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- 21.Freise D, Himmerkus N, Schroth G, Trost C, Weibgerber P, Freichel M, Flockerzi V. Biol Chem. 1999;380:897–902. doi: 10.1515/BC.1999.110. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka O, Sakagami H, Kondo H. Brain Res Mol Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- 23.Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. J Biol Chem. 1997;272:13877–13882. doi: 10.1074/jbc.272.21.13877. [DOI] [PubMed] [Google Scholar]
- 24.Schjott JM, Hsu SC, Plummer MR. J Biol Chem. 2003;278:33936–33942. doi: 10.1074/jbc.M302059200. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi H, Hara M, Strobeck M, Fukasawa K, Schwartz A, Varadi G. J Biol Chem. 1998;273:19348–19356. doi: 10.1074/jbc.273.30.19348. [DOI] [PubMed] [Google Scholar]
- 26.Dickie MM. Mouse News Lett. 1964;30:31. [Google Scholar]
- 27.Sidman RL, Green MC, Appel SH. Catalog of the Neurological Mutants of the Mouse. Cambridge, MA: Harvard Univ Press; 1965. p. 34. [Google Scholar]
- 28.Morrison DG, Moyer MP, Dung HC, Rogers W, Moyer RC. Dev Comp Immunol. 1984;8:435–442. doi: 10.1016/0145-305x(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 29.Burgess DL, Jones JM, Meisler MH, Noebels JL. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- 30.Murakami M, Fleischmann B, De Felipe C, Freichel M, Trost C, Ludwig A, Wissenbach U, Schwegler H, Hofmann F, Hescheler J, et al. J Biol Chem. 2002;277:40342–40351. doi: 10.1074/jbc.M203425200. [DOI] [PubMed] [Google Scholar]
- 31.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Nature. 1994;368:60–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 32.De Waard M, Pragnell M, Campbell KP. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 33.Dung HC. Am J Anat. 1976;147:255–264. doi: 10.1002/aja.1001470209. [DOI] [PubMed] [Google Scholar]
- 34.Mintz E, Guillain F. Biochim Biophys Acta. 1997;1318:52–70. doi: 10.1016/s0005-2728(96)00132-6. [DOI] [PubMed] [Google Scholar]
- 35.Rao A, Luo C, Hogan PG. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 36.Crabtree GR, Olson EN. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 37.Serfling E, Berberich-Siebelt F, Chuvpilo S, Jankevics E, Klein-Hessling S, Twardzik T, Avots A. Biochim Biophys Acta. 2000;1498:1–18. doi: 10.1016/s0167-4889(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 38.Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 39.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. Proc Natl Acad Sci USA. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 42.Xu W, Lipscombe D. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badou A, Basavappa S, Desai R, Peng YQ, Matza D, Mehal WZ, Kaczmarek LK, Boulpaep EL, Flavell RA. Science. 2005;307:117–121. doi: 10.1126/science.1100582. [DOI] [PubMed] [Google Scholar]
- 44.Flavell RA, Kaczmarek LK, Badou A, Boulpaep EL, Desai R, Basavappa S, Matza D, Peng YQ, Mehal WZ. Science. 2005;310:1903. doi: 10.1126/science.310.5756.1903b. (left) [DOI] [PubMed] [Google Scholar]
- 45.Yu H, Leitenberg D, Li B, Flavell RA. J Exp Med. 2001;194:915–926. doi: 10.1084/jem.194.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grynkiewicz G, Poenie M, Tsien RY. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.