Abstract
Mammalian cardiomyocytes have limited proliferation potential, and acutely injured mammalian hearts do not regenerate adequately. Instead, injured myocardium develops fibrosis and scarring. Here we show that FGF1/p38 MAP kinase inhibitor treatment after acute myocardial injury in 8- to 10-week-old rats increases cardiomyocyte mitosis. At 3 months after injury, 4 weeks of FGF1/p38 MAP kinase inhibitor therapy results in reduced scarring and wall thinning, with markedly improved cardiac function. In contrast, p38 MAP kinase inhibition alone fails to rescue heart function despite increased cardiomyocyte mitosis. FGF1 improves angiogenesis, possibly contributing to the survival of newly generated cardiomyocytes. Our data indicate that FGF1 and p38 MAP kinase, proteins involved in cardiomyocyte proliferation and angiogenesis during development, may be delivered therapeutically to enhance cardiac regeneration.
Keywords: cardiac regeneration, fractional shortening, Cyclin D2, Cyclin A, angiogenesis
The mammalian heart has little or no capacity to regenerate (1, 2). This is a major medical problem, because inadequate regeneration contributes to myocardial scarring, heart failure, arrhythmia, and death (3). As a single cause of death, ischemic heart disease is one of the leading causes of mortality worldwide among people ages 15–59. This disease results from occlusion of cardiac vessels leading to loss of cardiomyocytes causing >7 million deaths every year.
Observations from naturally occurring regeneration in zebrafish suggest that mammalian cardiac regeneration might be achieved through cardiomyocyte proliferation. Importantly, hearts of zebrafish carrying a mutation preventing proliferation do not regenerate; instead, they scar (4). It has been previously suggested that the mammalian heart induces cardiomyocyte proliferation after injury but the rate of proliferation is too low to repair the heart (5). In addition, we have recently shown that postnatal mammalian cardiomyocytes can proliferate in vitro by combining FGF1 stimulation and p38 MAP kinase (p38) inhibition (6). FGF1 and p38 inhibitor administration have been shown to be protective in ischemic heart disease, decreasing cardiomyocyte apoptosis (7, 8). However, constitutive cardiac-specific FGF1 overexpression only delayed myocardial infarct formation with no difference in maximal infarct size (9). The effects of p38 inhibition as well as FGF1 stimulation after permanent coronary occlusion are not well characterized.
Results
To determine whether FGF1/p38 inhibitor treatment can increase cardiomyocyte proliferation in vivo and improve heart function after cardiac injury, we performed a blinded and randomized study. We induced cardiac injury by permanent coronary artery occlusion, or myocardial infarction (MI), and treated animals with saline plus BSA (control), the p38 MAP kinase inhibitor SB203580 plus BSA (p38i), FGF1 plus saline (FGF1), or FGF1 plus SB203580 (FGF1/p38i) (n ≥ 9 per group). SB203580 and saline were injected i.p. once every 3 days. FGF1 and its carrier BSA were injected with self-assembling peptides once into the infarct border zone immediately after coronary artery ligation (10).
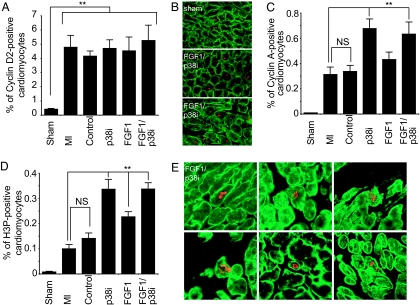
Recent data indicated that overexpression of Cyclin D2 as well as Cyclin A2 can enhance cardiac regeneration (11, 12). To determine whether FGF1/p38i treatment enhances Cyclin D2 and/or Cyclin A2 expression, we examined heart sections 2 weeks after treatment. Caveolin 3 staining was used to identify cardiomyocytes (see Fig. 5, which is published as supporting information on the PNAS web site). MI increased the number of Cyclin D2-positive cardiomyocytes by 11.5-fold within the infarct area and border zone (P < 0.01, Fig. 1A and B). This finding is in accord with the previous findings of an increase in cyclin expression and cell cycle activation after MI and during development of hypertrophy (13–15). Neither FGF1 nor p38i treatment increased the number of Cyclin D2-positive cardiomyocytes; this finding might be explained by increased myocardial growth factor production after infarction (6, 16). Our in vitro data indicated that FGF1 stimulation induces cell cycle reentry of cardiomyocytes but has only a minor effect on mitosis. In contrast, p38 inhibition increased cyclin A2 expression and progression through mitosis after growth factor stimulation (6). To determine whether FGF1/p38i treatment enhances Cyclin A expression and the progression of cardiomyocyte mitosis, we assayed for Cyclin A expression and histone H3 phosphorylation (H3P). Indeed, the number of Cyclin A- and H3P-positive cardiomyocytes within the infarct and the border zone were 2-fold (P < 0.01) and 3.4-fold (P < 0.0001) increased, respectively, in animals treated with FGF1/p38i compared with untreated MI (Fig. 1 C–E). p38i could, in contrast to our in vitro study, increase the number of Cyclin A-positive (2.2-fold, P < 0.001) and H3P-positive (3.4-fold, P < 0.0001) cardiomyocytes (Fig. 1 C and D), whereas FGF1 stimulation increased H3P-positive cardiomyocytes only 2.3-fold (P < 0.001, Fig. 1D). Mitosis was not detected 3 months after MI. This is most likely due to the fact that treatment was stopped after 1 month. Taken together, our data support the idea that endogenous growth factor signaling is present after MI, and indicate that p38 inhibition facilitates FGF1 and/or growth factor-mediated cardiomyocyte mitosis after MI within the infarct and the border zone.
Fig. 1.
FGF1/p38 inhibitor treatment induces cardiomyocyte mitosis in vivo. Rats were treated after myocardial infarction (MI) with saline plus BSA (control), SB203580 plus BSA (p38i), saline plus FGF1 (FGF1), or SB203580 plus FGF1 (FGF1/p38i). Two weeks after treatment, heart sections were analyzed for cell cycle activation (Cylin D2, Cyclin A) and mitosis (H3P) of cardiomyocytes within the scar area and the infarct border zone. (A) FGF1 and/or p38i significantly increased the number of Cyclin D2-positive cardiomyocytes. (B) Examples of heart section from sham or FGF1/p38i-treated animals stained for Caveolin 3 (green) and Cyclin D2 (red). (C) FGF1 and/or p38i significantly increased the number of Cyclin A-positive cardiomyocytes. (D) FGF1 and/or p38i significantly increased the number of H3P-positive cardiomyocytes. (E) Six examples of heart section after FGF1/p38i treatment stained for Caveolin 3 (green) and H3P (red). Data are ±SEM; n ≥ 9 in each group; NS, not significant; ∗∗, P < 0.01.
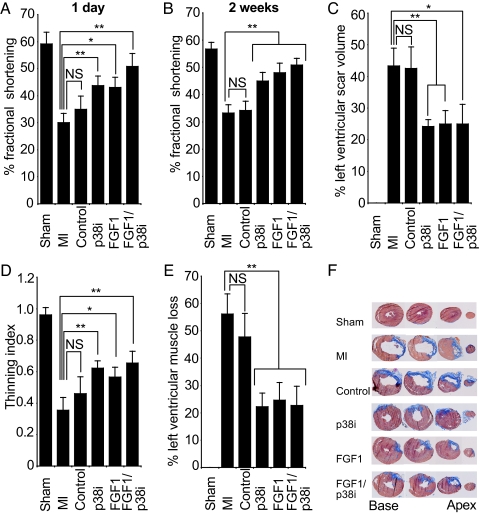
Injury from MI disturbs loading conditions within the heart, causes ischemic and oxidative stresses, and activates various local and systemic neurohormonal systems (17). These alterations to the extracellular environment trigger left ventricular (LV) remodeling characterized by necrosis and thinning of the infarcted myocardium, LV chamber dilation, fibrosis, and hypertrophy of viable cardiomyocytes. Persistent remodeling contributes to functional decompensation and eventually leads to heart failure (18). Because p38i improves cardiomyocyte proliferation more efficiently than FGF1, we tested whether this translates into a greater preservation of heart function. Cardiac function after MI was assessed by percentage left ventricular fractional shortening (%FS), end diastolic dimension (EDD), and end systolic dimension (ESD). Twenty-four hours after MI, %FS decreased from 59.1 ± 13.1% to 30.1 ± 10.9% (P < 0.0001, Fig. 2A). Injection of saline and BSA did not significantly improve %FS. In contrast, rats treated with p38i, FGF1, and FGF1/p38i significantly increased %FS (43.0 ± 11.4%, 44.3 ± 10.1%, 50.8 ± 15.2%, respectively; P < 0.05 over MI, Fig. 2A). This early improvement indicates that all treatments may have had an antiapoptotic effect as previously described (8, 19, 20). Two weeks after MI, %FS was maintained in animals that received FGF1 and/or p38i (P < 0.05 over MI; Fig. 2B). Apoptosis rates were low and did not differ between treated and untreated animals (data not shown). Consistent with the improvement of %FS after MI, injection of FGF1 and/or p38i prevented cardiac dilation as measured by EDD and ESD (P < 0.05, see Table 1, which is published as supporting information on the PNAS web site). Trichrome stain at seven levels of the heart from apex to base (1.2 mm apart) revealed that scar size at 2 weeks was reduced after all treatments by over 44% (P < 0.05, Fig. 2 C and F, see also Fig. 6, which is published as supporting information on the PNAS web site). In addition, we calculated the thinning index (ratio of minimal ventricular wall thickness to maximal thickness of the septum, using the top four levels from base). The thinning index decreased as expected from 0.96 ± 0.05 at 2 weeks in the sham group to 0.35 ± 0.08 in the MI group (P < 0.0001, Fig. 2D). Whereas the control group did not reduce thinning significantly, all other treatments reduced thinning by >34% (P < 0.05 for MI versus FGF1, P < 0.01 for p38i and FGF1/p38i versus MI; Fig. 2D). Because the left ventricular wall thins after injury, the amount of scar tissue does not reflect the amount of muscle loss. To determine the amount of muscle loss, we divided the circumference of left ventricular wall containing at least 75% scar by the circumference of left ventricular wall using all sections. This analysis revealed that the loss of left ventricular muscle at two weeks was reduced after all treatments by >56% (P < 0.01, Fig. 2E). There was no difference between p38i, FGF1, and FGF1/p38i therapy regarding %FS, thinning, scarring, and loss of left ventricular muscle. Taken together, these data indicate that FGF1 stimulation as well as p38 inhibition rescue cardiac structure and function after injury.
Fig. 2.
FGF1/p38 inhibitor treatment improves heart function. Rats were treated after myocardial infarction (MI) with saline plus BSA (control), SB203580 plus BSA (p38i), saline plus FGF1 (FGF1), or SB203580 plus FGF1 (FGF1/p38i). Hearts were analyzed by using echocardiography and trichrome stain of transverse heart sections. (A and B) FGF1 and/or p38i significantly improved left ventricular %FS 1 and 14 days after MI. %FS was calculated as (EDD − ESD)/EDD × 100%, where EDD is end-diastolic dimension and ESD is end-systolic dimension. (C) FGF1 and/or p38i significantly decreased left ventricular scarring. Percentage of scar volume was determined as fibrotic area/(fibrotic + nonfibrotic area) using seven sections from apex to base, 1.2 mm apart. (D) FGF1 and/or p38i significantly decreased ventricular wall thinning. The thinning index is the ratio of minimal infarct wall thickness and maximal septal wall thickness using sections 1–4 from base. (E) FGF1 and/or p38i significantly decreased left ventricular muscle loss. Percentage of left ventricular muscle loss was determined as circumference of the left ventricular wall containing at least 75% scar area/circumference of the left ventricular wall based on all sections. (F) Examples of heart sections stained for scar tissue (blue) and muscle tissue (brown) from base to apex in an interval of 2.4 mm 2 weeks after treatment. Data are ±SEM; n ≥ 9 in each group; NS, not significant; ∗, P < 0.05; ∗∗, P < 0.01.
To determine whether FGF1/p38i treatment has a long-term effect, we performed an additional and separate blinded and randomized study (n ≥ 7 per group). Because it has been shown that long-term exposure of humans to p38 inhibitors is toxic (21), we treated animals for 1 month and analyzed the effect of our treatment regimen after 3 months. Again, %FS 24 h after MI was significantly improved after injection of FGF1 and/or p38i (P < 0.05, Fig. 3A). At 3 months after injury, 4 weeks of FGF1 and FGF1/p38i therapy increased %FS by 25% and 30%, respectively (P < 0.05 over MI; Fig. 3B). Importantly, there was no significant difference in %FS to uninjured animals. In contrast, %FS of hearts treated with p38i were not significantly different from the MI group. Scar size was reduced in all rats treated with FGF1 and/or p38i (reduction of: p38i: 32.8%, FGF1: 43.7%, FGF1/p38i: 52.3%; P < 0.05 for p38i, FGF1, and P < 0.01 for FGF1/p38i over MI; Fig. 3C and F, see also Fig. 7, which is published as supporting information on the PNAS web site). However, ventricular wall thinning and left ventricular muscle loss were only significantly improved in rats treated with FGF1 (reduction of 34.0% and 51.0% respectively; P < 0.05) or FGF1/p38i (reduction of 54.6%, P < 0.01 and 61.7%, P < 0.05 respectively, Fig. 3 D and E). Treatment with p38i had no effect at this later stage. Taken together, these data indicate that FGF1 and FGF1/p38i treatment preserve wall thickness, reduce scaring, and improve function, whereas the effect of p38i is transient.
Fig. 3.
FGF1/p38 inhibitor improved heart function 3 months after myocardial infarction. Rats were treated after myocardial infarction (MI) with saline plus BSA (control), SB203580 plus BSA (p38i), saline plus FGF1 (FGF1), or SB203580 plus FGF1 (FGF1/p38i). Hearts were analyzed by using echocardiography and trichrome stain of transverse heart sections 3 months after treatment. Note that treatment was stopped after 1 month. (A and B) FGF1/p38i and FGF1 significantly improved %FS 1 day and 3 months after MI. In contrast, %FS after p38i treatment was only improved at day 1. (C) FGF1 and/or p38i significantly decreased scar formation. The percentage of scar volume was determined as fibrotic area/(fibrotic + nonfibrotic area) using nine sections from apex to base, 1.3 mm apart. (D) FGF1/p38i and FGF1 significantly decreased ventricular wall thinning but not p38i. The thinning index was determined by using sections 1–6 from base. (E) FGF1/p38i and FGF1 significantly decreased left ventricular muscle loss but not p38i. The percentage of left ventricular muscle loss was determined as circumference of the left ventricular wall containing at least 75% scar area/circumference of the left ventricular wall based on all sections. (F) Examples of heart sections stained for scar tissue (blue) and muscle tissue (brown) from base to apex in an interval of 2.6 mm 3 months after treatment. Data are ±SEM; n ≥ 9 in each group; NS, not significant; ∗, P < 0.05; ∗∗, P < 0.01.
Organ regeneration is a highly complex process. Studies in liver regeneration have shown that the process of regeneration depends on the injury and involves the cooperative effort of several cell types and secreted factors to rebuild a functional cytoarchitecture (22). Although our data indicate that p38 inhibition improves the proliferative potential of cardiomyocytes, newly generated cardiomyocytes can only survive if they are supplied with oxygen and nutrients in functioning heart muscle. Therefore, we speculated that FGF1 contributes to long-term improved heart function not only by inducing cardiomyocyte mitosis but also by improving angiogenesis in the heart (23, 24). Indeed, FGF1 and FGF1/p38i increased the capillary density in the scar by >40% at 3 months after injury (P < 0.05, Fig. 4, see also Fig. 5). In contrast, although contributing to the activation of cardiomyocyte mitosis, p38i had no effect on capillary density. This observation may explain why the overall effect of p38i treatment is transient. Taken together, our data indicate that FGF1 treatment increases angiogenesis in the scar area.
Fig. 4.
FGF1/p38 inhibitor treatment increases angiogenesis. (A) FGF1/p38i and FGF1, but not p38i, significantly increased angiogenesis of the scar area at 3 months. (B) Examples of vessel staining 3 months after MI using vessel markers smooth muscle actin (SMA, smooth muscle cells, fluorescent green) and von Willebrand factor (vWF, endothelial cells, red) and cardiomyocyte-specific marker Caveolin 3 (pale green). (C) Examples of vessel staining of heart sections from rats 3 months after coronary ligation treated with FGF1/p38i. Data are ±SEM; n ≥ 7 in each group; NS, not significant; ∗, P < 0.05; ∗∗, P < 0.01.
Discussion
Recent evidence suggests that a population of extracardiac or intracardiac stem cells may be feasible for cardiac repair (25–27), but this approach has been controversial and requires isolation of autologous stem cells or the use of donor cells along with immunosuppresion. The evidence presented here suggests that FGF1 and p38 MAP kinase, proteins involved in cardiomyocyte proliferation and angiogenesis during cardiac development, may be redeployed therapeutically to enhance cardiac regeneration after myocardial infarction. It has been suggested that the mammalian heart induces cardiomyocyte proliferation after injury, but the rate of proliferation is too low to repair the heart (5). Recent work had indicated that induction of cardiomyocyte proliferation by Cyclin D2 or Cyclin A2 overexpression has the potential to improve heart function after injury (11, 12). However, this work was based on transgenic technology requiring genetic engineering in the fetus or gene therapy. We have developed a pharmacological approach to treat cardiac injury in adults. To constrain adverse effects of systemic FGF1 therapy, we used a local delivery system (10, 28). This system is advantageous over prolonged overexpression by gene therapy vectors that can lead to excessive protein production and undesired effects. Local and pulsed delivery also is optimal for the p38 inhibitor SB203580; our previous data revealed that continued inhibition of p38 in vitro results in cell death of adult cardiomyocytes (F.B.E., unpublished observation).
Although our previous in vitro data suggest that FGF1/p38i treatment induces cardiomyocyte proliferation, it is also possible that FGF1/p38i treatment in vivo might increase proliferation of resident stem cells in the heart or peripheral stem cells recruited to the heart. In our proliferation assays, it is not 100% certain that the Cyclin D2-, Cyclin A, or H3P-positive cells are all proliferating adult cardiomyocytes, because it can be argued that they can also be proliferating stem cells that have already expressed cardiac markers. However, it has been reported that, 20 days after MI, cardiomyocytes derived from injected cardiac stem cells are much smaller (3,400 ± 560 μm3) than surviving cardiomyocytes (20,000–25,000 μm3) (29). In our study, >90% of Cyclin D2-, Cyclin A, or H3P-positive cells show no difference in size compared with surviving cardiomyocytes. Future studies will determine whether FGF1/p38i therapy has an effect on stem cells in the heart.
FGF1/p38i therapy consistently shows the best outcome regarding preservation of cardiac structure and function. Our data suggest that FGF1 improves heart function through enhancement of angiogenesis and cardiomyocyte mitosis. p38 inhibition further increases the effect on cardiomyocyte mitosis. Thus, FGF1/p38i therapy combines the effects of p38 inhibition on apoptosis and proliferation with the effects of FGF1 on apoptosis, proliferation, and angiogenesis to enhance cardiac regeneration. Given our findings, FGF1/p38 inhibitor treatment may prove useful for protecting patients from cardiac injury and therefore warrants further preclinical investigation.
Materials and Methods
MI and Delivery of FGF1 and p38 Inhibitor.
Animal experiments were performed in accordance with guidelines of Children's Hospital (Boston, MA) and were approved by the Harvard Medical School Standing Committee on Animals. MI was produced in ≈250-g male Sprague–Dawley rats [≈8–10 weeks old; Charles River (Wilmington, MA) and Harlan (Indianapolis, IN)] as described (10). Briefly, rats were anesthetized by pentobarbital and, after tracheal intubation, the hearts were exposed via left thoracotomy. The left coronary artery was identified after pericardiotomy and was ligated by suturing with 6-0 prolene at the location ≈3 mm below the left atrial appendix. For the sham operation, suturing was performed without ligation. Peptide nanofibers (peptide sequence AcN-RARADADARARADADA-CNH2 from Synpep, Dublin, CA) with BSA (0.1% in PBS) or 400 ng/ml bovine FGF1 (R & D Systems, Minneapolis, MN; diluted in 0.1% BSA/PBS) were dissolved in 295 mM sucrose and sonicated to produce 1% solution for injection. Eighty microliters of peptide nanofibers (NF) was injected into the infarcted border zone through three directions immediately after coronary artery ligation estimated to deliver an FGF1 concentration of ≈50–100 ng/ml to the cardiomyocytes (10). Subsequently, SB203580HCl (Tocris, Ellisville, MO, 2 mg/kg body weight) or saline was injected i.p., the chest was closed, and animals were allowed to recover under a heating pad.
Immunofluorescence Staining.
Hearts were embedded in tissue-freezing medium (Fisher, Hampton, NH) without fixation, frozen in 2-methylbutane (cooled in liquid nitrogen), stored at −80°C, and finally sectioned [20 μm; Leica, Bannockburn, IL) 3050S]. Staining was performed as described (see also Table 2, which is published as supporting information on the PNAS web site) (30). Immune complexes were detected with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies (1:400; Molecular Probes, Eugene, OR). DNA was visualized with 0.5 μg/ml DAPI (Sigma, St. Louis, MO).
Trichrome Stain.
Through each heart, seven to nine sections (1.2- or 1.3-mm interval) from apex to base were subjected to AFOG staining (4). Frozen sections were fixed at room temperature (RT) with 10% neutral buffered formalin (10–15 min). Sections were permeabilized (0.5% Triton X-100/PBS, 10 min), incubated in preheated Bouins fixative (2.5 h at 56°C, 1 h at room temperature), washed in tap water, incubated in 1% phosphomolybdic acid (5 min), rinsed with distilled water, and stained with AFOG staining solution (3 g of acid fuchsin, 2 g of orange G, 1 g of anilin blue dissolved in 200 ml of acidified distilled water, and pH 1.1 HCl, for 5 min). Stained sections were rinsed with distilled water, dehydrated with EtOH, cleared in Citrosolv, and mounted. This staining results in a blue coloration of the scar, and muscle tissue appears orange/brown. Images were taken for each section to calculate the fibrotic and nonfibrotic areas as well as ventricular and septal wall thickness.
Scarring, Thinning, and Muscle Loss.
Scarring was determined as fibrotic area/(fibrotic + nonfibrotic area), and muscle loss was determined as circumference of the left ventricular wall containing at least 75% scar area/circumference of the left ventricular wall based on all sections. The thinning index is a ratio of the amount of wall thinning in the infarct normalized to the thickness of the septum and is calculated by dividing the minimal infarct wall thickness with maximal septal wall thickness (2 weeks, sections 1–4; 3 months, sections 1–6 from base).
Echocardiography.
Echocardiographic acquisition and analysis were performed as previously described (31). Left ventricular fractional shortening was calculated as (EDD − ESD)/EDD × 100%.
Statistics.
Data are shown as mean ± SEM. Multiple group comparison was performed by one-way ANOVA followed by the Bonferroni procedure for comparisons of means. Comparison between two groups was analyzed by the one-tailed Student's t test because we hypothesized, based on our in vitro study, a positive effect of FGF1/p38 inhibitor treatment on heart function. Values of P < 0.05 were considered statistically significant. For infarct size, wall thickness, muscle loss measurements, and proliferation analysis, the observer was blinded to treatment group. Proliferation analysis was performed at two levels in each heart, and all cells inside the scar and in the border zone were analyzed.
Supplementary Material
Acknowledgments
We thank David Clapham, Yibin Wang, and Kyu-Ho Lee for critique of the manuscript and Keating laboratory members for helpful discussions. This work was supported by a grant from the Charles H. Hood Foundation (Boston, MA; Child Health Research grant to F.B.E.) and a fellowship from the American Heart Association (to P.C.H.H.).
Abbreviations
- MI
myocardial infarction
- EDD
end diastolic dimension
- ESD
end systolic dimension
- FS
fractional shortening.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rumyantsev PP. Int Rev Cytol. 1977;51:186–273. [PubMed] [Google Scholar]
- 2.von Harsdorf R, Poole-Wilson PA, Dietz R. Lancet. 2004;363:1306–1313. doi: 10.1016/S0140-6736(04)16006-6. [DOI] [PubMed] [Google Scholar]
- 3.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, et al. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 4.Poss KD, Wilson LG, Keating MT. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 6.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. Genes Dev. 2005;15:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuevas P, Reimers D, Carceller F, Martinez-Coso V, Redondo-Horcajo M, Saenz de Tejada I, Gimenez-Gallego G. Eur J Med Res. 1997;2:465–468. [PubMed] [Google Scholar]
- 8.Baines CP, Molkentin JD. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Buehler A, Martire A, Strohm C, Wolfram S, Fernandez B, Palmen M, Wehrens XH, Doevendans PA, Franz WM, Schaper W, Zimmermann R. Cardiovasc Res. 2002;55:768–777. doi: 10.1016/s0008-6363(02)00494-7. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh PC, Davis ME, Gannon J, Macgillivray C, Lee RT. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW. Circulation. 2006;114:I206–I213. doi: 10.1161/CIRCULATIONAHA.105.000455. [DOI] [PubMed] [Google Scholar]
- 12.Pasumarthi KBS, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Circ Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 13.Li JM, Poolman RA, Brooks G. Am J Physiol. 1998;275:H814–H822. doi: 10.1152/ajpheart.1998.275.3.H814. [DOI] [PubMed] [Google Scholar]
- 14.Soonpaa MH, Field LJ. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Reiss K, Cheng W, Giordano A, De Luca A, Li B, Kajstura J, Anversa P. Exp Cell Res. 1996;225:44–54. [PubMed] [Google Scholar]
- 16.Iwakura A, Fujita M, Ikemoto M, Hasegawa K, Nohara R, Sasayama S, Miyamoto S, Yamazato A, Tambara K, Komeda M. Heart Vessels. 2000;15:112–116. doi: 10.1007/pl00007264. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Braunwald E. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 18.Swynghedauw B. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 19.Palmen M, Daemen MJ, De Windt LJ, Willems J, Dassen WR, Heeneman S, Zimmermann R, Van Bilsen M, Doevendans PA. J Am Coll Cardiol. 2004;44:1113–1123. doi: 10.1016/j.jacc.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 21.Lee MR, Dominguez C. Curr Med Chem. 2005;12:2979–2994. doi: 10.2174/092986705774462914. [DOI] [PubMed] [Google Scholar]
- 22.Pawlowski R, Jura J. Mol Cell Biochem. 2006 doi: 10.1007/s11010-006-9133-7. in press. [DOI] [PubMed] [Google Scholar]
- 23.Geist A, Marx J, Muller S, Uzan A, von Specht BU, Haberstroh J. Eur Surg Res. 2005;37:191–198. doi: 10.1159/000087862. [DOI] [PubMed] [Google Scholar]
- 24.Safi J, Jr, DiPaula AF, Jr, Riccioni T, Kajstura J, Ambrosio G, Becker LC, Anversa P, Capogrossi MC. Microvasc Res. 1999;58:238–249. doi: 10.1006/mvre.1999.2165. [DOI] [PubMed] [Google Scholar]
- 25.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, et al. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leri A, Kajstura J, Anversa P. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 27.Laflamme MA, Murry CE. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 28.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 30.Engel FB, Hauck L, Boehm M, Nabel EG, Dietz R, von Harsdorf R. Mol Cell Biol. 2003;23:555–565. doi: 10.1128/MCB.23.2.555-565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT. Circulation. 2002;105:753–758. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.