Abstract
It has been proposed that bone marrow (BM) hematopoietic stem and progenitor cells are distributed along an oxygen (O2) gradient, where stem cells reside in the most hypoxic areas and proliferating progenitors are found in O2-rich areas. However, the effects of hypoxia on human hematopoietic stem cells (HSCs) have not been characterized. Our objective was to evaluate the functional and molecular responses of human BM progenitors and stem cells to hypoxic conditions. BM lineage–negative (Lin–) CD34+CD38– cells were cultured in serum-free medium under 1.5% O2 (hypoxia) or 20% O2 (normoxia) for 4 days. Using limiting dilution analysis, we demonstrate that the absolute number of SCID-repopulating cells (SRCs) increased by 5.8-fold in hypoxic cultures compared with normoxia, and by 4.2-fold compared with freshly isolated Lin–CD34+CD38– cells. The observed increase in BM-repopulating activity was associated with a preferential expansion of Lin–CD34+CD38– cells. We also demonstrate that, in response to hypoxia, hypoxia-inducible factor-1α protein was stabilized, surface expression of angiogenic receptors was upregulated, and VEGF secretion increased in BM Lin–CD34+ cultures. The use of low O2 levels to enhance the survival and/or self-renewal of human BM HSCs in vitro represents an important advance and could have valuable clinical implications.
Introduction
The effects of hypoxia on human hematopoietic progenitors and stem cells remain poorly understood. It has only recently been reported that, in normal volunteers, the partial pressure of oxygen (O2) and O2 saturation in human bone marrow (BM) are lower than in peripheral blood (1). The presence of hypoxic areas in the BM is determined by the architecture of medullary sinuses and the pattern of arterial blood flow in the marrow (2). It has been proposed that hematopoietic stem cells (HSCs) and progenitors are distributed along an O2 gradient, with stem cells residing in the most hypoxic areas and proliferating progenitors in O2-rich areas (3). The data in support of this model were generated using “closed systems” in which O2 consumption by cells results in a progressive decrease in O2 level below the initial 1%. In these conditions, Cipolleschi et al. (3) demonstrated that the ability of HSCs to repopulate the BM of lethally irradiated mice and give rise to myeloid colonies was better preserved in hypoxia (≤1.5% O2 for 5 days) than in normoxic conditions. In contrast, committed progenitors (GM-CFU) could not be preserved under the same low O2 levels. When an O2 regulation device and growth factor stimulation were used, Ivanovic et al. (4) reported a smaller expansion of mouse progenitors and a better maintenance of the number of myeloid progenitors in the recipients’ BM after 8 days in hypoxia compared with normoxia. In addition, we have shown that the proliferation of embryonic hematopoietic progenitors is regulated by a hypoxia-mediated signaling pathway (5).
The available data on the effects of hypoxia on human hematopoietic cells is limited to the clonogenic activity of umbilical cord blood, mobilized peripheral blood, or BM progenitor cells. Cipolleschi et al. (6) reported an increase in burst-forming unit, erythroid (BFU-E) and a decrease in GM-CFU colonies when cord blood CD34+ cells were cultured in a 14-day clonogenic assay under severe hypoxia (<1% O2). An increase in both BFU-E and GM-CFU activity in BM CD34+ cells cultured in 1.5% O2 for 6 hours has also been reported (7). In contrast, the culture of mobilized peripheral blood CD34+ cells under severe hypoxia (<1% O2) for 7 days resulted in a decrease in the total number of colony-forming cells (CFCs) (8). Although these in vitro assays provide clues on the effect of hypoxia on the clonogenic activity of human progenitors, they may not reflect its effects on human HSCs. It is necessary to use a xenotransplantation model, such as NOD/SCID mice (9), to evaluate the functional response of human BM-repopulating cells to culture under low O2 levels. Human HSCs capable of extensive proliferation and multilineage repopulation of the BM of NOD/SCID mice are defined as SCID-repopulating cells (SRCs) (10–12). SRCs are highly enriched in the BM lineage–negative (Lin–) CD34+CD38– fraction (10–12) and present at a higher frequency in umbilical cord blood than in adult BM (13, 14). We and others (15, 16–24) have examined various culture conditions for their ability to maintain or enhance SRC activity in vitro. From these studies, it has been shown that the ex vivo expansion of CFCs and long-term colony-initiating cells (LTC-ICs) is not necessarily associated with an increase in SRCs. Furthermore, limiting dilution analysis is required to quantitatively compare the effect of culture conditions on SRC activity (12, 17–20, 24).
The cellular mechanisms by which human hematopoietic progenitors and stem cells respond to hypoxia have not been characterized. However, the response to hypoxia has been investigated in a variety of other cell types and models (see refs. 25–29 for reviews). The hypoxia-inducible factor-1α (HIF-1α) protein, which is rapidly degraded under normoxic conditions, becomes stabilized (30) under low O2 levels (<5%) and forms a dimer with aryl hydrocarbon receptor nuclear translocator protein (ARNT). In contrast to HIF-1α, ARNT levels are not regulated by hypoxia. The heterodimer HIF-1α-ARNT is a transcriptional activator of genes encoding for a wide variety of genes including erythropoietin, VEGF, glucose transporters, and glycolytic enzymes (27, 31). HIF-2α (32–34) and HIF-3α (35) are closely related to HIF-1α but have a more restricted pattern of expression and partially overlapping functions (27).
The objectives of this study were to evaluate the functional and cellular responses of human BM hematopoietic progenitors and stem cells to hypoxia. We used normal adult BM as a source of human HSCs because these cells reside in a hypoxic environment in vivo (1) and therefore may represent a more relevant model than other sources of human HSCs such as cord blood or mobilized peripheral blood. In this study, we demonstrate quantitatively that adult BM SRCs can be expanded in vitro under hypoxic conditions. This increase in BM-repopulating activity was associated with the preferential expansion of Lin–CD34+CD38– cells, an upregulation of angiogenic receptors, and an increase in VEGF production. Our results suggest that hypoxia could play a critical role in regulating the self-renewal of human BM HSCs and that the increase in VEGF secretions induced by hypoxia could be involved in the maintenance of HSCs in vitro.
Methods
BM cell isolation.
BM aspirates (n = 137) were obtained from healthy volunteers in accordance with the guidelines of the University of Pennsylvania Institutional Review Board for Human Subjects. At least three BM samples from three different donors were pooled for the isolation of Lin– cells. Light-density mononuclear cells were isolated using Ficoll-Paque Plus (Amersham Biosciences Corp., Piscataway, New Jersey, USA), erythrocytes were lysed using ammonium chloride, and Lin– cells were isolated using immunomagnetic beads (StemCell Technologies Inc., Vancouver, British Columbia, Canada). Briefly, cells were incubated with a mixture of lineage-specific antibodies (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, CD41, and glycophorin A) followed by incubation with a secondary antibody conjugated to metal colloid. Cells were then eluted through a magnetized column to deplete the suspension from cells expressing lineage markers. Lin– cells were then stained with anti-human CD34-phycoerythrin (CD34-PE), anti-human CD38-allophycocyanine (both from Becton, Dickinson and Co., San Jose, California, USA), and propidium iodide (Molecular Probes Inc., Eugene, Oregon, USA) and sorted on a MoFlo cell sorter (Cytomation Inc., Fort Collins, Colorado, USA).
Liquid cultures.
BM Lin–CD34+ or Lin–CD34+CD38– cells (106/ml) were cultured in serum-free medium consisting of StemSpan SFEM medium (StemCell Technologies Inc.) supplemented with IL-3 (10 ng/ml), IL-6 (10 ng/ml), stem cell factor (SCF) (300 ng/ml), Flt-3 ligand (300 ng/ml), G-CSF (50 ng/ml), 1% HEPES, gentamicin, and LDL (10 μg/ml). Cells were cultured for 4–9 days at 37°C in 5% CO2-humidified incubators in normoxic (20% O2) or hypoxic (1.5% O2) conditions. Hypoxic cultures were performed in a two-gas incubator (Jouan Inc., Winchester, Virginia, USA) equipped with an O2 probe to regulate N2 levels. In some experiments, normoxic cultures of BM Lin–CD34+ cells were supplemented with human VEGF (100 ng/ml), angiopoietin-1 (100 ng/ml), or angiopoietin-2 (100 ng/ml), all obtained from R&D Systems Inc. (Minneapolis, Minnesota, USA).
Phenotypic analysis and cell division tracking.
Lin–CD34+ cells were harvested after 4 days of culture in hypoxic (1.5% O2) or normoxic conditions. Cell number and viability were evaluated using trypan blue exclusion. Cells were incubated with anti-human CD34 and the following antibodies: CD31, CD38, CD117 (c-kit), and HLA-DR (Becton, Dickinson and Co.), CD90 from Beckman Coulter (Miami, Florida, USA), CD133 from Miltenyi Biotec (Auburn, California, USA), CD135 and CD162 from Immunotech Inc. (Westbrook, Maine, USA), CXCR4, Tie-1, Tie-2, Flt-1, and VEGF receptor 2 (VEGFR2, also known as Kdr, Flk-1) from R&D Systems Inc., and vWF from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). In some experiments, freshly isolated Lin–CD34+ cells were labeled with CFSE (Molecular Probes Inc., Eugene, Oregon, USA) as previously described (15). Cells were cultured for 4 days in hypoxic (1.5% O2) or normoxic conditions and stained for CD34-allophycocyanin and either the appropriate isotype control or one of the following anti-human antibodies: CD38, CD90, CD117, CD133, CD135, or HLA-DR. Cells were then analyzed on a FACSCalibur flow cytometer (Becton, Dickinson and Co.).
Cell cycle analysis.
Lin–CD34+ cells cultured for 4 days in hypoxic (1.5% O2) or normoxic conditions were harvested and cell cycle analysis was performed as previously described (36). Briefly, cells were incubated with CD34-PE, then fixed with a 0.4% formaldehyde-buffered solution, washed, and permeabilized with a 0.2% Triton X-100 solution on ice. After two washes in PBS plus 2% FBS, cells were labeled with Ki-67–FITC (Becton, Dickinson and Co.). Finally, each sample was washed twice and resuspended in a 10 μM solution of DAPI (Molecular Probes Inc.) in PBS plus 2% FBS. Cells were analyzed using a Becton-Dickinson LSR flow cytometer equipped with a 325-nm helium-cadmium UV laser and a 488-nm argon-ion laser.
CFC and LTC-IC assays.
Human CFCs were assayed in semisolid methylcellulose medium in standard conditions (12). LTC-IC cultures were established on preformed M210B4 stroma layers using human myeloid long-term culture medium (MyeloCult 5100; StemCell Technologies Inc.) according to described methods (37, 38). Limiting dilutions (at least three replicates/dilution) were performed in 96-well plates in which 500–2,000 Lin–CD34+CD38– cells per well were plated. After 5 weeks in coculture with stroma cells, the content of each well was transferred in methylcellulose medium to reveal CFC activity.
Transplantation and analysis of NOD/SCID mice.
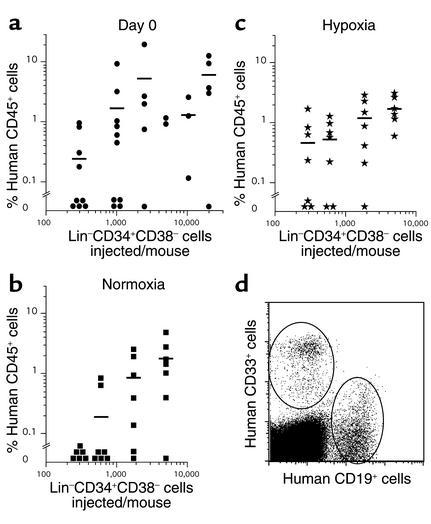
Eight-week-old sublethally irradiated (275 cGy at 240 cGy/min) NOD/LtSz-scid (NOD/SCID) mice were transplanted with fresh or cultured BM Lin–CD34+CD38– cells by lateral tail vein injection according to a standard protocol (12). Cultured Lin–CD34+CD38– cells were not resorted for this phenotype prior to transplantation. Lin–CD34+CD38– cells cultured for 0, 4, 6, and 9 days in hypoxic or normoxic conditions were injected into mice at doses ranging from 300 to 40,000 cells per mouse (at least three mice per cell dose per experiment). Lin–CD34+CD38– cells were coinjected with 0.5 million to 1 million accessory cells consisting of irradiated (1,500 cGy) BM or cord blood mononuclear or lineage-positive cells as previously described (39). Mice were sacrificed 8–10 weeks after transplant and BM from the femurs, tibiae, and iliac crests of each mouse were harvested. To prepare mouse BM cells for flow cytometry, BM cells were incubated with a 6% ammonium chloride solution, then washed and incubated with anti-human CD45-FITC, CD33-PE, (Beckman Coulter), and CD19-allophycocyanin (Becton, Dickinson, and Co.), and propidium iodide. For each group of mice analyzed, an aliquot of cells was also stained with matching isotype controls. For each sample, 100,000 scatter- and live-gated cells were acquired to determine engraftment. Transplanted mice showing at least 0.1% human CD45+ cells and both human myeloid (CD33+) and lymphoid (CD19+) cells were considered engrafted, as shown in Figure 3d.
Figure 3.
Quantitative analysis of the effect of hypoxia on SRC frequency. The frequency of SRCs in freshly isolated Lin–CD34+CD38– cells (a, day 0) or cells cultured for 4 days under normoxic (b) or hypoxic (1.5% O2) (c) conditions was determined by limiting dilution analysis. For each NOD/SCID mouse (n = 88), the level of human engraftment (percentage of CD45+ cells) is represented as a function of the number of Lin–CD34+CD38– cells transplanted. (d) Representative flow cytometry dot plot showing the presence of both human myeloid (CD33+) and lymphoid (CD19+) cells in NOD/SCID BM 6–8 weeks after transplantation.
RT-PCR.
RNA was isolated from freshly purified Lin–CD34+CD38– or Lin–CD34–CD38– cells using the RNAqueous isolation kit (Ambion Inc., Austin, Texas, USA). RNA extracted from K562 cells was used as a positive control for the PCR reactions. Standard reverse transcription was done using SuperScript II reverse transcriptase (Invitrogen Corp., San Diego, California, USA). The primers used were HIF-1α: 5′-AAGTCTCGAGATGCAGCCAGA and 5′-AGTTAGTTCAAA CTGACTTAATCC; HIF-2α: 5′-GCCCCTGCTGTCCTGCCTCATCA and 5′-ATCCGTCTGGGTACTGCATTGGTCCTT; and ARNT: 5′-AGGAATAGTGGCCTAGCCCCT and 5′-ATTGTTGTAGCTGTTGCTCTG. PCR was done using the Advantage-GC cDNA PCR kit (Clontech Laboratories Inc., Palo Alto, California, USA). The amplification cycles were: 94°C for 4 minutes followed by 35 cycles of 94°C for 10 minutes + 58°C for 30 seconds + 72°C for 2 minutes, and a final extension step at 72°C for 10 minutes was included before samples were cooled at 4°C.
Western blot analysis.
Equal numbers of Lin–CD34+ cells were cultured overnight in serum-free conditions (106/ml) at 1.5% O2 in a hypoxic workstation (Invivo2; Ruskinn Technology, Leeds, United Kingdom) or in normoxic conditions. Cells were directly lysed in 1× SDS loading buffer and boiled at 95°C for 10 minutes. The mouse anti–HIF-1α antibody used was from Becton, Dickinson and Co. (catalog no. H72320) and the rabbit anti-ARNT antibody was from Novus Biologicals Inc. (catalog no. NB 100-110; Littleton, Colorado, USA).
Human VEGF ELISA and lactate assay.
Conditioned media from overnight cultures (n = 5) of Lin–CD34+ cells were harvested and human VEGF and erythropoietin concentrations were determined by ELISA (R&D Systems Inc.) according to the manufacturer’s procedure. Lactate concentrations in the conditioned media were also determined after overnight culture in hypoxic (1.5% O2) and normoxic conditions using a colorimetric assay (Sigma-Aldrich, St. Louis, Missouri, USA) based on the enzymatic conversion of lactate to pyruvate and H2O2 by lactate oxidase.
Statistics.
Data are presented as mean ± SD or mean ± SEM. Statistical differences were evaluated using the Student t test. In the limiting dilution analysis used to determine SRC frequencies, mice with at least 0.1% human cells were considered engrafted. The data from limiting dilution experiments were analyzed using the single-hit Poisson model, and SRC frequencies were determined using the maximum likelihood estimator as previously shown (12, 13, 16–20, 24). We used χ2 analysis to verify the internal consistency of our data and validate the use of Poisson statistics.
Results
Preferential expansion of Lin–CD34+CD38– cells in hypoxia.
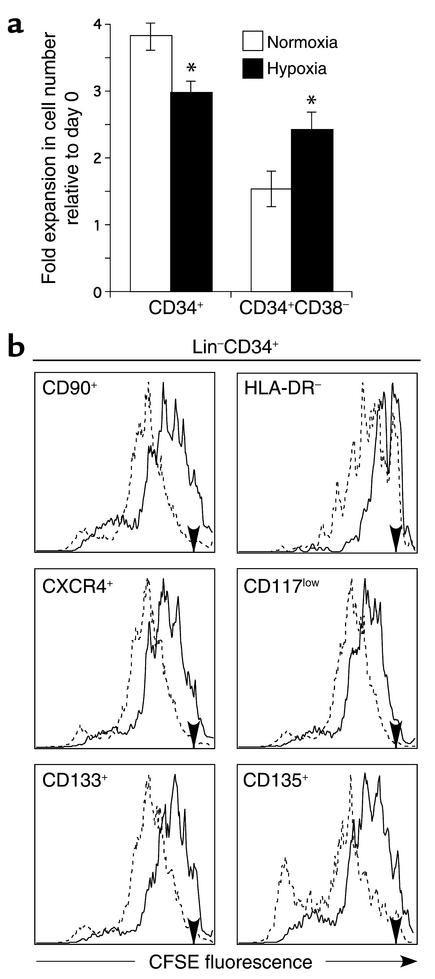
Lin–CD34+and Lin–CD34+CD38– cells were cultured in serum-free conditions for 4 days under normoxia (20% O2) or hypoxia (1.5% O2). We used this level of O2 based on previous reports indicating that mouse BM-repopulating cells can be maintained when cultured under 1% O2 (3, 4). As shown in Figure 1a, the level of expansion of Lin–CD34+ cells was decreased in hypoxia compared with normoxia. In contrast, the expansion of Lin–CD34+CD38– cells, a subpopulation enriched in primitive progenitors and stem cells, was greater in hypoxic conditions than in normoxia (2.4-fold vs. 1.5-fold, respectively). The differential effect of hypoxia on subsets of Lin–CD34+ cells was further investigated using CFSE to track the division history of each primitive subset (CD90+, HLA-DR–, CXCR4+, CD117low, CD133+, CD135+) during the 4-day culture (Figure 1b). Overall, CFSE profiles indicated that all examined subsets of Lin–CD34+ cells cultured in hypoxia divided at a slower rate than in normoxia. In particular, HLA-DR– cells divided only once or twice during the 4-day culture period. Despite this general slow proliferative rate, a small fraction (4–18%) of the cells in each subset was associated with a lower CFSE fluorescence, indicative of a rapid rate of division. These results show that a small subset of primitive BM CD34+ cells can rapidly proliferate under low O2 levels while most CD34+ cells have a decreased rate of division. This suggests that O2 levels can differentially regulate the proliferation of primitive subsets of human BM cells.
Figure 1.
Expansion and division history of BM cells after 4 days in culture in hypoxia or normoxia. Lin–CD34+ and Lin–CD34+CD38– cells were cultured in serum-free conditions for 4 days in the presence of IL-3, IL-6, SCF, Flt-3 ligand, and G-CSF. (a) Expansion. The fold increase in cell number relative to the initial cell number plated (day 0) is represented for each subpopulation cultured in normoxia (white bars) or hypoxia (black bars). *P < 0.05. (b) Division history of primitive subpopulations of Lin–CD34+ cells. Freshly isolated Lin–CD34+ cells were labeled with CFSE, cultured for 4 days in hypoxia (1.5% O2) or normoxia, and analyzed for CFSE fluorescence intensity and expression of markers associated with primitive progenitors and stem cells. Histograms (representative of three separate experiments) of CFSE fluorescence in various Lin–CD34+ cell subsets are shown after 4 days of culture in hypoxia (solid lines) or normoxia (dashed lines). Day 0 CFSE fluorescence intensity is indicated by an arrow on each histogram.
Hypoxia regulates the cell cycle of Lin–CD34+ cells.
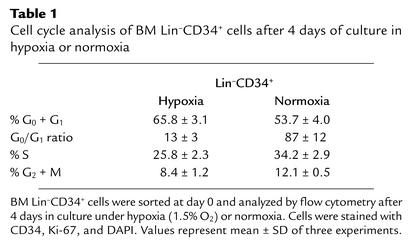
We evaluated the effect of hypoxia on the cell cycle of Lin–CD34+ cells after 4 days of culture in hypoxic or normoxic conditions. Before culture, less than 1% of sorted Lin–CD34+ cells was found in the S or G2/M fractions (data not shown). After 4 days under hypoxia, 34.2% ± 3.5% of CD34+ cells was found in S + G2/M phases compared with 46.3% ± 3.4% in normoxia (Table 1). A similar trend was observed for Lin–CD34– cells (data not shown). These results indicate that the slower rate of expansion of Lin–CD34+ cells under hypoxia is at least partially due to slower cell cycling and fewer cell divisions (as shown by DAPI and CFSE, respectively). We also examined the effect of hypoxia on the transition from G0 to G1 phase using Ki-67 to distinguish cells in G0 phase (Ki-67–) from those in G1 phase (Ki-67+). Table 1 shows that, in normoxic conditions, the G0/G1 ratio (% cells in G0 to % cells in G1) was 87 ± 12, indicating that most cells found in the G0 + G1 peak (as defined by DAPI staining) were resting. In hypoxic conditions, the G0/G1 ratio was only 13 ± 3 due to a 6.7-fold increase in cells in G1 phase and fewer cells in G0 phase. These results suggest that hypoxia preferentially promotes the transition of Lin–CD34+ cells from G0 to G1 and/or induces a cell cycle arrest in G1 phase.
Table 1.
Cell cycle analysis of BM Lin–CD34+ cells after 4 days of culture in hypoxia or normoxia
Effects of hypoxia on CFCs and LTC-ICs.
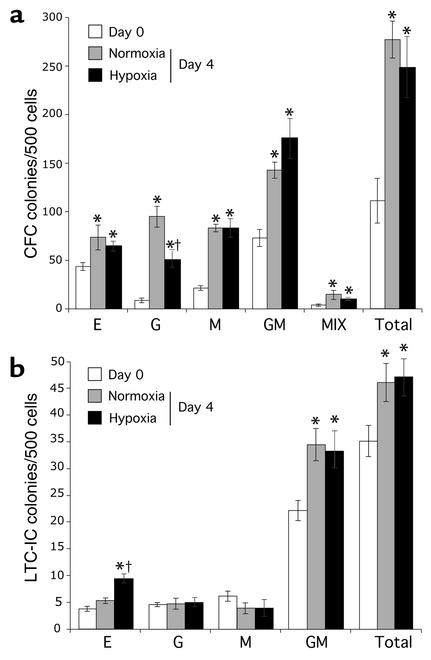
We evaluated the clonogenic activity of Lin–CD34+ cells after 4 days of culture in hypoxic or normoxic conditions (Figure 2). After 4 days of culture, the total number of CFCs was increased by twofold compared with freshly isolated Lin–CD34+ cells (Figure 2a). The level of O2 had no significant effect on lineage-committed progenitors, with the exception of granulocytic colonies (G-CFU), which decreased in number under hypoxic conditions. We also examined the effect of hypoxia on more primitive progenitors. LTC-ICs were equally expanded (1.4-fold) after 4 days of culture in hypoxia or normoxia (Figure 2b). In normoxia, only LTC-IC granulocyte-macrophage colonies significantly increased while, in hypoxia, both granulocyte-macrophage and erythroid colonies increased. This increase in BFU-E was the only hypoxia-specific effect on primitive progenitors we observed. These results indicate that a 4-day culture in hypoxia has a very limited effect on both committed and primitive progenitors.
Figure 2.
Effect of hypoxia on BM progenitor activity. The CFC (a) and LTC-IC (b) activity of BM Lin–CD34+CD38– cells was evaluated before (day 0; white bars) and after culture for 4 days under normoxic (gray bars) or hypoxic (1.5% O2; black bars) conditions. Data shown represent the number (mean ± SD) of BFU-E (E), granulocyte (G), monocyte (M), granulocyte-monocyte (GM), mixed (MIX, i.e., GM colonies with erythroid cells), and total number of colonies from three separate experiments. The paired Student t test was performed to compare day 0 with day 4 cells (*P < 0.05) and to compare hypoxic to normoxic conditions after 4 days of culture (†P < 0.05).
SCID-repopulating activity in cells cultured under hypoxic conditions.
First, we evaluated the effect of hypoxia on the SCID-repopulating ability of BM Lin–CD34+CD38– cells cultured for 4, 6, and 9 days in the presence of IL-3, IL-6, SCF, Flt-3 ligand, and G-CSF. In these conditions, SRCs could be maintained for only 4 days in culture, regardless of O2 levels. No human cells were detectable in the BM of mice (n = 16) transplanted with cells cultured for 6 or 9 days. These results indicate that in our conditions, hypoxia did not delay the loss of BM-repopulating activity observed after 4 days in normoxic cultures.
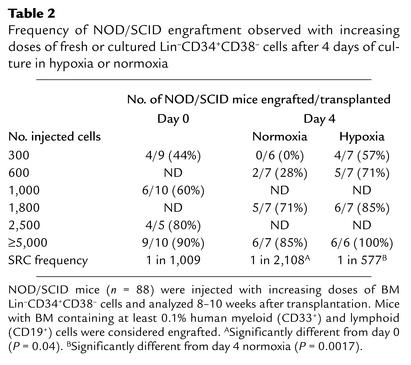
We used a limiting dilution analysis to quantitatively evaluate the effect of hypoxia on SCID-repopulating cells after 4 days. For this purpose, increasing numbers (ranging from 300 to 20,000) of fresh BM Lin–CD34+CD38– cells or cells cultured under hypoxia or normoxia for 4 days were transplanted at increasing doses into NOD/SCID mice (Figure 3). For each experimental condition (day 0, day 4 hypoxia, and day 4 normoxia), we estimated the number of Lin–CD34+CD38– cells required to statistically inject one SRC per mouse (12, 13, 20). Table 2 shows the frequencies of engrafted mice for each cell dose used in each experimental condition. We estimated that the frequency of SRCs in freshly purified Lin–CD34+CD38– cells (Table 2) was 1 in 1,009. After 4 days of culture, we determined that the frequency of SRCs in hypoxic conditions (1 in 577 Lin–CD34+CD38– cells) was significantly higher (P = 0.0017) than in normoxic cultures (1 SRC in 2,108 Lin–CD34+CD38– cells). Consequently, we observed a 3.6-fold increase in SRC frequency in hypoxic cultures compared with normoxia. In contrast, the apparent increase in SRC frequency between freshly isolated Lin–CD34+CD38– cells (1 SRC in 1,009 cells) and day 4 hypoxic cultures (1 in 577 cells) was not significant (P = 0.098). However, taking into account the expansion of Lin–CD34+CD38– CFCs after 4 days (Figure 1a), we estimate that in hypoxia, the absolute number of SRCs increased by 5.8-fold compared with the number in normoxia, and by 4.2-fold compared with freshly isolated Lin–CD34+CD38– cells. In contrast, in normoxic conditions, the absolute number of SRCs was just maintained in comparison with freshly isolated Lin–CD34+CD38– cells. In this study, we did not formally assess the effect of hypoxia on the self-renewal of human HSCs since no secondary transplants were performed. However, our results clearly demonstrate that low O2 levels can substantially increase the number of BM-repopulating cells compared with normoxic conditions.
Table 2.
Frequency of NOD/SCID engraftment observed with increasing doses of fresh or cultured Lin–CD34+CD38– cells after 4 days of culture in hypoxia or normoxia
Molecular response of Lin–CD34+CD38– cells to hypoxia.
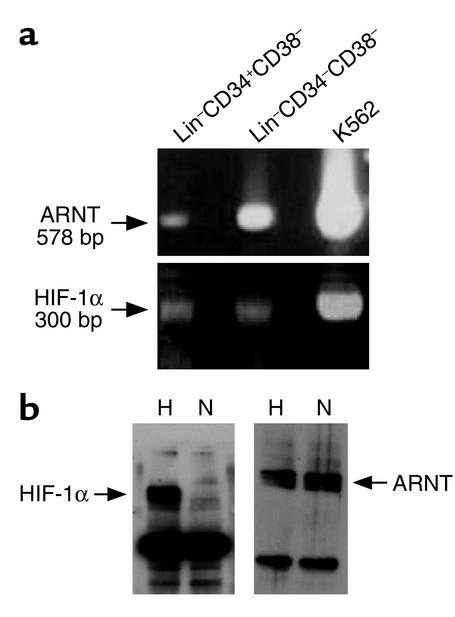
We examined whether human BM progenitors and stem cells express the critical components of the cellular response to hypoxia, such as HIF-1α and ARNT. Figure 4a shows that both HIF-1α and ARNT are constitutively expressed in purified Lin–CD34+CD38– cells. HIF-2α expression could not be detected in Lin–CD34+CD38– cells but was present at very low levels in Lin–CD34–CD38– cells, a subpopulation enriched in CD34– SRCs (data not shown). Since HIF-α subunits are regulated at the protein level, we evaluated the effect of hypoxia and normoxia on HIF-1α protein. Figure 4b shows that HIF-1α protein can be detected in cells cultured in hypoxia but not under normal O2 levels. In contrast, ARNT protein levels were unaffected by O2 levels. These results demonstrate that HIF can mediate the hypoxic response of human BM progenitors and stem cells.
Figure 4.
Expression of HIF in BM progenitors and stem cells. (a) RT-PCR analysis of ARNT and HIF-1α performed on RNA extracted from BM Lin–CD34+CD38–, Lin–CD34–CD38–, and K562 cells (as a positive control). (b) Western blot analysis of cell extracts from BM Lin–CD34+ cells cultured overnight under hypoxic (H) or normoxic (N) conditions using antibodies against ARNT and HIF-1α. All gels are representative of at least three experiments.
Hypoxia regulates expression of angiogenic factors in Lin–CD34+ cells.
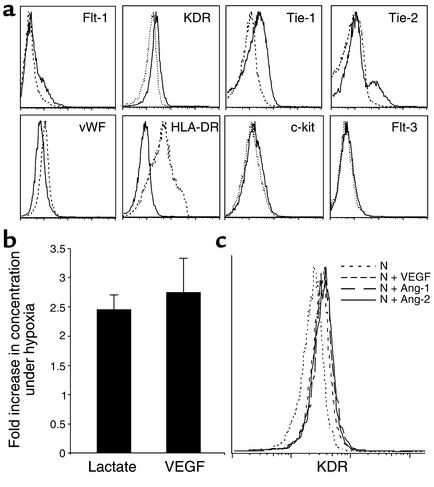
Angiogenic factors and receptors such as VEGF and Flt-1 are regulated by O2 levels (27). VEGF has been implicated in the regulation of HSC survival (40) and recruitment (41). To investigate whether the increase in SRC activity observed under hypoxia was associated with changes in the expression of angiogenic molecules, Lin–CD34+ cells cultured for 4 days at 1.5% O2 or at normoxia were analyzed by flow cytometry for cell surface expression of VEGF receptor 1 (VEGFR1, also known as Flt-1), VEGFR2, Tie-1, Tie-2, and vWF. Levels of VEGF cell surface receptors (VEGFR1 and VEGFR2) increased slightly, whereas angiopoietin receptors (Tie-1 and Tie-2) were more clearly upregulated in cells cultured in hypoxic conditions (Figure 5a). In contrast, c-kit and Flt-3 ligand were unchanged, and vWF and MHC class II (HLA-DR) expression was downregulated under the same conditions (Figure 5a). It is unlikely that the upregulation of VEGFR1, VEGFR2, Tie-1, and Tie-2 was due to a nonspecific response to low O2 levels since other surface molecules were either unchanged or downregulated. These results suggest that human progenitors and stem cells can specifically enhance their responsiveness to angiogenic factors under hypoxic conditions.
Figure 5.
Hypoxia regulates the expression of HIF target genes in BM progenitors and stem cells. (a) Representative histograms of cell surface expression of angiogenic and hematopoietic cytokine receptors in BM Lin–CD34+ cells after 4 days in culture under normoxic (dashed lines) or hypoxic (1.5% O2, solid lines) conditions. (b) The conditioned medium from BM Lin–CD34+ cells cultured overnight in normoxia or hypoxia (1.5% O2) was assayed for lactate and human VEGF. Bars represent the fold increase in lactate and VEGF concentrations measured in hypoxic cultures relative to normoxic conditions. Error bars show SD. (c) Effect of VEGF, angiopoietin-1 (Ang-1), and angiopoietin-2 (Ang-2) on cell surface expression of VEGFR2 in Lin–CD34+ cells after 4 days in culture under normoxic conditions (N).
Hypoxia-regulated genes include VEGF (42–44), erythropoietin (45), and glycolytic enzymes (46, 47) such as lactate dehydrogenase (48). To determine whether these HIF targets are upregulated in BM progenitors and stem cells, we cultured Lin–CD34+ cells for 18 hours under normoxic or hypoxic conditions and assayed VEGF, erythropoietin, and lactate concentrations in conditioned medium. Figure 5b shows that both VEGF and lactate secretions were increased by more than twofold, demonstrating that hypoxia upregulates VEGF production and glycolytic enzyme activity in human BM progenitors and stem cells. In contrast, erythropoietin was undetectable in the medium after 18 hours of culture regardless of O2 levels, suggesting that HIF targets may be differentially regulated in BM cells. Our results indicate that the hypoxic response of human progenitors and HSCs is characterized by a rapid increase in VEGF secretion and glycolytic activity. This rapid increase in VEGF secretion may play a critical role in the survival and expansion of human BM-repopulating cells under hypoxia.
To determine whether angiogenic cytokines play a role in the upregulation of angiogenic receptors observed under hypoxic conditions, Lin–CD34+ cells were cultured in normoxia in the presence of VEGF, angiopoietin-1, or angiopoietin-2 for 4 days. Interestingly, VEGFR2 surface expression was enhanced to levels comparable to those observed under hypoxia when cells were cultured in the presence of either VEGF, angiopoietin-1, or angiopoietin-2 (Figure 5c). In contrast, these cytokines had no effect on the expression or Flt-1, Tie-1, or Tie-2 (data not shown).
Discussion
In this report, we demonstrate for the first time that SRCs from human BM can be expanded in vitro under hypoxic conditions. We used BM Lin–CD34+CD38– cells, a population highly enriched in SRCs (12), and limiting dilution analysis to quantitatively evaluate the effect of hypoxia on SRCs. First, we determined that the frequency of SRCs in fresh adult BM is 1 in 1,009 Lin–CD34+CD38– cells. This result demonstrates that the frequency of SRCs in adult BM is only half of the SRC frequency that was reported previously for cord blood (1 in 617 Lin–CD34+CD38– cells) (12). This is consistent with other reports indicating that the frequency of SRC is higher in cord blood than in BM or mobilized peripheral blood (13, 14). To evaluate the effect of hypoxia on BM SRCs in vitro, we used culture conditions (IL-3, IL-6, SCF, Flt-3 ligand, and G-CSF in serum-free medium) capable of expanding SRCs in cord blood Lin–CD34+CD38– cells (16). Interestingly, after 4 days in the same culture conditions, SRCs in BM Lin–CD34+CD38– cells could not be expanded. The lack of SRC expansion after 4 days in culture under normoxic conditions suggests that human adult BM HSCs have different culture/cytokine requirements than cord blood HSCs. In contrast to normoxic conditions, we observed a significant increase in SRCs after 4 days in culture under 1.5% O2. Not only were SRCs increased relative to normoxia, but we also observed a 4.2-fold expansion of the number of SRCs in hypoxic cultures compared with freshly isolated BM Lin–CD34+CD38– cells. These results demonstrate that low levels of O2 can greatly enhance the survival and/or self-renewal of human BM-repopulating cells in vitro. This finding is consistent with a previous report indicating that mouse BM-repopulating cells were better maintained in culture under severe hypoxia than were more committed progenitors (3). We could no longer detect SRC activity after 6 or 9 days in culture under 1.5% or 3% (data not shown) or 20% O2. This finding indicates that, in the present culture conditions, which may not be optimal for BM stem cells, the positive effect of hypoxia on SRCs is short-lived.
The effect of hypoxia on clonogenic (CFC) and LTC-IC activity was limited to a reduction of granulocytic progenitors (G-CFU) and an increased number of LTC-ICs giving rise to BFU-E. The limited response of BM progenitors to hypoxia compared with the significant increase in SRCs observed in the same conditions demonstrates that the activity of human BM stem cells and progenitors is regulated differently by O2.
To further characterize the hypoxic response, we evaluated the effects of low O2 tension on the proliferation and cell cycle distribution of human BM progenitors and stem cells in vitro. The expansion of Lin–CD34+ cells was slightly reduced at 1.5% O2 compared with normoxia, indicating that most BM progenitors can survive and even proliferate under hypoxia. Interestingly, the expansion of purified Lin–CD34+CD38– cells was greater in hypoxic than in normoxic conditions. This positive effect of hypoxia on Lin–CD34+CD38– cells demonstrates that primitive progenitors and stem cells are particularly well adapted to proliferating in a low-O2 environment.
To further evaluate the effect of hypoxia on the proliferation of BM cells, we used CFSE to track the division history of primitive subsets (CD90+, CXCR4+, CD117low, CD135+, and HLA-DR–) of BM Lin–CD34+ cells cultured under hypoxia and normoxia. We show that under hypoxic conditions, the overall proliferative rates of Lin–CD34+ cells and subsets was slower in hypoxic conditions, resulting in fewer cells after 4 days in culture compared with normoxia. Hypoxia increased the percentage of the cells in G0 or G1 phase, while fewer cells were in found in S or G2/M. Furthermore, hypoxic conditions increased the proportion of cells in G1 phase relative to resting (G0) cells, resulting in a dramatically decreased G0 to G1 ratio. This observation suggests that low O2 levels can either promote the transition from a resting state to G1 phase and/or inhibit the transition from G1 to S phase in BM progenitors and stem cells. It has been shown that hypoxia can induce G1 arrest in tumor (49, 50) and endothelial cells (51). Gardner et al. (52) reported that, in fibroblasts, severe hypoxia can inhibit the G1/S transition through regulation of p27 expression. Wilpshaar et al. (53) found no difference in SCID-repopulating ability between uncultured cord blood CD34+ cells in G0 phase and those in G1 phase. However, in cultured mobilized peripheral blood CD34+ cells, the level of human chimerism in NOD/SCID BM was lower in mice transplanted with cells in G1 phase compared with G0 (54). The expansion of SRCs we observed after 4 days in hypoxia and the increased number of cells in G1 phase suggests that SRCs may be found in both G0 and G1 phases under low oxygen levels.
In this study, we also investigated the cellular mechanisms involved in the hypoxic response of BM hematopoietic progenitor and stem cells. We found that HIF-1α and ARNT are constitutively expressed in Lin–CD34+CD38– cells. However, HIF-2α transcripts could not be detected in Lin–CD34+CD38– cells. Our Western blot analysis of Lin–CD34+ cells shows that HIF-1α protein is present under hypoxic conditions and is undetectable in normoxia. In contrast, ARNT protein is detectable regardless of O2 levels. This is consistent with previous reports showing that, in normoxic conditions, HIF-1α is modified by a prolyl hydroxylase, an O2-dependent enzyme (55, 56). The modified form of HIF-1α can interact with the von Hippel-Lindau tumor suppressor protein (VHL). This interaction induces the ubiquitination of HIF-1α and its rapid degradation (57–60). Under hypoxic conditions, the hydroxylation no longer occurs, and HIF-1α remains stable and can upregulate expression of its target genes.
Among the genes induced by hypoxia (25–29), angiogenic factors and receptors appear to play a critical role in the regulation of HSC survival and self-renewal. In this report we show an increase in VEGF secretion and an upregulation of the cell surface expression of angiogenic receptors (Tie-2 and to a lesser extent VEGFR2 and Tie-1) under hypoxia. The hypoxia-induced increase in VEGF expression has been well characterized in other models (42–44). Recently, Gerber et al. (40) reported that VEGF regulates the survival of mouse HSCs through an internal autocrine loop. It has also been shown that human BM-repopulating cells express VEGF receptors (61, 62). It is thus tempting to speculate that a similar mechanism may be involved in regulating the survival of SRCs. Tie-2, the receptor for angiopoietin-1, has also been reported to be regulated by O2 levels (63) and can be found on HSCs (64). Thus, the combined effect of increased VEGF and enhanced responsiveness to angiogenic growth factors may play a role in the expansion of human BM-repopulating cells we observed. Future studies will be needed to further investigate the role of VEGF in the expansion of SRCs under hypoxia.
In conclusion, we show that the use of low O2 levels in vitro not only improves SRC survival compared with normoxia but also expands the number of human BM repopulating cells by 4.2-fold compared with freshly isolated Lin–CD34+CD38– cells. The present report also establishes that hypoxia increases both VEGF secretions and responsiveness to angiogenic factors in BM progenitors and stem cells. In addition, we show that stem cells and progenitors respond differently to hypoxic conditions. These findings suggest that the balance between the survival, self-renewal, and differentiation of human BM HSCs might be tightly regulated by O2 levels. The beneficial effect of hypoxia on human BM-repopulating cells may be mediated by angiogenic factors such as VEGF. Finally, the use of low O2 tension could represent an important advance in the ex vivo expansion of human HSCs and may have important translational and clinical implications.
Acknowledgments
We are grateful to Jennifer Sinibaldi, Matthew Lessie, Amy Cleck, and Martin Carroll from the Stem Cell and Leukemia Core at the University of Pennsylvania Cancer Center for providing the large number of normal BM samples necessary for this study. The authors also thank William Demuth at the Abramson Family Cancer Research Center (University of Pennsylvania) for cell sorting, Tammi Coleman and Derrick Dow for their help with the NOD/SCID mouse colony, and Diana Ramirez-Bergeron, Andy Arsham, Brian Keith, and other members of the Simon Laboratory for their valuable input. M.C. Simon is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Dominique A. Bonnet and M. Celeste Simon contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: bone marrow (BM); hematopoietic stem cell (HSC); burst-forming unit, erythroid (BFU-E); colony-forming cell (CFC); SCID-repopulating cell (SRC); bone marrow lineage–negative (Lin–); long-term colony-initiating cell (LTC-IC); hypoxia-inducible factor-1α (HIF-1α); aryl hydrocarbon receptor nuclear translocator protein (ARNT); phycoerythrin (PE); stem cell factor (SCF); VEGF receptor 2 (VEGFR2).
References
- 1.Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp. Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- 3.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- 4.Ivanovic Z, et al. Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br. J. Haematol. 2000;108:424–429. doi: 10.1046/j.1365-2141.2000.01842.x. [DOI] [PubMed] [Google Scholar]
- 5.Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipolleschi MG, et al. Severe hypoxia enhances the formation of erythroid bursts from human cord blood cells and the maintenance of BFU-E in vitro. Exp. Hematol. 1997;25:1187–1194. [PubMed] [Google Scholar]
- 7.Quinlan DP, Jr, et al. Effect of hypoxia on the hematopoietic and immune modulator preprotachykinin-I. Arch. Surg. 1998;133:1328–1334. doi: 10.1001/archsurg.133.12.1328. [DOI] [PubMed] [Google Scholar]
- 8.Ivanovic Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40:1482–1488. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 9.Dick JE. Normal and leukemic human stem cells assayed in SCID mice. Semin. Immunol. 1996;8:197–206. doi: 10.1006/smim.1996.0025. [DOI] [PubMed] [Google Scholar]
- 10.Larochelle A, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat. Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 11.Pflumio F, et al. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–3740. [PubMed] [Google Scholar]
- 12.Bhatia M, Wang JCY, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 14.Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp. Hematol. 1999;27:1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 15.Danet GH, Lee HW, Luongo JL, Simon MC, Bonnet DA. Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34(+) cells after ex vivo expansion. Exp. Hematol. 2001;29:1465–1473. doi: 10.1016/s0301-472x(01)00750-0. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia M, et al. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J. Exp. Med. 1997;186:619–624. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conneally E, Cashman J, Petzer A, Eaves C. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9836–9841. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piacibello W, et al. Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34(+) cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood. 1999;93:3736–3749. [PubMed] [Google Scholar]
- 19.Bhatia M, et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J. Exp. Med. 1999;189:1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda T, et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J. Clin. Invest. 2000;105:1013–1021. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryder D, Jacobsen SE. Interleukin-3 supports expansion of long-term multilineage repopulating activity after multiple stem cell divisions in vitro. Blood. 2000;96:1748–1755. [PubMed] [Google Scholar]
- 22.Kollet O, et al. The plant lectin FRIL supports prolonged in vitro maintenance of quiescent human cord blood CD34(+)CD38(-/low)/SCID repopulating stem cells. Exp. Hematol. 2000;28:726–736. doi: 10.1016/s0301-472x(00)00163-6. [DOI] [PubMed] [Google Scholar]
- 23.Shih CC, et al. A secreted and LIF-mediated stromal cell-derived activity that promotes ex vivo expansion of human hematopoietic stem cells. Blood. 2000;95:1957–1966. [PubMed] [Google Scholar]
- 24.Chute JP, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 25.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 26.Kaelin WG., Jr How oxygen makes its presence felt. Genes Dev. 2002;16:1441–1445. doi: 10.1101/gad.1003602. [DOI] [PubMed] [Google Scholar]
- 27.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez-Bergeron DL, Simon MC. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells. 2001;19:279–286. doi: 10.1634/stemcells.19-4-279. [DOI] [PubMed] [Google Scholar]
- 30.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J. Lab. Clin. Med. 1998;131:207–214. doi: 10.1016/s0022-2143(98)90091-9. [DOI] [PubMed] [Google Scholar]
- 32.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flamme I, et al. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech. Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 35.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 36.Guzman ML, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 37.Hogge DE, Lansdorp PM, Reid D, Gerhard B, Eaves CJ. Enhanced detection, maintenance, and differentiation of primitive human hematopoietic cells in cultures containing murine fibroblasts engineered to produce human steel factor, interleukin-3, and granulocyte colony-stimulating factor. Blood. 1996;88:3765–3773. [PubMed] [Google Scholar]
- 38.Sutherland HJ, Eaves CJ, Lansdorp PM, Thacker JD, Hogge DE. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991;78:666–672. [PubMed] [Google Scholar]
- 39.Bonnet D, Bhatia M, Wang JC, Kapp U, Dick JE. Cytokine treatment or accessory cells are required to initiate engraftment of purified primitive human hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant. 1999;23:203–209. doi: 10.1038/sj.bmt.1701564. [DOI] [PubMed] [Google Scholar]
- 40.Gerber HP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 41.Hattori K, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 43.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J. Biol. Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 45.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 46.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 47.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J. Biol. Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- 49.Graeber TG, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 50.Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol. Cell. Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iida T, et al. Hypoxia-inducible factor-1alpha induces cell cycle arrest of endothelial cells. Genes Cells. 2002;7:143–149. doi: 10.1046/j.1356-9597.2001.00512.x. [DOI] [PubMed] [Google Scholar]
- 52.Gardner LB, et al. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 53.Wilpshaar J, et al. Similar repopulating capacity of mitotically active and resting umbilical cord blood CD34(+) cells in NOD/SCID mice. Blood. 2000;96:2100–2107. [PubMed] [Google Scholar]
- 54.Gothot A, van der Loo JC, Clapp DW, Srour EF. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 55.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 56.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 57.Cockman ME, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 58.Ohh M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 59.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamura T, et al. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler BL, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285:1553–1558. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 62.Hattori K, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat. Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh H, et al. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J. Biol. Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 64.Hashiyama M, et al. Predominant expression of a receptor tyrosine kinase, TIE, in hematopoietic stem cells and B cells. Blood. 1996;87:93–101. [PubMed] [Google Scholar]