Abstract
Adiponectin has recently been shown to be a promising candidate for the treatment of obesity-associated metabolic syndromes. Replenishment of recombinant adiponectin in mice can decrease hyperglycemia, reverse insulin resistance, and cause sustained weight loss without affecting food intake. Here we report its potential roles in alcoholic and nonalcoholic fatty liver diseases in mice. Circulating concentrations of adiponectin decreased significantly following chronic consumption of high-fat ethanol-containing food. Delivery of recombinant adiponectin into these mice dramatically alleviated hepatomegaly and steatosis (fatty liver) and also significantly attenuated inflammation and the elevated levels of serum alanine aminotransferase. These therapeutic effects resulted partly from the ability of adiponectin to increase carnitine palmitoyltransferase I activity and enhance hepatic fatty acid oxidation, while it decreased the activities of two key enzymes involved in fatty acid synthesis, including acetyl-CoA carboxylase and fatty acid synthase. Furthermore, adiponectin treatment could suppress the hepatic production of TNF-α and plasma concentrations of this proinflammatory cytokine. Adiponectin was also effective in ameliorating hepatomegaly, steatosis, and alanine aminotransferase abnormality associated with nonalcoholic obese, ob/ob mice. These results demonstrate a novel mechanism of adiponectin action and suggest a potential clinical application of adiponectin and its agonists in the treatment of liver diseases.
Introduction
Alcoholic (ASH) and nonalcoholic (NASH) steatohepatitis are chronic liver diseases that are increasingly significant (1). ASH affects millions of patients worldwide and is one of the major causes of death in developed countries (2). The progression of ASH is characterized by steatosis (fatty infiltration), inflammation, necrosis, and finally fibrosis and cirrhosis; when severe hepatitis occurs, death is a common outcome (3). There are currently no effective pharmacological reagents that can prevent or reverse this disease.
The mechanisms underlying alcohol-induced hepatotoxicity are complex and multifactorial. Many recent studies suggest that several proinflammatory cytokines produced by activated Kupffer cells might be involved in the onset of alcoholic liver disease (4, 5). For instance, elevated circulating levels of TNF-α, IL-1β, and IL-6 have been observed in human patients and animal models of alcohol-induced liver injury (6, 7). The expression levels of these cytokines correlate well with the course of the disease. The pivotal role of these cytokines in the pathogenesis of alcoholic steatohepatitis are further supported by the finding that depletion of Kupffer cells prevents alcohol-induced early liver injury in an intragastric alcohol-fed model (8) and also suppresses alcohol-induced microvesicular and macrovesicular steatosis in an oral, alcohol-fed model (9). Alcoholic liver injury is significantly attenuated by administration of Ab’s against TNF-α (10) and is diminished in TNF-α receptor 1 knockout mice (11). Furthermore, treatment with thalidomide, a compound that inhibits TNF-α production in Kuppfer cells, completely prevents alcoholic liver injury in rats (12).
In contrast to proinflammatory cytokines, anti-inflammatory cytokines have been shown to have hepatoprotective effects (7). Thus, plasma levels of IL-10 in mice are decreased by alcohol, and IL-10 knockout mice are more susceptible to alcoholic liver injury (13). It has been postulated that an imbalance exists between pro- and anti-inflammatory cytokines in the liver during chronic exposure to alcohol.
NASH is a clinicopathological syndrome that is closely associated with obesity, insulin resistance, and type 2 diabetes (14). It is now recognized that NASH is a major contributor to overall obesity-related morbidity and mortality (15). At least 20% of patients with this condition develop cirrhosis, a leading cause of death in this population. Although the primary etiology of NASH is different from that of ASH, these two diseases show almost identical histology, including hepatic steatosis and lobular inflammation. There is also evidence suggesting that both diseases share similar pathogenesis and benefit from similar therapies (5, 16).
Adiponectin (also called ACRP30 and GBP28) is a protein exclusively secreted from adipose tissue (17). This protein shares sequence homology with a family of proteins that show modular design, a characteristic NH2-terminal collagen-like region and a COOH-terminal, complement factor C1q-like globular domain. Interestingly, although the three-dimensional structure of adiponectin closely resembles that of TNF-α (18), these two proteins have completely opposite effects. Both in vivo and in vitro experiments demonstrated that adiponectin and TNF-α suppress each other’s production and also antagonize each other’s action in their target tissues (19). Administration of adiponectin into mice has been shown to produce beneficial effects on lipid metabolism, such as enhancing lipid clearance from plasma and increasing fatty acid β oxidation in muscle (20, 21). Recent studies from Scherer’s group and our laboratory suggest that adiponectin can act directly on hepatic tissue and inhibit glucose production (22, 23). Adiponectin also has direct anti-inflammatory effects (24). These results collectively indicate that adiponectin might have hepatoprotective effects. Accordingly, we tested this hypothesis in mouse models with alcoholic and nonalcoholic liver diseases. Our results show that adiponectin is effective in alleviating both alcohol- and obesity-associated liver abnormality, including hepatomegaly, steatosis, and the elevated levels of serum alanine aminotransferase (ALT). The potential mechanisms include induction of hepatic fatty acid oxidation, inhibition of fatty acid synthesis, and suppression of TNF-α production in the liver.
Methods
Animals and diets.
Male FVB/n mice weighing 25–30 g were housed in stainless steel wire-bottom cages on a 12-hour light/12-hour dark cycle under the institutional guidelines for the humane treatment of laboratory animals. All the experimental protocols were approved by the Institutional Animal Ethics Committee. Mice were fed with a modified high-fat/low-carbohydrate liquid diet (25) containing 44% fat, 16% protein, 5.5% carbohydrate plus 34.5% ethanol or isocaloric maltose dextrin as the control. Ethanol concentration was gradually increased from 17% to 34% during the first week of feeding and then maintained at the same concentration for another 5 weeks. Mice that were offered ethanol-containing liquid diets as their sole food source consumed 15.0 ± 1.3 ml/d/mouse. The dietary intake of control groups was matched to that of the ethanol-fed group by pair-feeding the same volume of isocaloric liquid diet.
Adult (aged 8–10 weeks) ob/ob C-57BL-6 mice and their age-matched lean littermates were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). The mice were fed with standard rodent pellet food. All mice used in this study are male, and the body weight of each mouse was measured every 2 days for the duration of this study.
Measurement of circulating adiponectin, TNF-α, insulin, and ALT levels.
The protocol for collecting human blood samples from obese individuals was approved by the Ethics Committee of the Medical Faculty, University of Hong Kong. Blood was obtained after 12-hour overnight fasting. All subjects provided written informed consent before participation.
Serum levels of mouse and human adiponectin were determined using an in-house RIA assay, using a rabbit polyclonal Ab against mouse adiponectin and human adiponectin, respectively (23). Serum was diluted 1,000-fold before assay. One hundred microliters of diluted serum or standard samples of recombinant mouse or human adiponectin were then incubated with adiponectin tracer labeled with125I and rabbit antiadiponectin polyclonal Ab’s at room temperature overnight. The immunocomplexes were recovered by polyethylene glycerol (PEG) precipitation. Intra- and interassay coefficients of variance were 5.2% and 5.7%, respectively.
Circulating levels of mouse TNF-α and insulin were quantified using commercial ELISA kits from Chemicon International (Temecula, California, USA) and Mercodia AB (Uppsala, Sweden), respectively. Serum levels of ALT were determined using commercial reagents from Sigma-Aldrich (St. Louis, Missouri, USA).
Measurement of hepatic fatty acid oxidation.
Liver slices were taken at the time of slaughter and rapidly excised and cooled in ice-cold buffer consisting of 0.25 M sucrose, 2 mM Na2-EDTA, and 10 mM Tris-HCl (pH 7.4). Whole-tissue homogenate (5% wt/vol) was prepared in the same buffer using a glass homogenizer with an intervening space of 0.050 mm. The rates of palmitate oxidation were analyzed in tissue homogenate as described previously (26). Briefly, palmitate oxidation was performed in a total volume of 0.5 ml containing 25 μl of liver homogenates in an assay buffer with 25 mM sucrose, 75 mM Tris-HCl (pH 7.4), 10 mM K2HPO4, 5 mM MgCl2, 1 mM NAD+, 1 mM Na2-EDTA, 5 mM ATP, 25 μM cytochrome c, 0.1 mM coenzyme A, 0.5 mM L-malate, and 0.5 mM L-carnitine. Flasks were preincubated for 30 minutes at 37°C before adding 100 μl of 600 μM [1-14C]palmitate (Amersham Biosciences, Uppsala, Sweden) bound to albumin in a 5:1 ratio. Incubation was carried out for 30 minutes at 37°C with agitation and stopped by adding 0.2 ml of 3 M perchloric acid. The released 14CO2 was trapped by 0.3 ml ethanolamine/ethylene glycerol (1:2 vol/vol). After incubating for 90 minutes at 4°C, the acid incubation mixture was centrifuged for 5 minutes at 10,000 g, and the supernatant containing 14C-labeled perchloric acid–soluble products was subjected to liquid scintillation counting. The rates of palmitate oxidation were calculated from the sum of 14CO2 and 14C-labeled perchloric acid–soluble products. Blanks were prepared by adding 0.2 ml of 3 M perchloric acid to the liver homogenates before incubating with the assay buffer for 30 minutes.
Measurement of carnitine palmitoyltransferase I activity.
Primary hepatocytes from mice fed with liquid control diet, liquid ethanol diet, or liquid ethanol diet plus adiponectin treatment were isolated as we described previously (23). The enzyme activity was assessed in digitonin-permeablized cells by monitoring the incorporation of L-[1-3H] carnitine and palmitoyl-CoA into palmitoylcarnitine. Reactions were performed in 700 μl of assay buffer containing 50 mM imidazole, 70 mM KCl, 80 mM KCN, 1 mM ATP, 0.1% fatty acid–free BSA, 70 μM palmitoyl-CoA, 50 μg/ml digitonin, 0.5 mM 3H L-carnitine, with or without 50 μM malonyl-CoA. Further details of this assay were described elsewhere (27, 28).
Determination of the activities for hepatic acetyl-CoA carboxylase and fatty acid synthase.
The activity of hepatic fatty acid synthase (FAS) was analyzed by measuring the incorporation of [1-14C]-acetyl-CoA into fatty acids in the presence of malonyl-CoA (29). Liver slices were homogenized (25% homogenate wt/vol) in a buffer containing 10 mM HEPES (pH 7.5) and 0.25 mM sucrose and then centrifuged for 5 minutes at 12,000 g. The reactions were initiated by adding 0.5 mg proteins from the centrifuged (12,000 g) supernatant into a prewarmed buffer consisting of 200 mM HEPES, pH 6.8, 250 mM sucrose, 1 mM NADPH, 0.88 mM DTT, 2 mM EDTA, 2% BSA, 0.39 mM malonyl-CoA, and 0.124 mM [1-14C]-acetyl-CoA (Amersham Biosciences). Newly synthesized 14C-labeled fatty acids were extracted and quantified as described (30).
A cytosolic fraction containing acetyl-CoA carboxylase (ACC) was isolated from liver tissue according to the PEG precipitation method (31). The ACC activity in the PEG fraction was assessed using CO2 fixation technique (31). Briefly, 12.5 μg protein was added into a reaction buffer containing 60.6 mM Tris acetate (pH 7.5), 1 mg/ml BSA, 1.32 μM ATP, 2.12 nM β-mercaptoethanol, 5 mM magnesium acetate, 1.06 mM acetyl-CoA, 18.08 mM NaH14CO3 (Amersham Biosciences), and 2 mM magnesium citrate. Samples were incubated at 37°C for 4 minutes and stopped by adding 25 μl of 10% perchloric acid. Newly synthesized 14C-labeled malonyl-CoA was extracted and quantified as described (31).
Measurement of glucose, triglyceride, and FFA concentrations.
Blood glucose from the tail vein was measured using Accu-Chek Comfort Glucometer (Roche Diagnostics, Basel, Switzerland). The levels of plasma FFA and triglycerides (TGs) were determined using a Roche FFA kit and Triglyceride GPO reagent (Pointe Scientific Inc., Lincoln Park, Michigan, USA), respectively. Hepatic TG was extracted from frozen liver tissue using chloroform/methanol (2:1), separated, and then quantified colorimetrically as described (32).
Production of recombinant adiponectin from HEK293 cells.
Full-length adiponectin was expressed in HEK293 cells transiently transfected with a vector that encodes FLAG epitope-tagged mouse adiponectin, as described previously (23). Recombinant adiponectin was purified using anti-FLAG M2 affinity gel (Sigma-Aldrich) and was eluted with 150 μg/ml FLAG peptide. The eluted protein was concentrated using a concentrator with molecular-weight cut-off of 10,000 Da (Vivascience AG, Hannover, Germany). FLAG peptides were removed by an extensive wash with an excessive volume of saline. Highly sensitive matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass-spectrometric analysis confirmed that FLAG peptide was not detectable in the protein solution.
RNA extraction and Northern blot analysis.
Total RNA was extracted from liver tissue using Trizol reagent (Invitrogen Corp., San Diego, California, USA). Twenty micrograms of total RNA from each sample was separated by denaturing agarose gels, transferred onto nylon membrane, and probed with 32P-labeled cDNA fragments encoding mouse TNF-α, FAS, ACC, or CD36. The relative mRNA abundance of each gene was quantified using PhosphorImaging.
Histological analysis.
Liver specimens were fixed overnight in buffered formaldehyde (10%) and embedded in paraffin. H&E-stained sections were graded blindly for the degree of fatty change, inflammation, and necrosis. Ten low-power fields were examined per liver. The degree of lipid infiltration was graded from 0 to 4, with 0 indicating no fat present and 4 indicating that 75% or more of the cells contain fat.
Statistical analysis.
Experiments were performed routinely with five to six mice per group with values presented as mean plus or minus SD. All the studies were replicated with representative data shown. Statistical significance was determined by one-way ANOVA. In all statistical comparisons, a P value of less than 0.05 was used to indicate a significant difference.
Results
Chronic ethanol consumption causes significant decrease of circulating adiponectin in mice.
Mice fed with a modified high-fat liquid ethanol (LE) diet and pair-fed with high-fat liquid control (LC) diet (25) gained weight throughout the 6-week treatment period. Although the weight gain of LC mice (7.2 ± 0.6 g) was slightly higher, it was not significantly different from that of mice on the LE diet (6.8 ± 0.5 g). Mice fed with the LE diet consumed ethanol at approximately 17–19 g/kg body weight per day. At necropsy, liver-to-body weight ratios in mice receiving ethanol (8.3% ± 0.6%) were significantly higher than those in mice fed with the control diet (6.2% ± 0.4%).
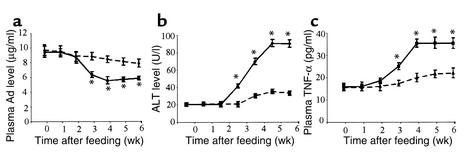
Chronic ethanol consumption caused a significant decrease in circulating concentrations of adiponectin. Plasma adiponectin decreased 32.1% ± 2.9% after 3 weeks and 40.3% ± 4.6% after 4 weeks of feeding with the LE diet (Figure 1a). Decreased adiponectin correlated closely with the development of liver injury, as judged by the plasma levels of ALT activity (Figure 1b). Moreover, there was an inverse relationship between circulating concentrations of adiponectin and TNF-α level following chronic consumption of the LE diet (Figure 1c).
Figure 1.
Chronic alcohol consumption decreases plasma adiponectin, increases TNF-α, and induces liver injury. Plasma samples were collected at different stages after mice had been fed with either high-fat LC diet (dashed line) or high-fat LE diet (solid line) and then were quantified for the levels of plasma adiponectin (a), ALT (b), and TNF-α (c), as described in the text. *P < 0.05 for ethanol diet versus control diet (n = 6).
Recombinant adiponectin markedly attenuated ethanol-induced liver injury.
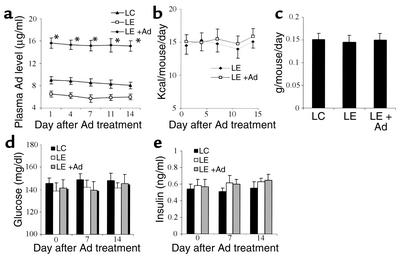
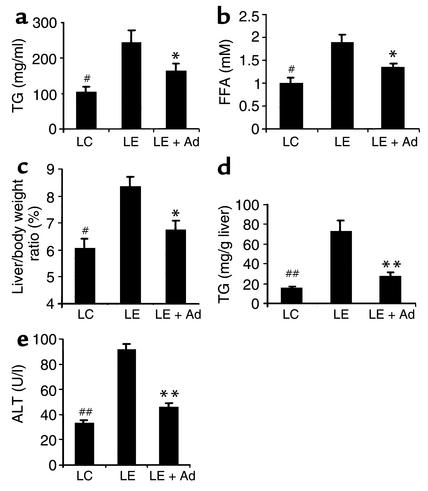
We next investigated the effect of adiponectin on alcohol-induced liver injury by infusion of full-length recombinant protein produced from HEK293 cells. Three weeks after being fed with the LE diet, the mice were surgically implanted with an ALZET osmotic pump (DURECT Corp., Cupertino, California, USA), which delivered 30 μg/d adiponectin or physiological saline as control. Delivery of adiponectin at this dosage caused approximately 2.7-fold elevation in the circulating concentration of adiponectin over that of untreated LE mice. The elevated concentration of plasma adiponectin remained constant throughout the course of treatment (Figure 2a). Adiponectin treatment had no obvious effects on food intake, body weight gains, and the levels of blood glucose and insulin (Figure 2, b–e), whereas it could decrease the elevated plasma concentrations of TG and FFA (Figure 3, a and b). Notably, continuous administration of adiponectin for 2 weeks significantly decreased the ratio of liver-to-body weight (Figure 3c), dramatically reduced hepatic lipid content (Figure 3d), and also markedly alleviated alcohol-induced elevation of serum ALT levels (Figure 3e).
Figure 2.
Effects of adiponectin treatment on food intake, body weight gains, and circulating levels of glucose and insulin. (a) Serum adiponectin levels in mice fed with LC diet, LE diet, or LE diet plus adiponectin infusion (LE + Ad), as described in the text (n = 5). Serum samples were collected at different times following adiponectin treatment. (b) Food intake in LE diet mice treated with or without adiponectin (n = 6). (c) Average daily body weight gains over 2 weeks of adiponectin treatment (n = 6). (d) Fasting glucose levels over adiponectin treatment period (n = 6). (e) Fasting plasma insulin levels over adiponectin treatment period (n = 6). Note that adiponectin and alcohol treatment also have no obvious effects on plasma levels of insulin and glucose in the fed state (data not shown). *P < 0.001 for adiponectin-treated group versus other two groups.
Figure 3.
Adiponectin treatment ameliorates alcohol-induced dyslipidemia and hepatic abnormality in mice. Serum samples were collected after 5 weeks of LC diet, LE diet, or LE + Ad diet in the last 2 weeks. Serum levels of TG (a) and FFA (b), liver-to-body weight ratio (c), hepatic TG contents (d), and plasma ALT levels (e) were determined at necropsy (n = 5). #P < 0.05, ##P < 0.01 for LC-treated mice versus LE-treated mice; *P < 0.05, **P < 0.01 for LE + Ad–treated mice versus LE-treated mice.
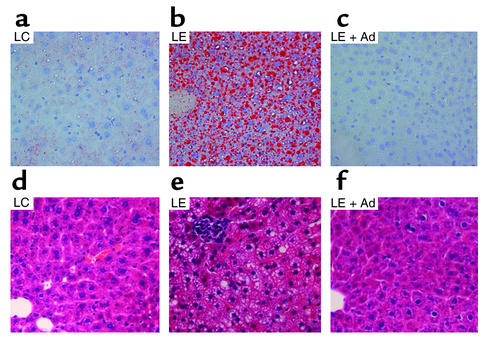
Histological evaluation of liver specimens demonstrated massive panlobular microvesicular and macrovesicular steatosis and occasional foci of inflammation in mice fed with LE diet alone (Figure 4). Administration of adiponectin dramatically decreased lipid accumulation to a background level and largely diminished inflammation (as judged by the absence of inflammatory foci under microscopy). Thus, our results demonstrated a protective role of adiponectin in alcohol-induced liver injury in mice.
Figure 4.
Effects of adiponectin on alcohol-induced steatosis and inflammation. Liver specimens were taken from mouse livers after 5 weeks of LC diet (a and d), LE diet (b and e), or LE + Ad diet (c and f) for the last 2 weeks and were stained with either red oil O (a–c) or H&E (d–f). The results are representative photomicrographs from six independent experiments.
Administration of adiponectin decreased hepatic expression of TNF-α and its plasma concentrations.
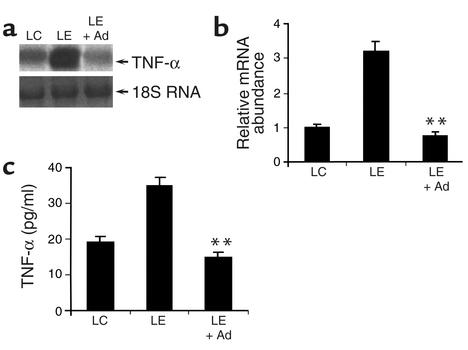
Elevated production of TNF-α from Kuppfer cells within the liver tissue is thought to be a key mediator of early alcohol-induced liver injury (5). A recent knockout study suggests that TNF-α and adiponectin may antagonize each other’s actions by each suppressing the other’s expression (19). We thus tested the hypothesis that adiponectin might alleviate alcohol-induced liver injury partly by suppressing alcohol-induced elevation of TNF-α production. Indeed, treatment of LE diet mice with recombinant adiponectin blunted the alcohol-induced increase of circulating TNF-α as well as mRNA production of this cytokine in the liver (Figure 5).
Figure 5.
Adiponectin treatment decreases alcohol-induced elevation of hepatic TNF-α expression and its plasma concentrations. (a) Total RNA from livers of mice treated with LC diet, LE diet, or LE + Ad diet was extracted and subjected to Northern blot analysis. (b) The results from a were quantified by PhosphorImaging (n = 5). All RNA levels are expressed relative to untreated LC pair-fed controls, after being normalized against the abundance of 18S RNA. (c) Plasma concentrations of TNF-α as measured at necropsy (n = 5). **P < 0.01 for LE + Ad–treated mice versus mice receiving LE diet alone.
Adiponectin treatment restored alcohol-induced suppression of CPT I activity and enhanced hepatic fatty acid oxidation.
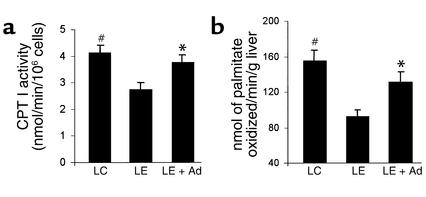
Impaired fatty acid oxidation plays a key role in alcoholic steatosis (33). Alcohol has been shown to decrease hepatic fatty acid oxidation in vivo and in vitro. Alcohol metabolism alters the intramitochondrial redox potential, which in turn impairs β oxidation and tricarboxylic acid cycle activity (34). In addition, both long-term and short-term alcohol consumption has been shown to suppress the activity of CPT I, a rate-limiting enzyme involved in the transport of long-chain fatty acids into mitochondrial matrix (35, 36). A truncated globular adiponectin can enhance fatty acid oxidation in muscle (21). We next investigated whether adiponectin-induced depletion of hepatic lipid accumulation was caused partly by enhancing fatty acid oxidation in this tissue. Indeed, consumption of LE diet significantly decreased the rate of hepatic fatty acid oxidation and also reduced CPT I activity (Figure 6). These ethanol-induced alterations were restored following adiponectin treatment.
Figure 6.
Effects of adiponectin on alcohol-induced suppression of hepatic CPT I activity (a) and fatty acid oxidation (b). Three groups of animals were treated with LC diet, LE diet, or LE + Ad diet as described in Figure 4. The activity of CPT I was determined by measuring 3H palmitoylcarnitine formed in digitonin-permeabilized hepatocytes. The rates of fatty acid oxidation were analyzed by monitoring nanomoles of [1-14C]palmitate oxidized per gram of liver tissue per minute. #P < 0.05 for LC-treated mice versus LE-treated mice; *P < 0.05 for LE + Ad–treated mice versus LE-treated mice.
Adiponectin treatment decreased the enzyme activities involved in fatty acid synthesis.
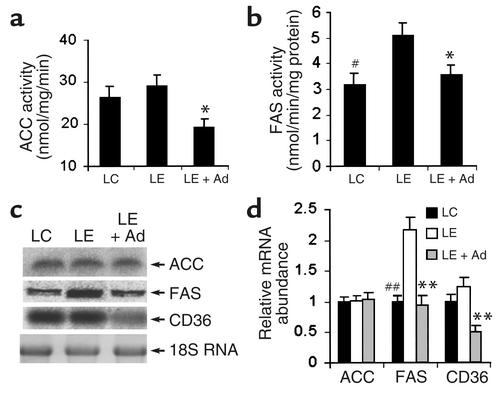
In addition to the suppression of fatty acid oxidation, enhanced fatty acid synthesis also contributes to alcohol-induced fatty liver, especially at the later stage of alcoholism. Chronic ethanol consumption can increase fatty acid synthesis in humans and rodents by inducing the expression of key enzymes in the lipogenic pathway (37, 38). We next investigated the effects of adiponectin on two key enzymes involved in hepatic lipogenesis, including ACC and FAS. Consumption of the LE diet caused a slight, but not significant increase of hepatic ACC activity (Figure 7a). Compared with the untreated LE diet mice, however, adiponectin caused a marked reduction of this enzyme activity. The activity of hepatic FAS was significantly increased following 5 weeks of LE diet, and the elevated enzyme activity was markedly decreased following adiponectin treatment (Figure 7b). Northern blot analysis revealed that the steady-state mRNA abundance of hepatic ACC was not altered following adiponectin treatment (Figure 7, c and d). Expression of hepatic FAS was significantly elevated following consumption of the LE diet, and adiponectin could suppress the hepatic mRNA expression of this enzyme. In addition, the hepatic expression of CD36, a fatty acid transport protein, was markedly inhibited following adiponectin treatment.
Figure 7.
Effects of adiponectin on proteins involved in fatty acid synthesis and uptake. Three groups of animals were treated with LC diet, LE diet, or LE + Ad diet as described in Figure 4. (a) Activity of hepatic ACC expressed as nanomoles of 14C malonyl-CoA produced per milligram of protein per minute. (b) Hepatic FAS activity expressed as nanomoles of 14C-labeled fatty acids produced per milligram of protein per minute. (c) Northern blot analysis of steady-state mRNA levels for ACC, FAS, and CD36. (d) The results from c were quantified by PhosphorImaging (n = 5). All mRNA levels are expressed relative to untreated LC pair-fed controls, after being normalized against the abundance of 18S RNA. #P < 0.05, ##P < 0.01 for LC-treated mice versus LE-treated mice; *P < 0.05, **P < 0.01 for LE + Ad–treated mice versus LE-treated mice.
Adiponectin treatment ameliorates nonalcoholic fatty liver disease in ob/ob mice.
NASH, which is often associated with obese individuals and patients with insulin resistance and type 2 diabetes, is an important feature of metabolic syndromes (1, 39). Anti-diabetes drugs might also improve fatty liver disease (40). Recombinant adiponectin has been shown to be effective in increasing insulin sensitivity and in decreasing hyperlipidemia in several animal models (20, 41). Therefore, we investigated the potential effects of adiponectin treatment on fatty liver disease in obese, ob/ob mice, which spontaneously develop hyperinsulinemia, insulin resistance, and steatosis owing to an inherited leptin deficiency (42). Adiponectin expression from adipose tissue is markedly reduced in ob/ob mice (43). Notably, overproduction of TNF-α in the liver tissue has been proposed to play a key role in the pathogenesis of fatty liver disease in this model (5). Fatty liver disease in ob/ob mice was significantly improved by inhibition of hepatic TNF-α overproduction or infusion of anti–TNF-α neutralizing Ab (16, 40).
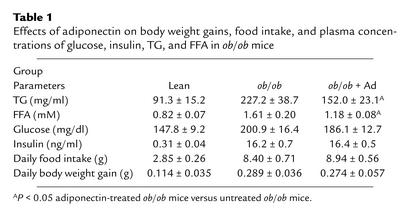
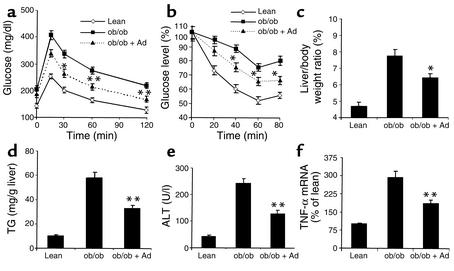
Consistent with those of LE diet–treated mice (Figure 2a), infusion of adiponectin using osmotic pumps (1.5 mg/kg body weight per day for 2 weeks) elevated plasma adiponectin levels approximately 2.7-fold throughout the course of the treatment. Adiponectin treatments had no obvious effects on body weight gains, food intake, and plasma levels of glucose and insulin, whereas circulating concentration of both TG and FFA were significantly decreased compared with untreated ob/ob mice (Table 1). In line with a recent study (44), infusion of adiponectin into obese ob/ob mice increased glucose tolerance as well as insulin sensitivity (Figure 8, a and b). Notably, adiponectin improved fatty liver disease in this animal model. Following 2 weeks of adiponectin treatment, the liver-to-body weight ratios were significantly reduced (Figure 8c), and this change was associated with the decreases in hepatic lipid contents (Figure 8d) and serum ALT levels (Figure 8e). The increased hepatic production of TNF-α in the obese mice was also significantly suppressed following adiponectin treatment (Figure 8f). Thus, adiponectin is an effective polypeptide for the treatment of obesity-associated nonalcoholic fatty liver disease.
Table 1.
Effects of adiponectin on body weight gains, food intake, and plasma concentrations of glucose, insulin, TG, and FFA in ob/ob mice
Figure 8.
Adiponectin increases insulin sensitivity and ameliorates fatty liver diseases in obese ob/ob mice. Age- and sex-matched lean control mice (lean), ob/ob mice (ob/ob), or ob/ob mice treated with adiponectin (ob/ob + Ad) for 2 weeks were subjected to a glucose-tolerance test (a) and insulin-tolerance test (b). The ratios of liver-to-body weight (c), hepatic TG contents (d), and serum ALT levels (e) were determined at necropsy. Hepatic mRNA expression of TNF-α (f) was analyzed as in Figure 5. The results were from five to seven mice per group. *P < 0.05, **P < 0.01 for adiponectin-treated ob/ob mice versus untreated ob/ob mice.
Adiponectin levels negatively correlate with serum ALT concentrations in morbidly obese Chinese individuals.
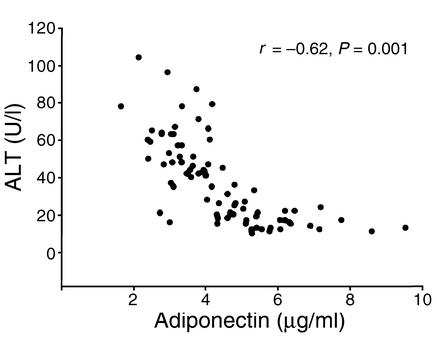
The results above suggest that adiponectin is associated with alcohol- and obesity-induced fatty liver diseases. To explore the clinical relevance of these findings, we next investigated the relationship between plasma levels of adiponectin and of ALT (a marker of liver injury) in 90 morbidly obese Chinese individuals (age 42 ± 9; BMI 40.1 ± 5.2). The rationale for this analysis is that there is a strong association between obesity and NASH. The prevalence of NASH among obese subjects is sixfold higher compared with lean individuals (45). Indeed, after adjustment for age, sex, and BMI, plasma levels of adiponectin inversely correlated with that of ALT (Figure 9), suggesting that hypoadiponectinemia may partly account for high incidence of NASH in obese individuals. Further prospective and cross-sectional studies are needed to ascertain the role of adiponectin in the cause of ASH and NASH in humans.
Figure 9.
Relationship between plasma levels of adiponectin and ALT in 90 morbidly obese Chinese individuals (45 male and 45 female). r, Spearman correlation.
Discussion
Recent studies from several independent laboratories have demonstrated that adiponectin and its agonists could be promising candidates for the treatment of obesity-associated metabolic syndromes (17). Adipose mRNA expression and plasma concentration of adiponectin are decreased dramatically in a variety of animal models as well as clinical patients with obesity, type 2 diabetes, and coronary heart disease (20, 46, 47). Replenishment of adiponectin can decrease hyperglycemia by inhibiting hepatic glucose production in mice (22). It can also improve dyslipidemia and decrease lipid accumulation in muscle, thus enhancing insulin sensitivity in this tissue (20). Chronic administration of a truncated COOH-terminal domain of recombinant adiponectin into mice consuming a high-fat diet has been shown to cause sustained and profound weight loss without affecting food intake (21). In addition to its antidiabetic and antiobesity effects, adiponectin has antiatherogenic potential. This protein accumulates in injured vessel walls and dose dependently inhibits TNF-α–induced cell adhesion in human aortic endothelial cells (24). These direct antidiabetic and antiatherogenic actions have been further confirmed by two recent independent knockout studies that demonstrated that depletion of adiponectin induced insulin resistance as well as neointimal formation after vessel injury (19, 41).
In this study, we showed that decreased expression of adiponectin might be partially responsible for alcohol-induced liver injury in mice (Figure 1). The mechanisms that underlie alcohol-induced reduction of adiponectin expression could be due to the elevated levels of TNF-α, which suppress adiponectin expression in adipose tissue through a paracrine or endocrine mechanism. Both circulating concentrations of TNF-α and local production of TNF-α in adipose tissue are increased at the early stage of alcoholic liver injury (48). Incubation of adipocytes with TNF-α markedly decreased expression of adiponectin (49). In addition, it is possible that alcohol may act directly on adipocytes and suppress adiponectin expression. Indeed, chronic consumption of alcohol in rats has been shown to increase relative expression of heterotrimeric guanosine triphosphate binding protein stimulatory α subunit (Gsα) to the inhibitory α subunit (Giα) in adipocyte membranes and to induce activation of the protein kinase A (PKA) pathway (50). PKA activation can dramatically decrease adiponectin expression in vivo as well as in vitro (51, 52).
Our results demonstrated that the protective role of adiponectin against liver injury is partly due to its antagonistic effect against TNF-α. Administration of adiponectin suppressed TNF-α expression in liver tissue and also decreased circulating concentrations of TNF-α (Figure 5). This finding is consistent with a recent report, which demonstrated that TNF-α production was markedly increased in adiponectin knockout mice (19). An in vitro study also indicated that treatment with adiponectin significantly inhibited LPS-induced TNF-α production in cultured macrophages (24).
In addition to suppressing TNF-α production, adiponectin may directly antagonize the damage-causing effects of TNF-α within the liver tissue. Indeed, adiponectin and TNF-α elicit many opposing effects. TNF-α is a causative factor of insulin resistance, whereas adiponectin increases insulin sensitivity (53). Adiponectin has antiatherogenic activity (54, 55), whereas TNF-α contributes to the onset of atherosclerosis (56). The direct antagonism between these two hormones has been demonstrated recently in muscle cells, where each impedes the other’s action in the regulation of glucose and lipid metabolism (19). In hepatic tissue TNF-α has been found to decrease insulin sensitivity and to enhance gluconeogenesis, whereas adiponectin has completely opposite functions (22). Thus, our findings further reinforce the concept that these two structurally similar hormones have opposite physiological effects and also suggest that increasing the ratio of plasma adiponectin/TNF-α will be useful for the treatment of alcoholic hepatitis.
It is important to note that the marked depletion of hepatic fat accumulation by adiponectin cannot be explained solely by its antagonistic effects against TNF-α. Growing evidence suggests that adiponectin can regulate hepatocyte metabolism. Treatment of primary rat hepatocytes with adiponectin in vitro dramatically increased the sensitivity of insulin to inhibit gluconeogenesis (23). Infusion of adiponectin into mice suppressed the expression of hepatic mRNAs corresponding to the gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (57). In the present study, we have demonstrated that administration of adiponectin can regulate hepatic fatty acid metabolism in vivo. Adiponectin infusion attenuated the activities of two rate-limiting enzymes involved in fatty acid synthesis, including FAS and ACC (Figure 7). Notably, hepatic mRNA abundance of FAS was suppressed following adiponectin treatment, whereas the expression of ACC was not affected. This result suggests that adiponectin suppresses these two hepatic enzymes via different pathways. The suppression of FAS is due to its decreased gene expression, whereas inactivation of ACC activity perhaps is caused by a posttranscriptional mechanism. Indeed, a more recent study has shown that full-length adiponectin can activate 5′-AMP–activated kinase in rat hepatocytes, which in turn will phosphorylate ACC and attenuate the activity of this enzyme (58).
A truncated COOH-terminal globular-region fragment of adiponectin has been shown to increase fatty acid β oxidation in muscle (21). In the present study, we showed that full-length adiponectin generated from mammalian cells could increase hepatic fatty acid oxidation in vivo, which is partly caused by activation of CPT I, a rate-limiting enzyme involved in this metabolic pathway (Figure 6). The mechanism underlying adiponectin-mediated elevation of CPT I activity is currently unclear. Hepatic expression of this enzyme was not altered following adiponectin treatment (data not shown). It is possible that adiponectin-mediated inactivation of ACC in the liver cells (Figure 7) will lead to a decrease in the concentration of its product malonyl-CoA (a potent inhibitor of CPT I) and will thus induce fatty acid oxidation in this tissue (59).
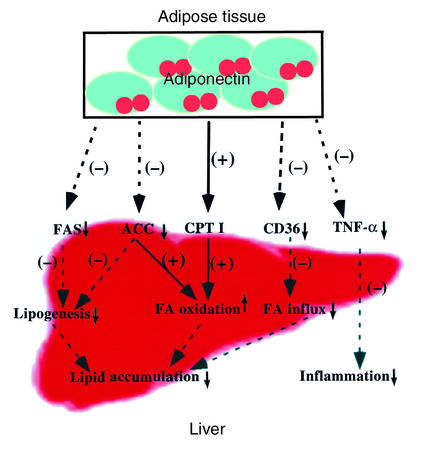
In addition to suppressing lipogenesis and activating fatty acid oxidation, other metabolic effects of adiponectin might also contribute to its lipid-depleting effects. For instance, adiponectin can enhance the clearance of circulating lipids by triggering its muscular combustion (21), which in turn will decrease the source of fatty acid influx into the liver. It can also downregulate the hepatic expression of CD36 (Figure 7), a protein responsible for transportation of fatty acids into tissues. These results collectively demonstrate that adiponectin abrogates alcohol-induced fatty liver by regulating multiple, coordinated, metabolic pathways (Figure 10).
Figure 10.
Schematic representation of the potential mechanisms that underlie the hepatoprotective actions of adiponectin.
The protective role of adiponectin against fatty liver diseases was confirmed in a nonalcoholic obese mouse model with insulin resistance and dyslipidemia (Figure 8). Although the primary etiology in the ob/ob mouse is due to the deficiency of another fat-derived hormone, leptin, adiponectin replacement therapy can partly compensate for leptin for alleviating hepatomegaly and steatosis and for reducing serum ALT levels. In addition, our results showed that infusion of adiponectin increased insulin sensitivity and alleviated hyperlipidemia in this model. This result is consistent with a recent transgenic study that demonstrated amelioration of insulin resistance and dyslipidemia after crossing ob/ob mice with globular domain adiponectin transgenic mice (44).
Fatty liver disease is a common finding in patients and various animal models with obesity, insulin resistance, diabetes, and dyslipidemia (60). Liver test abnormalities are found in up to 70% of diabetic patients, the majority attributable to fatty liver. Liver biopsies show steatosis in over 75% of morbidly obese subjects (61). In fact, hepatic steatosis has been proposed as an important feature of the insulin-resistance syndrome or syndrome X. A cross-sectional study of 551 severely obese individuals found that the risk of hepatic steatosis increased exponentially with each addition of the four components (type 2 diabetes, hyperlipidemia, visceral obesity, and hypertension) of the insulin-resistance syndromes (62). Notably, it has been reported that metformin, an oral antidiabetic drug that reduces hyperinsulinemia and improves hepatic insulin resistance, is effective in alleviating fatty liver diseases in patients and animal models (40, 63). The strong association between insulin resistance and fatty liver disease is further confirmed by our finding that adiponectin, which has insulin-sensitizing and glucose-lowering activities, can potently ameliorate hepatic steatosis. The fact that the plasma levels of adiponectin inversely correlate with that of ALT in morbidly obese subjects (Figure 9) suggests that hypoadiponectinemia is at least partly responsible for hepatic steatosis and liver injury in this population. This clinical finding is consistent with a recent study, which demonstrated that adiponectin deficiency is closely correlated with hepatic lipid accumulation in patients with insulin resistance (64).
In summary, our present study demonstrated, we believe for the first time, that the fat-derived hormone adiponectin is effective in alleviating alcohol- and obesity-induced hepatomegaly, steatosis, and serum ALT abnormality in mice. Notably, in CCl4–treated mouse liver, a large amount of adiponectin was found to accumulate and bind at the extracellular matrix of hepatocytes (65), suggesting that it may also act as an anti-inflammatory hormone that participates in the repair process of CCl4-induced liver injury. Therefore, in addition to its antidiabetic and antiatherogenic potentials, adiponectin or its agonists may represent a novel agent for the treatment of liver diseases.
Acknowledgments
We thank Anthony Phillips, Bernard Choong, and Beryl Davy for technical assistance. This work was supported by grants from Health Research Council of New Zealand, the New Zealand Lotteries Commission, and Auckland Medical Research Foundation. The clinical study was supported by a grant from Department of Medicine, The University of Hong Kong.
Footnotes
Aimin Xu and Yu Wang contributed equally to this work.
Conflict of interest: Garth J.S. Cooper is affiliated with Protemix Corp. in New Zealand.
Nonstandard abbreviations used: alcoholic steatohepatitis (ASH); nonalcoholic steatohepatitis (NASH); alanine aminotransferase (ALT); polyethylene glycerol (PEG); fatty acid synthase (FAS); acetyl-CoA carboxylase (ACC); carnitine palmitoyltransferase (CPT); triglyceride (TG); liquid ethanol (LE); liquid control (LC); protein kinase A (PKA).
References
- 1.Diehl AM. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am. J. Physiol. 2002;282:G1–G5. doi: 10.1152/ajpgi.00384.2001. [DOI] [PubMed] [Google Scholar]
- 2.Molina PE, et al. Molecular pathology and clinical aspects of alcohol-induced tissue injury. Alcohol. Clin. Exp. Res. 2002;26:120–128. [PubMed] [Google Scholar]
- 3.Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol. Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 4.Diehl AM. Cytokine regulation of liver injury and repair. Immunol. Rev. 2000;174:160–171. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N. Engl. J. Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 6.Thurman RG, et al. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front. Biosci. 1999;4:e42–e46. doi: 10.2741/A478. [DOI] [PubMed] [Google Scholar]
- 7.McClain CJ, et al. Cytokines in alcoholic liver disease. Semin. Liver Dis. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 8.Adachi Y, et al. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 9.Jarvelainen HA, et al. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J. Hepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 10.Iimuro Y, et al. Antibodies to tumor necrosis factor alpha attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 11.Yin M, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto N, et al. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization and TNF-alpha production. Gastroenterology. 2002;123:291–300. doi: 10.1053/gast.2002.34161. [DOI] [PubMed] [Google Scholar]
- 13.Hill DB, et al. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol. Clin. Exp. Res. 2002;26:74–82. [PubMed] [Google Scholar]
- 14.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin. Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 15.Matteoni CA, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, et al. Probiotics and antibodies to TNFα inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 17.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 19.Maeda N, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 21.Fruebis J, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Nat. Acad. Sci. U. S. A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg AH, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J. Biol. Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 24.Yokota T, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 25.Lindros KO, Jarvelainen HA. A new oral low-carbohydrate alcohol liquid diet producing liver lesions: a preliminary account. Alcohol Alcohol. 1998;33:347–353. doi: 10.1093/oxfordjournals.alcalc.a008403. [DOI] [PubMed] [Google Scholar]
- 26.Piot C, et al. Effects of dietary coconut oil on fatty acid oxidation capacity of the liver, the heart and skeletal muscles in the preruminant calf. Br. J. Nutr. 1999;82:299–308. [PubMed] [Google Scholar]
- 27.Adamo S, et al. Effect of L-carnitine on ethanol and acetate plasma levels after oral administration of ethanol in humans. Alcohol. Clin. Exp. Res. 1988;12:653–654. doi: 10.1111/j.1530-0277.1988.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Guzman M, Castro J. Ethanol increases the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA in short-term hepatocyte incubations. . Biochim. Biophys. Acta. 1989; 1002:405–408. doi: 10.1016/0005-2760(89)90358-5. [DOI] [PubMed] [Google Scholar]
- 29.Chang HC, et al. Liver acetyl CoA carboxylase and fatty acid synthetase: relative activities in the normal state and in hereditary obesity. Biochem. Biophys. Res. Commun. 1967;28:682–686. doi: 10.1016/0006-291x(67)90369-5. [DOI] [PubMed] [Google Scholar]
- 30.Bijleveld C, Geelen MJ. Measurement of acetyl-CoA carboxylase activity in isolated hepatocytes. Biochim. Biophys. Acta. 1987;918:274–283. doi: 10.1016/0005-2760(87)90231-1. [DOI] [PubMed] [Google Scholar]
- 31.Kudo N, et al. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. . Biochim. Biophys. Acta. 1996; 1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 32.Burant CF, et al. Troglitazone action is independent of adipose tissue. J. Clin. Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J. Lipid Res. 1979;20:289–315. [PubMed] [Google Scholar]
- 34.Eaton S, Record CO, Bartlett K. Multiple biochemical effects in the pathogenesis of alcoholic fatty liver. Eur. J. Clin. Invest. 1997;27:719–722. doi: 10.1046/j.1365-2362.1997.1780727.x. [DOI] [PubMed] [Google Scholar]
- 35.Guzman M, Geelen MJ. Effects of ethanol feeding on the activity and regulation of hepatic carnitine palmitoyltransferase I. Arch. Biochem. Biophys. 1988;267:580–588. doi: 10.1016/0003-9861(88)90065-3. [DOI] [PubMed] [Google Scholar]
- 36.Guzman M, Geelen MJ. Short-term inhibition of carnitine palmitoyltransferase I activity in rat hepatocytes incubated with ethanol. Biochem. Biophys. Res. Commun. 1988;154:682–687. doi: 10.1016/0006-291x(88)90193-3. [DOI] [PubMed] [Google Scholar]
- 37.Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am. J. Clin. Nutr. 1999;70:928–936. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 38.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J. Biol. Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 39.Chitturi S, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 40.Lin HZ, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 41.Kubota N, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002;277:35863–35866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 42.Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway-a link between adipose tissue mass and central neural networks. Horm. Metab. Res. 1996;28:619–632. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 43.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi T, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J. Biol. Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 45.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 46.Hotta K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 47.Hotta K, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 48.Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor-alpha and related cytokines in liver and adipose tissue. Alcohol. Clin. Exp. Res. 1998;22(Suppl.):231S–237S. doi: 10.1097/00000374-199805001-00004. [DOI] [PubMed] [Google Scholar]
- 49.Fasshauer M, et al. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 50.Wilkes JJ, DeForrest LL, Nagy LE. Chronic ethanol feeding in a high-fat diet decreases insulin-stimulated glucose transport in rat adipocytes. Am. J. Physiol. 1996;271:E477–E484. doi: 10.1152/ajpendo.1996.271.3.E477. [DOI] [PubMed] [Google Scholar]
- 51.Delporte ML, et al. Pre- and posttranslational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem. J. 2002;367:677–685. doi: 10.1042/BJ20020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fasshauer M, et al. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001;507:142–146. doi: 10.1016/s0014-5793(01)02960-x. [DOI] [PubMed] [Google Scholar]
- 53.Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol. Metab. 2002;13:18–23. doi: 10.1016/s1043-2760(01)00522-7. [DOI] [PubMed] [Google Scholar]
- 54.Ouchi N, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class Ascavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 55.Ouchi N, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 56.Ross R. Atherosclerosis: an inflammatory disease. New. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 57.Combs TP, et al. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 59.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 60.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin. Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 61.Falck-Ytter Y, et al. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin. Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 62.Marceau P, et al. Liver pathology and the metabolic syndrome X in severe obesity. J. Clin. Endocrinol. Metab. 1999;84:1513–1517. doi: 10.1210/jcem.84.5.5661. [DOI] [PubMed] [Google Scholar]
- 63.Marchesini G, et al. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 64.Funabiki A, et al. Adiponectin is associated with insulin resistance in liver. Diabetes. 2002;51(Suppl. 2):32a. (Abstr.) [Google Scholar]
- 65.Yoda-Murakami, et al. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochem. Biophys. Res. Commun. 2001;285:372–377. doi: 10.1006/bbrc.2001.5134. [DOI] [PubMed] [Google Scholar]