Abstract
Central thalamic electrical stimulation has been proposed as a method for remediation of acquired cognitive disability. Long-standing experimental and clinical observations indicate a key role for neurons within the central thalamus in maintaining the alert waking state and facilitating attended behaviors. Here, we show that continuous high frequency (100 Hz) electrical stimulation of the central thalamus generates widespread cortical activation of c-fos across all cortical layers and a selective pattern of regulation of zif268 within the supragranular, granular, and infragranular cortical laminae. Significant elevation of both immediate early genes also is seen in the dentate gyrus of the hippocampus. Use of the same stimulation parameters is shown to facilitate untrained goal-directed seeking behavior and object recognition memory in rodents. An overall increase of exploratory motor behaviors and grooming activity also is observed, consistent with a global increase in arousal. Taken together, these studies indicate that electrical stimulation of the central thalamus may enhance cognitive performance through neocortical and hippocampal neuronal activation and specific regulation of gene expression.
Keywords: attention, deep brain stimulation, gene expression, intralaminar thalamus, neuromodulation
Electrical stimulation of brainstem, thalamic, and basal ganglia structures is a rapidly emerging therapeutic technique for neuropsychiatric disorders, but knowledge of underlying mechanisms is limited (1, 2). Central thalamic stimulation has been proposed for the treatment of impaired cognitive function (3). Neurons within the intralaminar nuclei and paralaminar regions of the central thalamus link brainstem arousal systems to cerebral cortical and basal ganglia networks crucial to the organization of wakeful behaviors (4–8). To investigate the impact of central thalamic electrical stimulation on cognitive function, we characterize gene expression and behavioral effects of electrical stimulation centered on the central lateral (CL) nucleus of the rat anterior intralaminar thalamic nuclei (part of the central thalamus). We hypothesize that electrical stimulation of CL and surrounding regions may increase vigilance and cognitive performance in the intact animal. We assess functional activation associated with CL stimulation by using patterns of immediate-early gene expression in cortical and subcortical structures. Electrical stimulation of CL produced ipsilateral up-regulation of c-fos and zif268 expression with laminar specificity in the motor cortex (mCtx), anterior cingulate cortex (ACC), caudate-putamen (CP), and bilateral elevation in hippocampi at 2 hours after stimulation. In a separate series of experiments, unilateral high-frequency (100 Hz) electrical stimulation of CL in awake animals produced significant improvements in performance and learning of a visual object recognition task compared with control animals. These findings indicate that in vivo stimulation of central thalamus targeting CL activates a wide cerebral network and may influence basic cognitive processes associated with attention and memory.
Results
Experiment 1: Patterns of c-fos and zif268 Protein Expression After Central Thalamic Electrical Stimulation.
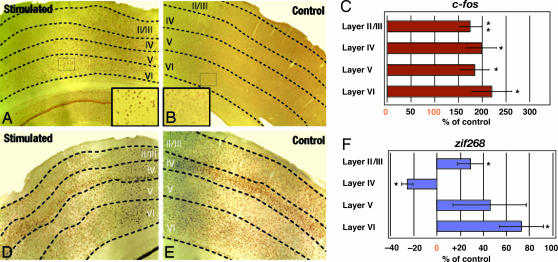
To characterize transynaptic activation of brain structures by electrical stimulation of CL, we evaluated expression of c-fos, the most studied immediate early gene in the brain. c-fos has been shown to reliably localize to the nucleus of neurons that are activated under varying conditions (9). Such data support the view that c-fos immunoreactivity (IR) can be interpreted as a sensible proxy for functional neuronal activity. Four different brain regions were prospectively chosen for study based on known anatomical projections of CL (5): mCtx, Dentate Gyrus (DG) of the hippocampus, the CP, and the ACC. We assessed gene expression effects after unilateral electrical stimulation of CL across six stimulation parameter sets by using 30-min stimulation periods. The stimulation protocols (SPs) used were based on known parameters effective for eliciting cerebral gene expression (9) and therapeutic effects of thalamic electrical stimulation (Volkman) (see Methods for further justification). These experiments identified 100 Hz stimulation at a current intensity of 1.5 mA as the most consistent parameter set producing a significant increase in cerebral c-fos expression. We observed elevated c-fos in layers II–VI of the ipsilateral mCtx after 2 h from stimulation onset (see Fig. 3A and Table 1). The layers of mCtx are heterogenous with respect to cell type and synaptic relationships, yet all showed comparable up-regulation in c-fos-expressing cells. The ACC of stimulated animals (n = 5) revealed a >2-fold increase (P < 0.015) in c-fos IR (Table 1) compared with sham-stimulated controls (n = 4). We also examined expression levels in subcortical regions critical for learning and memory. In the ipsilateral DG, CL-stimulated animals revealed a significantly elevated number of c-fos expressing cells. Although measurements were made only ipsilateral to stimulation, there was a qualitative observation of a uniform increase in bilateral c-fos IR in the DG and ACC (data not shown). The ipsilateral caudate-putamen, a region that receives dense input from CL (5), did not substantially differ in c-fos+ cell density between groups. To compare our findings with the effects of certain stimulating drugs we examined c-fos activation in the nucleus accumbens. Psychostimulants such as amphetamines and cocaine are known to increase arousal and to release dopamine in the nucleus accumbens (nAc), which is thought to be a critical step in the development of addictions (10). Activation of the nAc by these substances is accompanied by up-regulation of c-fos expression. After CL stimulation, however, the nucleus accumbens showed equal levels of activation in both stimulated and control animals. We also examined c-fos expression in the amygdala to assess the possible involvement of stress or anxiety (11) and found no difference between control, tethered animals, and stimulated rats' c-fos expression in the amygdala. This finding correlates with observed behavior of the rats in the open field that did not reflect anxiety.
Fig. 3.
C-fos and zif-268 expression are modified after thalamic electrical stimulation. (A–C) C-fos cortical expression, as assessed by immunocitochemistry, was elevated in rats that received central thalamic electrical stimulation. A significant difference was observed in the number of nuclei positive for c-fos ICC in layers II–VI. There was an increase in c-fos+ nuclei throughout the neocortex. (D–F) Zif-268 cortical expression, as assessed by ICC was differentially regulated in the different cortical layers after thalamic DBS. Layers II/III, V, and VI showed a significant increase in the number of ICC-labeled nuclei. However, layer IV had a significant decrease in the number of ICC-stained nuclei. ∗, P < 0.05. ∗∗, P < 0.01.
Table 1.
Expression of c-fos and zif268 in selected areas of the brain after thalamic electrical stimulation
| Brain region | Gene | Immunoreactivity ± SE, % of control |
|---|---|---|
| Anterior cingulate cortex (ACC) | c-fos | 203.0 ± 25.3* |
| zif268 | 174.9 ± 16.3* | |
| Caudate-putamen (CP) | c-fos | 136.6 ± 9.4 |
| zif268 | 153.6 ± 16.0 | |
| Dentate gyrus (DG) | c-fos | 121.5 ± 9.4 |
| zif-268 | 157.2 ± 16.9* | |
| Neocortex | ||
| Layer II/III | c-fos | 174 ± 22.9† |
| Layer IV | 198 ± 32.43* | |
| Layer V | 184.2 ± 30* | |
| Layer VI | 220.1 ± 42.3* | |
| Layer II/III | Zif-268 | 128 ± 11* |
| Layer IV | 74 ± 4.8‡ | |
| Layer V | 145 ± 31.8 | |
| Layer VI | 173.13 ± 19.0‡ |
*, P < 0.05;
†, P < 0.01;
‡, P < 0.001.
The broad increase of c-fos seen across cortical laminae II–VI provides evidence for an overall increase in cortical synaptic firing rates and neuronal activity (9). We examined another regulatory transcription factor, zif268, known to interact with c-fos. Zif268 is a regulatory immediate-early gene that activates downstream target genes and is up-regulated during associative learning and after tetanic stimulation that induces long-lasting long-term potentiation (LTP). Rat brains were evaluated for expression levels of zif268, which has a high baseline expression in the brain, in the same brain regions as c-fos. Whereas a significant increase in zif268+ cell density was seen in mCtx layer II/III and layer VI for stimulated rats (Fig. 3 and Table 1), a significant decline was observed for layer IV (Fig. 3 and Table 1). This laminar specific distribution of zif268 expression in mCtx is consistent with the known projection of CL into the cortical microarchitecture (5). A similar laminar pattern of neocortical zif268 expression has been observed after LTP induction followed by a period of rapid eye movement sleep (14). Also consistent with the neocortical data, ACC of CL-stimulated animals displayed (Table 1) a significant increase in zif268 expression. In the ipsilateral DG, zif268 expression was elevated significantly in CL-stimulated rats. Together with previous studies (14, 15), increased zif268 expression in the DG suggests that stimulation of CL may help to establish a local environment in the DG that is conducive to enhanced facilitation of LTP or memory reconsolidation (see Discussion). CP revealed an increase in zif268-expressing cells, but this did not meet statistical significance. Our estimate of activated cells in this structure is affected possibly by stereological regional sampling, as studies indicate that the dorsolateral subregion of CP receives a disproportionately large number of axons from CL compared with other subregions that are more diffusely innervated (5). As with c-fos (10), zif268 did not appear to be up-regulated in the nucleus accumbens or amygdala (16).
Experiment 2: Enhancement of Object Recognition Performance with Central Thalamic Electrical Stimulation Is Timing-Specific.
We tested animals across 3 days on a visual novel object recognition (NOR) task. Animals were shown one of two unique pairs of identical objects on any given day. After a 2-h interval, only one of the initially shown objects was paired with one novel object (taken from the not-shown pair). The task exploits an intrinsic tendency to explore novel stimuli. In our experiments, these stimuli were neither a reward nor a punishment, therefore allowing us to exclude, up to a certain extent motivation or fear in this complex behavior (Fig. 1E) (17). We tested rats after single, randomized periods of stimulation [during sample or object presentation (OP), interval (I) or during the object recognition (OR) testing period] over 3 consecutive days. These experiments demonstrated that stimulated rats increased their exploration of the novel object compared with tethered sham-stimulated animals (Fig. 2B). NOR performance measured as the time spent with the novel object minus the time with the old object was significantly enhanced for rats that were stimulated (n = 5) during OR itself compared with tethered controls (n = 4), (tethered, −0.5 sec ± 6.9; stimulated, +24.5 ± 8.3; P < 0.05) as shown in Fig. 2 C. As stimulation in this condition began ≈13.5 min before OR testing (see Fig. 1), enhanced performance represents a relatively prompt (minutes) and transient effect of CL stimulation rather than an accumulative effect (days) due to changes in gene expression patterns. Neither stimulation during OP nor during the interval resulted in statistically different performance than tethered controls. Increased performance during OR stimulation was not sustained in the following days with stimulation applied during the OP or I periods, suggesting a reversible effect of stimulation on cognition. Animals handled for stimulation during the I period or sham stimulation during either the I or OP period showed a reduced performance on the task compared with similar handling during the OR period (Fig. 2C). This difference suggests that the handling process after the OP period may have interfered with the memory task; the marked improvement with OR stimulation, however, was significant in within-animal comparisons to both baseline performance in the pretesting period (Fig. 2A) and the OP performance for both groups (Fig. 2C). In light of these observations, we continued to study the effect of the OR stimulation protocol on OR performance.
Fig. 1.
Experimental designs. (A) Stimulation of CL. All rats underwent implantation of a bipolar electrode in the right CL thalamic nucleus (in green). For all experiments reported herein, rats received bipolar electrical stimulation (ES) at 100 Hz, with an intensity of 1.5 mA and for a duration of 30 min. (B) Experiment 1, gene expression. Approximately 1 week after surgical implantation and habituation to the experimental chamber, animals received ES. Animals were intracardially perfused 2 h after initiating ES (90 min after finishing) and processed for gene expression immunostaining. (C) Nissl staining confirmed electrode placement in the brains of all of the animals. (D) Object recognition test. Animals are shown two identical objects (A and B) for a 3-min duration. After a 2-h interval, one of the objects initially presented is substituted by a different object. Time spent exploring the novel object is taped and then measured. (E) Experiment 2. Animals are randomly assigned over 3 consecutive days to one of three different SPs: SP1, electrical or sham stimulation during object presentation; SP2, stimulation during the 2-h interval; and SP3, stimulated during the OR testing phase. Protocols and pairs of objects are also randomly assigned without repetition for any given animal. (F) Experiment 3. A new cohort of animals underwent 3 consecutive days of SP3.
Fig. 2.
Effect of CL stimulation on NOR. (A) Animals were randomized to either the stimulation or the sham-stimulation group. We quantified the time spent with the novel object (Tnovel) minus the time spent with the old object (Told). In the training phase (no stimulation), animals in the control and in the stimulated group scored very similarly. (B) In Experiment 2, all animals were stimulated or sham-stimulated during OP, I, or OR across 3 consecutive days. The order was randomly assigned. Animals stimulated during OR performed significantly better than those stimulated during OP or I. The graph shows that Tnovel − Told pooled across the 3 consecutive days is ≈100% higher in stimulated animals than in sham-stimulated. (C) All animals were stimulated or sham-stimulated during OP, I, or OR across 3 consecutive days with randomly assigned order. Animals stimulated during OR performed significantly better than those stimulated during OP or I. (D) Experiment 3: progressive improvement in OR with repeated stimulation. Increased performance is observed in rats after repeated daily stimulation during OR. ∗, P < 0.05.
Experiment 3: Object Recognition Performance Is Enhanced by Central Thalamic Electrical Stimulation over Consecutive Days.
NOR.
How does continuous CL stimulation during testing affect cognition? To address this question, we conducted larger follow up experiments by using OR stimulation exclusively over 3 successive days. (Fig. 2 A and B). Rats stimulated during OR (n = 10) showed significantly better performance in Tnovel − Told, than tethered controls (n = 12) over all days (Fig. 3A; 15.1 ± 2.7 sec vs. 7.6 ± 1.4 sec, respectively; P < 0.05). Measurements of OR performance across 3 days allowed us to examine whether stimulation of CL modified sequential daily performance. After day 3 (n = 10), the stimulated rats showed significantly enhanced novel object recognition over day 1 (n = 10) of testing compared with controls (20.1 ± 4.7 vs. 8.1 ± 2.5, respectively; P = 0.01, Fig. 3B). We interpret these findings as evidence that CL stimulation may enhance object recognition during performance and has a cumulative effect across days. Although it appears that stimulated animals exhibit accelerated learning it is not certain whether memory acquisition, storage or retrieval is specifically influenced (13, 14).
Arousal.
We further examined effects of CL stimulation on overall behavioral activation, as indicated by nonspecific exploration and grooming behaviors. Animals stimulated in either the OP or OR period showed significantly increased activity (n = 10) compared with controls (n = 12) across evaluations of several measures of general arousal (Fig. 4, which is published as supporting information on the PNAS web site). This finding is consistent across days as shown in Fig. 4. Measured changes of total object exploration time and task performance therefore reflect both alteration of overall level of motor activity and intentional or goal-directed behavior (Fig. 3; see Fig. 5, which is published as supporting information on the PNAS web site). Exploratory activity relevant to the goal (measured in seconds as time spent with both the novel and the old object) significantly increased in stimulated animals (P < 0.01 across days; and, 50.4 ± 6.8 vs. 33.9 ± 3.0, P < 0.05, stimulated versus control within day 3) across 3 days, indicating that repeated stimulation of CL specifically influences intentional activity related to task performance. The observations are consistent with previous demonstrations that increased motor activity reflects a change in generalized arousal (18). Note that stimulation did not induce seizure-like behavior (see Methods) or impairment in general motor function. The lack of c-fos IR consistent with seizure activity lends further support to this claim (see below).
Thus, the time course of observed effects on task performance and global activity is consistent with both a transient effect of stimulation, lending support to enhanced retrieval abilities, and with a carry-over effect of the previous day's stimulation episode suggesting an influence on consolidation mechanisms. The priming of molecular memory networks may be a product of increased generalized arousal (19). Specifically, elevations of arousal levels may result in increased attentional effort and stronger engagement of neocortical memory systems with progressive changes occurring over multiple sleep-wake periods.
Discussion
Arousal underlies all mammalian behaviors. A recent operational definition states that a more aroused animal or human being (i) is more alert to sensory stimuli in all modalities, (ii) emits more voluntary motor activity, and (iii) is more emotionally reactive (22). Higher levels of arousal are associated with increased cognitive performance. Early studies (23) demonstrated changes in arousal level during electrical stimulation of the central thalamus (intralaminar nuclei including CL). Detailed electroanatomical studies and clinical observations further indicate a key role of these neuronal populations in maintaining the alert wakeful state (6, 24–26). Here, we show that central thalamic electrical stimulation can modulate cognitive function in awake behaving rats. The results provide insight into the underlying mechanisms of behavioral effects produced by central thalamic electrical stimulation and support further studies of effects on cognition in intact and brain-injured animals. These studies also complement ongoing efforts to develop central thalamic stimulation as a therapeutic strategy for acquired cognitive disabilities associated with nonprogressive brain injuries (3).
Stimulation parameters used in our studies produced significant increases in the time that rats spent with a novel object compared with a previously presented object, enhancing an intrinsic, unrewarded behavior. Broad bilateral cerebral activation as evidenced by the patterns of up-regulation of c-fos and zif268 expression after the unilateral electrical stimulation of CL suggests an enhancement of global arousal. Previous experimental studies have shown that CL projections play an important role in supporting the state changes of corticothalamic systems that underlie sleep-wake cycles but do not necessarily drive these state changes (24). The anterior intralaminar nuclei (including CL) and adjacent paralaminar regions of thalamic association nuclei receive the strongest innervation from brainstem cholinergic neuronal populations (28) and cholinergic populations from the basal forebrain (29) as well as heavy innervation from noradrenergic afferents from the locus ceruleus (30) and serotoninergic afferents from the medial raphe (30). Thus, the brainstem and basal forebrain arousal systems converge on the anterior intralaminar neurons allowing for a key role in arousal regulation (5). Our observations support this recognized role for neurons within and surrounding CL in generalized arousal (4, 5, 28). We recognize that the macrostimulation currents produced likely generate contributions from nearby neuronal populations, although the behavioral effects can be explained primarily through CL activation.
The measured increases in intentional exploratory behavior seen in the stimulated rats can be compared with studies that demonstrate deficits in initiating motor behaviors after CL lesions (31). Importantly, the effects demonstrated above are reward-independent, indicating that CL stimulation can influence behavior without the use external incentives consistent with a generalized arousal effect producing increase behavioral responsiveness (19, 32). The high-frequency (100 Hz) stimulation of CL may facilitate spontaneous activity within the 20–100 Hz range of firing frequencies known to exist in these cell populations during alert wakefulness (33). Selective increases of central thalamic local field potential activity at these frequencies correlates with short-term focusing of attention and continuous stimulation of central thalamic neurons at ≈50 Hz has been shown to facilitate performance of vigilance tasks in primates.§ More recently, a pilot clinical study of central thalamic electrical stimulation in a single-subject demonstrated improvements of behavioral responsiveness and arousal regulation despite several years of remaining in a minimally conscious state after a severe brain injury.¶ Taken together, the present results suggest that CL stimulation may influence cognitive performance through alteration of an endogenous arousal set point, possibly accessing a cognitive reserve proposed in earlier studies of attentional effort (36).
Recent in vivo, in vitro, and modeling studies show a strong convergence of evidence that continuous brain electrical stimulation as used in clinical applications (37) activates target structures, typically driving neuronal firing rates at the stimulation frequency (20, 21). Consistent with these other experimental studies, our findings of c-fos up-regulation indicate that areas with known monosynaptic connection with CL (5) show evidence that neurons are transynaptically activated. Llinas et al. (8) demonstrated that in vitro stimulation of CL produces strong excitation of supragranular and infragranular cortical layers that potentiate inputs to cortical granular layers with increasing activity associated with increasing stimulation duration. These calbindin positive thalamic neurons are known to selectively and strongly project to supergranular cortical layers and are proposed to facilitate large-scale synchronization within the corticothalamic system (38, 39). Although the stimulation intensities used here would necessary allow for some activation of nearby thalamic structures (see Methods), the specific laminar pattern of neocortical zif268 expression that we observe directly correlates with the findings of Llinas et al. (8) and the known anatomical specialization of calbindin positive thalamic neurons, which although concentrated in CL, are also present in paralaminar and other thalamic nuclei (5, 38). Taken together, the neocortical activation patterns seen in our gene expression studies are consistent with activation of CL neurons by electrical stimulation.
Activation of CL targets may also promote changes in synaptic function arising over the sleep-wake cycle (27) that may help account for changes in effects observed here over days of stimulation. Recent studies indicate that zif268 plays a specific role in both consolidation of memory and reconsolidation arising when long-term memories are reactivated (14, 15). Induction of hippocampal LTP is associated with a similar pattern of laminar neocortical zif268 formation during subsequent rapid eye movement periods and has been proposed to correlate with instructional signals to create cortical memory (15). Thus improvement of task performance over time observed here may reflect facilitation of neocortical learning and memory processes.
Although the present experiments do not evaluate a causal role for the gene product associated with zif268 activation, the correlation with improved memory performance may index recruitment of specific memory related systems through CL stimulation. Mair (12) had earlier proposed CL lesions as the origin of diencephalic amnesia and suggested three general mechanisms for the participation of CL in cortical memory function: a role in setting the overall level of cortical activation (a generalized arousal effect), maintenance of sustained neuronal firing in the neocortex (persistent activity, cf. ref. 13), and facilitation of cortical LTP process through different molecular mechanisms. Our results are potentially consistent with all three hypotheses, but do not allow us to specifically support one particular mechanism. As an important extension, we demonstrate that CL stimulation is sufficient to recruit remote brain regions that enhance goal-oriented behavior and learning through repeated exposures.
Finally, based on the above findings we propose that stimulation of CL and related central thalamic structures may improve cognitive disabilities and enhance directed awareness in damaged as well as intact brains. If similar cognitive improvements can be seen with brain-injured animals after electrical stimulation, our experimental paradigm could be used to examine the interaction of electrical stimulation and function of damaged cerebral networks.
Methods
Experimental Design.
Experiment 1: Gene expression after thalamic electrical stimulation.
Rats had an electrode implanted on the right central lateral nucleus of the right thalamus as described below. Seven days after surgery, including 3 days for habituation to the chamber and manipulation, rats were stimulated at 0.25, 1 or 1.5 mA, 50 or 100 Hz, 50 μsec per phase pulse duration, for 30 min. Perfusion occurred 2 h after stimulation with tissue processing as described below. Transynaptic cortical c-fos up-regulation and zif-268 expression was observed consistently with a 1.5 mA, 100 Hz stimulation. All of the experiments were then done with these parameters.
Experiment 2: Multiperiod stimulation study.
After surgery, habituation and training animals were tested in an object recognition task on 3 consecutive days (n = 5 stimulated, 4 controls). (Fig. 1 and below). During the OP trial of the behavioral testing, animals were shown one of two unique pairs of identical objects. Later, during the OR trial the animal was presented with one of the objects previously encountered during the OP trial and one novel object (taken from the not-shown pair). After presentation of the pair of objects for 3 min (OP), animals were taken back to their cages for a 2-h I and then returned to the chamber where an old object and a new object were already placed. Animals then would spend time exploring the old and the novel object. More time with the novel object is associated with better recollection of the old object (17). On each day of testing the animals received random assignment to be stimulated during the OP time SP1, during the 2-h I period (SP2) or during OR (SP3). These experiment identified the protocol most effective in enhancing performance on this task
Experiment 3.
Based on the results of Experiment 2, we tested a new cohort of rats (n = 10 stimulated, 12 controls) assigned on 3 consecutive days to the SP3. Surgery habituation and training were identical to those applied in Experiment 2.
Surgical Techniques.
Male Sprague–Dawley rats (see respective Methods sections for sample sizes) between 300–450 g were fed ad libitum and kept in a 12-h light-dark cycle. During the third day of housing, animals were operated on under anesthesia achieved with an 87 mg/kg ketamine, 13 mg/kg xylazine i.p. injection. Animals were then positioned in a stereotaxic device (TSE instruments) and an incision was made between the ears; the skin of the scalp was pulled back to expose the dorsal surface of the skull around bregma. Four stainless steel screws were screwed half-way into the skull to enlarge the surface area for a skull cap and a small hole was drilled to pass the electrode (MS303/3; Plastics One, Roanoke, VA). The electrode was then lowered and implanted into CL at the following coordinates: 2.8 mm posterior to bregma, 1.25 mm lateral to the midline, and 5.5 mm ventral to the dural surface. All electrodes were implanted on the right side of the brain. Placements were verified histologically.
Stimulation Parameters.
Animals were allowed to recover for at least 7 days after surgery before commencing stimulation (Experiment 1). Initial training in the NOR task included varying intervals between initial presentation and recognition. We chose the 2-h interval to optimize task difficulty and the number of animals able to perform at this interval. Rats for the histology study were habituated to a new cage, identical to their own, for a period of 15 min for 3 days. On the fourth day their electrode was connected to a digital bipolar stimulator (Pulsar, 6 bp) via a spring shielded bipolar stimulating cable (Plastics One, Roanoke, VA). The stimulator was then switched on for a period of 30 min to deliver a 100 Hz bipolar stimulus with the following parameters: 1.5 mA, 10 msec cycle time, 50 μsec per phase pulse duration, 1 pulse per cycle, bipolar stimulation. These stimulation parameters were chosen to reflect the upper range of charge densities used in thalamic deep brain stimulation in clinical settings and after the preliminary results from the gene expression experiment; this permitted us to model potential effects within the available parameter range of the present applications of the technique (37). The current intensities for macrostimulation, although large compared with microstimulation methods, are balanced by the very brief (50 μsec) duration per phase. After 30 min, stimulation was terminated; animals were removed, placed back into their housing cage and killed 90 min later (2 h after onset of stimulation). A 2-h time point was chosen to maximize yield of c-fos expression in the cortex (9). Histological examination of electrode tracks showed no evidence of tissue damage at the stimulation sites for any animal.
Immunocytochemistry.
Animals were killed by pentobarbital overdose [150 mg/kg followed by intracardial perfusion of 4% paraformaldehyde (PFA); Sigma, St. Louis, MO]. Brains were removed and kept in PFA 4% in PB 0.1 M for 24 h and then switched to a PB 0.1 M solution. Coronal sections, 40-μm thick, were obtained by using a Vibratome (Leica Microsystems, Bannockburn, IL). Sections were serially kept in a 24-well tray. Electrode placement was verified histologically by Nissl staining in all of the brains for the animals used in this experiments. Sections spanning the diencephalon to the dorsal hippocampus were processed for imnmunostaining as previously described (40). For Experiment 1, every 12th section was selected for either zif268 or c-fos immunostaining. The following antibodies were used: c-fos (Santa Cruz Biotechnology, Santa Cruz, CA; 1:2,000) and EGR-1(zif268) (Santa Cruz Biotechnology; 1:5,000), secondary Ab (IgG, goat anti-rabbit: Jackson ImmunoResearch, West Grove, PA; 1:400 in glycerol).
Stereological Quantification.
For unbiased stereological counting, a stereological Nikon (Melville, NY) Eclipse E600 microscope with a ×10 Plan objective lens was used in concert with Stereo Investigator analysis software (Microbright Field, Williston, VT). Contours were drawn around individual regions of interest to determine areas (motor cortex, dentate gyrus, caudate putamen and anterior cingulate; Paxinos and Watson year). Cell population and density estimates were made by using the optical dissector and fractionator probes. Because some sections were processed on separate occasions, experimental groups were pair-matched to processing event, and scores were normalized and expressed as % compared with control.
Behavioral Testing and Stimulation.
Surgeries were performed as above. Rats for behavioral studies were habituated to the open-field box for 10 min for 1 day. NOR task was performed while rats (Experiment 2: n = 5 + stimulation, n = 4 ctrl; Experiment 3: n = 9 stimulation, n = 10 ctrl) were tethered to stimulator (or sham) via a 2-m shielded cable (Plastics One) passed through an overhead guide-loop. Sessions consisted of two 3-min trials, an OP trial and an OR test trial. The trials were separated by 5 min, 10 min, 30 min, 60 min (for training), and 2-h I. During OP, the rat were introduced into an 80 × 80 × 80 cm black Plexiglas testing box and exposed to two identical objects that were equally spaced in each trial. During OR, one old object was replaced by a novel object (left-right, and new vs. old objects were counterbalanced across groups and days) and the duration spent sniffing (rat's nose within 2 cm) the novel object was recorded. Rats were graduated to the 2-h time delay when performance ratios (Tnovel/Ttotal) were >0.5 after a 60-min training interval (17).
To ensure adequate motivation and baseline behavior, animals were excluded from trial analysis if they failed to explore either object during OP or OR. This occurred on only three occasions throughout Experiment 2 and on two occasions in Experiment 3. We used a numerical handicapping method to compensate for variations in the salience of objects presented during the behavioral tasks for individual animals (see below). Data from animals that exhibited persistent unilateral rotation after surgery were removed from analysis (n = 2 rats per trial). Observers remained blinded during manipulation of animals and assessments of treatment tapes.
Electrical SP (see Fig. 1) rats were stimulated (or sham-tether) for 30 min/day on 3 consecutive days in one of three task periods: SP1, during OP (electrical stimulation started 13.5 min before OP and continued during the 3 min of OP and the following 13.5 min to complete a 30-min stimulation period); SP2, electrical stimulation occurred during the median 30 min of the 2-h interval; SP3, stimulation occurred during OR (starting 13.5 min before OR continue during the 3 min of OR and the following 13.5 min to complete a 30-min stimulation period). Rats were randomized with respect to the consecutive daily order of stimulation/tether period.
In the last experiment, a different cohort of animals underwent electrode implantation, habituation and trainin as reviewed above. Animals were then assigned to a T3 protocol for 3 consecutive days.
Statistics.
Statistical analyses were performed with MATLAB software (Mathworks, Natick, MA). Data are presented as mean ± SE. Statistical analyses for Experiment 1 were made by using ANOVA, and Fisher's test was used for multiple comparison analysis, unless mentioned otherwise. For the behavioral experiments, analyses were made by using ANOVA with repeated measures to account for asphericity in the data, and post hoc Bonferroni correction to evaluate multiple comparisons. For Experiment 2, trial was used as the within-subject variable (OP, I, OR) and group as the between-subject variable (stim vs. control). For Experiment 3, day of stimulation was used as the within-subject variable, and group as the between-subject variable.
A Note on the Handicap-Ratio Correction.
The following statistical correction was applied to correct any behavioral bias in the animals' performance resulting from the intrinsic salience or ‘attractiveness’ of a particular testing object pair. As explained before, during the OP trial in behavioral testing on any given day, animals were shown one of two unique pairs of identical objects. Later, the OR trial contained one of the objects that the animal encountered during the OP, and one novel object (taken from the not-shown pair). Animals were deemed to have adequately explored the presented items during OP if they explored the pair of identical objects for a time >1 sec each, and did not show a consistent bias toward one of the two pairs across animals. The actual identity of the pair shown to animals during the OP trial varied randomly across all animals.
Time spent exploring a particular pair of identical objects during the animals' OP trial was then analyzed by ANOVA with Fisher's test by using the identity of the presented object pair as the between-group variable (i.e., ceramic block pair vs. soda can pair). If there was a significant difference in the time spent exploring between particular object pairs during OP, the following correction was applied. The object pair which elicited a greater total exploration time during the OP was considered to be of greater intrinsic interest to the animals. Thus, the average time spent exploring the object pair of lesser interest was divided by average time spent exploring the more interesting pair, to yield a handicap-ratio (each group had an n = 5–7). This ratio was then multiplied to the raw time spent exploring the more interesting object during the OR trial. This resulted in variably enhancing or suppressing the animals performance measured as Tnovel – Told. Effectively, the analysis creates a more conservative performance criterion for all animals, based on previous population performance. The main reason we did this was because we found out that there was a large difference in the attractiveness of object pairs in only one instance on day 3 of experiment 2. In the following cases, we used two pairs with close average exploration times.
Supplementary Material
Acknowledgments
D.G.H. was supported by grants from the Weill Cornell Center for Aging Research and Clinical Care, Reader's Digest Foundation, and the American Federation for Aging Research.
Abbreviations
- ACC
anterior cingulate cortex
- CL
central lateral
- CP
caudate-putamen
- I
interval
- IR
immunoreactivity
- mCtx
motor cortex
- NOR
novel object recognition
- OP
object presentation
- OR
object recognition
- SP
stimulation protocol.
Footnotes
Conflict of interest statement: N.D.S. is an inventor on the patents that have been issued to Cornell University for central thalamic brain stimulation for impaired cognitive function.
This article is a PNAS direct submission.
§Schiff, N. D., Hudson, A. E., Purpura, K. P., 32nd Annual Meeting of the Society of Neuroscience, November 2–7, 2002, Orlando, FL, program 62.12 (abstr.).
¶Schiff, N. D., Giacino, J., Kalmar, K., Kobylarz, E., Baker, K., Farris, S., Machado, A., Victor, J., McCagg, C., Plum, F., et al., 36th Annual Meeting of the Society of Neuroscience, October 14–18, 2006, Atlanta, GA, program 182.4 (abstr.).
References
- 1.McIntyre CC, Savasta M, Walter BL, Vitek JL. J Clin Neurophysiol. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, Vercucil L, Benazzouz A, Koudsie A, Chabardes S, Minotti L, Kahane P, Gentil M, Lenartz D, Andressen C, et al. Adv Neurol. 2003;91:293–302. [PubMed] [Google Scholar]
- 3.Schiff ND, Plum F, Rezai AR. Neurol Res. 2002;24:116–124. doi: 10.1179/016164102101199576. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M. In: Thalamus. Steriade M, Jones E, McCormick D, editors. Amsterdam: Elsevier; 1997. pp. 721–742. [Google Scholar]
- 5.Van der Werf YD, Witter MP, Groenewegen HJ. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 6.Schiff ND, Purpura KP. Thalamus Relat Syst. 2002;2:51–69. [Google Scholar]
- 7.Schiff ND, Plum F. J Clin Neurophysiol. 2000;17:438–452. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Llinas RR, Leznik E, Urbano FJ. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrera DG, Robertson HA. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Sandner G, Oberling P, Silveira MC, Di Scala G, Rocha B, Bagri A, Depoortere R. Behav Brain Res. 1993;58:9–18. doi: 10.1016/0166-4328(93)90086-6. [DOI] [PubMed] [Google Scholar]
- 12.Mair RG. Rev Neurosci. 1994;5:105–140. doi: 10.1515/revneuro.1994.5.2.105. [DOI] [PubMed] [Google Scholar]
- 13.McCormick DA, von Krosigk M. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. J Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JL, Everitt BJ, Thomas KL. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 16.Malkani S, Rosen JB. Brain Res. 2000;860:53–63. doi: 10.1016/s0006-8993(00)01976-4. [DOI] [PubMed] [Google Scholar]
- 17.Bowman RE, Beck KD, Luine VN. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 18.Pfaff D, Westberg L, Kow LM. J Comp Neurol. 2005;493:86–91. doi: 10.1002/cne.20720. [DOI] [PubMed] [Google Scholar]
- 19.Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, Korach KS, Ogawa S, Pfaff DW. Proc Natl Acad Sci USA. 2003;100:11019–11022. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. J Neurosci. 2003;23:8743–8751. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaff DW. Brain Arousal & Information Theory: Neural, Genetic and Hormonal Analyses. Cambridge, MA: Harvard Univ Press; 2005. [Google Scholar]
- 23.Moruzzi G, Magoun HW. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 24.Glenn LL, Steriade M. J Neurosci. 1982;2:1387–1404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steriade M, Glenn LL. J Neurophysiol. 1982;48:352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- 26.Plum F, Posner J. The Diagnosis of Stupor and Coma. Philadelphia: F.A. Davis; 1980. [Google Scholar]
- 27.Tononi G, Cirelli C. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Pare D, Smith Y, Parent A, Steriade M. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 29.Kolmac C, Mitrofanis J. Neurosci Lett. 1999;272:151–154. doi: 10.1016/s0304-3940(99)00614-x. [DOI] [PubMed] [Google Scholar]
- 30.Krout KE, Belzer RE, Loewy AD. J Comp Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- 31.Burk JA, Mair RG. Behav Brain Res. 2001;123:49–63. doi: 10.1016/s0166-4328(01)00202-9. [DOI] [PubMed] [Google Scholar]
- 32.Pfaff D. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard Univ Press; 2005. [Google Scholar]
- 33.Steriade M, Contreras D, Amzica F, Timofeev I. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahnemann D. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- 35.Volkmann J, Herzog J, Kopper F, Deuschl G. Mov Disord. 2002;17(Suppl 3):S181–S187. doi: 10.1002/mds.10162. [DOI] [PubMed] [Google Scholar]
- 36.McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Cereb Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- 37.Jones EG. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 38.Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.