Abstract
Graft-versus-host disease (GVHD) represents a major cause of morbidity and mortality following conventional allogeneic hematopoietic stem cell transplantation (HSCT). A study in mice (see related article on pages 101–108) demonstrates that the selective administration of donor memory CD4+ T cells results in immune reconstitution without GVHD, a result that, if translatable to humans, has important clinical implications for HSCT.
Clinical transplantation of hematopoietic stem cells (HSCs) was first successful in 1968 (1, 2) and has provided life-saving therapy for potentially fatal diseases. These include acquired marrow aplasia, inherited dysfunction of hematopoietically-derived elements, and the iatrogenic marrow failure caused by supra-lethal chemo- or radiotherapy in the treatment of neoplasms. The goal of HSCT is stable engraftment of donor-derived hematopoietic cells, allowing them to differentiate and function normally, while not causing destruction of host tissues by donor-derived immune cells, resulting in GVHD.
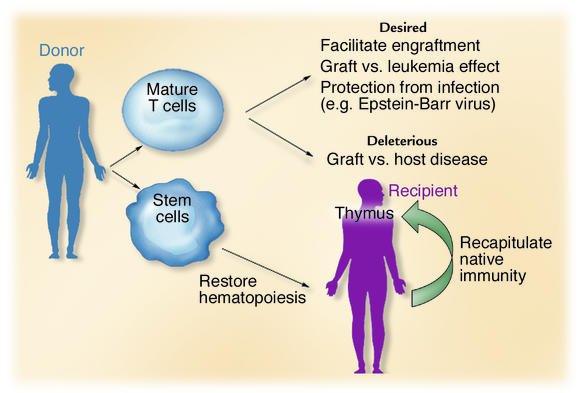
The presence of minor histocompatibility antigen (miHA) disparities between allogeneic individuals may induce GVHD, even when donor and recipient are matched at all MHC loci. Immunosuppressive drugs given after HSCT, or T cell depletion of the graft, can decrease the incidence and severity of GVHD (3). However, in the absence of mature T cells, many months are required to re-create an intact T cell immune system (4), leaving the recipient at great risk for opportunistic infection while a new T cell system arises from HSCs and undergoes thymic education (Figure 1). The ability to infuse donor T cells that protect against infection and provide an antineoplastic effect, while excluding those T cells responsible for damaging normal tissues (observed in GVHD), would have important clinical implications.
Figure 1.
The donor graft contains both mature immune cells and primitive HSCs. T cells facilitate engraftment, protect against infection, and mediate an antileukemic effect. However, they may also initiate GVHD, a potentially fatal transplant complication. HSCs restore hematopoiesis and native immunity, the latter process requiring education of immune cells in the host thymus.
Memory T cells do not cause GVHD in a miHA-disparate murine model
In this issue of the JCI, Anderson et al. (5) report on the coinfusion of purified CD4+ memory T cells in an MHC-matched, miHA-disparate, T cell–depleted murine HSCT model. Their results conclusively show that unpurified CD4+ T cells cause GVHD, while the memory cells (defined here as CD44+CD62L–CD4+ T cells) do not. Furthermore, memory CD4+ T cells taken from a donor immunized to chicken γ-globulin (CGG) retain their CGG-specific memory when the recipient of those memory T cells is immunized with CGG. In other words, these CD44+CD62L–CD4+ memory T cells remember how to respond to antigens to which they were primed but have “forgotten” how to react to allogeneic miHAs that are the targets of GVHD mediated by unfractionated or naive CD4+ T cells.
Applicability to other strain combinations
This finding provides some insight on the nature of alloreactivity. In this HSCT model, the CD4+ T cells responsible for GVHD are not in the CD44+CD62L–CD4+ population (5). As such, the components of the T cell receptor (TCR) repertoire that recognize the miHAs may not be in the CD44+CD62L–CD4+ memory T cell population. If so, the present study would suggest that the degeneracy of TCR recognition (6) does not extend to a cross-reactivity between the environmental antigens to which the donor’s T cells have been primed and the miHAs on allogeneic tissues of the host in this model system. It is essential that the GVHD-inducing potential of CD44+CD62L–CD4+ memory T cells be examined in a variety of other miHA- and MHC-mismatched donor-host murine strain combinations. It seems likely that CD44+CD62L–CD4+ memory T cells will still mediate GVHD in MHC-disparate hosts. This is because of the strength of the spontaneous T cell response to MHC-incompatible tissue and the postulated immunologic cross-reactivity between allogeneic MHC molecules and environmental antigens being presented by autologous MHC molecules (7).
Effector capabilities of CD44+CD62L–CD25–CD4+ memory T cells
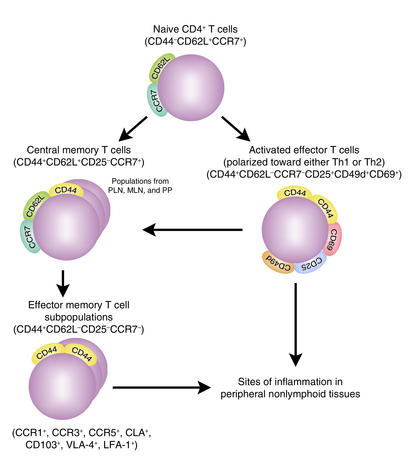
Alternatively, it remains possible that the CD44+CD62L–CD4+ memory T cells retain their ability to recognize miHAs but are missing the ability to mediate important effector functions involved in the initiation of GVHD. Using the landmark studies and memory cell differentiation pathways described by Lanzavecchia, Sallusto, and colleagues (8, 9), the population of CD44+CD62L–CD25–CD4+ memory T cells selectively infused by Anderson et al. (5) would be designated “effector memory cells” (Figure 2, lower left). These differ from the “activated effector cells” (Figure 2, upper right) by their less frequent expression of activation markers (i.e., CD25, CD49d, and CD69), and they differ from the central memory cells (Figure 2, upper left) by their loss of CD62L (L-selectin) and loss of CCR7, a chemokine receptor that facilitates homing to central lymphoid tissues. Thus the CD44+CD62L–CD4+ effector memory T cells are considered to be terminally differentiated cells that mediate some effector functions in the peripheral tissues but do not readily proliferate and do not induce central lymphoid activation (8, 10). In the absence of the other three T cell populations shown in Figure 2, these cells might not be potent inducers of tissue destruction because of their in vivo pattern of distribution (8–10), or the nature of the cytokines they release. These CD44+CD62L–CD4+ effector memory T cells appear to secrete more IFN-γ, IL-4, and IL-10 (inhibitory cytokines), and less IL-2 (an activating cytokine), than do central memory T cells (10).
Figure 2.
Stepwise differentiation of CD4+ T cells from naive CD4+ T cells to activated effector CD4+ T cells, and then to subsets of memory CD4+ T cells. These are divided into central memory CD4+ T cells (CD44+CD62L+CD25–CCR7+) and effector memory CD4+ T cells (CD44+CD62L–CD25–CCR7–). CD44+CD62L–CD25–CCR7–CD4+ effector memory T cells represent a pool of terminally differentiated cells with heterogeneous immunophenotypes that express multiple patterns of chemokine receptors (i.e., CCR1, 3, or 5), tissue-specific homing receptors (i.e., CD103 and the cutaneous lymphocyte antigen [CLA]), and adhesion molecules (i.e., leukocyte function–associated antigen-1 [LFA-1] and very late antigen-4 integrin [VLA-4]), facilitating migration of these cells into different peripheral nonlymphoid tissues. The unique expression pattern and level of chemokine receptors, homing receptors, and adhesion molecules depend on the lymphoid origin (i.e., peripheral lymph nodes [PLNs], mesenteric lymph nodes [MLNs], or Peyer’s patches [PPs]) of the effector memory T cells and the duration of TCR and cytokine stimulation. These features determine the migratory and homing preferences and functional capabilities of CD44+CD62L–CD25–CCR7–CD4+ effector memory T cells.
Therefore the demonstration of retained CGG-induced proliferation by donor-derived reconstituted CD44+CD62L–CD4+ effector memory T cells in the host (5) does not necessarily translate to retention of protective immunity. It will be important to determine whether the retained CD44+CD62L–CD4+ effector memory T cells from a donor immunized to pathogenic infection can mediate protective immunity to a challenge of the pathogen after HSCT (11, 12).
Presently, the major indication for allogeneic HSCT is the treatment of chronic myeloid leukemia and high-risk acute leukemias. The importance of a graft-versus-leukemia (GVL) effect (13, 14) has been documented by the higher risk of leukemic recurrence after T cell–depleted HSCTs, and the successful treatment of post-HSCT relapse with donor lymphocyte infusions (15). Retaining the GVL effect while eliminating GVHD has been an elusive clinical goal. Additional testing is needed to determine whether CD44+CD62L–CD4+ effector memory T cells might retain GVL activity, and whether specific immunization strategies of the donor might enhance transfer of protective immunity and GVL.
Translation to clinical testing
Finally, as pointed out by the authors (5), “if these murine results are applicable to human alloSCT, selective administration of memory T cells could greatly improve post-transplant immune reconstitution.” Before this is attempted clinically, murine CD44+CD62L–CD4+ effector memory T cells still need to be tested in other strain combinations, evaluated for transfer of protective immunity, and tested for GVL potential. In addition, the immune capabilities of human CD44+CD62L–CD4+ effector memory T cells must be studied in vitro and in adoptive transfer models (i.e., in immunodeficient or humanized mice) to determine whether their behavior parallels that of murine CD44+CD62L–CD4+ T cells.
There is still much to do before we can forget about GVHD. The work of Anderson et al. (5) may be an important step toward that goal.
Acknowledgments
I.N. Buhtoiarov is supported by an International Union against Cancer–American Cancer Society international fellowship for beginning investigators.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: graft-versus-host disease (GVHD); hematopoietic stem cell (HSC); HSC transplantation (HSCT); minor histocompatibility antigen (miHA); chicken γ-globulin (CGG); T cell receptor (TCR); graft-versus-leukemia (GVL).
References
- 1.Bach FH, Albertini RJ, Joo P, Anderson JL, Bortin MM. Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet. 1968;2:1364–1366. doi: 10.1016/s0140-6736(68)92672-x. [DOI] [PubMed] [Google Scholar]
- 2.Hong R, et al. Immunological restitution in lymphopenic immunological deficiency syndrome. Lancet. 1968;1:503–506. doi: 10.1016/s0140-6736(68)91468-2. [DOI] [PubMed] [Google Scholar]
- 3.Trigg ME, et al. Clinical trial depleting T lymphocytes from donor marrow for matched and mismatched allogeneic bone marrow transplants. Cancer. Treat. Rep. 1985;69:377–386. [PubMed] [Google Scholar]
- 4.Mackall C, Gress R. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol. Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson BE, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J. Clin. Invest. 2003;112:101–108. doi:10.1172/JCI200317601. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leng Q, Bentwich Z. Beyond self and nonself: fuzzy recognition of the immune system. Scand. J. Immunol. 2002;56:224–232. doi: 10.1046/j.1365-3083.2002.01105.x. [DOI] [PubMed] [Google Scholar]
- 7.Mandell RB, Sondel PM. Allele-specific alien-driven diversity, immune responsiveness and MHC-restriction. Immunol. Today. 1985;6:321–323. doi: 10.1016/0167-5699(85)90126-4. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia A, Sallusto F. Dynamics of T cell lymphocyte responses: intermediates, effectors and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Lenig D, Reihold F, Lipp M, Lanzavecchia A. Two subsets of memory T cells with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 10.Hengel RL, et al. Cutting edge: L-selectine (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J. Immunol. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Hogan RJ, et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lung. J. Immunol. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen P, Smedegaard B. CD4+ T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect. Immun. 2000;68:621–629. doi: 10.1128/iai.68.2.621-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz MM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 14.Sondel, P.M. 2000. The graft vs. leukemia effect. In Allogeneic immunotherapy for malignant diseases. J. Barrett and Y.Z. Jiang, editors. Marcel Dekker Inc. New York, New York, USA. 1–12.
- 15.Slavin S, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87:2195–2204. [PubMed] [Google Scholar]