Abstract
Recently, decreased activity levels have been observed in pigs treated postoperatively with transdermal delivery of fentanyl (TD-fentanyl) after isoflurane anaesthesia. Whether the change in behaviour is related to opioid-induced sedation or to insufficient pain relief remains to be investigated. This study was therefore undertaken to evaluate the effect of TD-fentanyl 50 μg h-1 on the activity level with and without isoflurane anaesthesia. Eight pigs (25.4 ± 5.2 kg) were submitted to a cross-over study and given two treatments; 1) fentanyl patch applied after 30 minutes of anaesthesia (treatment A/F) and 2) fentanyl patch without anaesthesia (treatment F). The pigs' behaviour was observed from a video recording instantaneously every 10 minutes for 24 h before treatments and up to 72 h after the patch attachment. Venous blood samples were taken 1, 6, 12, 24, 48 and 72 h after the patch application. The behaviour recordings showed that TD-fentanyl did not produce sedation in any pig. No differences were found between the two treatments in activity level, weight gain or serum fentanyl concentration. This concentration measured after 24 h was 0.27 ± 0.11 ng ml-1 and 0.47 ± 0.40 ng ml-1 in the A/F and F group, respectively.
In conclusion, transdermal delivery of 50 μg h-1 fentanyl did not cause inactivity in growing pigs. However, the large variations in serum fentanyl concentration indicate that drug absorption from transdermal patches is unpredictable and sometimes deficient.
Keywords: swine, analgesia, opioids, patch, behaviour, pain, sedation, activity
Introduction
Opioids have short serum half-lives in pigs, leading to a need for repeated restraint and drug administration to achieve adequate analgesia [11]. Both restraint and injections are potential acute stressors in pigs and may result in physiological and behavioural changes. These changes can influence animal research outcomes, as stress factors have been reported to cause a rise in plasma catecholamines (i.e. epinephrine and norepinephrine), β-endorphin and cortisol in pigs [23,9]. It is therefore important to study different methods to administer drugs to pigs in an attempt to decrease the number of restraints and the need for animal handling during the postoperative period.
In veterinary medicine, the main routes of drug administration are oral, intramuscular, subcutaneous and intravenous. Recently, transdermal patches have been considered as an alternative method of drug application in animals [21,16]. One of the advantages of transdermal patches is that they can minimise the risk of adverse effects of the drug by decreasing large fluctuations in plasma concentration [3].
Fentanyl is a synthetic opioid at least 75–100 times more potent than morphine and has been selected over other opioids for use as a transdermal delivery system both for pre-emptive and postoperative analgesia in humans [1,5], dogs [20,19], cats [4], pigs [27,11], goats [3] and horses [28]. Also, efficient transdermal delivery of fentanyl (TD-fentanyl) in pigs can provide continuous and systemic delivery of fentanyl up to three days per patch application [11,30,28]. However, there is still a lack of information about the optimal dosing and the analgesic and behavioural effects of fentanyl when given as a transdermal patch to pigs [24,30]. In an attempt to elucidate these issues, Malavasi and co-workers (2004) studied the effect of TD-fentanyl on the activity level in pigs after abdominal surgery. In addition to the analgesia provided with medetomidine and tiletamine/zolazepam anaesthesia, fentanyl patches were used postoperatively. During this period, the activity level decreased significantly compared to preoperative recordings. This observed inactivity could either be associated with sedation produced by the opioid or to insufficient pain relief. Fentanyl is a full opioid agonist that acts mainly on mu-receptors, which are responsible for producing analgesia as well as having side effects e.g. respiratory depression [18,17,20,2]. Additionally, fentanyl is known to produce narcosis in dogs, rabbits, rats and primates. In contrast, excitement is reported in horses and mice [17].
In the present study we have tested the hypothesis that growing pigs treated with a fentanyl patch delivering a dose of 50 μg h-1 will display inactivity due to the opioid effect. Further, there is a possibility that inhalation anaesthesia can influence the serum fentanyl concentration [4,19]. Thus, the aim of the present study was to video-record and analyse behaviour pattern, and assay serum fentanyl by gas chromatography in a longitudinal cross-over study. It was considered that any interaction between fentanyl and isoflurane, as observed previously in studies on dogs and cats [4,19], could be identified in this way.
Materials and methods
Animals
Eight crossbreed pigs (Swedish Landrace × Yorkshire) from a conventional gilt-producing herd were purchased from a farm which has been under our swine specialist supervision for many years. On arrival, the pigs were five weeks old and clinically healthy, and there were an equal number of males and females. They were housed at the Department of Clinical Sciences, in individual pens (approximately 3.5 m2 each) with a solid concrete floor and straw as bedding. They were kept within sight and sound of one another with a light regime of 8 h light/16 h dark. The pigs were fed twice daily (8:00 h and 15:00 h) with a commercial finisher diet (Singel Flex®, Odal, Sweden) and had free access to water. They were allowed a five-week acclimatisation period. When the experiment started the animals weighed 25.4 ± 5.2 kg (mean ± SD).
The experimental protocol was approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden.
Anaesthesia and analgesia
Before anaesthesia the pigs were fasted for 12 h but supplied with water ad libitum. The total duration of the experiment was three weeks: two weeks of treatment (week 1 and week 3) and one week of washout in between (week 2). The animals were randomly chosen in pairs for a cross-over test in which the same animal would receive one treatment during week 1 and the other treatment during week 3. The treatments with TD-fentanyl were: 1) one fentanyl patch (50 μg h-1; Durogesic, Janssen-Cilag AB, Sollentuna, Sweden) for 60 h applied after 30 minutes of general anaesthesia (treatment A/F); and 2) one fentanyl patch for 60 h without general anaesthesia (treatment F). The plasters were attached to the skin behind the ear. This skin area was shaved with care to avoid abrading the skin but it was not washed prior to attachment of the patch. The attachment procedure was in accordance with the manufacture's recommendations for humans. In order to protect the patches and prevent their being rubbed off, a piece of canvas was sutured onto the skin and checked at least twice a day. In the awake pigs, a local anaesthetic (EMLA cream; AstraZeneca Läkemedel AB, Södertäjle, Sweden) was applied to the suture sites on the skin approximately 60 minutes prior to the suturing of the canvas.
Anaesthesia was induced with a combination of medetomidine hydrochloride at a dosage of 0.05 mg kg-1 (Domitor®vet 1 mg ml-1; Orion, Espoo, Finland) and tiletamine-zolazepam (Zoletil forte vet; Virbac, Carros, France) at a dosage of 5 mg kg-1 (2.5 mg kg-1 of zolazepam and 2.5 mg kg-1 of tilazamine), given intramuscularly. Ten minutes after injection of the anaesthetic drug, the animal was intubated with an endotracheal tube 6–8 mm in diameter for maintenance of inhalation anaesthesia with 1% isoflurane during 30 minutes (Isoflo™vet; Schering-Plough, Kent, UK), supplied with oxygen and air (vaporizer Isotec 5; Datex-Ohmeda, Helsinki, Finland). At the end of the general anaesthesia the fentanyl patch was attached to the skin of the pig.
During the fentanyl patch treatments, all pigs were clinically examined at least twice a day, with special regard to any physiological side effects or manifestations of narcotisation.
Fentanyl measurement
Blood samples (4 mL) were collected from the external jugular vein into vacutainer tubes without additives at six different time points: 1, 6, 12, 24, 48 and 72 h after application of the fentanyl patch. The animals were restrained with a nose twitch during the blood sampling procedure. Serum was separated by centrifugation at + 4°C within ten minutes after blood sampling, and stored at -70°C until analysed at the University Hospital of Linköping. The serum fentanyl concentration was measured by gas chromatography with mass-selective detection as described by [27]. Fentanyl was extracted from 1 mL of serum by using 2-octanol, and deuterium-labelled fentanyl was used as internal standard (Fentanyl-D5). This method was calibrated to detect fentanyl concentrations above 0.05 ng ml-1.
Ethogram
An ethogram based on earlier observations of four pigs of the same age, breed and weight, was used [16]. The animal's spontaneous behaviour was recorded and the different activities were divided into active and inactive behaviours. The following behaviours were regarded as active: standing up, walking, running, jumping, interacting with blanket or straw, rooting, eating and drinking. The inactive behaviours comprised: lying down quietly, lying down agitatedly and sitting position.
To minimize human disturbance on the pig behaviour, animal care taking, feeding and videotape changing were executed daily at same time (8:00 h and 15:00 h).
Behavioural recordings
Each animal was videotaped for a total of 168 hours. The recordings covered the periods 24 hours before the treatments and 72 hours after each treatment, beginning when the animal was left alone in its pen after completion of the treatment. Two black and white video cameras with a wide-angle lens (Computar CE IP66, Italy) were positioned approximately one meter in front of each pen. Behaviour was recorded with a time-lapse video cassette recorder (Panasonic, AG-TL350) and a video multiplexer (Panasonic, WJ-FS409). The picture-sampling interval was 0.18 second, and the time code in hours/minutes/seconds was recorded on the tape.
A researcher, blinded to the treatments, watched all videotapes and manually recorded the pig's behaviour, using instantaneous sampling at intervals of ten minutes troughout the 168 h period.
Body weight
The pigs were weighed before the treatments and once a day for three consecutive days after the treatments. The weighing procedure was performed before the first feeding time and a wooden box was used to restrain the pigs. The box was positioned in front of the door of the animal pen and the pig was oriented to walk to the inside of the box. When the pig had entered the box this was lifted onto a digital scale and the body weight was measured. The entire procedure was carefully executed to avoid excessive animal stress.
Statistical analysis
The data from the pre-treatment period were used as control data. The activity/inactivity levels and each specific activity were analysed by repeated measurements ANOVA. The weight gains were calculated as the difference between the pre-treatment value and the values obtained 24, 48 and 72 hours after the application of the patch and analysed with repeated measurements ANOVA. Throughout the study, serum fentanyl values were analysed by a General Linear Model. All results were produced by statistical software (Minitab, Inc., Coventry, UK.) and presented as mean values ± SD. Statistical significance was defined as p < 0.05.
Results
Behavioural observations
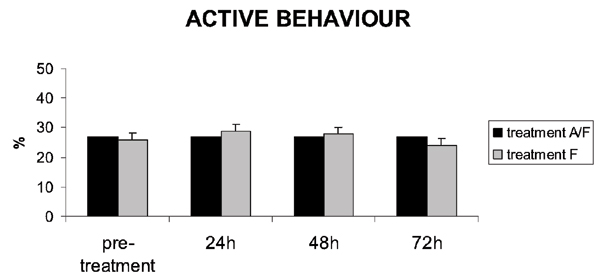
The activity level in all eight pigs during the pre-treatment period was 27 ± 6%. Throughout the experiment, comprising week 1 and week 3, pigs from treatment group A/F had an activity level of 27 ± 0.2% and pigs from treatment group F had an activity level of 27 ± 2%. No significant difference in activity level was found between treatment groups or between time points (Fig. 1). Among all pigs treated, the TD-fentanyl did not induce any signs of narcotisation. The percentage of each specific activity did not differ significantly between groups or between time points. For instance, eating behaviour in treatment group A/F was 10 ± 3% on the pre-treatment day, 9 ± 2% 24 h after the patch application, 8 ± 2% at 48 h and 8 ± 2% at 72 h. In treatment group F, the eating behaviour was 10 ± 3% on the pre-treatment day, 9 ± 3% 24 h after the patch application, 8 ± 3% at 48 h and 7 ± 12% at 72 h.
Figure 1.
Percentage (mean ± SD) numbers of observations of active behaviours in pigs treated with a fentanyl patch with (treatment A/F) and without isoflurane anaesthesia (treatment F), during the pre-treatment day and 24, 48 and 72 h after application of the patch. (n = 8)
Both treatment groups showed a similar weight gain throughout the experiment.
Fentanyl measurement
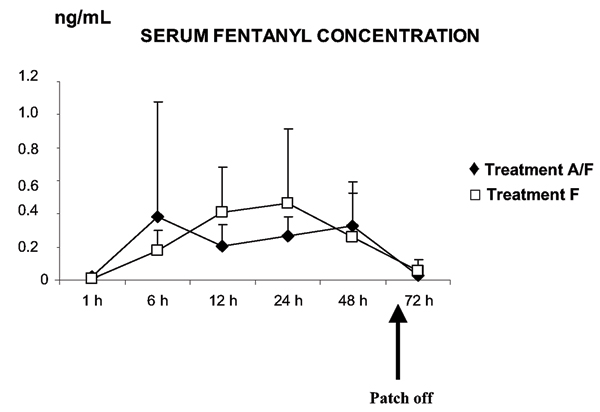
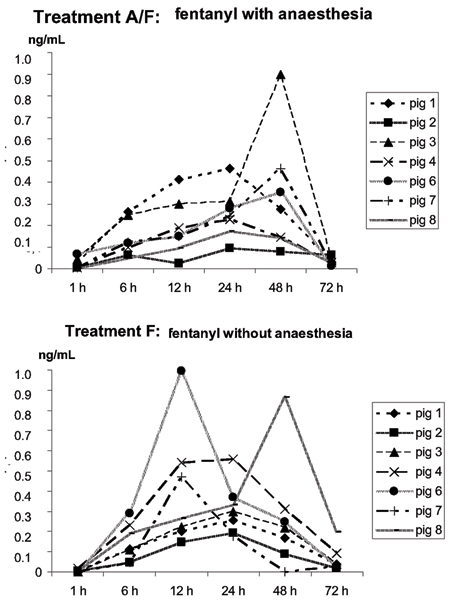
There were no significant differences in mean serum fentanyl concentrations between the treatment groups as shown in Figure 2. The serum fentanyl range 24 h after the patch application in treatment group A/F was 0.09–0.46 ng mL-1 and in treatment group F 0.17–1.00 ng mL-1. Transdermal delivery of fentanyl resulted in large interindividual differences in serum concentrations (Fig. 3). Pig 5 was excluded from this analysis because of unreliable serum values.
Figure 2.
Mean serum fentanyl concentrations ± SD (ng/mL) in pigs treated with a fentanyl patch with (treatment A/F) and without isoflurane anaesthesia (treatment F). Values obtained 1, 6, 12, 24, 48 and 72 hours after the patch application are shown. The fentanyl plasters were taken off 60 hours after application. (n = 8)
Figure 3.
Serum fentanyl concentrations (ng/mL) in individual pigs treated with a fentanyl patch with (treatment A/F) and without isoflurane anaesthesia (treatment F). Values obtained 1, 6, 12, 24, 48 and 72 hours after the patch application are shown. (n = 7)
Discussion
Transdermal delivery of fentanyl at 50 μg h-1 in pigs weighing 25 kg was not associated with any measurable behavioural consequences. All pigs displayed a constant activity level of approximately 27%, which was significantly higher than the activity levels of 16% in pigs treated with TD-fentanyl postoperatively [16]. In that study the activity level of pigs treated with postoperative TD-fentanyl as additional analgesia to medetomidine and tiletamine/zolazepam was compared with the behaviour recorded during the pre-operative day. The lower activity level could thus be related to insufficient pain relief or sedation caused by the opioid.
Regarding the isoflurane factor in the present study, this inhalatory drug is only metabolised to a minor extent [10]. However, it may affect hepatic function and blood flow, hence interfering with the clearance of fentanyl, which largely takes place in the liver [19]. Inhalant anaesthetics may increase the vascular uptake of fentanyl from the dermal depot, as a result of the peripheral vasodilatation induced by these agents [4,19]. Also, induction agents such as medetomidine and tiletamine/zolazepam produce cardiovascular changes which may have an effect on the uptake of transdermal drugs. Medetomidine has been reported to cause vasoconstriction in cats [8] and dogs [14], and tiletamine/zolazepam has been reported to have direct vasodilatory effect in cats [12] and dogs [13]. However, neither the average serum fentanyl concentration nor the activity level was affected by the isoflurane variable during the experiment in the present study.
Additionally to the behavioural observations, the weight of the pigs was measured daily throughout the experiment. In the present study, the pigs from both treatment groups exhibited a weight gain at least similar to that of conventional herds [29]. A quick return to homeostasis is crucial for a fast recovery, as it allows initiation of the wound-healing process [25]. Hence, the fact that the pig is gaining weight can be correlated to the acquisition of energy for homeostasis and may be a useful indicator of post surgical pain [15].
In the present study, no true steady state of fentanyl concentration was achieved in either of the treatment groups, on account of the high individual variability and the absence of a steady continuity in fentanyl concentration. This variability is in accordance with the interindividual variation described in dogs [19], cats [4], horses [28] and goats [3]. Various suggestions have been made in attempts to explain the variability in plasma fentanyl concentrations, such as drug release from the patch, variable absorption across the epidermis and dermis, variable uptake by the cutaneous vasculature, and drug distribution depending on the animal's volume and metabolic clearance [3,21,4]. The serum fentanyl concentration required for optimal analgesia in pigs is not yet known. However, the level producing analgesia in humans (0.5 to 2.0 ng mL-1) is commonly extrapolated to animal species. In cats subjected to onychectomy, fentanyl patches delivering 25 μg h-1 have been compared with intramuscular administration of butorphanol. Together with evaluation of clinical variables (e.g. appetite, heart rate and respiratory rate) and subjective evaluation of response to handling the feet, the researchers concluded that serum concentrations ranging from 0.3 to 7.0 ng mL-1 were associated with an analgesic effect [6]. In dogs undergoing abdominal surgery, fentanyl patches delivering 25 and 50 μg h-1 produced serum levels ranging from 0.11 to 1.08 ng mL-1; on the basis of a Simple Descriptive Scale pain assessment method, the authors considered that all animals achieved an analgesic level [7]. In domestic pigs with a body weight of 25–30 kg, [11] found that 50 μg h-1 fentanyl patches after thoracotomy resulted in serum concentrations ranging from 0.3 to 0.6 ng mL-1, which they associated with adequate analgesia. In the present study, 50 μg h-1 fentanyl patches in growing pigs yield serum concentrations in the range of 0.01 to 0.99 ng mL-1. These levels did not produce any behavioural effect in our pigs. However, in a previous study by [16], pigs treated with TD-fentanyl after abdominal surgery showed decreased activity postoperatively compared to the preoperative behavioural. Taken this into consideration, we conclude that fentanyl patches delivering 50 μg h-1 might not be reliable for producing analgesia in growing pigs. Allowing for variation with factors such as the invasiveness of surgery, severity of pain and morbidity, and physical condition of the animal, TD-fentanyl analgesia will require supplementation with parenteral opioids, local analgesics (e.g. epidural morphine) and non-steroidal anti-inflammatory drugs (NSAIDs). Further studies regarding higher dosage and patch technology may improve transdermal delivery of fentanyl in pigs.
The site of patch attachment, behind the ear, in the current study was chosen because of the smaller amount of fat and the high blood flow in this area, and it is expected that the fentanyl would have been efficiently absorbed [21]. However, during the experiment, a few patches were found to be attached inappropriately to the skin. Consequently, the absorption of fentanyl would be interrupted and the serum level would decrease, as mentioned in the literature [11,21]. Thus, despite the canvas used in the present study to protect against moisture, dust and rubbing off of the patch, it is recommended that an occlusive dressing over the patch/canvas is placed to ensure good skin attachment. Secure placement of the TD-fentanyl patch is also of importance in view of the danger of accidental ingestion, which could result in intoxication of the animal and gastro-intestinal obstruction [26,22].
In conclusion, transdermal delivery of 50 μg h-1 fentanyl in growing pigs did not cause inactivity. However, the large variation in serum fentanyl concentrations indicates unpredictable or possibly deficient drug absorption from transdermal patches when applied according to the manufacturer's instructions for humans. Further studies under clinical conditions are therefore required before transdermal delivery of fentanyl in pigs can be recommended for postoperative treatment of pain.
Acknowledgments
Acknowledgements
The Swedish Research Council (VR) and the Swedish Research Council for the Environment, Agricultural Sciences and Spatial Planning (FORMAS) supported this study financially. The authors also thank Dr. Arne Eklund (University Hospital of Linköping) for measuring the serum fentanyl concentrations and Patrik Öhagen at the Department of Clinical Sciences (SLU) for statistical assistance.
References
- Bowdle TA. Adverse effects of opioid agonists and Agonist-Antagonists in anaesthesia. Drug Safety. 1998;19(3):173–189. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- Branson KR, Gross ME. Opioid agonists and antagonists. In: Adams HR, editor. Veterinary Pharmacology and therapeutics. Ames: Iowa State University press; 2001. pp. 268–98. [Google Scholar]
- Carroll GL, Hooper RN, Boothe DM, et al. Pharmacokinetics of fentanyl after intravenous and transdermal administration in goats. Am J Vet Res. 1999;60(8):986–991. [PubMed] [Google Scholar]
- Egger CM, Glerum LE, Allen S, et al. Plasma fentanyl concentrations in awake cats and cats undergoing anesthesia and ovariohysterectomy using transdermal administration. Vet Anaesth Analg. 2003;30:229–236. doi: 10.1046/j.1467-2995.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- France CP, Ahn SC, Brockunier LL, et al. Behavioral effects and binding affinities of the fentanyl derivate OHM3507. Pharmacol Biochem Behav. 1998;59(2):295–303. doi: 10.1016/S0091-3057(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Gellasch KL, Kruse-Elliot KT, Osmond CS, et al. Comparison of transdermal administration of fentanyl versus intramuscular administration of butorphanol for analgesia after onychectomy in cats. J Am Vet Med Assoc. 2002;220(7):1020–1024. doi: 10.2460/javma.2002.220.1020. [DOI] [PubMed] [Google Scholar]
- Gilbert DB, Motzel SL, Das SR. Postoperative pain management using fentanyl patches in dogs. Contemp Top Lab Anim Sci. 2003;42(4):21–26. [PubMed] [Google Scholar]
- Golden AL, Bright JM, Daniel GB, et al. Cardiovascular effects of the a2-adrenergic receptor agonist medetomidine in clinically normal cats anesthetized with isoflurane. Am J Vet Res. 1998;59(4):509–513. [PubMed] [Google Scholar]
- Gonyou HW. Pig behavior and biomedical research. In: Tumbleson, Schook, editor. Advances in swine in biomedical research. Plenum Press: New york; 1996. pp. 485–490. [Google Scholar]
- Harvey CR, Walberg J. Principles and Practice of Veterinary Anesthesia. Williams and Wilkins:Baltimore; 1987. Special considerations for anesthesia and analgesia in research animals; pp. 380–394. [Google Scholar]
- Harvey-Clark CJ, Gilespie K, Riggs KW. Transdermal fentanyl compared with parenteral buprenorphine in post-surgical pain in swine: a case study. Lab Anim. 2000;34:386–398. doi: 10.1258/002367700780387750. [DOI] [PubMed] [Google Scholar]
- Hellyer P, Muir WW, III, Hubbell JA, et al. Cardiorespiratory effects of the intravenous administration of tiletamine-zolazepam to cats. Vet Surg. 1988;17(2):105–110. doi: 10.1111/j.1532-950x.1988.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Hellyer P, Muir WWW, III, Hubbell JA, et al. Cardiorespiratory effects of the intravenous administration of tiletamine-zolazepam to dogs. Vet Surg. 1989;18(2):160–165. doi: 10.1111/j.1532-950x.1989.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Keegan RD, Greene SA, Bagley RS, et al. Effect of medetomidine administration on intracranial pressure and cardiovascular variables of isoflurane-anesthetized dogs. Am J Vet Res. 1995;56(2):193–198. [PubMed] [Google Scholar]
- Liles JH, Flecknell PA. The effects of buprenorphine, nalbuphine and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movements in rats. Lab Anim. 1992;26:180–189. doi: 10.1258/002367792780740558. [DOI] [PubMed] [Google Scholar]
- Malavasi LM, Nyman G, Augustsson H, et al. Preoperative epidural morphine and postoperative transdermal fentanyl analgesia for abdominal surgery in pigs: a behavioral study. Unpublished. 2004.
- Martin RF. An introduction to veterinary pharmacology. 4. Longman: London; 1985. Depressants of the CNS; Narcotics; anticonvulsants; analgesics; pp. 130–155. [Google Scholar]
- Nolan AM. Pain management in animals. W. B. Saunders, London, UK; 2001. pp. 21–52. [Google Scholar]
- Pettifer GR, Hosgood G. The effect of inhalant anesthetic and body temperature on peri-anesthetic serum concentrations of transdermally administered fentanyl in dogs. Vet Anaesth Analg. 2004;31:109–120. doi: 10.1111/j.1467-2987.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM. Pharmacology. 3. Churchill Livingstone, London, UK; 1996. pp. 609–633. [Google Scholar]
- Riviere JE, Papich MG. Potential and problems of developing transdermal patches for veterinary applications. Adv Drug Deliv Rev. 2001;50:175–203. doi: 10.1016/S0169-409X(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Taylor PM. Pain management in cats – past, present and future. Part 2. Treatment of pain-clinical pharmacology. J Feline Med Surg. 2004;0:1–13. doi: 10.1016/j.jfms.2003.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen AWM, Tsuma VT, Magnusson U. Effects of short-term restraint stress on plasma concentrations of catecholamines, β-endorphin, and cortisol in gilts. Am J Vet Res. 1995;56(9):1225–1227. [PubMed] [Google Scholar]
- Sanford J, Ewbank R, Molony V, et al. Guidelines for the recognition and assessment of pain in animals. Vet Rec. 1986;118:334–338. doi: 10.1136/vr.118.12.334. [DOI] [PubMed] [Google Scholar]
- Short CE. Textbook of pain. Edinburgh: Churchill Livingstone, UK; 1999. pp. 1007–15. [Google Scholar]
- Swindle MM. Technical bulletin: Anesthesia, analgesia andperioperative techniques in swine. 2002. http://www.sinclairresearch.com
- Szeit A, Riggs KW, Harvey-Clark C. Sensitive and selective assay for fentanyl using gas chromatography with mass selective detection. J Chromatogr B Biomed Appl. 1996;675:33–42. doi: 10.1016/0378-4347(95)00350-9. [DOI] [PubMed] [Google Scholar]
- Thomasy SM, Slovis N, Maxwell LK, et al. Transdermal fentanyl combined with nonsteroidal anti-inflammatory drugs for analgesia in horses. J Vet Intern Med. 2004;18:550–554. doi: 10.1892/0891-6640(2004)18<550:TFCWNA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wallgren P, Vallgårda J, Söderström P, et al. Infektioners inflytande på till-växthastigheten hos svin. Svenk Veterinärtidning. 1993;45(16):727–732. [Google Scholar]
- Wilkinson AC, Thomas ML, III, Morse BC. Evaluation of a transdermal fentanyl system in Yucatan miniature pigs. Contemp Top Lab Anim Sci. 2001;40(3):12–16. [PubMed] [Google Scholar]