Abstract
This study continues analysis from a companion paper on over 350,000 insured Swedish dogs up to 10 years of age contributing to more than one million dog-years at risk during 1995–2000. The age patterns for total and diagnostic mortality and for general causes of death (trauma, tumour, locomotor, heart and neurological) are presented for numerous breeds. Survival estimates at five, eight and 10 years of age are calculated. Survival to 10 years of age was 75% or more in Labrador and golden retrievers, miniature and toy poodles and miniature dachshunds and lowest in Irish wolfhounds (91% dead by 10 years). Multivariable analysis was used to estimate the relative risk for general and more specific causes of death between breeds accounting for gender and age effects, including two-way interactions. Older females had tumour as a designated cause of death more often than males in most breeds, but not in the Bernese mountain dog. Information presented in this and the companion paper inform our understanding of the population level burden of disease, and support decision-making at the population and individual level about health promotion efforts and treatment and prognosis of disease events.
Keywords: competing risk analysis, database, survival analysis.
Introduction
The burden of disease within the purebred dog population has become the focus of discussions on welfare considerations and with regard to the ethics of breeding practices [8]. There is a risk that breeding to eliminate specific genetic defects, without clear understanding of the inter-relationships between risk factors and disease expression could do more harm than good [6]. Improving the overall health and welfare of the dog population requires that we quantify and monitor disease and death across breeds and over time.
Identifying those breeds with significantly increased risk of certain health problems is important for dog breeders, researchers and the veterinary community. In the accompanying paper, we presented mortality rates for various breeds of insured dogs under 10 years of age [2]. Rates were presented using the exact time at risk for over 350,000 dogs contributing to over a million dog-years at risk (DYAR) during six years for total mortality (all deaths), diagnostic mortality (those deaths for which an insurance claim was processed), for deaths within various diagnostic categories and for some specific diagnoses. Breed rates for total mortality ranged from 230 to 1,574 and diagnostic mortality from 168 to 1,319 deaths per 10,000 DYAR.
In addition to mortality rates (MRs), proportional mortality estimates were presented. At the population level it was informative to see that, for example, German shepherd dogs accounted for 7% of the insured population and almost 12% of deaths. Bernese mountain dogs were over-represented in mortality at twice their occurrence in the population. Golden retrievers, on the other hand, accounted for less than 4% of the deaths while being more than 6% of the population.
Owner-pet mismatch has been suggested as an important cause of pet relinquishment. Specific information about expected longevity [7] and disease risks should be part of an informed choice for prospective dog owners, as individuals may have differing preferences and concerns. Decisions regarding highly invasive and costly veterinary care in older dogs should include consideration of both the likely duration and quality of life to be expected. Although many anecdotal reports on average age at death are available, and there are some better-quantified statistics, good estimates on rates of disease and expected longevity are not available for most breeds.
In addition to breed differences it is well recognized that the occurrence of many causes of death vary by age and other host factors. Differences between males and females for the rate of death due to certain conditions have been shown (e.g. [2]). In order to examine the combined effects of breed, gender and age, multivariable modelling techniques are useful.
This study is a further analysis of the database presented in the companion paper. The objectives of this paper are to present the age pattern for various causes of death for numerous breeds of Swedish dogs insured between 1995 and 2000, to calculate survival estimates at five, eight and 10 years of age and to present the relative risk for general and more specific causes of death between breeds accounting for gender and age effects.
Materials and methods
Data
Details on the database and analysis, including calculation of rates and proportional mortalities have been presented in the companion paper [2]. Briefly, dogs covered by life insurance from 1995 to 2000 in a Swedish animal insurance company (Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden) were included in this study. Most dogs were enrolled as puppies and life insurance was, because of the terms of the insurance policy, terminated by 10 years of age. Deaths may be claimed, in which case there is a recorded diagnosis for the cause of death or euthanasia, usually made by a veterinarian, but which might be 'dead-no diagnosis'. These deaths are included in 'diagnostic' MRs. Deaths are often recorded, even when not claimed and there is no diagnosis. Deaths with or without claims are included in 'total' MR calculations. Variables downloaded from the insurance company database were breed, date of birth, date of death, gender (male/female, not neuter status), diagnostic code for death, date when the dog entered or left the insurance program and information on the type of insurance for which the dog was enrolled.
A hierarchical diagnostic registry is used by veterinarians and at the insurance company to assign causes which are classified within organ system and process [9]. For this study, the registered causes of death were partitioned into six diagnostic categories – tumours, trauma, locomotor disorders, heart, neurological and other problems. Tumours were those diagnoses listed under process neoplastic. The diagnostic category trauma included all diagnoses that were listed as traumatic processes; locomotor disorders were all those in systems skeletal, muscles and joints, except for the traumatic or neoplastic processes. Similarly, the diagnostic category heart consisted of all heart diagnoses, and neurological disorders included all diagnoses said to emanate from the nervous system, except for those said to be neoplastic or traumatic in origin. Cases with the diagnosis epileptiforme seizures were included under the diagnostic category neurological (from system unspecified). The specific diagnosis cruciate rupture was included under the diagnostic category locomotor and gastric dilatation/volvulus was included under the diagnostic category other (both from the traumatic process). All diagnoses that did not belong in any of the preceding categories were said to be in the diagnostic category other.
Analysis
Breed-specific survival, for total and diagnostic mortality, to ages five, eight and 10 years has been estimated using Cox regression, using the baseline survival statement. There were no independent variables; each dog was entered the 1st of January 1995 or the day of enrolment. If not a case, nor censored during the year, it was censored the 31st of December 2000. For example, a dog that died at five years of age was coded as "failed" at five years of age. In addition, the survival to eight years for those alive at five, and to 10 years for those alive at eight was calculated.
The age- and breed-specific and age-, breed-and diagnostic category-specific MRs (hazards) were constructed for common and high-risk breeds (for breed groupings see table 1) using the SMOOTH macro [1], which creates age-specific hazards from the survival function computed by the SAS (SAS Institute Inc., Cary, NC, 27513, USA) procedure PHREG. The macro provides a smoothed estimate of the hazard curve using a kernel smoothing method. This involves arbitrarily setting the WIDTH parameter, which influences the degree of smoothing, to achieve a reasonable curve; in this case one-fifth of the range of event times was chosen. The mortality rates were plotted against age using different scales to adjust for marked differences across breeds. Poisson regression was used regressing total deaths, all deaths with a diagnosis and death due to specific diagnostic categories on age, gender and breed. In these analyses 20 dummy variables were created for the 20 breeds (common and high-risk) using 'other' breeds as the initial baseline. Age was entered as a continuous variable, after verifying that age could be considered adequately linearly related to the outcome. Two-way interactions were tested, one at a time, upon the unreduced main effect models. Interactions that were significant at P ≤ 0.05 were kept for further modelling. Models were reduced based on the type 3 criterion. A P-value of 0.001 was considered significant. For the gender-breed and age-breed interactions only those terms that were statistically significant were kept in the models. Model fit was inspected by plotting standardised deviance residuals against covariates and predicted values. Over-dispersion was not evaluated as each dog belonged to multiple records in the dataset. The statistical software program SAS (SAS Institute Inc., Cary, NC, 27513, USA) was used to analyse the data. The PHREG procedure was used for Cox regression and the GENMOD procedure for Poisson regression.
Table 1.
Probability of death (1-survival) using Cox regression in dogs life-insured at Agria2 during 1995–2000.
| Group Breed | Probability (%) of death by | Given survival to 5 years probability (%) of death by 8 | Given survival to 8 years, probability (%) of death by 10 | ||
| 5 years | 8 years | 10 years | |||
| CKC spaniel3a | 7 | 23 | 48 | 17 | 33 |
| German shepherd | 20 | 35 | 51 | 19 | 25 |
| Drever | 18 | 32 | 45 | 17 | 19 |
| Dachshund | 9 | 19 | 28 | 11 | 11 |
| Labrador retriever | 7 | 14 | 25 | 8 | 13 |
| Springer spaniel3b | 9 | 18 | 29 | 10 | 13 |
| Mongrel | 16 | 26 | 35 | 12 | 12 |
| Golden retriever | 7 | 13 | 22 | 7 | 10 |
| Poodle (min/toy) | 10 | 17 | 25 | 8 | 10 |
| Min dachshund3c | 9 | 17 | 25 | 9 | 10 |
| Total COMMON4 | 12 | 23 | 35 | 13 | 16 |
| Irish wolfhound | 28 | 63 | 91 | 49 | 76 |
| St Bernard | 30 | 52 | 74 | 31 | 46 |
| Great Dane | 28 | 59 | 83 | 43 | 59 |
| Bernese mtn dog3d | 17 | 45 | 72 | 34 | 49 |
| Newfoundland | 22 | 42 | 62 | 26 | 35 |
| Dobermann | 22 | 44 | 68 | 28 | 43 |
| Leonberger | 13 | 41 | 74 | 32 | 56 |
| Boxer | 11 | 28 | 50 | 19 | 31 |
| Greyhound | 23 | 44 | 60 | 27 | 29 |
| Pyrenees | 24 | 41 | 58 | 22 | 29 |
| Total HIGH-RISK5 | 19 | 42 | 66 | 28 | 41 |
| OTHER6 | 11 | 21 | 33 | 11 | 15 |
| All males | 13 | 25 | 38 | 14 | 17 |
| All females | 10 | 20 | 33 | 11 | 16 |
| TOTAL all breeds | 22 | 23 | 35 | 13 | 16 |
1 – Deaths for which a diagnosis was recorded for cause of death
2 – Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden
3 – Breed names: 3a – Cavalier King Charles spaniel, 3b – English springer spaniel, 3c – miniature dachshund, 3d – Bernese mountain dog
4 – 10 most common breeds in the database
5 – 10 breeds with highest diagnostic mortality, among breeds with at least 1,800 DYAR
6 – All breeds not included in common or high risk
For the presentation of the Poisson models' breed-specific mortality rate ratios (MRR), estimates and SE's have been reported for all the included effects. Single and multiple effect MRR's were constructed based on the formula (for a single effect):
![]()
which shows the rate ratio for a one-unit change in xi and where E is the size of the exposure. The 99.9% confidence intervals (99.9% CI) were constructed taking βi ± 3.291 × SE, and then exponentiating these limits. Given the size of the data base, 99.9% limits were chosen to yield conservative results. The point estimate for a given age, for example a 9 < 10 year old female of a specific breed is: exp(estimate for breed + estimate for gender + estimate for age × 9 + the gender-age interaction for age × 9 + the breed-age interaction for age × 9 + the gender-breed interaction). This gives the MRR with respect to breed, age and gender simultaneously and expresses the risk relative to the baseline.
Results
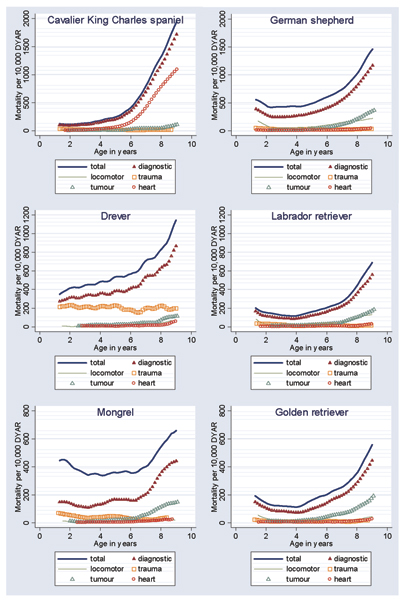
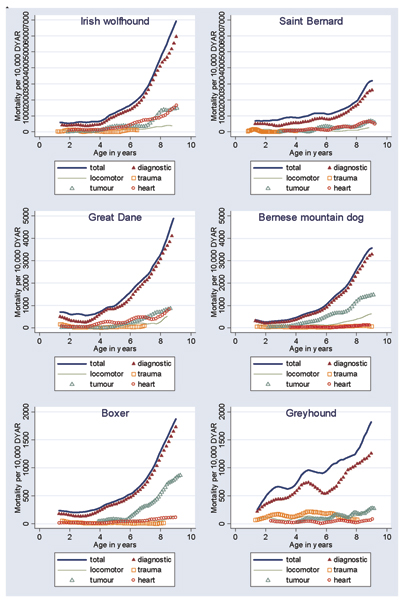
Figures 1 and 2 present the MRs from Cox regression (hazards) for the total and diagnostic mortality, as well as diagnostic category-specific mortality in six selected common breeds of dogs and six selected high-risk breeds respectively. Note that the scales on the y-axis vary from 7,000 in the Irish wolfhound and St Bernard to 800 in the mongrel and golden retriever. It is evident that in different breeds, different cause-specific mortality curves depart more clearly from the cluster of causes with lower MRs.
Figure 1.
Mortality rates, per 10,000 dog-years at risk (DYAR), developed using Cox regression for six common breeds. Shown are mortality rates for total and diagnostic mortality, as well as for the categories tumours, trauma, locomotor problems and heart. Note that scales differ between breeds.
Figure 2.
Mortality rates, per 10,000 dog-years at risk (DYAR), developed using Cox regression for six higher-risk breeds. Shown are mortality rates for total and diagnostic mortality, as well as for the categories tumours, trauma, locomotor problems and heart. Note that scales differ between breeds.
The probability of death (1-survival) to five, eight and 10 years of age for total and diagnostic mortality is presented for all breeds and groups, and for all dogs by gender in Table 1. For example, 91% of wolfhounds were dead by 10 years of age and given that an Irish wolfhound reached the age of eight years, the probability that it would be dead by 10 years of age was 76%.
Table 2 shows MRRs, with 99.9% CIs, the estimates and standard errors from the Poisson regressions for total and diagnostic mortality and tables 3 and 4 show the same for the models by diagnostic category. Model validation plots of standardised deviance residuals against predicted values demonstrated in general a satisfactory fit. However, some deaths with low predicted values had large residuals and did not fit the models. There was no pattern in the plots of deviance residuals against different covariates. In all models all main effects were statistically significant, although the insignificant terms within main effects were successively removed. For the trauma model younger animals were at increased risk; for all other models mortality rates increased with age. In all models the main effect for gender was negative indicating that, overall, females had lower mortality compared to males.
Table 2.
Multivariable estimates, standard errors (SE's) and mortality rate ratios (MRR), with 99.9% confidence intervals (99.9% CI), developed using Poisson regression for total and diagnostic mortality in dogs insured at Agria1 from 1995 to 2000.
| Total mortality2 | Diagnostic Mortality2 | |||
| MRR (99.9% CI)3 | Estimate (SE) | MRR(99.9% CI) | Estimate (SE) | |
| Intercept | - | -3.4983 (0.0313) | - | -4.2076 (0.0401) |
| Gender (female) | 0.7(0.6–0.7) | -0.4071 (0.0203) | 0.8(0.7–0.8) | -0.2536 (0.0254) |
| Age (in years) | 1.1(1.1–1.2) | 0.1245 (0.0057) | 1.2 (1.2–1.2) | 0.1690 (0.0070) |
| Breed | ||||
| CKC spaniel4a | 0.4(0.3–0.6) | -0.8627 (0.0815) | 0.5(0.4–0.7) | -0.6043 (0.0899) |
| German shepherd (GS) | 2.2(2.0–2.4) | 0.7852 (0.0300) | 2.7(2.2–3.3) | 0.9873 (0.0622) |
| Drever | 2.0(1.7–2.4) | 0.6924 (0.0497) | 2.5(2.1–3.0) | 0.9075 (0.0576) |
| Dachshund | 0.9(0.8–0.9) | -0.1521 (0.0257) | 1.2(0.9–1.5) | 0.1631 (0.0696) |
| Labrador retriever | 0.7 (0.7–0.8) | -0.3373 (0.0269) | 0.8(0.7–0.9) | -0.1983 (0.0301) |
| Springer spaniel4b | 0.9(0.8–1.0) | -0.1501 (0.0359) | 0.9(0.7–1.0) | -0.1519 (0.0430) |
| Mongrel | 2.1(1.8–2.4) | 0.7251 (0.0420) | 1.0(0.8–1.3) | 0.0459 (0.0691) |
| Golden retriever | 0.6(0.6–0.7) | -0.4576 (0.0254) | 0.7(0.6–0.7) | -0.3898 (0.0293) |
| Poodle (min/toy) | 0.7(0.6–0.8) | -0.4091 (0.0417) | 0.6(0.5–0.8) | -0.4365 (0.0504) |
| Min dachshund4c | 0.8(0.7–0.9) | -0.2271 (0.0383) | 1.1(0.8–1.4) | 0.0799 (0.0759) |
| Irish wolfhound (IW) | 2.0(1.2–3.2) | 0.6828 (0.1459) | 2.3(1.3–3.9) | 0.8173 (0.1671) |
| St Bernard | 3.4(2.7–4.1) | 1.2144 (0.0620) | 3.5(2.8–4.5) | 1.2637 (0.0723) |
| Great Dane | 2.9(2.1–4.0) | 1.0495 (0.1005) | 3.1(2.1–4.6) | 1.1266 (0.1192) |
| Bernese mtn dog4d | 1.3(1.0–1.7) | 0.2497 (0.0798) | 1.7(1.3–2.3) | 0.5231 (0.0875) |
| Newfoundland | 2.5(2.1–2.8) | 0.8987 (0.0405) | 2.8(2.4–3.2) | 1.0166 (0.0456) |
| Dobermann | 1.9(1.4–2.6) | 0.6482 (0.0945) | 1.6(1.0–2.3) | 0.4418 (0.1218) |
| Leonberger | 0.8(0.5–1.2) | -0.2580 (0.1249) | 0.8(0.5–1.4) | -0.1725 (0.1452) |
| Boxer | 0.8(0.6–1.1) | -0.2198 (0.1039) | 1.0(0.6–1.4) | -0.0452 (0.1166) |
| Greyhound | 2.4(1.9–2.9) | 0.8569 (0.0603) | 2.5(2.0–3.2) | 0.9203 (0.0705) |
| Pyrenees | 2.3(1.7–2.9) | 0.8114 (0.0806) | 2.2(1.6–3.1) | 0.8103 (0.0966) |
| Interactions | ||||
| Age-gender | 1.0(1.0–1.1) | 0.0386 (0.0037) | 1.0(1.0–1.0) | 0.0258 (0.0044) |
| Age- IW | 1.2(1.1–1.3) | 0.1838 (0.0251) | 1.2(1.1–1.3) | 0.1897 (0.0281) |
| Age- great Dane | 1.1(1.0–1.2) | 0.0789 (0.0201) | 1.1(1.0–1.2) | 0.0943 (0.0228) |
| Age- boxer | 1.1(1.1–1.2) | 0.1354 (0.0164) | 1.2(1.1–1.2) | 0.1402 (0.0181) |
| Age- dobermann | 1.1(1.0–1.1) | 0.0690 (0.0163) | 1.1(1.1–1.2) | 0.1227 (0.0197) |
| Age-leonberger | 1.3(1.2–1.4) | 0.2361 (0.0203) | 1.3(1.2–1.4) | 0.2449 (0.0230) |
| Age- BMD4d | 1.2(1.1–1.2) | 0.1565 (0.0140) | 1.2(1.1–1.2) | 0.1465 (0.0151) |
| Age-CKC spaniel4a | 1.2(1.2–1.3) | 0.2237 (0.0125) | 1.2(1.2–1.3) | 0.2111 (0.0137) |
| Age- GS | 1.0(0.9–1.0) | -0.0397 (0.0057) | 1.0(0.9–1.0) | -0.0370 (0.0069) |
| Age-drever | 0.9(0.9–1.0) | -0.0481 (0.0098) | 0.9(0.9–1.0) | -0.0805 (0.0113) |
| Age-mongrel | 0.9(0.9–0.9) | -0.1271 (0.0092) | 0.9(0.9–1.0) | -0.0601 (0.0135) |
| Age-dachshund | NA5 | NA | 1.0(0.9–1.0) | -0.0416 (0.0118) |
| Age-min dachshund4c | NA | NA | 0.9(0.9–1.0) | -0.0638 (0.0181) |
| Gender-GS | NA | NA | 0.9(0.8–1.0) | -0.1274 (0.0357) |
1 – Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden
2 – Separate models were run for each heading
3 – MRR ratio from Poisson regression = EXP(estimate), interpreted as risk for that variable (eg. breed) relative to the baseline (all breeds not in the model), adjusted for gender and age
4 – Breed names: 4a – Cavalier King Charles spaniel, 4b – English springer spaniel, 4c – miniature dachshund, 4d – Bernese mountain dog (BMD)
5 – Not applicable
Table 3.
Multivariable estimates, standard errors (SE's) and mortality rate ratios (MRR), with 99.9% confidence intervals (99.9% CI), developed using Poisson regression on diagnostic categories tumour and locomotor in dogs insured at Agria1 from 1995 to 2000.
| Diagnostic Category2 | ||||
| Tumour | Locomotor | |||
| Variable | MRR(99.9% CI)3 | Estimate (SE) | MRR(99.9% CI) | Estimate (SE) |
| Intercept | - | -7.5762 (0.1424) | - | -6.4992 (0.0957) |
| Gender (female) | 0.8(0.6–1.1) | -0.2434 (0.0904) | 0.9(0.7–1.1) | -0.1327 (0.0586) |
| Age (in years) | 1.5(1.4–1.6) | 0.4006 (0.0209) | 1.2(1.2–1.3) | 0.2186 (0.0183) |
| Breed | ||||
| CKC spaniel4a | 0.5(0.4–0.8) | -0.6247 (0.1251) | 1.0 | - |
| German shepherd (GS) | 1.7(1.5–2.0) | 0.5548 (0.0450) | 8.3(6.6–10) | 2.1168 (0.0707) |
| Drever | 1.05 | - | 1.05 | - |
| Dachshund | 0.4(0.3–0.5) | -0.9778 (0.1009) | 1.7(1.3–2.1) | 0.5012 (0.0739) |
| Labrador retriever | 1.05 | - | 4.2(3.0–5.9) | 1.4349 (0.1056) |
| Springer spaniel4b | 1.05 | - | 0.5(0.3–0.9) | -0.6732 (0.1785) |
| Mongrel | 1.05 | - | 0.5(0.3–0.8) | -0.6625 (0.1465) |
| Golden retriever | 1.05 | - | 3.3(2.2–4.9) | 1.2020 (0.1194) |
| Poodle (min/toy) | 0.3(0.2–0.6) | -1.0843 (0.1570) | 0.5(0.3–0.9) | -0.7120 (0.1874) |
| Min dachshund4c | 0.3(0.1–0.5) | -1.3715 (0.2301) | 0.7(0.3–1.4) | -0.4041 (0.2218) |
| Irish wolfhound | 8.9(5.7–14) | 2.1823 (0.1324) | 6.7(3.6–12) | 1.9023 (0.1874) |
| St Bernard | 3.9(2.2–6.6) | 1.349 (0.1652) | 5.2(2.7–9.9) | 1.6491 (0.1941) |
| Great Dane | 4.6(2.7–7.8) | 1.5173 (0.1632) | 6.1(3.6–11) | 1.8111 (0.1644) |
| Bernese mtn dog4d | 17(9.4–31) | 2.8382 (0.1804) | 7.1(5.4–9.4) | 1.9645 (0.0826) |
| Newfoundland | 2.2(1.5–3.2) | 0.7664 (0.119) | 15 (8.6–26) | 2.7003 (0.1657) |
| Dobermann | 4.0(2.9–5.5) | 1.3857 (0.0990) | 5.0(3.4–7.5) | 1.6117 (0.1205) |
| Leonberger | 5.5(4.1–7.6) | 1.7128 (0.0947) | 3.9(2.4–6.2) | 1.3615 (0.1423) |
| Boxer | 4.2(3.3–5.3) | 1.4336 (0.0687) | 2.2(1.4–3.4) | 0.7977 (0.1305) |
| Greyhound | 1.05 | - | 4.8(2.8–8.2) | 1.5584 (0.1642) |
| Pyrenees | 2.7(1.3–5.5) | 0.9926 (0.2138) | 5.7(3.0–11) | 1.7342 (0.1977) |
| Interactions | ||||
| Age-gender | 1.1(1.0–1.1) | 0.0551 (0.0132) | 1.0(0.9–1.0) | -0.0407 (0.0116) |
| Age- Newfoundl. | NA6 | NA | 0.8 (0.8–0.9) | -0.1707 (0.0355) |
| Age-GS | NA | NA | 0.9(0.9–0.9) | -0.1102 (0.0143) |
| Age-Labrador retr. | NA | NA | 0.9(0.8–1.0) | -0.1176 (0.0212) |
| Age- Golden retr. | NA | NA | 0.8(0.7–0.8) | -0.2674 (0.0278) |
| Age- Min dachshund4c | NA | NA | 1.2(1.1–1.4) | 0.2221 (0.0409) |
| Gender-BMD4d | 0.7(0.5–1.0) | -0.3941 (0.1153) | NA | NA |
1 – Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden
2 – Separate models were run for each heading
3 – MRR ratio from Poisson regression = EXP(estimate), interpreted as risk for that variable (eg. breed) relative to the baseline (all breeds not in the model), adjusted for gender and age
4 – Breed names: 4a – Cavalier King Charles spaniel, 4b – English springer spaniel, 4c – miniature dachshund, 4d – Bernese mountain dog (BMD)
5 – Breed is included in baseline category for this model
6 – Not applicable
Table 4.
Multivariable estimates, standard errors (SE's) and mortality rate ratios (MRR), with 99.9% confidence intervals (99.9% CI), developed using Poisson regression on diagnostic categories trauma, heart and neurological in dogs insured at Agria1 from 1995 to 2000.
| Diagnostic category 2 | ||||||
| Trauma | Heart | Neurological | ||||
| Variable | MRR (99N.9% CI)3 | Estimate (SE) | MRR (99.9% CI) | Estimate (SE) | MRR (99.9% CI) | Estimate (SE) |
| Intercept | - | 4.8732 (0.0464) | - | -7.4284 (0.0929) | - | -6.1979 (0.0805) |
| Gender (female) | 0.8(0.8–0.9) | -0.1961 (0.0277) | 0.5(0.5–0.6) | -0.6040 (0.0426) | 0.7(0.6–0.8) | -0.3586 (0.0469) |
| Age (in years) | 0.9(0.9–0.9) | -0.069 (0.0053) | 1.4(1.4–1.5) | 0.3552 (0.0111) | 1.1(1.1–1.1) | 0.0945 (0.0091) |
| Breed | ||||||
| CKC spaniel4a | 0.5(0.3–0.8) | -0.7135 (0.1314) | 3.0(1.7–5.5) | 1.1074 (0.1793) | 1.05 | - |
| German shepherd (GS) | 0.7(0.6–0.9) | -0.3225 (0.0637) | 2.1(1.0–4.4) | 0.7300 (0.2277) | 0.5(0.2–0.9) | -0.7643 (0.1977) |
| Drever | 4.4(3.8–5.1) | 1.4848 (0.0447) | 1.05 | - | 1.05 | - |
| Dachshund | 2.3(2.0–2.7) | 0.842 (0.0493) | 0.1(0.01–0.4) | -2.5337 (0.5188) | 0.3(0.2–0.6) | -1.1793 (0.1943) |
| Labrador retriever | 0.5(0.3–0.6) | -0.7764 (0.0941) | 0.5(0.3–0.8) | -0.7959 (0.1677) | 0.6(0.4–1.0) | -0.4432 (0.1276) |
| Springer spaniel4b | 0.5(0.3–0.8) | -0.7061 (0.1303) | 1.05 | - | 1.05 | - |
| Mongrel | 1.05 | - | 0.5(0.3–1.0) | -0.7037 (0.2027) | 1.05 | - |
| Golden retriever | 0.3(0.2–0.4) | -1.1771 (0.1046) | 0.5(0.3–0.8) | -0.7061 (0.1401) | 1.05 | - |
| Poodle (min/toy) | 1.05 | - | 0.4(0.2–0.9) | -0.9602 (0.2693) | 1.05 | - |
| Min dachshund4c | 1.7(1.4–2.1) | 0.5237 (0.0654) | 0.3(0.1–1.0) | -1.1053 (0.3352) | 0.3(0.1–0.7) | -1.1682 (0.2597) |
| Irish wolfhound | 1.05 | - | 29 (19–44) | 3.3645 (0.1294) | 1.05 | - |
| St Bernard | 1.05 | - | 12 (6.5–20) | 2.4438 (0.1746) | 5.1(2.4–11) | 1.6204 (0.2309) |
| Great Dane | 1.05 | - | 21 (13–32) | 3.0210 (0.1371) | 3.5(1.6–7.8) | 1.2455 (0.2442) |
| Bernese mtn dog4d | 1.05 | - | 1.05 | - | 1.05 | - |
| Newfoundland | 1.05 | - | 10 (7.3–14) | 2.3268 (0.1044) | 1.05 | - |
| Dobermann | 1.05 | - | 6.4(4.0–10) | 1.8546 (0.1439) | 1.05 | - |
| Leonberger | 1.05 | - | 8.6(5.6–13) | 2.1565 (0.1344) | 1.05 | - |
| Boxer | 0.4(0.2–0.8) | -0.9084 (0.2243) | 2.7(1.7–4.5) | 1.0094 (0.1527) | 1.1(0.4–3.3) | 0.0995 (0.3296) |
| Greyhound | 2.8(1.8–4.5) | 1.0461 (0.1411) | 3.3(1.3–8.2) | 1.1839 (0.2794) | 1.05 | - |
| Pyrenees | 1.05 | - | 1.05 | - | 1.05 | - |
| Interactions | ||||||
| Age-CKC spaniel | NA6 | NA | 1.3(1.2–1.4) | 0.2667 (0.0253) | NA | |
| Age-GS | NA | NA | 0.9(0.8–1.0) | -0.1500 (0.0409) | 1.2(1.1–1.3) | 0.1812 (0.0341) |
| Age-Dachshund | NA | NA | 1.5(1.2–1.9) | 0.4194 (0.0678) | NA | |
| Age-Boxer | NA | NA | NA | NA | 1.3(1.1–1.5) | 0.2237 (0.0523) |
1 – Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden
2 – Separate models were run for each heading
3 – MRR ratio from Poisson regression = EXP(estimate), interpreted as risk for that variable (eg. breed) relative to the baseline (all breeds not in the model), adjusted for gender and age
4 – Breed names: 4a – Cavalier King Charles spaniel, 4b – English springer spaniel, 4c – miniature dachshund, 4d – Bernese mountain dog
5 – Breed is included in baseline category for this model
6 – Not applicable
However, positive age-gender interactions were found for total mortality, diagnostic mortality, and tumours, indicating that for these conditions older females had a significantly increased risk compared to males. The age-gender interaction term for locomotor was negative; i.e. older males had higher relative mortality.
There were several significant age-breed interaction terms in several models, namely for total and diagnostic mortality, for locomotor, heart and neurological. In all but the neurological model these interactions were both positive and negative. For example in the heart model, the age-breed interactions for Cavalier King Charles spaniels and dachshunds were positive, while the one for German shepherds was negative (further illustrated below).
Statistically significant terms for certain breed-gender interactions were found in the diagnostic mortality and the tumour model (see below). For trauma, only main effect variables were significant, and, therefore, the interpretation of the breed-specific MRR is the effect of breed after adjusting for age and gender differences.
To be able to interpret the multivariable results from the Poisson regression more completely with respect to all of the variables, including the interactions, figures 3, 4, 5 have been constructed. These exemplify how the MRRs vary across breeds, genders and ages for the models with respect to diagnostic mortality, tumours and heart.
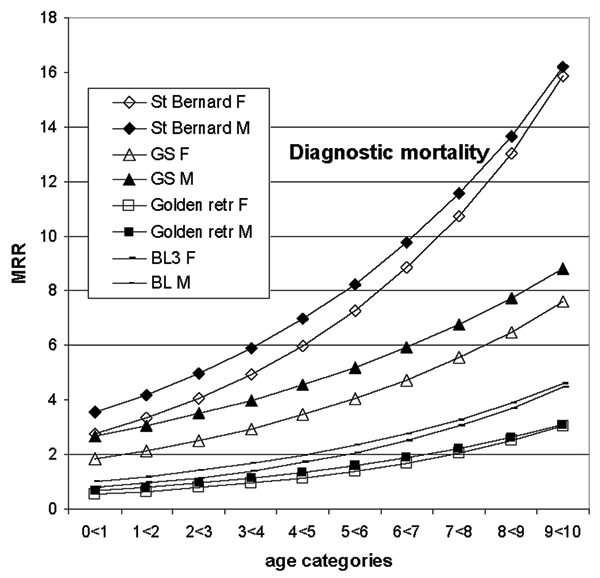
Figure 3.
Mortality rate ratios (MRR) from the diagnostic mortality are shown by breed1, age and gender (F-female, M-male) for a few selected breeds, as well as for the baseline. The data are on dogs with life insurance at Agria2 during years 1995 to 2000. The MDR are derived using the estimates in table 2. For example, the MRR for female German shepherds (GS) at age category 9 < 10 is constructed taking the exponent of (the breed estimate+ the gender estimate + (the age estimate × 9) + (the gender-age estimate × 9) + (the age-breed estimate × 9) +gender-GS estimate). 1GS- German shepherd, Golden retr- golden retriever 2Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden 3BL- baseline- all breeds not included in the model
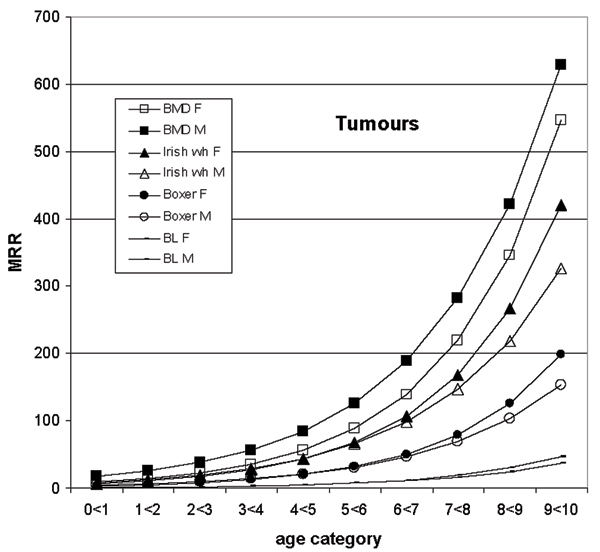
Figure 4.
Mortality rate ratios (MRR) from the tumour model are shown by breed1, age and gender (F-female, M-male) for a few selected breeds, as well as for the baseline. The data are on dogs with life insurance at Agria2 during years 1995 to 2000. The MRR are derived using the estimates in table 3. For example, the MRR for female Bernese mountain dogs (BMD) at age category 9 < 10 is constructed taking the exponent of (the breed estimate+ the gender estimate + (the age estimate × 9) + (the gender-age estimate × 9) + the gender-BMD estimate). 1BMD- Bernese mountain dog, Irish wh- Irish wolfhound 2Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden 3BL- baseline- all breeds not included in the model
Figure 5.
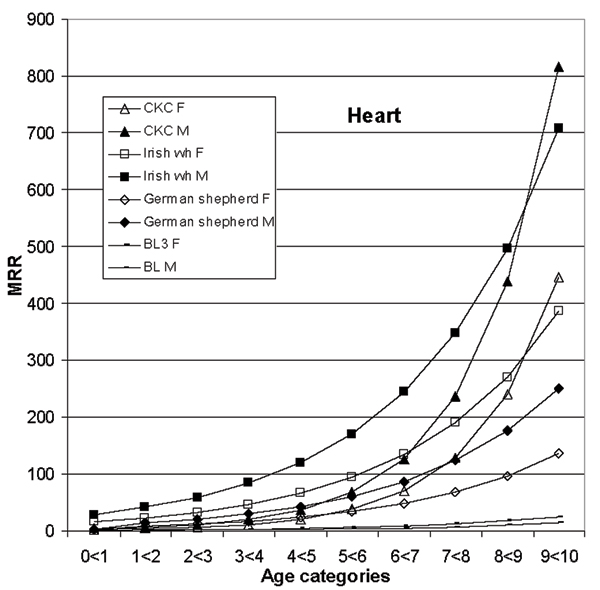
Mortality rate ratios (MRR) from the heart model are shown by breed1, age and gender (F-female, M-male) for a few selected breeds, as well as for the baseline. The data are on dogs with life insurance at Agria2 during years 1995 to 2000. The MRR are derived using the estimates in table 3. For example, the MRR for female Cavalier King Charles spaniel (CKC) at age category 9 < 10 is constructed taking the exponent of (the breed estimate + the gender estimate + (the age estimate × 9) + (the age-breed estimate × 9)). 1CKC- Cavalier King Charles spaniel, Irish wh- Irish wolfhound 2Agria Insurance, PO 70306, SE-107 23 Stockholm, Sweden 3BL- baseline- all breeds not included in the model
In figure 3, lines are shown for three breeds and the baseline for diagnostic mortality. One of the lines, for the German shepherd, is shown because there was interaction between breed and gender. By comparing the St Bernards and the baseline breeds to the breed with the breed-gender interaction, German shepherds, it is evident that the MR in females is consistently lower in male German shepherds, while in the other breeds there is no difference between genders in the older age categories.
In figure 4, comparing the Bernese mountain dogs with the Irish wolfhounds and the other breeds (boxer and baseline), it can be seen that the male Bernese mountain dogs have a higher MRR for tumours at all ages, whereas for the other breeds the tumour rate is actually higher in the females at higher ages.
Figure 5 shows results for the heart category. The positive age-breed interaction for the Cavalier King Charles spaniel clearly makes the curves bend upwards, while the negative breed interaction in the German shepherd visually has a smaller effect. There are large MRRs by breed and gender for a few breeds (e. g. Cavalier King Charles spaniels and Irish wolfhounds) compared to baseline, especially for males at older ages.
Discussion
Analysis
Age patterns of death are informative both within and across breeds. This paper presents comprehensive information on mortality by including individual breed patterns of death for overall mortality and by category-specific causes. Survival estimates are clinically useful information within a breed or for contrasting across breeds. Finally, multivariable models quantify the relative rate between breeds adjusted for effects of age and gender for different causes of death. Most of the age effects are, presumably, largely biological, however the model also adjusts for potential differences in age distributions between breeds that might be due to differences in enrolment in insurance or other factors. Although this study presents appropriate quantitative measures, the output can be somewhat overwhelming. Exhaustive presentation of relative rates of death for every breed, both genders and at all ages would be beyond the scope of a research publication. Therefore, estimates and standard errors from the modelling procedures have been presented to allow readers to calculate specific MRRs for any gender-age-breed combination in which they are interested. Graphic figures have been included to provide a more obvious display of some relationships across breeds.
In addition to differences between breeds, it was shown in the companion paper that for some conditions there was a statistically significant effect of gender [2]. For example, males tended to have higher MRs for trauma and females had higher rates of cancer, except in the Bernese mountain dog, where males had twice the rate of tumours. Such complex relationships can be examined using statistical modelling. As the goal of this study was to produce clinically relevant statistics on death in dogs, a practical and parsimonious approach to modelling was taken.
Cox regression was used for the survival analysis as an appropriate technique to model time to death. Multivariable Cox regression models were initially attempted but proved unmanageable in terms of computer and time constraints, therefore Poisson regression was used for the calculation of MRRs, allowing inclusion of a primary baseline category of all other breeds.
Exploring the fit of the models several large residuals were found, mainly for low predicted values, however this was not related to low age categories. Also, the model fit was worst in the total and diagnostic mortality models (data not shown), likely partly because these contained larger numerators, and accordingly by chance some more deaths will be ill-fitting (and more deaths will be well-fitting). Further, all types of deaths were included in the outcomes and, as only a few variables were available to model, it is likely that important predictors of deaths were not included, thus limiting the fit of the model.
Unfortunately over-dispersion could not be evaluated because multiple records belonged to each dog, as the dogs moved through the age categories. However, in previous work over-dispersion has never been a problem working with the Agria insurance database ([5], in press, unpublished observations).
Age patterns and survival
Different breeds age at different speed, however in many breeds the mortality starts to increase after about six to seven years of age, similar to findings of increased morbidity and mortality seen in previous work by these authors [4]. Superficially, this may be seen as supporting anecdotal suggestions that 'geriatric profiling' be started on all breeds at seven years of age. However, this increase must be considered together with the magnitude of mortality at specific ages in individual breeds of dogs. There is no accepted mortality level at which we designate dogs to be geriatric, or even senior. However, considering that for some breeds over 50% of dogs are dead by eight years of age (e.g. Irish wolfhounds, St. Bernard, great Dane) whereas in other breeds less than 25% have died (Labrador and golden retrievers, poodle, miniature dachshund), it is inappropriate to consider them as having equivalent biological age or similar risk of age-related diseases that might be detected by screening programs.
For tumours, the mortality rate starts to increase after six or seven years in Labrador retrievers and mongrels, although it is still below 200 deaths per 10,000 DYAR. In Bernese mountain dogs tumour deaths are common starting by four years of age and account for over 2,000 deaths per 10,000 DYAR by eight years of age. For boxers there is a sharp increase to very high levels after the age of six years. These findings highlight the probability that some previously published breed risk estimates are misleading. Proportional estimates of death from data sources with no population at risk (e.g. postmortem registries) have likely made erroneous conclusions about cancer risks across breeds [3].
The age patterns are reflected in the survival statistics and highlight differences across breeds. For Cavalier King Charles spaniels there is a very low early mortality with a sharp rise after five years of age. Only 7% of Cavalier King Charles spaniels are dead by five years of age, but 48% are dead by 10 years. This is in contrast to a breed like the German shepherd, where the early mortality is high (20% mortality by five years) but by 10 years of age the survival (49%) is quite similar to the Cavalier King Charles spaniel. Boxers have relatively low early mortality – one in nine will die before five years of age. One in five will die between five and eight and of those alive at eight, one-third will die by 10.
Information on the average survival pattern for different breeds is useful clinical information. Prospective owners should understand that in getting, for example, either a Bernese mountain dogs or a golden retriever, the likelihood of the dog living past 10 years of age is very different. Most owners, once their dog becomes seriously ill or dies, are keen to know if the condition is rare or common, in general or in their breed. Many owners are especially concerned if they feel their dog has died prematurely, that is at a younger than average age. Veterinarians and their clients can also use survival statistics as part of informed decision-making regarding expensive veterinary care and for conditions in which quality of life is an issue. For example, an Irish wolfhound that has survived to eight years has a 76% chance of being dead before 10, whereas an eight-year old golden retriever has a 90% chance of surviving to 10.
Prospective owners may wish to consider breed-specific patterns of death or disease before choosing a breed. Owner preferences and expectations about, for example, longevity, may influence their satisfaction with a chosen pet [7]. For health management at the individual dog level, it is also useful for veterinarians to have quantified estimates of the increased risk certain breeds have for certain conditions. These findings inform diagnostic decision-making and facilitate communication with owners.
Multivariable analysis
The MRRs highlight both the relative magnitude and breed differences for total and diagnostic mortality. Differences between the total and diagnostic mortality rates within breeds were discussed in the companion paper [2]. Elective euthanasia for behaviour problems or dog-owner mismatch does not get reimbursed. Total mortality statistics probably best reflect overall mortality across breeds. However, for examining disease conditions as causes of death, the diagnostic mortality statistics are more informative, even though these are only available for the subset of dogs for which claims have been submitted.
Risk of tumour, in general was higher in males (MRR < 1), however the interaction term (age-gender, > 1) tells us that older females were at higher risk of tumours. This is likely due to the high rate of mammary tumours in females ([5] in press). Most interesting in the tumour model was the interaction term for Bernese mountain dogs. Overall this breed is at a much greater risk of dying from tumour than the baseline breeds and the interaction term tells us that the effect at older ages is the opposite in the Bernese mountain dog compared to other breeds, i.e. at older ages males have a higher risk of death from tumours than females. Note that confidence intervals are not included in figures 3, 4, 5.
Dachshunds (both breed groups) and poodles were at decreased risk of death due to tumours, as were Cavalier King Charles spaniels. One explanation might be a lower biological age, for example, in poodles. As only one quarter of poodles die before 10, poodles in this study population must be considered to have not even reached 'middle age'.
Traumatic deaths were more common in males and at younger ages (although there was no age-gender interaction). As was discussed in the companion paper, drevers had a high rate of death due to traumatic causes, and that was reflected in the multivariable models, where they were at 4.5 times increased risk than the baseline group. The risk for dachshunds and greyhounds were also high, although from different causes, i.e. mainly car accidents for the former and a great deal of fractures in the latter (data not shown). Interestingly German shepherds, Cavalier King Charles spaniels, Labradors, springer spaniels and golden retrievers had a significantly reduced risk of death due to trauma compared to baseline breeds. Reasons for this are unknown, but could relate to training and use of these animals and behavioural characteristics of the dogs or their owners.
Golden retrievers were at low risk for mortality in this study – only 22% died before 10 years. Golden retrievers were significantly less likely to die of trauma and heart disease and were in the baseline (average) risk group for neurological and tumour causes of death. They were at increased risk in the first age category for locomotor problems, but this effect waned with age as demonstrated by a negative age-breed interaction.
Overall, males were at increased risk and risk increased with age for locomotor causes of death. The interaction term indicates that the difference between males and females was more pronounced at higher ages. Many breeds were at increased risk of death from locomotor problems, but springer spaniels, mongrels and poodles had a significantly decreased risk. In five breeds (table 3) there were negative age-breed interactions. In four of these the risk in these breeds were pronounced at early age, but the risk waned with age. However, in the miniature dachshund, the risk was similar to baseline at low ages and less than baseline in older ages. Females were at reduced risk of dying from heart diseases compared to males. Risk increased quite sharply with increasing age. Several breeds had markedly increased risk of death due to heart causes, for example, Irish wolfhounds and great Danes were at 29 and 21 times increased risk, respectively, compared to baseline breeds. The Cavalier King Charles spaniel had a low MRR of 3, but also a positive age-breed interaction. This made the MRR in the first age category low, 1.7 and 3.0 for females and males respectively, but high, 446 and 816 in the last age category (fig 5). The miniature breeds (poodle and dachshund), the two retriever breeds and mongrels were at decreased risk.
The risk of death due to neurologic causes increased with age and males were 1.4 times more likely to die from neurologic causes compared to females. There were significant age-breed interactions in two breeds, boxers and German shepherds. Both were positive. For example, in boxers the risk in the lowest age category was not significantly different from baseline, but with age the MRR increased to 13.5 and 19 in females and males respectively (using the formulas from the part of materials and methods). In baseline breeds these MRR were 1.6 and 2.3 and females and males respectively. Further, St. Bernards and great Danes were 3.5 to five times more likely to die from neurological causes, and both regular and miniature dachshunds were over three times less likely to die of neurologic causes than other breeds (baseline). Labradors were also significantly less likely to die of neurologic causes than baseline breeds. As was seen and discussed in the companion paper, a large proportion of neurologic deaths were due to epileptic disease.
Conclusion
Information presented in this and the companion paper inform our understanding of the population level burden of disease and support decision-making at the population and individual level about health promotion efforts and treatment and prognosis of disease events. Findings such as these provide some new insights and, even where they confirm clinical impressions, offer the advantage of both relative and absolute quantification of the direction and magnitude of breed differences and the effects of age and gender. Population statistics on health and disease of dog populations will be a crucial part of breeding strategies and monitoring as we enter into a new era with knowledge of the canine genome. Increased disease in purebred populations has been identified as a welfare issue and as stewards of animal health the veterinary profession should work with other stakeholders in animal health to reduce the occurrence of preventable disease.
Acknowledgments
Acknowledgements
This work has been supported by grants from the Foundation for Research, Agria Insurance and the Swedish Kennel Club.
References
- Allison P. Survival analysis using the SAS® system: a practical guide. Cary; 1995. [Google Scholar]
- Bonnett BN, Egenvall A, Olson P, Hedhammar Å. Mortality in over 350,000 insured Swedish dogs from 1995–2000: I. Breed-, gender-, age- and cause-specific rates. Acta vet scand. 2005;46:105–120. doi: 10.1186/1751-0147-46-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig LE. Cause of death in dogs according to breed: a necropsy survey of five breeds. J Am Anim Hosp Assoc. 2001;37:438–443. doi: 10.5326/15473317-37-5-438. [DOI] [PubMed] [Google Scholar]
- Egenvall A, Bonnett BN, Olson P, Hedhammar Å. Gender, age, breed and geographic pattern of morbidity and mortality in insured dogs during 1995 and 1996. Vet Rec. 2000;146:519–525. doi: 10.1136/vr.146.18.519. [DOI] [PubMed] [Google Scholar]
- Egenvall A, Bonnett BN, Öhagen P, Olson P, Hed-hammar Å, von Euler H. Incidence of and survival after mammary tumours in a population of over 80,000 insured female dogs in Sweden from 1995–2002. Prev Vet Med. [DOI] [PubMed]
- Meyers-Wallen VN. Ethics and genetic selection in purebred dogs. Reprod Domest Anim. 2003;38:73–76. doi: 10.1046/j.1439-0531.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Michell A. Longevity of British breeds of dogs and relationships with sex, size, cardiovascular variables and disease. Vet Rec. 1999;145:625–629. doi: 10.1136/vr.145.22.625. [DOI] [PubMed] [Google Scholar]
- Ott RS. Animal selection and breeding techniques that create diseased populations and compromise welfare. J Amer Vet Med Ass. 1996;12:1969–1974. [PubMed] [Google Scholar]
- Svenska Djursjukhusföreningen (Swedish animal hospital association) Diagnosregister för häst, hund och katt. Diagnostic registry for the horse, the dog and the cat. Taberg. 1993. In Swedish.