Abstract
Culicoides biting midges serve as vectors of pathogens affecting humans and domestic animals. Culicoides sonorensis is a vector of several arboviruses in North American that cause substantial economic losses to the US livestock industry. Previous studies showed that C. sonorensis saliva, like the saliva of many hematophagous arthropods, contains numerous pharmacological agents that affect hemostasis and early events in the inflammatory response, which may enhance the infectivity of Culicoides-borne pathogens. This paper reports on the immunomodulatory properties of C. sonorensis salivary gland extracts on murine immune cells and discusses the possible immunomodulatory role of C. sonorensis saliva in vesicular stomatitis virus infection of vertebrate hosts. Splenocytes treated with C. sonorensis mitogens were significantly affected in their proliferative response, and peritoneal macrophages secreted significantly less NO. A 66-kDa glycoprotein was purified from C. sonorensis salivary gland extract, which may be in part responsible for these observations and may be considered as a vaccine candidate.
INTRODUCTION
Several species of biting midges (Diptera: Ceratopogonidae) serve as vectors of diseases affecting humans and domestic animals. Culicoides paraensis is the primary vector of Oropouche virus to humans in Neotropical urban cycles.1 Culicoides sonorensis is the primary vector of several economically important livestock diseases in North America.2,3
Hemostasis, inflammation, and immunity are major obstacles that hematophagous arthropods must circumvent when taking a blood meal on the vertebrate host. These physiologic barriers are inactivated by bioactive substances of hematophagous arthropods that are inoculated into the host skin through saliva during blood feeding.4 Hematophagous arthropods share the ability to modulate the host’s blood flow and the immune response through the effect of bioactive salivary factors. Bioactive salivary factors have been identified for mosquitoes, sand flies, hard ticks, black flies triatomine bugs, and biting midges.4–14 These factors have been shown to play active roles during transmission and pathogenesis in experimental models of arthropod-borne diseases caused by arboviruses, bacteria, and protozoans.4
Culicoides sonorensis is the primary vector of the blue-tongue viruses in the United States,2 and it has been implicated in the transmission of vesicular stomatitis virus (VSV) in the southwestern United States.15–18 In addition to antihe-mostatics,19–21 preliminary findings indicated the presence of salivary immunomodulatory factors in C. variipennis (C. sonorensis sensu stricto).22 The objective of this study was to confirm and evaluate in detail the effects of C. sonorensis salivary gland extracts on murine immune cells. Here, we report the anti-proliferative effects on B and T cells and the reduced NO production by macrophages associated with exposure to C. sonorensis salivary factors and discuss their potential role in arbovirus transmission.
MATERIALS AND METHODS
Mice
Young adult C57BL/6 mice 4–6 weeks of age were purchased from the National Cancer Institute (Bethesda, MD). The maintenance and care of experimental animals complied with the National Institutes of Health guidelines for the humane use of laboratory animals. All studies requiring mice were performed in accordance with protocols approved by the Animal Care and Use Committee at Colorado State University.
Culicoides sonorensis and salivary gland extracts. Culicoides sonorensis salivary glands were dissected from 2- to 4-day-old C. sonorensis adult females from colony AK23 and reared under conditions described elsewhere.23 Salivary glands were dissected, collected in phosphate-buffered saline (PBS) and stored at −70°C before use. The glands were lysed by freezing and thawing in PBS. A pair of female salivary glands averaged 0.21 μg of soluble protein.21 This estimate was used to calculate the amount of salivary extract protein used in experiments.
T- and B-cell proliferation assays
Optimal concentrations for Concanavalin A (Con A) with T cells and lipopolysaccha-ride (LPS) with B cells and macrophages were determined. Spleen cell suspensions were prepared from C57BL/6 mice in Dulbecco minimal essential medium (DMEM; GIBCO BRL, Gaithersburg, MD) supplemented with 5 × 10−5 mol/L 2-mer-captoethanol and 0.5% normal mouse serum.24 Aliquots containing 2 × 105 cells were added to the wells of 96-well culture plates (Costar, Corning, NY). The cells were pre-incubated for 2 hours in medium (final volume 100 μL) with or without C. sonorensis salivary gland extract (SGE).
Cells were stimulated with the mitogens, either T-cell mitogen Con A (Miles Laboratories, Naperville, IL) or B-cell mitogen LPS (Escherichia coli 055:B55W; Difco Laboratories, Detroit, MI), for 18 hours at specific concentrations. The degree of proliferation was determined by pulsing cultures with 1 μCi/well of 3H-thymidine (Amersham, Arlington Heights, IL) for 8 hours followed by scintillation counting.24
To determine that there was no effect by SGE on the baseline proliferation of murine splenocytes, splenocytes were placed into culture with and without C. sonorensis SGE, and 3H-thymidine was added at 16, 24, or 48 hours of culture to determine the degree of proliferation of the cells.
NO assays
Peritoneal exudate cells (PECs) were obtained from C57BL/6 mice and plated at 1 × 106/mL in 96-well culture plates.25 After 2-hour incubation, non-adherent cells were removed by rinsing with medium, and the adherent cells, which were 95% macrophages, were incubated for 2 hours with medium or medium plus C. sonorensis SGE.25,26 LPS was added to a final concentration of 10 ng/mL, and nitrite production (which reflects NO production by macrophages) was assessed at 72 hours thereafter by the Griess reaction.24
Lectin affinity chromatography
Purified salivary glycoproteins from C. sonorensis SGE were obtained from the PBS extract from 76 pairs of glands passed through a PBS-equilibrated 0.1-mL column of Con A immobilized on aga-rose beads (Vector Laboratories, Burlingame, CA). After incubation on ice for 1 hour, the flow through was collected, and the beads were washed three times with PBS before eluting the glycoproteins with 200 mmol/L methyl α-D-mannopyranoside (αMMP) in PBS. The eluate was processed for lectin-blot analysis and the B-cell proliferation assay. The B-cell assay was chosen to test glycoprotein fractions of C. sonorensis SGE because 1) Con A, a known T-cell mitogen, present in the eluate would stimulate the proliferation of T cells, and 2) B cells showed the greatest degree of proliferative inhibition. Therefore, CBP66 binding to Con A would not interfere with interpretation of results in the B-cell assay.
Lectin blot analysis
The electrophoretic separation of the fractions was conducted under reducing conditions on precast 4–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Invitrogen, Carlsbad, CA) in MOPS buffer, at 200 V for 50 minutes. Pre-stained rainbow protein mix (Amersham Biosciences, Piscataway, NJ) was used as molecular weight standards. The proteins were electrotransferred to nitrocellulose paper (Biorad, Hercules, CA) in tris-HCl glycine-methanol buffer at 100 V for 1 hour and stained with reversible stain MemCode (Pierce, Rockford, IL). After verifying protein transfer with MemCode, the membrane was destained and blocked with 1% bovine skin gelatin (Sigma, St. Louis, MO) in PBS. The membrane was incubated with biotinylated lectin (Vector, Burlingame, CA) diluted at 1 μg/mL in TST (0.05 mol/L Tris, pH 7.5, 0.15 mol/L sodium chloride, 0.1% Tween 20) for 1 hour at room temperature on a rocking platform. After washing three times with TST buffer, the membrane was incubated with neutra-vidin (Vector) 1 μg/mL and biotinylated alkaline phosphatase (Vector) 1 μg/mL, for 1 hour at room temperature. The membrane was incubated in BCIP/NBT (KPL, Gaithersburg, MD) enzyme substrate after washing three times with TST buffer. For the purpose of comparison, the equivalent of 10 pairs of C. sonorensis salivary glands was loaded on a 4–12% SDS-PAGE gel, and the proteins were separated as above and visualized using silver stain.
Statistical analyses
Data were analyzed using a Student t test, and differences were considered significant when P < 0.05 (SigmaPlot 9.0, Point Richmond, CA).
RESULTS
Inhibition of the Con A–driven mitogenic response by murine splenic T cells
Figure 1 shows that pre-incubation with C. sonorensis medium containing SGE significantly inhibited the proliferation of splenic T cells compared with cells pre-incubated in medium alone. Cells stimulated with 0.5 and 1.0 μg Con A/mL showed 66% inhibition (P < 0.00001) and 40% inhibition (P < 0.001), respectively.
Figure 1.
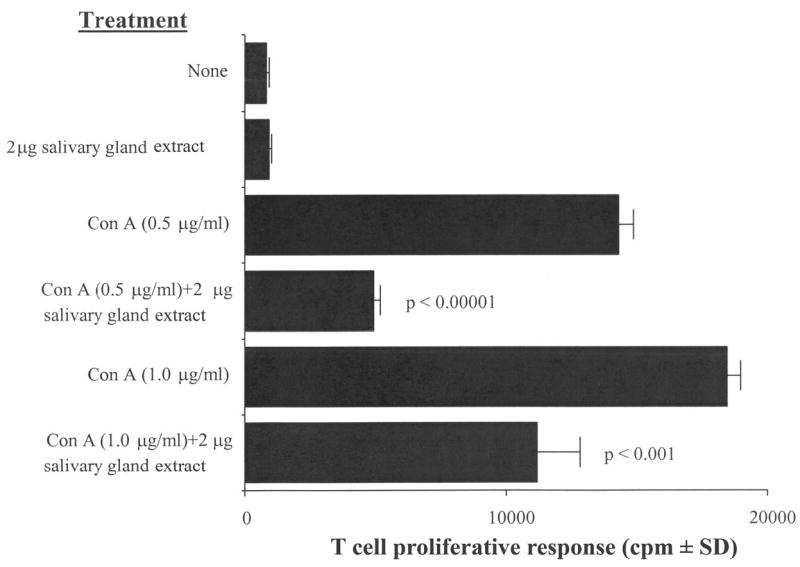
Culicoides sonorensis salivary gland extract inhibits the Con A–driven mitogenic response of murine splenic T cells. C57BL/6 spleen cells were pre-incubated with 2 μg of C. sonorensis salivary gland extract for 2 hours and were stimulated with the indicated concentrations of Con A for 18 hours. The degree of proliferation by the splenocytes was assessed by scintillation counting. Salivary gland extract significantly inhibited proliferation; P values are indicated in the figure. The figure is representative of four independent experiments.
Inhibition of the LPS-driven mitogenic response by murine splenic B cells
Figure 2 shows that C. sonorensis SGE significantly inhibited the polyclonal response of B cells. Cells stimulated with 10 μg LPS/mL plus 2 μg SGE showed 42% inhibition (P < 0.01), and 76% inhibition (P < 0.00001) was seen when the cells were stimulated with 30 μg LPS/mL plus 2 μg SGE.
Figure 2.
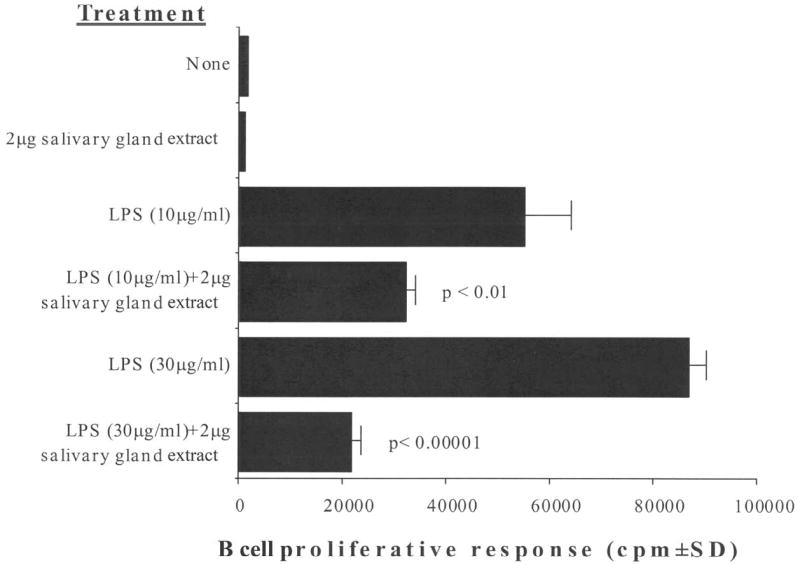
Culicoides sonorensis salivary gland extract inhibits the LPS-driven mitogenic response of murine splenic B cells. C57BL/6 spleen cells were pre-incubated with 2 μg of C. sonorensis salivary gland extract for 2 hours and were stimulated with the indicated concentrations of LPS for 18 hours. Proliferation was assessed as in Figure 1. Salivary gland extract significantly inhibited proliferation; P values are indicated in the figure. The figure is representative of four independent experiments.
Inhibition of NO production by murine macrophages
Peritoneal macrophages were pre-treated with C. sonorensis SGE for 2 hours before stimulation with LPS 10 ng/mL for 72 hours to induce the production of NO. Figure 3 shows that treatment with C. sonorensis SGE reduced the amount of NO secreted by LPS-stimulated macrophages by 59% (P < 0.03).
Figure 3.
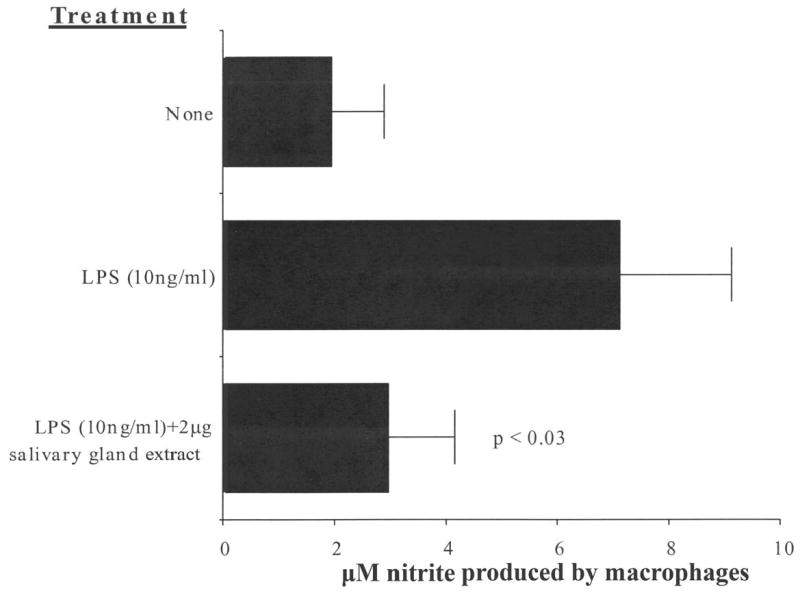
Culicoides sonorensis salivary gland extract inhibits NO production by murine macrophages. C57BL/6 macrophages were pre-incubated with 2 μg of C. sonorensis salivary gland extract for 2 hours and were stimulated with 10 ng/mL of LPS for 72 hours. Nitrite production by the cells was assessed. Salivary gland extract significantly inhibited nitrite production; P values are indicated in the figure. The figure is representative of six independent experiments.
Lack of effect on the baseline proliferation of murine sple-nocytes
The immunoinhibitory effects of C. sonorensis SGE was not caused by a non-specific anti-proliferative effect of the extract on target cells (Figure 3). The viability of extract-treated cells did not differ significantly from control cells as assessed by trypan blue exclusion. The results shown in Figure 4 show that there is no significant anti-proliferative effect of SGE on murine splenocytes. The cells most likely to proliferate in response to LPS stimulation are B cells. By 48 hours of culture, there was a modest augmentation of proliferation at the higher dose of 2 μg SGE. These results suggest that a non-specific anti-proliferative effect was not responsible for the inhibitory effects that C. sonorensis SGE had on the functions of murine splenocytes.
Figure 4.
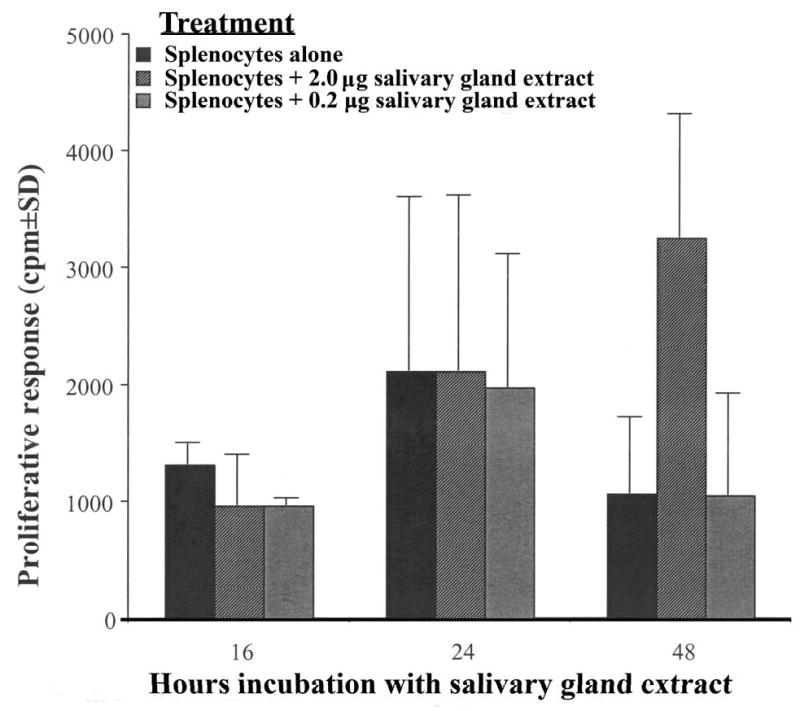
Culicoides sonorensis salivary gland protein is not directly toxic for murine splenocytes. C57BL/6 spleen cells were incubated with the indicated amounts of C. sonorensis salivary gland protein for 16, 24, or 48 hours and were assessed for proliferation as in Figure 1. The figure is representative of two independent experiments.
A 66-kDa glycoprotein in the Con A–binding fraction retains inhibitory activity
Lectin affinity chromatography selectively purified glycoproteins present in the SGE that interact with Con A. Figure 5B shows a major 66-kDa Con A–binding protein (CBP66) eluted from the column. Incubation of murine splenocytes with dilutions of the CBP fraction, derived from the PBS extract of C. sonorensis salivary glands, inhibited the proliferation of splenocytes induced by LPS as indicated by a decrease in the incorporation of 3H-thymidine (Figure 6). An identical affinity chromatography column was processed in parallel with only PBS in place of SGE as a negative control (data not shown). The 1:10 and 1:50 dilutions of CBP66 fractions significantly inhibited the proliferation of spleen cells by 71% (P = 0.028) and 75% (P = 0.037), respectively. A protein profile of C. sonorensis salivary glands in Figure 5A is shown for comparison. Approximately 20 silver-stained proteins are observed in this extract.
Figure 5.
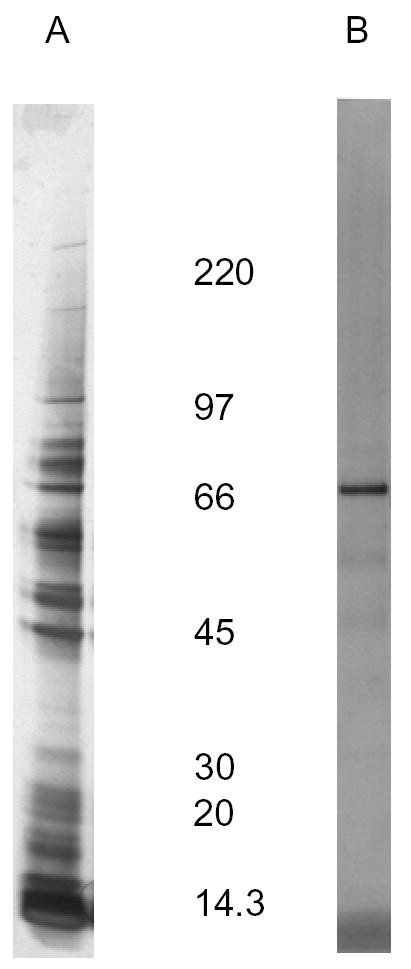
Culicoides sonorensis Con A binding protein and SGE. (A), The equivalent of 10 pairs of salivary glands (2 μg) was loaded on a reducing SDS-PAGE gel, and the proteins were stained with silver stain. (B), Con A–binding protein of 66 kDa, eluted from a lectin affinity chromatography column, reacts with biotinylated Con A on a lectin blot. An identical affinity chromatography column was processed in parallel with only PBS in place of SGE as a negative control (data not shown). (B) is representative of two independent experiments.
Figure 6.
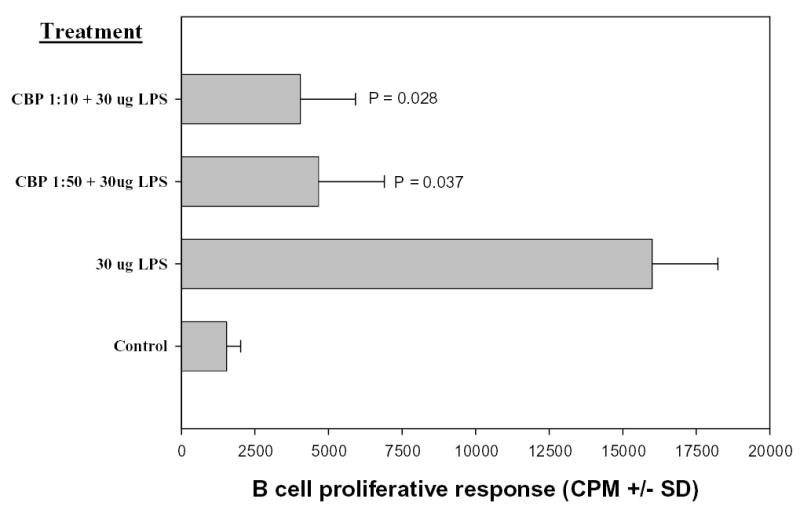
Culicoides sonorensis CBP fraction inhibits proliferation of C57BL/6 splenic B cells. C57BL/6 spleen cells were treated with dilutions of CBP (1:10 or 1:50). After 2 hours, LPS was added to all wells with the exception of the negative control. Proliferation was assessed as in Figure 1. Both dilutions of CBP significantly inhibited proliferation. (CBP 1:10, P = 0.028; CBP 1:50, P = 0.037). This figure is representative of two independent experiments.
DISCUSSION
The in vitro data reported here extend previous observations from a variety of hematophagous arthropods showing that salivary gland extracts modulate the immune response of the vertebrate host. Two levels of normal immune cell function were negatively affected by Culicoides extracts. The expansion of both T and B mitogen-stimulated splenocytes was inhibited. These cells are capable of mounting a specific immune response to antigens/pathogens by the production of secreted products such as antibody by B cells. Second, macrophages were inhibited in their ability to produce NO in response to LPS. Macrophages can act as antigen-presenting cells and can be critical for the activation of T cells. In addition, they are phagocytic and are involved in killing intracellular pathogens including viruses, bacteria, and protozoans. One of the mechanisms by which macrophages kill intracellular L. major parasites is through the production of NO.27–29 Moreover, NO has an effect on VSV infection in vivo that is mediated in the murine central nervous system by interleukin (IL)-12, interferon (IFN)γ, and NO synthase.17,18 These observations and the results reported in this study are consistent with evidence that Culicoides saliva may have play a role in the establishment of VSV infection in vertebrate hosts.11 The observations that C. sonorensis transmission of VSV to cattle and guinea pigs is more efficient than direct syringe inoculation of higher titer inoculum support the hypothesis that C. sonorensis saliva increases the likelihood of VSV infection in susceptible vertebrate hosts.17,18 The influence of Culicoides saliva in arbovirus infection requires further study.
Salivary gland components can be used as protective immunogens as shown in animal models.12,30,31 It should be noted that chronic exposure of vertebrate hosts to vector saliva in endemic areas might be one of the factors involved in their age-related resistance to arthropod-borne diseases.32,33 This is an intriguing observation; however, immunization with arthropod saliva or SGE is not a viable option. Therefore, specific molecules of the C. sonorensis salivary gland need to be purified for consideration as immunogens for a vaccine.
There are other abundant components derived from epithelial cells that are secreted with the saliva, and these can have profound effects on the immune system.34–36 Cytoplasmic components not destined for secretion are not covalently modified with the N-linked glycans that are synthesized in the Golgi system. In arthropods, most N-linked glycans are of the paucimannosidic structure, which can be recognized by man-nose-specific lectins such as Con A.37 Therefore, it is possible to affinity purify C. sonorensis SGE inhibitors using Con A, which recognizes mannose-rich N-linked glycans. Interfering with salivary immunomodulators might reduce the establishment of infection in vertebrate hosts. CBP66 is likely present in the saliva of C. sonorensis. Therefore, it could be a candidate antigen to control some of the microorganisms that C. sonorensis transmits to its vertebrate hosts. Furthermore, given the immunogenicity and antigenicity of arthropod glycans, the N-linked glycan attached to CBP66 may be the target of a vaccine.38 Our research on C. sonorensis salivary gland pharmacology provides the foundation to explore the role of salivary factors in Culicoides vectors of animal diseases.
Acknowledgments
The authors thank B. Drolet and M. A. Stuart, for providing some of the biting midges used in this study and B. Drolet, C. Campbell, and D. Gillespie for reading the manuscript and making valuable comments.
Footnotes
Authors’ addresses: J. V. Bishop, J. S. Mejia, and R.G. Titus, Colorado State University, Department of MIP, 1619 Campus Delivery, Fort Collins, CO 80523, Telephone: 970-491-1607, Fax: 970-491-0603, E-mails: Jeanette.Bishop@colostate.edu, Santiago.Mejia@colostate.edu, Richard.Titus@colostate.edu. A. A. Pérez de León, SCYNEXIS, PO Box 12878, Research Triangle Park, NC 27709-2878, E-mail: tobe.mexfra@scynexis.com. W. J. Tabachnick, Florida Medical Entomology Laboratory, Department of Entomology and Nematology, University of Florida, IFAS, Vero Beach, FL 32962, Telephone: 772-778-7200, Fax: 772-778-7205, E-mail: wjt@ufl.edu.
Financial support: This work was supported by grants from the National Institutes of Health (AI 27511 and AI 065784). Portions of this study were part of the PhD dissertation of A.A.P. at the University of Wyoming and supported by the USDA, Agricultural Research Service.
References
- 1.Mercer DR, Castillo-Pizango MJ. Changes in relative species compositions of biting midges (Diptera: ceratopogonidae) and an outbreak of Oropouche virus in Iquitos, Peru. J Med Entomol. 2005;42:554–558. doi: 10.1093/jmedent/42.4.554. [DOI] [PubMed] [Google Scholar]
- 2.Tabachnick WJ. Culicoides variipennis and bluetongue—virus epidemiology in the United States. Annu Rev Entomol. 1996;41:23–43. doi: 10.1146/annurev.en.41.010196.000323. [DOI] [PubMed] [Google Scholar]
- 3.Price DA, Hardy WT. Isolation of the bluetongue virus from Texas sheep—Culicoides shown to be a vector. J Am Vet Med Assoc. 1954;124:255–258. [PubMed] [Google Scholar]
- 4.Titus RG, Bishop JV, Mejia JS. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006;28:131–141. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen JF, Gudderra NP, Francischetti IM, Ribeiro JM. The role of salivary lipocalins in blood feeding by Rhodnius prolixus. Arch Insect Biochem Physiol. 2005;58:97–105. doi: 10.1002/arch.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champagne DE. Antihemostatic strategies of blood-feeding arthropods. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:375–396. doi: 10.2174/1568006043335862. [DOI] [PubMed] [Google Scholar]
- 7.de Almeida MC, Vilhena V, Barral A, Barral-Netto M. Leishmanial infection: analysis of its first steps. A review. Mem Inst Oswaldo Cruz. 2003;98:861–870. doi: 10.1590/s0074-02762003000700001. [DOI] [PubMed] [Google Scholar]
- 8.Nuttall PA, Labuda M. Dynamics of infection in tick vectors and at the tick-host interface. Review. Adv Virus Res. 2003;60:233–272. doi: 10.1016/s0065-3527(03)60007-2. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 10.Schoeler GB, Wikel SK. Modulation of host immunity by haematophagous arthropods. Ann Trop Med Parisitol. 2001;95:755–771. doi: 10.1080/0003498012011118. [DOI] [PubMed] [Google Scholar]
- 11.Tabachnick WJ. Pharmacological factors in the saliva of blood-feeding insects. Ann N Y Acad Sci. 2000;916:444–452. doi: 10.1111/j.1749-6632.2000.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 12.Kamhawi S. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2000;2:1765–1773. doi: 10.1016/s1286-4579(00)01331-9. [DOI] [PubMed] [Google Scholar]
- 13.Bowman AS, Coons LB, Needham GR, Sauer JR. Tick saliva: Recent advances and implications for vector competence. Med Vet Entomol. 1997;11:277–285. doi: 10.1111/j.1365-2915.1997.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 14.Cupp EW, Cupp MS. Black fly (Diptera: Simuliidae) salivary secretions importance in vector competence and disease. J Med Entomol. 1997;34:87–94. doi: 10.1093/jmedent/34.2.87. [DOI] [PubMed] [Google Scholar]
- 15.Drolet BS, Campbell CL, Stuart MA, Wilson WC. Vector competence of Culicoides sonorensis (Diptera:Ceratopogonidae) for vesicular stomatitis virus. J Med Entomol. 2005;42:409–418. doi: 10.1603/0022-2585(2005)042[0409:vcocsd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Nunamaker RA, Pérez de León AA, Campbell CL, Lonning SM. Oral infection of Culicoides sonorensis (Diptera:Ceratopogonidae) by vesicular stomatitis virus. J Med Entomol. 2000;37:784–786. doi: 10.1603/0022-2585-37.5.784. [DOI] [PubMed] [Google Scholar]
- 17.Pérez de León AA, Tabachnick WJ. Transmission of vesicular stomatitis virus to cattle by the biting midge, Culicoides sonorensis (Diptera: Ceratopogonidae) J Med Entomol. 2006a;43:323–329. doi: 10.1603/0022-2585(2006)043[0323:tovsnj]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Pérez de León AA, O’Toole D, Tabachnick WJ. Infection of guinea pigs with vesicular stomatitis virus transmitted by the biting midge, Culicoides sonorensis (Diptera: Ceratopogonidae) J Med Entomol. 2006;43:568–573. doi: 10.1603/0022-2585(2006)43[568:iogpwv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Pérez de León AA, Ribeiro JM, Tabachnick WJ, Valenzuela JG. Identification of a salivary vasodilator in the primary North American vector of bluetongue viruses, Culicoides variipennis. Am J Trop Med Hyg. 1997;57:375–381. doi: 10.4269/ajtmh.1997.57.375. [DOI] [PubMed] [Google Scholar]
- 20.Pérez de León AA, Valenzuela JG, Tabachnick WJ. Anticoagulant activity in salivary glands of the insect vector Culicoides variipennis sonorensis by an inhibitor of factor Xa. Exp Parasitol. 1998;88:121–130. doi: 10.1006/expr.1998.4210. [DOI] [PubMed] [Google Scholar]
- 21.Pérez de León AA, Tabachnick WJ. Apyrase activity and adenosine diphosphate induced platelet aggregation inhibition by the salivary gland proteins of Culicoides variipennis, the North American vector of bluetongue viruses. Vet Parasitol. 1996;61:327–338. doi: 10.1016/0304-4017(95)00828-4. [DOI] [PubMed] [Google Scholar]
- 22.Pérez de León AA, O’Toole TD, Schmidtmann ET, Titus RG, Tabachnick WJ. Insect blood-feeding and the transmission of arboviruses and vesiculoviruses. Proc US Animal Health Assoc. 1997;101:29–34. [Google Scholar]
- 23.Hunt G. A procedural manual for the large-scale rearing of the biting midge, Culicoides variipennis (Diptera: Ceratopogonidae) US Dep Agric Research Service Tech Bull. 1994;121:1–68. [Google Scholar]
- 24.Urioste S, Hall LR, Telford SR, Titus RG. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–1086. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titus RG, Kelso A, Louis JA. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984;55:157–165. [PMC free article] [PubMed] [Google Scholar]
- 26.Chakkalath HR, Titus RG. Leishmania major-parasitized macrophages augment Th2-type T cell activation. J Immunol. 1994;153:4378–4387. [PubMed] [Google Scholar]
- 27.Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen oxides in macrophages: A protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Liew FY, Cox FE. Nonspecific defense mechanism: The role of nitric oxide. Immunol Today. 1991;12:A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- 29.Mauel J, Corradin SB, Buchmuller Rouiller Y. Nitrogen and oxygen metabolites and the killing of Leishmania by activated murine macrophages. Res Immunol. 1991;142:577–580. doi: 10.1016/0923-2494(91)90106-s. [DOI] [PubMed] [Google Scholar]
- 30.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 31.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Bor-relia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies CR, Mazloumi Gavgani AS. Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology. 1999;119:247–257. doi: 10.1017/s0031182099004680. [DOI] [PubMed] [Google Scholar]
- 33.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, Valenzuela JG, Barral-Netto M, Barral A. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 34.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci USA. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 37.Hollister J, Grabenhorst E, Nimtz M, Conradt H, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinglasan RR, Valenzuela JG, Azad AF. Sugar epitopes as potential universal disease transmission blocking targets. Insect Biochem Mol Biol. 2005;35:1–10. doi: 10.1016/j.ibmb.2004.09.005. [DOI] [PubMed] [Google Scholar]


