Abstract
To examine the neural basis of route knowledge by which one can reach one's destination, we recorded the activity of 580 neurons in the monkey medial parietal region (MPR) while monkeys actively navigated through a virtual environment. One hundred eighty of these neurons (31%) showed significant responses to the monkeys' movements in the virtual environment. Of these responsive neurons, 77% (139/180) showed responses associated with a specific movement at a specific location (navigation neurons), 8% (14/180) showed responses associated with a specific movement (movement-selective neurons), and the remaining 27 neurons (15%) were nonselective. We found navigation neurons whose responses to the same movement at the same location were modulated depending on the route that the monkey was currently taking, that is, in a route-selective manner (32 of 59 tested neurons among 139 navigation neurons, route-selective navigation neurons). The reversible inactivation of MPR neurons by muscimol resulted in a monkey becoming lost during the navigation task trial. These results suggest that MPR plays a critical role in route-based navigation by integrating location information and self-movement information.
Keywords: route knowledge, virtual environment, cognitive map
A cognitive map is a stored representation of a large-scale environment in the brain. When we behave in a large-scale environment, a cognitive map is necessary but not sufficient, because it is too abstract to plan a specific route map for our navigation. When we drive to our office, we can take the correct route subconsciously, making a turn or going straight at each intersection. This phenomenon suggests we may have an internal list of what we have to do at a given location in addition to a “cognitive map” in our brain. This internal list is known as “route knowledge” and is accessed to be able to navigate ourselves in a large-scale environment (1, 2). Lesion and neuroimaging studies of humans suggest that the medial parietal region (MPR), including the retrosplenial and posterior cingulate cortices, is critically involved in navigation (3–8) based on route knowledge. To study the neural mechanisms of navigation in a large environment in primates, a large environment within the experimental setup needs to be built. In this study, we used a virtual reality technique to overcome this problem and recorded a single unit activity from the monkey MPR while the monkey navigated in the virtual environment without performing any actual movement.
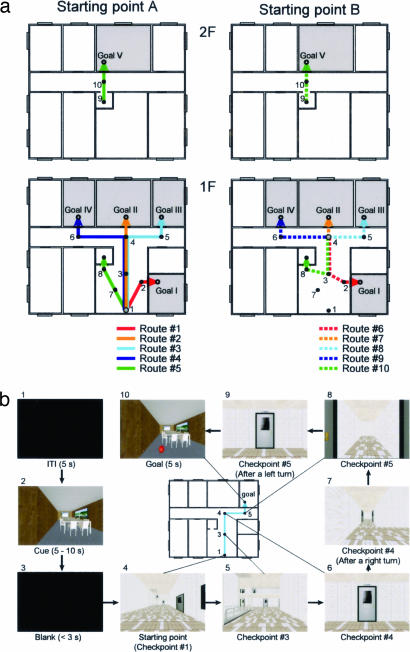
Two Japanese monkeys were trained to perform a navigation task in a virtual reality building (Fig. 1; see also Fig. 6 and Movie 1, which are published as supporting information on the PNAS web site). The destination room was presented to the monkeys at the beginning of each trial, and then they controlled their virtual movement using a joystick from a starting point (SP) to the destination room through a series of checkpoints (CPs). At each CP, the monkeys chose one of three joystick operations to move to the next CP, tilting the joystick forward to move forward and tilting it to the left or right to make a left or a right rotation, respectively. The operation of the joystick evoked the motion of the scene on the screen that gave the sensation, to the monkey, of moving inside the virtual building. There was no whole-body movement associated with the movement in the virtual environment. The routes from SPs to the destination rooms were predetermined. The monkeys could not stray from the predetermined routes during the task (see Experimental Setup and Navigation Task in Materials and Methods for more detail). Our navigation task had more than one destination in a large-scale space and thus required a planned route from each SP to each destination (9). This property of our task focuses on route knowledge among navigational strategies.
Fig. 1.
Navigation task in a virtual environment. (a) Floor plan of virtual building used in present study. The black dots indicate CPs, and the lines connecting them indicate the routes. The monkeys were required to navigate from one of the SPs (shaded circles) to one of the goal rooms. When starting from either of the SPs, the initial direction was toward CP no. 3. (b) Time sequence of trial (route no. 3, from SP A to Goal III). Each box indicates the image projected on the screen. The image was projected stereoscopically. The number on the upper left side of each box indicates the temporal order (see also Movie 1).
Results
We recorded the activity of 580 neurons in the MPR of four hemispheres (Fig. 2) while the monkeys were performing the navigation task. One hundred eighty of these neurons (31%) showed significant responses to the monkeys' movements during navigation in the virtual reality building. We analyzed the mean discharge rate of any movement period at each CP (during the 1-s period from the onset of movement at each CP). Of these responsive neurons, 77% (139/180) showed responses associated with a specific movement at a specific location (navigation neuron), 8% (14/180) showed responses associated with a specific movement (movement-selective neuron), and the remaining 27 neurons (15%) were nonselective (see Materials and Methods for more detail).
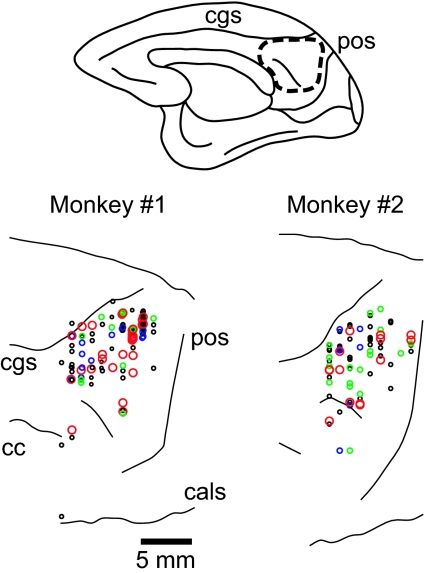
Fig. 2.
Recording site. (Upper) Medial view of a monkey's brain. The area enclosed by the dashed line indicates the recording area. The recording site was reconstructed on magnetic resonance images of each monkey. (Lower) Recording sites of navigation (red, green, and black circles) and movement-selective (blue circles) neurons in monkeys nos. 1 (Left) and 2 (Right). Red and green circles indicate the recording sites of route- and location-selective navigation neurons, respectively. The data obtained from both hemispheres are superimposed on those obtained from the right hemisphere. cgs, cingulate sulcus; pos, parietooccipital sulcus; cc, corpus callosum; and cals, calcarine sulcus.
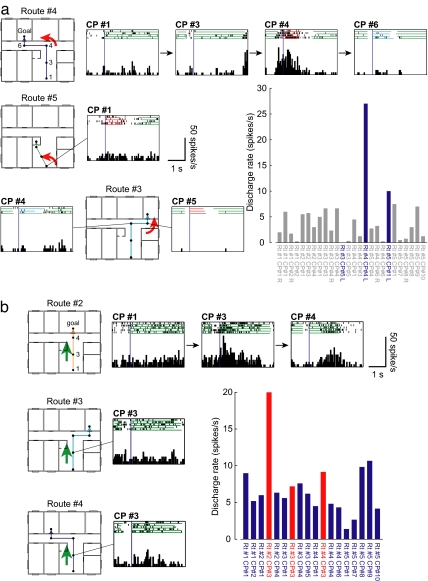
The neuron shown in Fig. 3a is an example of a navigation neuron. This neuron responded while the monkey made a left turn at CP no. 4 on route no. 4. This neuron did not respond to a left turn at other locations (Fig. 3a Middle and Bottom) or other movements (forward, right turn; Fig. 3a, bar graph).
Fig. 3.
Examples of navigation neurons. (a) (Top) Neuronal activity on route no. 4. Each histogram and raster diagram were aligned at the onset of movement at each CP or SP (vertical dark-blue line). The color used to underline each raster diagram indicates the type of movement during that period: green, forward movement; red, left turn; and blue, right turn. This neuron became active when the monkey made a left turn (red underline) at CP no. 4. (Middle and Bottom) This neuron did not respond when the monkey made a left turn at the other locations (CP no. 1 on route no. 5 and CP no. 5 on route no. 4). (Right) Discharge rates during all movement periods. The blue bars indicate neuronal activity during the left turn period. The response at CP no. 4 on route no. 4 was significantly stronger than those at the other CPs (P <0.05). The selectivity and discrimination indices for this neuron were 1.00 and 0.72, respectively. Rt, route; L, left turn; and R, right turn. (b) An example of route-selective navigation neuron. (Top) Neuronal activity on route no. 2. Note that this neuron became active when the monkey made a forward movement (green underline) from CP no. 3 on route no. 2. (Middle) Responses during forward movement from CP no. 3 on route no. 3. (Bottom) Responses during forward movement from CP no. 3 on route no. 4. Note that this neuron did not respond when the monkey made the same movement at the same location on these two routes. (Right) Discharge rate during all movement periods when the monkey made a forward movement. The red bars indicate the response to the same movement at the same CPs. Note that this neuron showed the strongest response at CP no. 3 on route no. 2; the response was significantly stronger than at other CPs (P <0.05). The selectivity and discrimination indices for this neuron were 1.00 and 0.67, respectively.
As a result of route setting, there were movement periods with the same movement at the same location on different routes (e.g., the movement period at CP no. 3, forward movement on routes nos. 2–4). If a neuron showed the best response to such a route segment, we could test the route selectivity of the neuron. Fifty-nine of 139 navigation neurons met the requirements for this analysis. Of the 59 navigation neurons, 54% (32/59) responded in a route-selective manner (route-selective navigation neuron). Among the 32 route-selective navigation neurons, four neurons were found responsive at CP no. 3, 3 at CP no. 2, 6 at CP no. 3, 9 at CP no. 4, 2 at CP no. 6, 7 at CP no. 8, and 1 at CP no. 9. Fig. 3b shows a typical activity of a route-selective navigation neuron. This neuron responded to a specific movement at a particular CP (forward movement at CP no. 3); furthermore, this response was observed only when the monkey was moving toward goal II (route no. 2). A similar response was not observed when the monkey was moving toward goal III (route no. 3, Fig. 3b Middle) and goal IV (route no. 4, Fig. 3b Bottom). This neuron did not respond during the forward movement from any other CPs (Fig. 3b, bar graph). Thus, the discharge of this route-selective navigation neuron appeared to correlate with a specific segment of a specific route, indicating an association with the representation of route knowledge. The remaining neurons (46%, 27/59) responded with a nonroute-selective manner (Fig. 7, which is published as supporting information on the PNAS web site) and may represent a certain location (location-selective navigation neuron).
As mentioned above, we found neurons whose responses were associated with a specific movement (14/180). Neurons of this type responded while the monkey made a particular movement (e.g., right turn) irrespective of CP on any route (Fig. 8, which is published as supporting information on the PNAS web site). We could not find consistent differences in the recording sites of each neuron type in the MPR (Fig. 2).
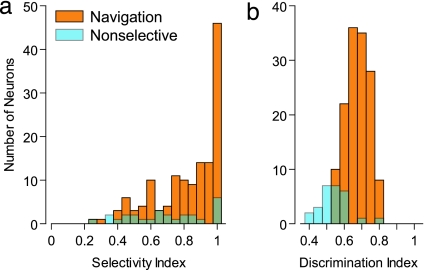
We assessed the degree of response selectivity of navigation neurons using selectivity index (see Materials and Methods). The selectivity index will be high when the best response to a movement at a given location is prominently strong. The mean selectivity indices for the navigation and nonselective neurons were 0.81 ± 0.20 and 0.67 ± 0.24 (mean ± SD), respectively (Fig. 4). The selectivity index for the navigation neurons was significantly higher than for the nonselective neurons [t (164) = 3.24, P < 0.005]. The selectivity index does not take response variability into account. Thus, we also calculated the discrimination index to characterize the ability of a neuron to discriminate a stimulus difference relative to its intrinsic variation (10). The discrimination indices for the navigation and nonselective neurons were 0.65 ± 0.06 and 0.51 ± 0.08 (mean ± SD), respectively (Fig. 4). The discrimination index for the navigation neurons was significantly higher than for the nonselective neurons [t (164) = 9.77, P < 0.001]. The selectivity and discrimination indices for the neuron shown in Fig. 3a were 1.00 and 0.72, respectively, and those for the neuron shown in Fig. 3b were 1.00 and 0.67, respectively. It was suggested that these navigation neurons had typical selectivity. We next analyzed the distribution of the preferred route segment of the navigation neurons (see Materials and Methods). We observed that the preferred route segment of the navigation neurons was not concentrated on a specific route segment (χ2 test, P > 0.1). Thus, the preferred route segment of navigation neurons was equally distributed on all routes, and it seemed that all route knowledge was represented by navigation neurons without bias.
Fig. 4.
Distribution of selectivity (a) and discrimination indices (b) of navigation (orange) and nonselective (cyan) neurons.
We analyzed the effects of the monkey's eye movement in the response of the 70 navigation neurons (including 14 route- and 20 location-selective neurons) from which we obtained a complete data set. First, we calculated the correlation coefficient between the number of saccades and the discharge rate for each neuron during the movement period and found that 65 neurons of investigated 70 neurons (93%) did not show significant correlation (P >0.1). Next, we investigated the effects of eye position. The visual field was divided into four quadrants (left or right and upper or lower) and examined the bias of time spent on each quadrant using the χ2 test. If we observed any biases in time spent, we could attribute the change in neuronal activity to the bias of the eye position. However, in 69 of the 70 neurons (99%), we did not find such a tendency (χ2 test, P >0.1). It is unlikely that the responses of the navigation neurons are explained only by their correlation to eye movement.
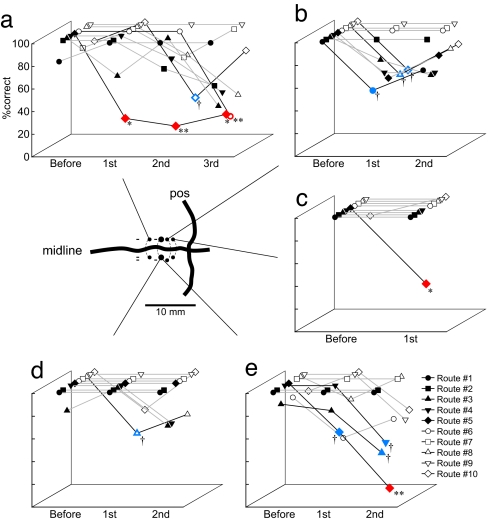
To confirm that our recording site in MPR was involved in navigation, we carried out inactivation experiments by the bilateral microinjection of muscimol, a GABA A receptor agonist. Of 15 inactivation sessions, the performance in the navigation task was significantly impaired in three sessions (Fig. 5a, c, and e; P <0.05 using the χ2 test) and tended to be impaired in two sessions (Fig. 5 b and d, P <0.1). The effects were observed in a route-selective manner. In one session (Fig. 5c), the performance for only one route was selectively impaired. In some sessions, navigation error tended to occur at a specific CP. This was in line with the finding that most MPR neurons represented a specific movement at a specific CP. These results suggest MPR is involved in generating a route and plays a critical role in navigation. The impairments caused by the inactivation of MPR did not seem to be due to simple deficits in sensory or motor processes. The total time required by a monkey to reach its destination and the averaged time required to move through one segment were measured, and they were analyzed by using two-way ANOVA with the times of injection (30 min before injection and the first 30 min after injection) and the route (10 routes) as factors. In both total time [F (9, 276) = 5,060.67, P < 0.001] and averaged required time [F (9, 278) = 1,820.45, P < 0.001], the significant main effect was the route factor. There were no significant main effects of the time of injection factor and the interaction (P >0.1).
Fig. 5.
Results of muscimol injection experiments. Each graph indicates the performance of the monkey during each experimental session. Performances were evaluated every 30 min after muscimol injection. The data are shown for each route. (Inset) The injection site. Closed circles indicate the sites where the effects of the injection could be observed. Sites with the minus sign are those showing no effects. ∗∗, P < 0.01; ∗, P < 0.05; †, P < 0.1.
Discussion
This work demonstrates cortical neuronal representation of route knowledge. Our findings strongly suggest that MPR neurons as a population represent route knowledge and are indeed involved in navigation based on route knowledge. The existence of location-selective navigation neurons and movement-selective neurons suggests that information regarding a location and a movement is independently represented in some MPR neurons. Route-selective navigation neurons may integrate information from these neurons. The navigation neurons responded to a unique combination of a specific movement and location.
Although the virtual-reality system used in this study is advantageous for overcoming difficulty in executing this type of experiment, navigation in this experimented setup is different from that in the real world. Because there were no actual whole-body or chair movements associated with movement in the virtual environment, information about self movement came from the optic flow of the scene on the screen. Thus, the responses of navigation neurons could be explained as responses to a specific optic flow pattern, which was the combination of a specific scene (location) and its specific motion (movement). However, the responses of the route-selective navigation neurons (Fig. 3b) could not be explained by such visual responses alone, because these neurons were not active in other routes, even though the pattern of optic flow was the same. In our preliminary experiments, we tested the passive response of navigation neurons. We presented two types of movie, and the monkey watched only without joystick manipulation. One was a movie of the entire route from the SP to the destination room, which included the most-preferred route segment of that neuron; the other was a movie that included the most-preferred route segment. Thirty-nine percent of 46 tested navigation neurons, including the route-selective navigation neurons, responded to both movies; however, 22% of those did not respond to both movies. Furthermore, 35% of neurons responded only to the most-preferred segment that was presented in the movie that showed the entire route. These results are preliminary; however, they suggest that the response of route-selective navigation neurons was affected by route knowledge. Thus, route-selective navigation neurons in MPR could represent route knowledge particularly associated with the route that one is currently taking.
A possible criticism of our interpretation of navigation neurons is that these activities were associated with specific movements of body parts. As mentioned in Results, analyses of eye movements suggest that the number of saccades and eye positions did not affect the activity of almost all navigation neurons. We allowed the monkey to move its eyes freely, and the navigation task was long; thus, it was difficult to analyze precisely the temporal pattern of eye movement. However, when we roughly compared these results, we could not find a specific relationship between the temporal pattern of eye movement and neuronal activity. Thus, we are convinced the activity of navigation neurons was not associated with a specific eye movement. Regarding body movement, the monkey was seated with its head fixed on a primate chair, similar to a high chair for children at a restaurant, with its body facing the front. We could not observe a marked body movement that seemed associated with the monkey's behavior in the task except its arm movement. We did not record body electromyograms, and there is a possibility of a relationship at a more fundamental level. However, if MPR neurons are associated with a specific body movement, such neurons are expected to show the same activity during the specific movement. However, the percentage of movement-selective neurons among MPR neurons, which showed activation in response to the same movement, was very low (8%). The majority of neurons showed differential activation for the same movement; furthermore, the route-selective navigation neurons showed differential activation for the same movement at the same location. Thus, it is unlikely that the activity of navigation neurons was associated with specific body movements.
We found no neurons that were active either during the initial route-planning period or during the entire navigation period toward a given goal. Furthermore, the preferred route segments were equally distributed. Thus, navigation along a route may be processed by an ensemble of neurons in the MPR. Each route-selective navigation neuron may represent knowledge of a route segment, which is combined with knowledge of other route segments sequentially to represent knowledge of the complete route.
The involvement of the MPR in navigation based on route knowledge is also suggested by the finding that muscimol affects the ability of the monkey to generate a route. Errors caused by muscimol injection occurred in a route-selective manner (for example, on route no. 5) and tended to occur at a specific CP. Thus, these findings suggest that neurons with the same properties are clustered in the MPR. However, bilateral injections were more effective than a hemilateral injection, and a distinct topographical organization of cell location based on their properties was not clear in this study. We need to further examine the distribution and clustering of neurons in the MPR. The effects of muscimol injections may not be explained by a fundamental impairment of movement of specific body parts, because we could not find a substantial difference in time data before and after muscimol injection. As mentioned above, very clear deficits tended to be observed in route no. 5. Indeed, this could be explained by the clustering of neurons related to this route. However, this route had specific properties, such as the route to the second floor with an elevator and one of the longest routes. Thus, there is a possibility that these properties may have an impact on the effects of muscimol.
Our results are consistent with case reports on topographically disorientated patients (4). Usually, patients with damage in the MPR can recognize the buildings and landscape around them and determine their current location. However, they cannot determine which direction they should take to reach their destination. Our results indicate the possibility that route-selective navigation neurons in the MPR integrate information of location and that of movement at that location. Thus, deficits in navigation in humans may be caused by an impairment of the function of route-selective navigation neurons.
The next question is the source of information for MPR neurons. As mentioned above, the responses of navigation neurons could be explained as responses to a specific optic flow pattern (combination of a specific movement and location). Neurons in the medial superior temporal (MST) area represent a heading direction based on optic flows (11, 12), not only forward movement but also turning. Furthermore, these neurons represent a location as well (13). This, together with anatomical connections between MPR and MST (14, 15), suggests that MST is one of the candidates for information sources for MPR neurons in the dorsal visual system. The medial temporal region, including the hippocampus, is another strong candidate. The medial temporal regions also have direct connections with MPR (14, 15). Place cells are in the hippocampus of rodents (16, 17) and macaques (18). Furthermore, in macaques, hippocampal neurons were found to respond to spatial views (19) and whole-body movement (20). Recent studies have shown that hippocampal neurons in rats exhibit differential activities depending on the direction of movement (21) or on an upcoming movement (left or right turn) (22) even at the same place. Task demands for animals are different between those studies and ours; however, the properties of neuronal activity are similar to those of MPR neurons we recorded. Authors of those studies focused more on memory aspects of these neurons. We need to carry out further experiments to clarify functional differences between the hippocampus and the MPR; however, differences in species of experimental animals and conditions should be taken into consideration.
Human imaging studies (5–8) showed the importance of the neural network among the hippocampus, parietal cortex, and retrosplenial cortex in navigation. In those studies, Maguire and colleagues (5–8) hypothesized that the hippocampus provides an allocentric representation of space from a stored cognitive map for an initial route planning, the parietal cortex computes an egocentric representation to enable movement toward the goal from the allocentric representation, and then the retrosplenial cortex integrates these allocentric and egocentric representations for ongoing route planning during navigation. Our results support their concept, that is, MPR neurons integrate location information (allocentric) with movement direction information (egocentric) at that location to reach the final destination.
Materials and Methods
Subjects.
The subjects were two male Japanese monkeys (Macaca fuscata), which were cared for in accordance with the guidelines outlined in the “Guide for the Care and Use of Laboratory Animals” from the National Research Council (1996). The project was approved by the Ethical Committee of the Nihon University School of Medicine.
Experimental Setup.
During the experiments, the monkey was seated on a primate chair, similar to a high chair for children at a restaurant, with its head fixed (Fig. 6). Its lower legs were strained lightly to make its body face the front. Thus, its body movement was rather restricted except for its arms and eyes. The monkey could move its eyes freely, and the position of one eye was monitored routinely during unit recording using an infrared eye movement recording system (sampling rate 250 Hz, RMS, Hirosaki, Japan). On a 100-inch tangential screen, a virtual environment generated by computer graphics (hardware, SGI, Tokyo, Japan; software, Solidray, Yokohama, Japan) was projected stereoscopically by two liquid crystal projectors (Victor, Tokyo, Japan). The monkey wore polarized glasses and was able to see the stereoscopic images. The monkey manipulated a joystick attached to the chair to move in a virtual environment (see below).
Navigation Task.
Floor plan.
A virtual building with two floors was prepared for this experiment (Fig. 1). The building included eight rooms on the first floor and seven rooms on the second, which opened onto a corridor. All rooms had a door. An elevator connected the first and second floors. When the monkey reached the elevator, it automatically moved to the other floor.
Route setting.
Two SPs and five goals were prepared, so the monkey learned a total of 10 routes (Fig. 1). The routes from SPs to the destination rooms were predetermined. The monkey was overtrained to trace the route and could not stray from the predetermined routes during the task during both training and experiment.
Joystick operation.
The monkey controlled its virtual movement using a joystick from SP to the destination room through a series of CPs (Movie 1). At each CP, the monkey chose one of three joystick operations to move to the next CP, tilting the joystick forward to move forward and tilting it to the left or right to make a left or a right rotation, respectively. The monkey was to keep tilting the joystick during the movement. The operation of the joystick evoked an optic flow of a scene on the screen, making the monkey sense its corresponding movement. Thus, when the monkey moved to the next CP at the right side, as in the case when the monkey has arrived at CP no. 4 then moved on to CP no. 5, the monkey first kept the joystick tilted to the right until the scene stopped its left rotation, then released the joystick once and again kept it tilted forward until the optic flow, which gave the sensation of moving forward, stopped.
Trial.
In each trial, the route was selected pseudorandomly. When the monkey reached the goal room, a drop of juice was delivered as a reward. It took ≈14 s on average for the monkey to reach the goal room. If the monkey moved to a wrong CP, the trial was aborted, and a time-out period (10–30 s) was imposed. Each route was generally repeated five times in a recording session. The two monkeys performed the task correctly in 87 ± 14% and 94 ± 8% (means ± SDs) of the test trials. It took ≈30 min to finish all trials in the recording session.
Data Acquisition and Analysis.
We recorded single-unit activity from the MPR. The details of the method of single-unit recording were described previously (23). The recording site included area 7m and the posterior cingulate and retrosplenial cortices (Fig. 2).
Classification.
We considered a neuron to be responsive when the mean discharge rate for any movement period (during the 1-s period from the onset of movement) was significantly higher than that during the control period (during the 1-s period before the cue presentation; paired t test). We identified navigation neurons using the following analyses. First, the movement period that elicited the strongest response among all movement periods was determined (the best movement period). The mean activity for the best movement period was compared with that for the other movement periods, including the same movement at different locations (ANOVA and posthoc pair-wise comparison; Newman–Keuls test). If the mean activities were significantly different (P < 0.05), the neuron was classified as a navigation neuron. Subsequently, for the remaining neurons, all movement periods were pooled into three movement categories (forward movement and left and right turns) and compared. If the mean activities were significantly different among these movement categories (P < 0.05), the neuron was classified as a movement-selective neuron. Finally, the remaining neurons were classified as nonselective neurons. As a result of the route setting, there were movement periods with the same movement at the same location on different routes (e.g., the movement period at CP no. 3 and forward movement on routes nos. 2–4). If that was the case with the best movement period, comparisons were made among them. If the mean neuronal activities were significantly different (P < 0.05), the neuron was classified as a route-selective navigation neuron. If not, the neuron was classified as a location-selective navigation neuron.
Selectivity Index.
To describe the selectivity of navigation neurons, we calculated the selectivity index using the following formula. For the calculation of the index, the square root of the discharge rate was used:
Here, Rmax is the mean discharge rate of the movement period of the preferred route segment, and Rmin is the lowest mean discharge rate among all movement periods.
Discrimination Index.
The discrimination index was calculated from the square root of the discharge rate using the following formula:
Here, Rmax is the mean discharge rate of the movement period of the preferred route segment, and Rmin is the lowest mean discharge rate in all movement periods. MSerror is the mean square of the variance of all of the movement periods. This index characterizes the ability of a neuron to discriminate a stimulus difference relative to its intrinsic variation (10) and will approach one, if the best responses to a route segment are larger than the responses to any other route segments.
Preference Tendency of MPR Neurons.
We analyzed the distribution of the preferred route segments of MPR neurons. For each route segment, the number of navigation neurons that most preferred that route segment was counted. This gave us the actual distribution of the preferred route segment. Then, the expected number of preferred neurons for each route segment was calculated using the following formula:
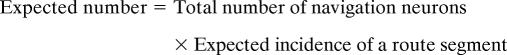 |
 |
This gave us the expected distribution of the preferred route segment. For example, in the case of CP no. 3, the “number of a combination of a movement and a CP” was 3, such as forward movement on routes nos. 2–4. The “number of all possible route segments” was 51, which was the total number of route segments on all routes. In the case of monkey no. 1, we found 67 navigation neurons, thus the “expected number” is 67 × 3/51 = 3.94. Thus, we expected 3.94 neurons that would show a selective response at CP no. 3. Finally, the actual and the expected distributions were statistically compared by using the χ2 test. If the actual and expected distributions are significantly different, it means there is some populational tendency in preference for a movement at a location.
Muscimol Injection.
We injected muscimol bilaterally into the MPR using an injection-recording device (a stainless steel injection cannula containing a Teflon-coated tungsten wire that served as a neuronal recording electrode). The tip of the injection cannula and that of the electrode were ≈0.5 mm apart. The cannula was attached to the electrode manipulator and inserted into the recording site. To confirm that the tip of the cannula was in the gray matter, extracellular neuronal recordings were carried out through the attached electrode. In one experimental session, the microinjection of muscimol (5 μg/μl) was carried out in three to five sites (1 mm apart) of each hemisphere at a speed of 0.5 μl/min. After the injection into both hemispheres, the monkeys were required to perform the task. The performances evaluated every 30 min (the monkey carried out three to five trials for each route) after the injection were statistically compared with those evaluated before the injection (χ2 test).
Supplementary Material
Acknowledgments
We thank Dr. Charles J. Duffy for helpful comments on the earlier version of this manuscript. We also thank Solidray for their assistance in developing the computer programs and contents used in presenting the virtual environment. This work was supported by a Grant-in-Aid for Japan Society for the Promotion of Science fellows, the Academic Frontier Project for Private Universities; “Brain Mechanisms for Cognition, Memory and Behavior” at Nihon University; a matching fund subsidy from Ministry of Education, Culture, Sports, Science, and Technology (MEXT), a Grant-in-Aid for Scientific Research on Priority Areas (higher-order brain functions) (Grant 17022038), and a Grant-in-Aid for Scientific Research (B) (Grant 17300131) from MEXT.
Abbreviations
- MPR
medial parietal region
- SP
starting point
- CP
checkpoint.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Siegel AW, White SH. In: Advances in Child Development and Behavior. Reese HW, editor. Vol 10. New York: Academic; 1975. pp. 9–55. [DOI] [PubMed] [Google Scholar]
- 2.Thorndyke PW, Hayes-Roth B. Cognit Psychol. 1982;14:560–589. doi: 10.1016/0010-0285(82)90019-6. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre GK, D'Esposito M. Brain. 1999;122:1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Neurology. 1997;49:464–469. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- 5.Maguire EA. Scand J Psychol. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- 6.Maguire EA, Frackowiak RSJ, Frith CD. J Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Firth CD, O'Keefe J. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 8.Spisrs H, Maguire EA. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Sakata H, Tanaka Y, Taira M. Behav Brain Res. 2004;153:287–291. doi: 10.1016/j.bbr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Prince SJD, Pointon AD, Cumming BG, Parker AJ. J Neurophysiol. 2002;87:191–208. doi: 10.1152/jn.00465.2000. [DOI] [PubMed] [Google Scholar]
- 11.Duffy CJ, Wurtz RH. J Neurophysiol. 1991;65:1346–1359. doi: 10.1152/jn.1991.65.6.1346. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Yukie M, Tanaka K, Hikosaka K, Fukada Y, Iwai E. J Neurosci. 1986;6:145–157. doi: 10.1523/JNEUROSCI.06-01-00145.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froehler MT, Duffy CJ. Science. 2002;295:2462–2465. doi: 10.1126/science.1067426. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi Y, Amaral DG. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 15.Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. J Comp Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- 16.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford, UK: Clarendon; 1978. [Google Scholar]
- 17.O'Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura N, Nishijo H, Tamura R, Eifuku S, Endo S, Ono T. J Neurosci. 1999;19:2381–2393. doi: 10.1523/JNEUROSCI.19-06-02381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolls ET, O'Mara SM. Hippocampus. 1995;5:409–424. doi: 10.1002/hipo.450050504. [DOI] [PubMed] [Google Scholar]
- 20.O'Mara SM, Rolls ET, Berthoz A, Kesner RP. J Neurosci. 1994;14:6511–6523. doi: 10.1523/JNEUROSCI.14-11-06511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 22.Loren M, Frank LM, Brown EN, Wilson M. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsui K, Jiang M, Yara K, Sakata H, Taira M. J Neurophysiol. 2001;86:2856–2867. doi: 10.1152/jn.2001.86.6.2856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.