Abstract
Reexpression of the fetally expressed β-myosin heavy chain (β-MHC) gene is a well documented marker of pathological cardiac hypertrophy and normal aging in many experimental models. To gain insights into factors affecting this reexpression of β-MHC within the complex anatomical structure of the heart, we investigated the spatial pattern of its expression at the level of single cells during aging and hypertrophy. We generated mice that express yellow fluorescent protein fused to the N terminus of the β-MHC and examined its expression pattern during normal aging and in mice with hypertrophy induced by constitutive expression of a renin transgene. The localization of fibrosis within the hearts also was determined by using a fluorescent lectin. The results show that reexpression of β-MHC occurs in discrete subsets of myocytes within the subendocardium rather than uniformly throughout the heart, that β-MHC induction is not an obligatory consequence of cellular hypertrophy, and that β-MHC-expressing cells in the normal aging heart and the hypertrophic heart are distributed predominantly in clusters within and surrounding foci of fibrosis. We conclude that β-MHC gene expression in the normal aging adult and hypertrophic mouse heart is a marker of fibrosis rather than of cellular hypertrophy.
Keywords: genetic reprogramming
The adult myocardium responds to extrinsic forms of stress, such as hypertension, myocardial infarction, and pressure overload, by a hypertrophic growth response (reviewed in ref. 1). Left ventricular hypertrophy caused by chronic hypertension is accompanied by two key pathological processes: myocyte hypertrophy and fibrosis. Hypertrophic growth of myocytes is initiated by physical stress and by endocrine, paracrine, and autocrine factors that activate a variety of receptors (reviewed in refs. 2–4). These receptors trigger multiple cytoplasmic signal transduction cascades that regulate gene expression (5), resulting in increased cell size due to increased synthesis of sarcomeric and other proteins together with reorganization of myofibrillar structures
Fibrosis is a complex multifactorial process involving reciprocal interactions between stimulatory and inhibitory factors that lead to increased collagen deposition (6–8). Myocardial fibrosis caused by hypertension is associated with increased accumulation of type I and III collagens within the adventitia of coronary arteries (perivascular fibrosis), which progressively extends into the neighboring interstitial spaces (interstitial fibrosis). Myocardial fibrosis also results from ischemia due to insufficient coronary reserve, involves the actions of plasma and local peptides, and usually accompanies nonphysiological left ventricular hypertrophy; in addition, its consequences include loss of functionality of the myocardium due to abnormal diastolic stiffness. When severe, myocardial fibrosis contributes to fatal cardiac arrhythmias due to interruptions in the normal propagation of electrical impulses (9).
A molecular feature common to cardiac hypertrophy and heart failure is the induction of a fetal gene program (reviewed in refs. 10 and 11). In the mouse and rat, cardiac β-myosin heavy chain (β-MHC) is a fetal-specific isoform whose expression in ventricular myocytes is down-regulated to low levels soon after birth. However, in normal animals, β-MHC expression gradually increases in an age-dependent manner (12). Increased expression of the β-MHC gene also is observed in most experimental models of cardiac hypertrophy and heart failure. β-MHC expression increases may directly affect cardiac function, as judged by a reduction in myofibrillar ATPase activity and reduced shortening velocity of cardiac myofibers in animals that express β-MHC in place of α-MHC (13). The up-regulation of β-MHC can be reversible in conditions, such as the regression of hypertrophy (14, 15), the rescue of murine models of heart failure (16), and in humans who respond favorably to beta blocker treatments (17–19). Recent studies suggest that reexpression of β-MHC may contribute to the overall poor functioning of the hypertrophic ventricle (13, 20).
Surprisingly little is known, however, about the manner in which individual myocytes respond during the perinatal decrease and subsequent increase in β-MHC expression accompanying normal aging or hypertrophy. Thus, it is not clear whether this up-regulation is mediated by an increase in expression levels in all myocytes or by an increase in expression levels in only discrete subsets. Also lacking is an understanding of the precise relationship between the presence or absence of hypertrophy of individual cells and the state of their β-MHC expression. Thus, it is not known whether reexpression of β-MHC is an indicator of increased cell size that leads to cardiac hypertrophy or a different event that accompanies cardiac hypertrophy. Accordingly, we investigated the precise distribution of β-MHC expression at the level of single cells within the three-dimensional space of the normal perinatal heart, in the normal adult heart, and in the hypertrophic heart. The resulting data show that reexpression of β-MHC occurs in only a subset of myocytes, is not obligatorily linked to cellular hypertrophy, and occurs predominantly in myocytes associated with regions of fibrosis.
Results
Generation of Yellow Fluorescent Protein (YFP)–β-MHC Animals.
To identify individual β-MHC expressing cells, we used gene targeting to mark the native β-MHC gene by appending the coding sequence of YFP to the 5′ end of the coding sequence of β-MHC, thereby generating a YFP–β-MHC fusion protein with YFP joined to the N terminus of β-MHC (Fig. 1). Previous work has indicated that a comparable N-terminal fusion between GFP and MHC results in a functional protein with WT enzymatic activity in vitro and can replace the normal MHC gene in Dictostylium in vivo (21). To minimize potential differences between native β -MHC and YFP–β-MHC genes, we ensured that the Kozak sequence and the intron/exon boundaries of the β-MHC gene remained unchanged. We also used a valine–proline sequence between YFP and β-MHC by following published accounts that this type of MHC fusion gene is appropriately folded in vivo (22).
Fig. 1.
Generation of the β-MHC fusion allele. (Top) WT β-MHC gene (upper line) and targeting construct (lower line). (Middle) Targeted allele in ES cells. (Bottom) YFP–β-MHC fusion allele after germline transmission. Open boxes represent 5′ UTRs, and solid boxes represent translated exons. The coding sequence of YFP is shown as a shaded box within exon 4, and loxP sites are shown as triangles. A selection cassette (23) containing a testis promoter-driving CRE (tACE-CRE) and a Neo gene is inserted into intron 1; the selection cassette is not drawn to scale.
The selection cassette (23) consists of two linked genes as shown in Fig. 1: CRE (driven by the testis-specific promoter tACE), and Neo, both flanked by loxP sites. With this selection cassette, transmission of a targeted allele through the male germline activates CRE expression, causing deletion of the entire selection cassette and leaving only a single 34-bp loxP site at the insertion site (23). We inserted the selection cassette within intron 1 of the targeting construct to minimize any potential deleterious effects of this remaining loxP site. All factors controlling expression of the normal β-MHC gene are expected to be preserved because the fusion gene is at the normal chromosomal position of the β-MHC and differs only by the presence of the loxP sequence. Heterozygous transgenic animals carrying one copy of the fusion gene and one copy of the WT β-MHC gene (Yb/+, referred to below as Yb) were used for our studies. These animals were born at the expected Mendelian ratios and show no detected abnormalities.
Developmental Expression of YFP–β-MHC.
To determine the expression pattern of the Yb allele perinatally, ventricular sections from neonatal-day-1 hearts were examined by using confocal microscopy. Fig. 2A shows the YFP fluorescence of a transverse heart section from a Yb, neonatal-day-1 pup. As illustrated, the 1-day-old Yb heart shows high expression of YFP throughout the tissue. In higher-magnification images, the presence of fluorescent A bands within sarcomeres (Fig. 2B) clearly show that the YFP–β-MHC polypeptide retains all features necessary for incorporation into sarcomeres.
Fig. 2.
Developmental regulation of the YFP–β-MHC fusion protein. Confocal yellow fluorescent images of ventricular sections from a 1-day-old neonate (A and B), 8-day-old pup (C and D), and 9-month-old adult (E and F) imaged with a ×20 objective (A, C, and E), a ×60 objective (D and F), or a ×60 objective with 3.5× optical zoom (B; final magnification, ×210). Identical settings were used to procure images for the series of ×20 or ×60 objectives. (Scale bars: 20 μm.)
To determine the postnatal changes in the distribution of cells expressing YFP–β-MHC and the levels of its expression, we examined ventricular sections of Yb animals from neonatal day 8 by using YFP fluorescence. Fig. 2C shows that expression of the fusion protein is reduced to low levels by day 8 and that, at this age, most myocytes show either low or undetectable levels of YFP–β-MHC. However, despite the overall low level of YFP–β-MHC expression, high-resolution confocal images show a few sparsely scattered cells that retain a high level of expression (Fig. 2D). These perinatal changes in YFP–β-MHC expression are in agreement with the known expression pattern of WT β-MHC in the mouse (24).
To investigate the cellular pattern of β-MHC reexpression that occurs during normal aging, we examined the expression of the Yb fusion protein at 5 weeks and 9 months of age. By 5 weeks, expression of YFP–β-MHC is essentially off, with almost all ventricular myocytes having levels of yellow fluorescence indistinguishable from the low levels of autofluorescence observed in WT animals, although a few cells with fluorescence are occasionally detected in some Yb animals (data not shown). By 9 months, the vast majority of cells still show undetectable levels of YFP fluorescence, but there were some clearly fluorescent cells distributed in small clusters (Fig. 2 E and F). Thus, in a normal animal the age-related increase in β-MHC expression is mediated by a large increase in β-MHC expression levels in clusters of a few cells, rather than by a uniform small increase in expression of most cells.
YFP–β-MHC Expression During Hypertrophy Occurs in Discrete Subsets of Myocytes.
To determine the pattern of β-MHC reexpression during hypertrophy, we mated Yb mice to mice carrying a single copy of a renin transgene (RenTg) inserted at the ApoAI CIII locus. This transgene directs the chronic constitutive secretion of renin from the liver (25, 26), leading to high plasma renin levels (≈6-fold of those in WT mice) and to cardiac hypertrophy. RenTg animals have chronic hypertension and develop concentric cardiac hypertrophy, and more than half die before 9 months of age (25, 26). For our experiments, we used 7- to 9-month-old male and female mice of a mixed 129/B6 genetic background that have one copy of the YFP–β-MHC gene and one copy of RenTg (referred to below as Yb/RenTg).
The Yb/RenTg animals, as expected, show significant increases in the heart weight/body weight ratio relative to the littermate Yb animals without the RenTg as well as increases in mRNA levels for atrial natriuretic peptide (ANP) and β-MHC (Fig. 3A–C). These increases are virtually identical in magnitude to those observed for RenTg alone (25, 26). To test whether expression of YFP–β-MHC allele parallels expression of the WT β-MHC allele, we used quantitative RT-PCR to determine the levels of YFP and WT β-MHC mRNAs in the ventricles of 7- to 9-month-old animals. Fig. 3D illustrates the resulting data as a scatterplot matrix of the expression levels of Yb and β-MHC from individual animals and demonstrates that expression of YFP is highly correlated (r = 0.82, P < 0.002), with expression of the WT β-MHC allele over a 20-fold range. Thus, the YFP–β-MHC fusion allele faithfully tracks the expression of β-MHC. We also determined whether expression of the YFP–β-MHC allele parallels expression of ANP, a well conserved marker of cardiac hypertrophy. As shown in Fig. 3E, YFP expression also is highly correlated with that of ANP (r = 0.92, P < 0.0001).
Fig. 3.
Induction of hypertrophy. (A–C) Comparisons of heart weight/body weight ratios (Hw/Bw) (A) and mRNA expressions relative to mean of control (Relative Exp) for β-MHC (b MHC; B) and ANP (C) of 7- to 9-month-old control animals (Yb) and Yb/RenTg animals. (D and E) Bivariate analyses between mRNA levels as the log10 of the relative expression of YFP [Log (Rel YFP)] and log10 of the relative expression of WT β-MHC [Log (Rel wt bMHC)] (D) or ANP [Log (Rel ANP)] (E). Circles denote control animals (Yb); triangles denote Yb/RenTg animals.
To assess the cellular distribution pattern of YFP–β-MHC re-expression in the Yb/RenTg animals, ventricular sections were analyzed by confocal microscopy. Fig. 4 A and B compare the expression patterns of the fusion protein within a typical Yb control (heart weight/body weight ratio = 5.6 mg/g; Fig. 4A) and Yb/RenTg heart (heart weight/body weight ratio = 7.8 mg/g; Fig. 4B) at 7 to 9 months of age. In the animal made hypertrophic with the RenTg, a marked increase in YFP–β-MHC expression is readily observed, with many but not all cells expressing high levels of the YFP–β-MHC protein. Nevertheless, although some cells show high levels of expression of the fusion protein, many other cells show undetectable levels of expression. The YFP–β-MHC-expressing cells are predominantly within the left ventricular subendocardium, with the subepicardium and right ventricle showing few YFP–β-MHC-expressing cells. Analyses of ventricular sections from Yb/RenTg animals cut in the coronal plane (Fig. 4C) show that the cells expressing YFP–β-MHC within the subendocardium extend from the base to the apex of the left and right ventricle. Thus, reexpression of YFP–β-MHC occurs in discrete subsets of myocytes rather than uniformly in all. These cells are more frequent in the subendocardium, which is consistent with previous observations (27).
Fig. 4.
Distribution of YFP–β-MHC-expressing cells. Yellow fluorescence confocal images of ventricular sections from Yb control mice (A, transverse) and Yb/Rentg mice (B, transverse; and C, coronal). (D) A fluorescent image (pseudocolored blue) of the same section as for C that visualizes a lectin stain. Overlapping images were acquired with a ×4 objective and were stitched together by using ImageJ. The separation of individual images is illustrated in Fig. 9, which is published as supporting information on the PNAS web site. High levels of staining of WGA around the edges of the tissue in D are due to lectin binding to the pericardium.
Reexpression of YFP–β-MHC Is Associated with Fibrosis.
To gain further insights into the nature of the distribution of YFP–β-MHC-expressing cells within the hearts of Yb/RenTg animals, we examined whether the clustered expression pattern of YFP–β-MHC is correlated with the focal myocardial fibrosis that is known to occur in the RenTg animals (25). Masson's trichrome staining of paraffin sections is a conventional stain for fibrosis, but sections prepared in this manner lose their YFP fluorescence. We therefore used fluorescent wheat germ agglutinin (WGA), a lectin that binds to sugar residues and has previously shown to demonstrate the presence of the highly glycosylated protein collagen in tissue sections (28, 29). We confirmed that WGA binds to collagen by showing that it binds to the same interstitial structures that stain blue with Masson's trichrome in serial sections of a hypertrophic heart (Fig. 10, which is published as supporting information on the PNAS web site). Increases in WGA lectin binding consequently provide a surrogate for increased fibrosis. Fig. 4D shows the WGA fluorescence (pseudocolored blue) of the same section whose Yb fluorescence (yellow) was shown in Fig. 4C. Comparison of these two images clearly demonstrates that the distribution of increased WGA staining coincides remarkably well with the distribution of the cells reexpressing YFP–β-MHC. Thus, YFP–β-MHC-expressing cells surround or are included within areas of increased WGA stain. Indeed the areas of fibrosis can be predicted from the clustering of yellow cells or vice versa. Fig. 5 A–C illustrates high-magnification confocal images that show markedly increased interstitial fibrosis surrounding myocytes expressing YFP–β-MHC (Fig. 5 A–C, areas encircled by solid ovals) but not around neighboring areas that do not express YFP–β-MHC (Fig. 5 A–C, areas encircled by dashed ovals). Similar correlation is also seen in Yb homozygous animals (animals harboring two copies of the YFP–β-MHC fusion allele) made hypertrophic by aortic banding (data not shown). We next quantified the association between YFP–β-MHC expression and fibrosis. Confocal images of left ventricle from a 6-month-old Yb/RenTg animal were digitally dissected into fibrotic and nonfibrotic areas, and fibrosis (mean WGA pixel intensity) and the YFP expression (mean YFP pixel intensity) of each area were quantified. Fig. 5D illustrates the resulting data in the form of a scatterplot matrix and demonstrates a high and significant correlation between WGA staining and YFP expression (r = 0.77, P < 0.0001). Thus, fibrosis and YFP expression are closely associated within the hypertrophic heart.
Fig. 5.
Association of β-MHC expression with fibrosis. (A–C) Confocal images of transverse sections from a Yb/RenTg heart imaged with a ×20 objective. Yellow fluorescent images for YFP (A) and TRITC (B) for WGA lectin (pseudocolored blue) were acquired from the same section and merged (C) with ImageJ. (Scale bar: 100 μm.) (D) Bivariate analysis between YFP expression and fibrosis as log10 of mean YFP pixel intensity [Log (Av. YFP intensity)] and log10 of mean WGA pixel intensity [Log (Av. WGA intensity)].
One of the hallmark of hypertensive myocardial fibrosis is perivascular fibrosis, which is obvious in RenTg mice, as previously described (25). We therefore asked whether β-MHC reexpression is associated with perivascular fibrosis as well as with interstitial fibrosis. Fig. 6A shows that clusters of YFP–β-MHC cells also are found surrounding coronary vessels, demonstrating that YFP–β-MHC reexpressing myocytes are associated with perivascular and interstitial fibrosis. RenTg animals display a well documented age-dependent increase in the levels of myocardial fibrosis (30). To determine whether this increase in fibrosis is associated with an increase in the number of YFP–β-MHC cells, we compared ventricular sections from 5-month-old Yb/RenTg animals that show low levels of fibrosis with those from 15-month-old Yb/RenTg animals that show markedly higher levels of fibrosis. Fig. 11, which is published as supporting information on the PNAS web site, shows the results of this comparison and demonstrates that the increased area of fibrosis is associated with a clear increase in the number of YFP–β-MHC-expressing cells (Fig. 11 A and C). Thus, the age-dependent increases in fibrosis in RenTg animals are closely paralleled by increases in the number of YFP–β-MHC-expressing cells.
Fig. 6.
YFP–β-MHC expression in individual cells. Shown are merged images for YFP and fluorescent WGA lectin (pseudocolored blue) of ventricular sections from hypertrophic Yb/RenTg hearts with a ×20 objective. The image in B was taken with a ×3 optical zoom (final magnification of ×60). (Scale bars: 50 μm.)
Diffuse Scattering of YFP–β-MHC-Expressing Cells.
We also find, in addition to the YFP–β-MHC cells associated with areas of fibrosis, a few YFP–β-MHC-expressing cells (<10% of the expressing cells) that are distributed singly and not associated with any increase in WGA staining. These cells are present in low numbers in control Yb animals and in higher numbers in Yb/RenTg animals. These singletons contact cells that do not express YFP–β-MHC and are otherwise indistinguishable from these nonexpressing cells (Fig. 6B). Note that the nonexpressing cells adjacent to the expressing cells show no yellow fluorescence, not just a reduced level. Indeed, the fluorescence of these cells is indistinguishable from background fluorescence in animals that have no YFP–β-MHC gene.
Cellular Hypertrophy Can Occur Without YFP–β-MHC Expression.
We tested whether the reexpression of YFP–β-MHC in only some of the cells was due to a greater degree of hypertrophy of the YFP–β-MHC-expressing cells compared with nonexpressing cells in the same region of the heart. Heart sections were stained with fluorescent WGA, and morphometrical analyses of the cross-sectional areas of cells within the left ventricular free wall from Yb control and Yb/RenTg animals were conducted. Fig. 7A shows that Yb/RenTg animals, as expected from their overall hypertrophic state, show a highly significant increase in the average cross-sectional areas of cardiac myocytes in the subendocardium compared with control animals. We then asked whether in Yb/Rentg animals the YFP–β-MHC-expressing cells were larger than the nonexpressing cells in the same regions by simultaneously assessing their cross-sectional areas and scoring them for their YFP fluorescence. Fig. 7B shows the resulting data, which clearly demonstrate that the average cross-sectional areas of YFP–β-MHC-expressing cells are not significantly different from the cross-sectional areas of non-YFP–β-MHC-expressing cells in the same region of the heart. Thus, YFP–β-MHC-expressing cells are no more hypertrophic than non-YFP–β-MHC-expressing cells. Taken together, these data demonstrate that the YFP–β-MHC-expressing cells in RenTg animals are a subset of hypertrophic cells and that the increase in cell size accompanying hypertrophy is associated with but not sufficient to induce reexpression of YFP–β-MHC.
Fig. 7.
Morphometeric analyses. (A) Cross-sectional areas of myocytes from Yb control (open bar) and Yb/RenTg hearts (black bars) irrespective of their fluorescence. (B) Cross-sectional areas of the same myocytes from Yb/RenTg animals used in A but grouped according to their yellow fluorescence (Yellow cells or Non-Yellow cells). A total of 300 cells were analyzed for each genotype.
Reexpression of YFP–β-MHC During Normal Aging Is Associated with Fibrosis.
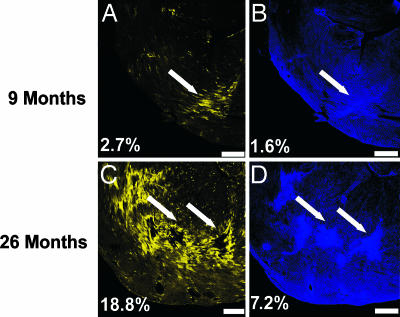
We noted above (Fig. 2E) that normal control animals show an increase in the number of YFP–β-MHC-expressing cells as they age. We therefore asked whether the YFP–β-MHC-reexpressing cells in these nonhypertrophic but aging hearts were also associated with the fibrosis that is known to occur in aging mice (31). We compared ventricular sections from 9- and 26-month-old Yb animals. Fig. 8 shows the result of this comparison, which demonstrates that increases in fibrosis [from 1.6% of tissue in the 9-month-old animal (Fig. 8B) to 7.2% of total tissue in the 26-month-old animal (Fig. 8D)] are associated with increases in YFP–β-MHC expression [from 2.7% of tissue in the 9-month-old animal (Fig. 8A) to 18.8% of tissue in the 26-month-old animal (Fig. 8C)] and that the YFP–β-MHC-expressing cells cluster around these fibrotic areas (Fig. 8, white arrows) as well as coronary vessels (Fig. 12, which is published as supporting information on the PNAS web site). Furthermore, the sizes of these cells are not significantly different from cells in the same region that do not express YFP–β-MHC (data not shown).
Fig. 8.
Increased numbers of β-MHC-expressing cells are associated with increased fibrosis during normal aging. Confocal images of ventricular sections from 9-month-old (A and B) and 26-month-old (C and D) Yb animals. (A and C) YFP. (B and D) Fluorescent WGA lectin (pseudocolored blue). For each animal, the same ventricular section was imaged for YFP and WGA lectin. Percentages in the lower left corner of each image shows the fraction of tissue positive for YFP expression (A and C) or WGA lectin staining (B and D). (Scale bars: 500 μm.)
Discussion
We have described here the generation of a genetic model having a YFP–β-MHC fusion gene that allows a precise and accurate assessment of the cellular distribution of β-MHC expression in adult mouse hearts. We have shown that the fusion gene follows the expected regulation during perinatal development and accurately tracks the expression of WT β-MHC expression during hypertrophy.
Our investigations demonstrate that increases in β-MHC expression that occur during cardiac hypertrophy as well as during normal aging are mediated by increases in expression levels in subsets of myocytes, rather than by increases in the expression of β-MHC in all myocytes. The YFP–β-MHC-expressing cells show markedly stronger yellow fluorescence compared with nonexpressing cells in the same animals, which show a level of fluorescence indistinguishable from cells of animals that do not have the Yb fusion gene.
Our investigations show that reexpression occurs predominantly in hypertrophic cells clustered close to or within areas of focal and interstitial fibrosis rather than in all hypertrophic cells. Areas within the heart that are free from fibrosis include only a small number of β-MHC-expressing cells that are isolated rather than clustered (see below). Thus, we find that many hypertrophic myocytes in Yb/RenTg animals have undetectable levels of YFP–β-MHC expression, but the hypertrophic myocytes expressing YFP–β-MHC always are associated with areas of fibrosis. The predominant subendocardial pattern of YFP–β-MHC reexpression also reflects this association, because myocardial fibrosis is known to occur more frequently in this region (32). The perivascular fibrosis found in hypertensive hypertrophy also is faithfully mirrored by the reexpression of YFP–β-MHC in myocytes around coronary vessels, which is likewise consistent with previous work showing β-MHC reexpression around coronary arteries in hypertrophic hearts (27). Of considerable interest is our finding that the age-dependent reexpression of YFP–β-MHC in myocytes in normal control animals also is associated with fibrosis (perivascular as well as interstitial fibrosis). Thus, we find that, in the absence of fibrosis, β-MHC reexpression in myocytes does not occur in normally sized or hypertrophic myocytes. Experimental models of hypertrophy in which substantial fibrosis does not occur, for example, hypertrophy due to either volume overload or subpressor doses of ang II, are not associated with changes in β-MHC reexpression (33–35). In addition, recent studies with mice heterozygous for the disruption of the gene for the major myocyte sodium channel SCN5A show that mice reexpress β-MHC and develop fibrosis without the induction of cardiac hypertrophy (36). Together, these associations suggest that β-MHC reexpression does not develop without fibrosis. A similar statement can be made with respect to ventricular reexpression of ANP; increased ANP levels do not invariably correlate with cardiac hypertrophy but are observed always in the presence of cardiac pathology and accompanied by fibrosis (interstitial and parivascular) (37–39).
It is not clear whether the association of β-MHC-expressing myocytes with areas of fibrosis is because of a cause-and-effect relationship in either direction or because both are different consequences of a common precursor event. However, it is unlikely that simply the reexpression of β-MHC in myocytes is sufficient to induce fibrosis, given that chronic expression of the β-MHC transgene in adult hearts does not lead to fibrosis (13). Furthermore, mechanical stretch of cultured rat neonatal ventricular myocytes is sufficient to induce the expression of β-MHC without the need for or the development of fibrosis (11). Schultz et al. (40) found that after aortic coarctation there was increased β-MHC in fgf2-null mice compared with WT mice but significantly less fibrosis or hypertrophy, suggesting that neither hypertrophy nor fibrosis causes β-MHC expression. It is possible that the same signal induces hypertrophy, fibrosis, and β-MHC expression but that β-MHC induction does not require FGF2.
Some comment is required on our finding a few β-MHC-expressing cells scattered throughout the heart but not associated with notable increases in collagen deposition. These cells are frequently singletons and are present in low numbers in normal Yb control animals and in higher numbers in Yb/RenTg animals, possibly because RenTg animals are already hypertrophic at birth (30). Because these cells are usually surrounded by cells that do not express β-MHC and are indistinguishable from them except for their expression of YFP–β-MHC, they are unlikely to be a consequence of differential mechanical stress. We suspect that they are left-over cells that failed to switch off expression of β-MHC in the manner that normally occurs subsequent to birth.
In summary, we have generated a genetic model that allows a precise and accurate assessment of the cellular distribution of β-MHC expression in normal or hypertrophic heart. Our analyses reveal that reexpression of β-MHC in hearts that are hypertrophic is due to the occurrence of strong expression in a subset of hypertrophic cardiac myocytes rather than to a general increase of expression in all hypertrophic cells. We demonstrate an essentially invariant association of β-MHC reexpression in the hypertrophic hearts with areas of perivascular and interstitial fibrosis rather than with hypertrophy per se. This association also is seen in the normal aging heart without the need for RenTg. Thus, β-MHC expression in the adult mouse heart is an indicator of fibrosis rather than of hypertrophy.
Materials and Methods
Generation of Mice with the YFP–β-MHC Fusion Allele.
Enhanced YFP coding sequence with a valine–proline linker at the 3′ end was PCR-amplified and inserted at the start codon of a 2.7-kb EcoRI β-MHC fragment subcloned from a λ-phage library. The resulting fragment was cloned into a targeting vector (41), containing a 6.5-kb Xho/BamHI 5′ homology fragment (kind gift from Jeffrey Robbins, University of Cincinnati, Cincinnati, OH). Electroporation and selection of ES cells (E14) were performed as described (42). Male chimeras generated from targeted ES cells were bred to C57/Bl6 females, and progeny carrying the targeted allele were bred to RenTg mice (for details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). Animals were handled according to procedures approved by the Institutional Animal Care and Use Committee.
RNA Analysis.
RNA extraction and gene quantification were carried out as described (43) by using published PCR primers and probes (25) (for the sequences, see Supporting Materials and Methods).
Tissue Processing.
Hearts were procured as described (25) and sectioned with a vibratome at 150-μm thickness. Individual sections were treated with sodium borohydride (1 mg/ml in PBS) for 30 min at room temperature to reduce fixative-induced fluorescence.
Confocal Analyses.
Sections were stained with fluorescent TRITC- or Alexa Fluor 633-labeled 50 μg/ml WGA and analyzed with a FV500 confocal microscope (Olympus, Tokyo, Japan) (see Supporting Materials and Methods for details on lasers, image acquisition, and morphometric analyses). To quantify the association between YFP expression and fibrosis, confocal sections from the left ventricle of a Yb/Rentg animal were digitally dissected into fibrotic and nonfibrotic areas (total number of areas = 44), and fibrosis (mean WGA pixel intensity) and the YFP expression (mean YFP pixel intensity) of each area were measured by using ImageJ.
Data Analysis.
Values are reported as means ± SEM. Bivariate analyses were performed with JMP software (SAS institute, Cary, NC). Unpaired two-tailed Student's t test (Prism; GraphPad, San Diego, CA) were used for comparisons between control and RenTg groups, and differences were considered to be statistically significant with P < 0.05.
Supplementary Material
Acknowledgments
We thank T. Doetschman and H. Rockman for helpful suggestions; N. Maeda, N. Malouf, N. Takahashi, M. Altenberg, and A. Pendse for critical reading of the manuscript; R. Bagnell for excellent assistance with microscopy and imaging; D. Winkelmann for helpful technical advice; and J. Robbins for mouse β-MHC promoter. This work was supported by National Institutes of Health Grants HL71266 and HL49277 (to O.S.) and American Heart Association Grants 0325655U and 0525594U (to K.P.).
Abbreviations
- MHC
myosin heavy chain
- YFP
yellow fluorescent protein
- WGA
wheat germ agglutinin
- ANP
atrial natriuretic peptide
- RenTg
renin transgene.
Footnotes
The authors declare no conflict of interest.
References
- 1.Swynghedauw B. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 2.Copper GT. Annu Rev Med. 1997;48:13–23. doi: 10.1146/annurev.med.48.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Chien KR. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Chien KR, Olson EN. Cell. 2002;110:153–162. doi: 10.1016/s0092-8674(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 5.Molkentin JD, Dorn IG., II Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 6.Weber KT. Curr Opin Cardiol. 2000;15:264–272. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT, Brilla CG, Janicki JS. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 8.Cuspidi C, Ciulla M, Zanchetti A. Nephrol Dial Transplant. 2006;21:20–23. doi: 10.1093/ndt/gfi237. [DOI] [PubMed] [Google Scholar]
- 9.Assayag P, Carre F, Chevalier B, Delcayre C, Mansier P, Swynghedauw B. Cardiovasc Res. 1997;34:439–444. doi: 10.1016/s0008-6363(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 10.Sadoshima J, Izumo S. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 11.Komuro I, Yazaki Y. Annu Rev Physiol. 1993;55:55–75. doi: 10.1146/annurev.ph.55.030193.000415. [DOI] [PubMed] [Google Scholar]
- 12.Lompre AM, Nadal-Ginard B, Mahdavi V. J Biol Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- 13.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, Osinska HE, Lorenz JN, Brosseau C, Federico A, et al. J Biol Chem. 2003;278:17466–17474. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- 14.Gupta M, Zak R. Am J Physiol. 1992;262:R346–R349. doi: 10.1152/ajpregu.1992.262.3.R346. [DOI] [PubMed] [Google Scholar]
- 15.Saadane N, Alpert L, Chalifour LE. Br J Pharmacol. 1999;127:1165–1176. doi: 10.1038/sj.bjp.0702676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaxall BC, Spang R, Rockman HA, Koch WJ. Physiol Genomics. 2003;15:105–114. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- 17.Yasumura Y, Takemura K, Sakamoto A, Kitakaze M, Miyatake K. J Cardiovasc Failure. 2003;9:469–474. doi: 10.1016/s1071-9164(03)00581-5. [DOI] [PubMed] [Google Scholar]
- 18.Abraham WT, Gilbert EM, Lowes BD, Minobe WA, Larrabee P, Roden RL, Dutcher D, Sederberg J, Lindenfeld JA, Wolfel EE, et al. Mol Med. 2002;8:750–760. [PMC free article] [PubMed] [Google Scholar]
- 19.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, et al. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 20.Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Am J Physiol. 2000;278:H412–H419. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- 21.Moores SL, Sabry JH, Spudich JA. Proc Natl Acad Sci USA. 1996;93:443–446. doi: 10.1073/pnas.93.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow D, Srikakulam R, Chen Y, Winkelmann DA. J Biol Chem. 2002;277:36799–36807. doi: 10.1074/jbc.M204101200. [DOI] [PubMed] [Google Scholar]
- 23.Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss A, Leinwand LA. Annu Rev Cell Dev Biol. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- 25.Caron KM, James LR, Kim HS, Knowles J, Uhlir R, Mao L, Hagaman JR, Cascio W, Rockman H, Smithies O. Proc Natl Acad Sci USA. 2004;101:3106–3111. doi: 10.1073/pnas.0307333101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caron KM, James LR, Kim HS, Morham SG, Sequeira Lopez ML, Gomez RA, Reudelhuber TL, Smithies O. Proc Natl Acad Sci USA. 2002;99:8248–8252. doi: 10.1073/pnas.112222199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiaffino S, Samuel JL, Sassoon D, Lompre AM, Garner I, Marotte F, Buckingham M, Rappaport L, Schwartz K. Circ Res. 1989;64:937–948. doi: 10.1161/01.res.64.5.937. [DOI] [PubMed] [Google Scholar]
- 28.Soderstrom KO. Histochemistry. 1987;87:557–560. doi: 10.1007/BF00492470. [DOI] [PubMed] [Google Scholar]
- 29.Parikka V, Lehenkari P, Sassi ML, Halleen J, Risteli J, Harkonen P, Vaananen HK. Endocrinology. 2001;142:5371–5378. doi: 10.1210/endo.142.12.8533. [DOI] [PubMed] [Google Scholar]
- 30.Caron KM, James LR, Lee G, Kim HS, Smithies O. Physiol Genomics. 2005;20:203–209. doi: 10.1152/physiolgenomics.00221.2004. [DOI] [PubMed] [Google Scholar]
- 31.Dhahbi JM, Tsuchiya T, Kim HJ, Mote PL, Spindler SR. J Gerontol A Biol Sci Med Sci. 2006;61:218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- 32.Jantunen E, Collan Y. Appl Pathol. 1989;7:179–187. [PubMed] [Google Scholar]
- 33.Schultz JJ, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namba T, Tsutsui H, Tagawa H, Takahashi M, Saito K, Kozai T, Usui M, Imanaka-Yoshida K, Imaizumi T, Takeshita A. Circulation. 1997;95:2448–2454. doi: 10.1161/01.cir.95.10.2448. [DOI] [PubMed] [Google Scholar]
- 35.Weber KT, Pick R, Silver MA, Moe GW, Janicki JS, Zucker IH, Armstrong PW. Circulation. 1990;82:1387–1401. doi: 10.1161/01.cir.82.4.1387. [DOI] [PubMed] [Google Scholar]
- 36.Royer A, van Veen TA, Le Bouter S, Marionneau C, Griol-Charhbili V, Leoni AL, Steenman M, van Rijen HV, Demolombe S, Goddard CA, et al. Circulation. 2005;111:1738–1746. doi: 10.1161/01.CIR.0000160853.19867.61. [DOI] [PubMed] [Google Scholar]
- 37.Vikstrom KL, Bohlmeyer T, Factor SM, Leinwand LA. Circ Res. 1998;82:773–778. doi: 10.1161/01.res.82.7.773. [DOI] [PubMed] [Google Scholar]
- 38.Kruse JJ, Strootman EG, Bart CI, Visser A, Leer JW, Wondergem J. Int J Radiat Biol. 2002;78:297–304. doi: 10.1080/09553000110102021. [DOI] [PubMed] [Google Scholar]
- 39.Takemura G, Fujiwara H, Yoshida H, Mukoyama M, Saito Y, Nakao K, Fujiwara T, Uegaito T, Imura H, Kawai C. J Pathol. 1990;161:285–292. doi: 10.1002/path.1711610404. [DOI] [PubMed] [Google Scholar]
- 40.Schultz JE, Witt SA, Nieman ML, Reiser PJ, Engle SJ, Zhou M, Pawlowski SA, Lorenz JN, Kimball TR, Doetschman T. J Clin Invest. 1999;104:709–719. doi: 10.1172/JCI7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai YS, Maeda N. Trends Cardiovasc Med. 2005;15:81–85. doi: 10.1016/j.tcm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Proc Natl Acad Sci USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HS, Lee G, John SW, Maeda N, Smithies O. Proc Natl Acad Sci USA. 2002;99:4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.