Abstract
Slx9p (Ygr081cp) is a nonessential yeast protein previously linked genetically with the DNA helicase Sgs1p. Here we report that Slx9p is involved in ribosome biogenesis in the yeast Saccharomyces cerevisiae. Deletion of SLX9 results in a mild growth defect and a reduction in the level of 18S rRNA. Co-immunoprecipitation experiments showed that Slx9p is associated with 35S, 23S, and 20S pre-rRNA, as well as U3 snoRNA and, thus, is a bona fide component of pre-ribosomes. The most striking effects on pre-rRNA processing resulting from deletion of SLX9 is the accumulation of the mutually exclusive 21S and 27SA2 pre-rRNA. Furthermore, deletion of SLX9 is synthetically lethal with mutations in Rrp5p that block cleavage at either site A2 or A3. We conclude that Slx9p has a unique role in the processing events responsible for separating the 66S and 43S pre-ribosomal particles. Interestingly, homologs of Slx9p were found only in other yeast species, indicating that the protein has been considerably less well conserved during evolution than the majority of trans-acting processing factors.
Keywords: yeast, ribosome biogenesis, pre-rRNA processing
INTRODUCTION
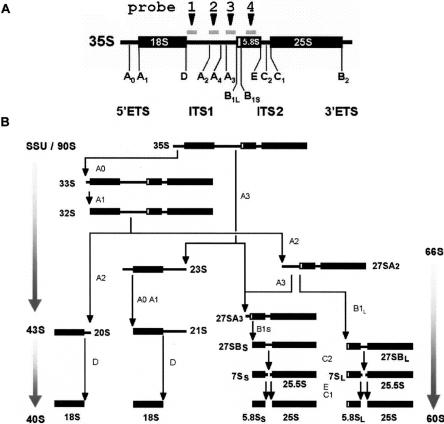
In eukaryotes, biogenesis of ribosomes starts in the nucleolus with transcription by RNA polymerase I of a large precursor RNA molecule, called 35S pre-rRNA in yeast, in which the 18S, 5.8S, and 25S mature rRNAs reside, while RNA polymerase III transcribes a 3′-extended pre-5S rRNA. The 35S precursor also contains external transcribed spacer elements (5′- and 3′-ETS) at either end as well as internal transcribed spacers (ITS1 and ITS2) that separate the mature sequences (Fig. 1A). During transcription many nonribosomal (trans-acting) factors as well as some ribosomal proteins start to associate with the nascent transcript, eventually forming a large pre-ribosomal complex called the small subunit processome (SSU) or 90S pre-ribosome (for reviews, see Fromont-Racine et al. 2003; Granneman and Baserga 2004; Raué 2004). The nonribosomal factors include small nucleolar RNAs (snoRNA), exo- and endonucleases, RNA helicases, and a host of other protein factors, whose exact role is still unclear. However, individual inactivation of any of these components in most cases inhibits one or more specific steps in the ordered removal of the ITS and/or ETS sequences from the 35S precursor, causing accumulation of intermediate pre-rRNAs at the expense of downstream products.
FIGURE 1.
Processing of pre-rRNA in S. cerevisiae. (A) Structure of the 35S pre-rRNA. The mature sequences 18S, 5.8S, and 25S rRNA (thick bars) are separated and flanked by transcribed spacers (thin bars). The positions of the processing sites and the probes used in this study are indicated. (B) The-rRNA processing pathway. The sedimentation coefficients of the pre-ribosomal complexes are indicated at the left and right. Note that the order of the cleavages at the A sites is flexible to some extent (see text). The precise role of the cleavage at site A4 remains to be established.
The first processing steps occurring within the 90S complex are cleavages at sites A0 and A1 that remove the 5′-ETS (Fig. 1B). Then, endonucleolytic cleavage of the resulting 32S precursor at site A2 splits the complex into a 43S and a 66S particle containing the 20S and 27SA2 pre-rRNA, respectively. The 43S particle is exported to the cytoplasm where its 20S precursor RNA is further matured by cleavage at site D to generate the mature 18S species. Within the 66S pre-ribosomal complex the 27SA2 pre-rRNA is further processed into 25S and 5.8S rRNA by an ordered series of exo- and endonucleolytic digestion steps. The majority of the 27SA2 molecules are cleaved at site A3, producing 27SA3 pre-rRNA, which is subsequently processed exonucleolytically to sites B1S and B2 to render the 27SBS precursor. A minor portion of the 27SA2 pre-rRNA enters an alternative route involving endonucleolytic cleavage at site B1L that produces the 5′-extended 27SBL pre-rRNA (Faber et al. 2006). The 27SBS and 27SBL precursor are then matured into 25S as well as 5.8SS or 5.8SL rRNA by endonucleolytic cleavage at site C1, followed by exonucleolytic digestion to sites C2 and E, respectively.
Recent experiments using mutant yeast strains have shown that the order of processing events described above is not a strictly obligatory one. For instance, processing of 32S pre-rRNA in ITS1 can start at site A3 instead of at A2, generating the normal 27SA3 intermediate and an alternative 21S precursor that is efficiently converted into 18S rRNA (Vos et al. 2004b). Processing of 35S pre-rRNA can skip the early cleavages at A0 and A1 and commence instead at site A3 (for review, see Venema and Tollervey 1999). This generates 27SA3 and a 23S precursor, whose fate is still in dispute. Although initially considered to be a dead-end product (Venema and Tollervey 1999; Allmang et al. 2000), recent evidence indicates that it may be further processed into 18S rRNA (Granneman and Baserga 2004). ITS1 also contains a further endonucleolytic processing site A4, that has so far been detected only in particular mutant yeast strains in which cleavage at site A3 is blocked (Eppens et al. 2002; Faber et al. 2004). It is as yet unclear whether this site is used in wild-type cells.
SLX9 was identified in a synthetic lethality (sl) screen searching for genes that interact with SGS1 (Ooi et al. 2003), a 3′→5′ DNA helicase that is involved in DNA damage repair and replication (Bennett et al. 1998). Double-mutant Δsgs1Δslx9 cells displayed a reduced growth rate. Although this demonstrates a genetic interaction between SGS1 and SLX9, their functional relationship remains to be clarified. In contrast, in large-scale affinity purifications Slx9p copurifies with Enp1p and Tsr1p (Gavin et al. 2002), two proteins that are involved in processing of precursor rRNA (Gelperin et al. 2001; Chen et al. 2003). Both Enp1p and Tsr1p associate with pre-rRNA as well as proteins previously characterized as components of 90S and 40S pre-ribosomal particles. Furthermore, GFP-tagged Slx9p was found to localize to the nucleolus (Ghaemmaghami et al. 2003). These observations suggest that, although Slx9p is a nonessential protein (Giaever et al. 2002; Steinmetz et al. 2002: Ooi et al. 2003; Deutschbauer et al. 2005), it may have a role in ribosome biogenesis.
The biochemical and genetic data reported in this paper demonstrate that Slx9p is present in pre-ribosomes from an early stage and implicate the protein in the processing events that remove the ITS1 spacer sequences.
RESULTS
Slx9p is specific to yeast species
SLX9 encodes a protein of 210 amino acids and has been identified as a nonessential gene in Saccharomyces cerevisiae by several genome-wide screens (Giaever et al. 2002; Steinmetz et al. 2002: Ooi et al. 2003; Deutschbauer et al. 2005). To assess its evolutionary conservation we performed a BLAST search in the SwissProt/TrEMBL proteome database using the amino acid sequence of S. cerevisiae Slx9p. Except for other yeast species, no homologs of Slx9p could be found in prokaryotes, archea, or higher eukaryotes. Figure 2 shows a sequence comparison of S. cerevisiae Slx9p with several of its counterparts in other yeasts species. In addition to a conserved putative N-terminally located nuclear localization sequence (KRRxxLRxK), several highly conserved regions can be recognized. However, none of these domains shows any similarity to known functional protein motifs. Slx9p thus appears to be unique to yeast species, and its sequence does not give any hints regarding its precise function.
FIGURE 2.
Amino acid alignment of Slx9p with yeast homologs. Sequence alignments were carried out using CLUSTAL W and BOXSHADE at www.ch.EMBnet.org. Accession numbers for the sequences are as follows: Sc S. cerevisiae Slx9p (UniProt P53251), Sp S. pombe (Q9C0Z3), Cg C. glabrata (Q6FNC2), Kl K. lactis (Q6CT43), Ag A. gossypii (Q758S2), Dh D. hansenii (Q6BMZ5). Identical amino acids are shaded in black, while similar amino acids are in gray.
Slx9p is associated with pre-rRNA species and U3 snoRNA
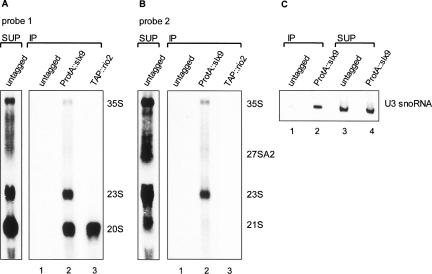
In previous large-scale affinity purifications Slx9p was found to co-precipitate with Tsr1p and Enp1p, two proteins that are part of 90S and 43S pre-ribosomes (Gelperin et al. 2001; Gavin et al. 2002; Chen et al. 2003; Schafer et al. 2003). To determine whether Slx9p also is a component of these pre-ribosomes we tested its association with pre-rRNA molecules by performing co-precipitation experiments. To this end, we used the slx9::KAN strain (obtained from EUROSCARF) transformed with a plasmid encoding Slx9p that carried a ProtA tag at its N terminus. As a positive control, we used strain SC1413 that expresses TAP-tagged Rio2p, a trans-acting factor present in 43S pre-ribosomes (Vanrobays et al. 2003). Extracts were prepared from exponentially growing cells and treated with IgG-agarose beads. The bound material was eluted from the beads, after which the protein and RNA were separated by phenol extraction and the latter was analyzed by Northern hybridization. In agreement with earlier findings, Rio2p–TAP exclusively co-precipitates 20S pre-rRNA (Fig. 3A, lane 3). In the precipitate of ProtA–Slx9p, however, a broader spectrum of pre-rRNAs is present (Fig. 3A,B). Apart from 20S pre-rRNA, we also detected the 35S and 23S precursor species. Furthermore, ProtA–Slx9p co-precipitates substantial amounts of U3 snoRNA (Fig. 3C). Only trace amounts of 27SA2 are detectable (Fig. 3B). Northern analysis of the small pre-rRNA species demonstrated the absence of any significant amounts of 7S pre-rRNA (data not shown). Previously, we have used high-level expression of ProtA-Rrp5p and shown a complete absence of 20S precursors in the immunoprecipitation (Vos et al. 2004a), which ensures further supports that the ProtA–Slx9p precipitation is specific. We conclude that Slx9p is a bona fide component of the 90S pre-ribosome, and upon ITS1 processing remains associated with 43S, but not the 66S, particle.
FIGURE 3.
Identification of RNA species co-immunoprecipitated by ProtA–Slx9p. Total cell extracts from exponentially growing strains slx9::KAN (Y14711), slx9::KAN+pURA3–ProtA::slx9, and SC1413 (TAP::Rio2) were prepared and treated with IgG beads. (A,B) Northern analysis of co-precipitated RNA (IP) or nonbound RNA (SUP) on agarose gels using probe 1 and 2 (see Fig. 1A). (C) Northern analysis of co-precipitated RNA separated on a 8% polyacrylamide gel using a probe complementary to the C′ box of U3 snoRNA.
Slx9p is required for normal pre-rRNA processing in ITS1
To identify potential defects in pre-rRNA processing in cells carrying an slx9 deletion, we performed Northern hybridization experiments on total RNA that was extracted from exponentially growing slx9::LEU2 cells. As controls, we used RNA from the slx9::LEU2 strain supplemented with a plasmid-borne copy of the SLX9 gene, as well as the wild-type parent strain. The slx9 deletion strain is sensitive to a high concentration of 600 ng/mL cycloheximide, which is rescued upon introduction of the plasmid-borne copy (data not shown).
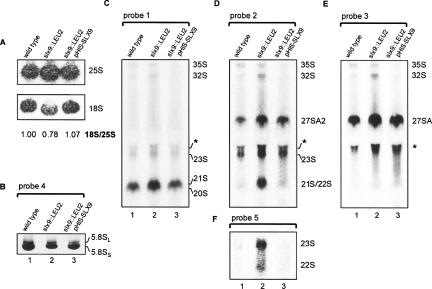
As is evident from Figure 4A, the level of 25S rRNA in cells lacking Slx9p is very similar to that in cells expressing the protein, either from a genomic or a plasmid-borne gene. Figure 4B shows that the lack of Slx9p also has no significant effect on the relative level of the long and short forms of 5.8S rRNA. In contrast, the level of 18S rRNA in slx9::LEU cells is reduced by about 20%, as is evident from quantification of Northern blots. This effect is abolished by introduction of a plasmid-borne copy of the gene into the mutant cells (Fig. 4A; cf. lanes 2 and 3). These findings indicate that inactivation of SLX9 does not inhibit processing in the large subunit pathway, leading to mature 25S and 5.8S rRNA, but does have a negative effect on one or more of the processing steps required for formation of the small subunit. The limited reduction in 18S rRNA is in accordance with the roughly 20% slower growth rate we observed for the slx9::LEU cells in rich medium (data not shown). There were no specific growth effects at either low (16°C) or high (37°C) temperature (data not shown).
FIGURE 4.
Northern analysis of pre-rRNA in Δslx9 cells. Total RNA was isolated from exponentially growing YJV140 (wild-type), strain slx9::LEU2 (YCV711) and slx9::LEU2 carrying a plasmid-borne SLX9 gene. The RNA was separated on denaturing 1.2% agarose gels (A,C–E) or on 8% polyacrylamide gels (B). See Figure 1A for the position of the different probes. (A) Northern analysis to visualize 25S and 18S rRNA. The 18S: 25S ratio was determined by Northern analysis using probes that detect 18S and 25S rRNA. The signals obtained by phosphor imaging were quantitated using the ImageQuant software tools. (B) Northern analysis of 5.8S rRNA using probe 4. (C–F) Northern analysis with probes 1–3 and 5 (see Fig. 1A). The same blot was used for the different hybridizations. The asterisk indicates a nonspecific hybridization signal.
To assess the nature of this defect in more detail, we determined the levels of various processing intermediates by Northern hybridization using a selection of probes complementary to different regions in ITS1 as indicated in Figure 1A. Analysis with probe 1, complementary to the sequence shortly downstream from site D, revealed that 35S pre-rRNA is present at normal levels in Slx9p-deficient cells (Fig. 4C). However, we noticed a strong accumulation of an intermediate that, on the basis of its mobility, could be either 20S or 22S/21S pre-rRNA. To distinguish between these possible products, we rehybridized the blot with probe 2, located just downstream from site A2. We obtained a strong signal at the same position (Fig. 4D), indicating the accumulation of substantial amounts of 22S/21S pre-rRNA in the slx9::LEU2 strain. Using probe 5 specific for RNA sequences between sites A0 and A1, we found only minor amounts of 22S pre-rRNA (Fig. 4F). Thus, the signal observed in the slx9 deletion strain predominantly represents the 21S species. Furthermore, we observed an increase in 23S pre-rRNA (also visible in Fig. 4C,E) as well as 27SA2 pre-rRNA (Fig. 4D) and 32/33S pre-rRNAs in these cells (best visible in Fig. 4D,E).
The simultaneous increase in the levels of both the 27SA2 as well as the 21S and 23S pre-rRNAs is quite surprising considering that these two processing intermediates overlap each other (Fig. 1B). In previously studied mutants an increase in 21S and/or 23S pre-rRNA resulted from a defect in cleavage at site A2 (as well as A0 and A1 in the case of 23S), and was accompanied by a decrease in the level of the 27SA2 precursor (Jansen et al. 1993; Lee and Baserga 1997; Lafontaine and Tollervey 1999; Gallagher et al. 2004). Inactivation of SLX9, thus, appears to have complex consequences for processing in ITS1, resulting in a phenotype that has not been observed before.
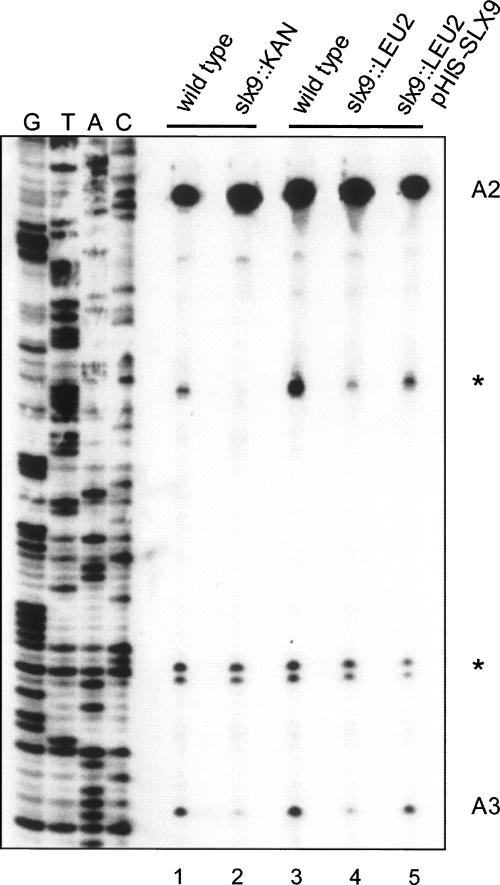
To further refine our picture of the effect of the slx9 deletion on ITS1 processing, we performed a reverse transcription assay on total RNA extracted from slx9::LEU2 and slx9::KAN cells and their corresponding wild-type strains, using probe 3, which is complementary to a sequence between sites A3 and B1L. The results displayed in Figure 5 show an increase in the primer extension stop at A2 upon inactivation of SLX9 (cf. lanes 2,3 and lanes 1,4), thus confirming the data obtained by Northern hybridization (Fig. 4). In stark contrast, the signal corresponding to the stop at A3 is substantially reduced. The wild-type processing phenotype is restored when a plasmid-encoded SLX9 is introduced (Fig. 5; cf. lane 4 and lane 1). Several arguments support the conclusion that the reduction in the signal representing cleavage at site A3 is not due to inhibition of processing at this site. First, the Δslx9 mutant cells accumulate the 21S and 23S pre-rRNA products of this cleavage (Fig. 4D). Second, as shown in Figure 4B, inactivation of SLX9 does not cause the shift in the production of 5.8S rRNA toward the long form that invariably results from inhibition of processing at site A3 (Eppens et al. 2002; Henry et al. 1994). Finally, we failed to observe a primer extension stop corresponding to site A4 in the Δslx9 cells. This stop does appear as a consequence of mutations in either the RRP2 or RRP5 gene that lead to loss of cleavage at site A3 (Eppens et al. 2002; Faber et al. 2004).
FIGURE 5.
Reverse transcription analysis of pre-rRNA processing intermediates. RNA was isolated from strains Y10000 (EUROSCARF wild-type, lane 1), slx9::KAN (Y14711) (lane 2), slx9::LEU2 (YCV711) (lane 3), and slx9::LEU2+pHIS–SLX9 (lane 4) and used as a template for reverse transcription using probe 3 (see Fig. 1A). The bands indicated by the asterisks represent artificial stops.
Taken together, these data therefore suggest that lack of Slx9p does not have a major impact on the efficiency of the various processing cleavages taking place in ITS1, but affects the further maturation of the different precursor species resulting from these cleavages.
Slx9p is essential when cleavage at either site A2 or A3 is impaired
To try to clarify the possible role of Slx9p in ITS1 processing further, we decided to study the effect of combining the slx9 deletion with two mutants of the trans-acting factor Rrp5p that affect processing at sites A2 and A3, respectively. In the deletion, mutant rrp5-Δ6 processing of 32S pre-rRNA at site A2 is completely inhibited while cleavage at site A3 proceeds unhindered to produce 21S and 27SA3 pre-rRNA. The 21S precursor behaves as a normal intermediate and is efficiently processed at site D to 18S rRNA. The growth rate of rrp5-Δ6 mutant cells is not affected (Vos et al. 2004b). In the rrp5-Δ3 deletion mutant, on the other hand, cleavage at site A3 is blocked and processing is redirected to site A4 (Eppens et al. 2002).
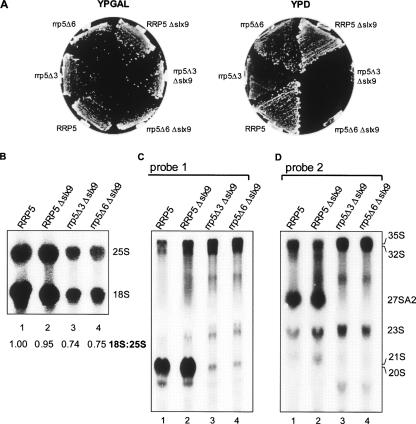
To combine the Δslx9 allele with the respective rrp5 deletion mutations, we used yeast strain YJV154 in which the genomic RRP5 gene is under control of the GAL promoter (Venema and Tollervey 1995). In this strain SLX9 was deleted by homologous recombination with an slx9::LEU2 construct. The resulting strain YRB154 was transformed with a plasmid carrying either the rrp5-Δ3, rrp5-Δ6, or the wild-type RRP5 allele under control of the wild-type RRP5 promoter. The strains were grown on YPD plates to deplete wild-type Rrp5p and to observe the effect of deletion of SLX9 on growth. Surprisingly, the absence of Slx9p results in a synthetic lethality with each of the two RRP5 mutants (Fig. 6A) due to a reduction in both 18S and 25S rRNA production. Moreover, 18S rRNA levels appear to be more strongly affected than 25S rRNA levels (Fig. 6B).
FIGURE 6.
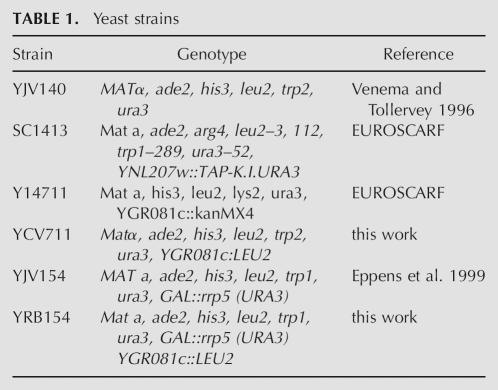
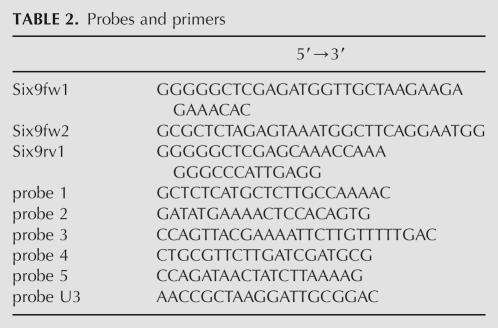
Effect of the deletion of SLX9 in cells expressing the mutant genes rrp5-Δ3 and rrp5-Δ6. (A) Growth. Cells of strain YJV306 (rrp5-Δ3), YJV207 (rrp5-Δ6), YJV163 (wild-type RRP5), rrp5-Δ3Δslx9 (YRB154Δ3), rrp5-Δ6Δslx9 (YRB154Δ6), and RRP5Δslx9 (YRB154), were streaked out on YPGAL and YPD plates and grown at 30°C for 3–4 d. (B–D) Effect on pre-rRNA processing. The strains were grown on YPGAL to mid-exponential phase and then shifted to YPD medium for 24 h. Total RNA was isolated from equal amounts of cells, separated on 1.2% agarose gels, and subjected to ethidium bromide staining and Northern analysis. (B) Northern analysis to visualize mature 18S and 25S rRNA. The 18S:25S ratio was established by Northern analysis using probes that detect 18S and 25S rRNA. The signals obtained by phosphor imaging were quantitated using the ImageQuant software tools. (C) Northern analysis using probe 1. (D) Northern analysis using probe 2. See Figure 1A for the positions of the probes.
We next analyzed the levels of different precursor intermediates in the double mutant cells by first growing them to mid-exponential phase on liquid-selective galactose-based medium and then shifting them to YPD medium for 24 h to make them dependent upon the mutant Rrp5p. Total RNA isolated from equal amounts of cells as determined by the OD600 of the cultures was subjected to Northern analysis using probes 1 and 2 (cf. Fig. 1A). SLX9 cells dependent upon either Rrp5Δ3p or Rrp5Δ6p displayed the processing phenotypes observed previously (data not shown; Eppens et al. 2002; Vos et al. 2004b). In the first case, the cells still produce 27SA2 and 20S pre-rRNA, albeit at a reduced level in accordance with their reduced growth rate. The rrp5-Δ6 mutation caused depletion of 27SA2 and 20S pre-rRNA on one hand, and increased production of 21S pre-rRNA on the other. Disruption of the SLX9 gene in the GAL::RRP5 strain shows only a mild increase of 21S pre-rRNA (Fig. 6D, lane 2) and considerably less decrease in 18S rRNA compared to the YCV711 strain used above (Fig. 6B), suggesting that the effects of loss of Slx9p depend upon genetic background, a conclusion stressed by the synthetic lethality shown. Inactivation of SLX9 in combination with either rrp5 deletion mutation resulted in loss of detectable levels of 20S and 27SA2 pre-rRNA in rrp5-Δ3 (Fig. 6C,D, lane 3) and 21S as well as 27SA2 in rrp5-Δ6 cells (lane 4). The relatively high levels of the 35S and 32S pre-rRNA species in both double mutants indicate that RNA polymerase I still transcribes the rDNA unit (cf. lanes 3,4 and lane 2). Thus, the lack of the 20S/21S and 27SA2 precursor species must be due to a complete failure of the processing machinery to cleave the primary precursor. We conclude that Slx9p, although not essential under normal conditions, plays a crucial role in ensuring the flexibility in ITS1 processing that guarantees production of both ribosomal subunits when cleavage at either site A2 or A3 is inhibited. Note that the ratio 18S to 25S rRNA is decreased, consistent with an initial shift from a high level of Rrp5p to a normal level of Rrp5p, followed by a complete dependence on the mutant protein and a complete block in ribosome biogenesis.
DISCUSSION
The yeast gene SLX9 was first identified in a synthetic lethality screen with a null mutant of SGS1, which encodes a 3′→5′ DNA helicase that monitors movements of replication forks (Ooi et al. 2003). However, accumulating evidence generated by proteomics pointed toward an additional role for Slx9p in ribosome biogenesis: Slx9p is enriched in the nucleolus (Ghaemmaghami et al. 2003), and the protein co-precipitates with the nonribosomal, trans-acting proteins Tsr1p and Enp1p (Gavin et al. 2002).
Our co-immunoprecipitation data using epitope-tagged Slx9p (Fig. 3) show that the protein is associated with 35S, 23S, and 20S pre-rRNA, as well as U3 snoRNA, demonstrating that it is indeed present in pre-ribosomes from the early 90S particle to the 43S precursor of the small subunit. Slx9p, therefore, belongs to a small group of trans-acting factors, also including Enp1p and Noc4p, that associate early and remain attached to pre-40S ribosomal subunits after their separation from the 66S pre-ribosomes (Grandi et al. 2002; Chen et al. 2003; Milkereit et al. 2003; Leger-Silvestre et al. 2005). This was confirmed by a recent computational analysis of proteomic data, which showed that six out of eight of the protein complexes that were co-precipitated by tagged Slx9p also featured well-established components of the SSU processome or the 43S pre-ribosome (Gavin et al. 2006). The sensitivity of the slx9 deletion strain toward cycloheximide also corroborates a role of Slx9p in ribosome biogenesis.
The RNA profiles depicted in Figure 4 provide direct evidence for a role of Slx9p in production of 40S ribosomal subunits since disruption of SLX9 decreases synthesis of 18S rRNA, while the levels of the large subunit 25S, 5.8SS, and 5.8SL rRNA remain normal. However, we found that Δslx9 cells possess an extraordinary profile with regard to levels of a number of pre-rRNA intermediates (Fig. 4C–F). The mutant cells accumulate 27SA2 pre-rRNA as well as the upstream 21S and 23S precursor species that extend beyond A2 to site A3. Second, reverse transcription data indicate a decrease in the steady-state level of 27SA3 pre-rRNA, the downstream product of cleavage at site A3 (Fig. 5). We, therefore, conclude that the processing phenotype caused by lack of Slx9p is probably not due to any major negative effects on the efficiency of the cleavages at sites A2 and A3. This conclusion is supported by the fact that the shift in 5.8S rRNA production from the short to the long form as the dominant species, which generally results from inhibition of cleavage at site A3 (Henry et al. 1994; Eppens et al. 2002), does not occur in Δslx9 cells (Fig. 4B). Instead, we propose that in cells lacking Slx9p, the rate at which the products of ITS1 processing are converted further is altered. It would appear that processing of 27SA3 pre-rRNA proceeds more rapidly than in wild-type cells, whereas maturation of 21S pre-rRNA, which may be a minor alternative product to the 20S precursor in wild-type cells (Vos et al. 2004b), is slowed down. Unfortunately, direct experimental evidence for these conclusions is difficult to obtain because of the low levels of both precursors and the fact that Δslx9 cells also still produce the 20S precursor to 18S rRNA. In order to try to circumvent this problem we decided to analyze the effect of the slx9 deletion in cells that carry either of two different mutant forms of Rrp5p, a trans-acting factor whose N-terminal domain consists of 12 S1 RNA-binding motifs. Deletion of nonoverlapping sets of these motifs completely blocks processing at either site A2 (mutant rrp5-Δ3) or A3 (mutant rrp5-Δ6) (Eppens et al. 2002; Vos et al. 2004b), which would enable us to analyze the effect of the Δslx9 mutation on each of the individual cleavages and further processing of the respective products. Surprisingly, however, both rrp5 deletion mutations proved to be synthetically lethal with the deletion of SLX9, apparently because lack of Slx9p prevents the processing machinery from carrying out the cleavage in ITS1 still allowed by the mutant Rrp5p in question (Fig. 6). While these results defy the original purpose of the approach, they do provide additional strong support for a role of Slx9p in ITS1 processing. An attractive hypothesis is that in wild-type cells Slx9p acts as a chaperone, ensuring an architecture of the late 90S pre-ribosome that is optimal for ITS1 processing. However, because of the natural flexibility of this processing, lack of Slx9p does not have any drastic negative consequences for ribosome biogenesis. The need for Slx9p becomes compelling, however, when the normal architecture of the late 90S pre-ribosome is compromised of mutations in other components such as Rrp5p. Slx9p, therefore, resembles the Ssf1p/Ssf2p proteins that are important in determining the order of ITS1 and ITS2 processing in pre-60S complexes. Premature cleavage of 27SA2 pre-rRNA at site C2 within ITS2 in the absence of Ssf1p/Ssf2p, is suggested to result from an improper transitional composition of the early large pre-ribosomal subunit (Fatica et al. 2002).
Interestingly, Slx9p does not have any readily identifiable orthologs in other eukaryotes apart from other yeast species. This is in clear contrast to the majority of trans-acting factors involved in ribosome biogenesis, which are generally found to be well conserved during evolution (Jansen et al. 1993; Kaser et al. 2001; Pestov et al. 2001; Wehner and Baserga 2002). The reason for the existence of such a yeast-specific factor could lay in the somewhat different manner in which ITS1 processing proceeds in yeast compared to higher eukaryotic cells (Borovjagin and Gerbi 2005).
The genetic interaction of SLX9 with SGS1, a gene encoding a nucleolar 3′→5′ DNA helicase that is involved in removal of stalled replication forks and DNA repair (Ooi et al. 2003), indicates a further role for Slx9p and a possible link between pre-rRNA processing and DNA integrity. Such a dual role is not without precedent (for review, see Dez and Tollervey 2004). Ribosome synthesis has, for example, previously been linked with DNA replication via Yph1p, Rrb1p, and Noc3p (Du and Stillman 2002; Zhang et al. 2002; Killian et al. 2004). Slx9p may be a further member of this group of proteins responsible for integrating ribosome biogenesis with other processes vital for survival.
MATERIALS AND METHODS
Strains and plasmids
The yeast strains that were used are listed in Table 1. The oligonucleotides used are listed in Table 2.
TABLE 1.
Yeast strains
TABLE 2.
Probes and primers
The ORF of SLX9 was amplified by PCR, using genomic DNA as a template and the primers Slx9fw1 and Slx9rv1 to introduce flanking XhoI sites. After digestion with XhoI, the resulting fragment was cloned into the corresponding sites of pET15b (Invitrogen) and pBS(KS+) (Stratagene).
To construct the slx9::LEU2 allele, the BamHI fragment that contains the LEU2 marker from vector YDp-L (BCCM/LMBP) was cloned into the BglII-site located in SLX9 in pBS–SLX9 to give pBS–slx9::LEU2. The slx9::LEU2 allele on this plasmid was then amplified by PCR using primers Slx9fw1 and Slx9rv1. The PCR product was transformed into the strains, YJV140 and YJV154, to allow homologous integration into the genome. Transformants were selected on Leu plates and screened by PCR to check for disruption of the genomic wild-type SLX9 gene.
For complementation of the SLX9 disruption in YCV711, the SLX9 gene including its promoter region was amplified from wild-type genomic DNA using primers Slx9fw2 and Slx9rv1. The former primer introduced an XbaI site at the 5′ end of the promoter region, the latter an XhoI site after the open reading frame of SLX9. The PCR product was cloned into the corresponding sites of pRS313 (Sikorski and Hieter 1989).
SLX9 was fused in-frame with the coding sequence of the two IgG-binding domains of Streptococcus aureus Protein A. To this end, an NcoI fragment containing the ProtA sequence was cloned into the NcoI site of pET15b–SLX9. For expression in yeast, the ProtA–SLX9 fragment was cloned blunt as an XbaI–EcoRI fragment into the BamHI site of pRS–PGK. Finally, the ClaI fragment from pRS–PGK–ProtA::slx9 was cloned into the ClaI site of pRS316. The resulting construct pRS316–PGK–ProtA::slx9 was transformed into Y14711 and selected on Ura plates.
Immunoprecipitation, isolation, and analysis of RNA
Immunoprecipitation was performed as described previously (Vos et al. 2004a). RNA isolation, and Northern and primer extension analyses were carried out as described previously (Venema and Tollervey 1996; Venema et al. 1998). The positions of the probes are indicated in Figure 1A.
ACKNOWLEDGMENTS
This work was supported by a grant from the Council for Chemical Sciences (CW) with financial aid from The Netherlands Foundation for Scientific Research (NWO).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.159406.
REFERENCES
- Allmang, C., Mitchell, P., Petfalski, E., Tollervey, D. Degradation of ribosomal RNA precursors by the exosome. Nucleic Acids Res. 2000;28:1684–1691. doi: 10.1093/nar/28.8.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, R.J., Sharp, J.A., Wang, J.C. Purification and characterization of the Sgs1 DNA helicase activity of Saccharomyces cerevisiae . J. Biol. Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- Borovjagin, A.V., Gerbi, S.A. An evolutionary intra-molecular shift in the preferred U3 snoRNA binding site on pre-ribosomal RNA. Nucleic Acids Res. 2005;33:4995–5005. doi: 10.1093/nar/gki815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Bucaria, J., Band, D.A., Sutton, A., Sternglanz, R. Enp1, a yeast protein associated with U3 and U14 snoRNAs, is required for pre-rRNA processing and 40S subunit synthesis. Nucleic Acids Res. 2003;31:690–699. doi: 10.1093/nar/gkg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschbauer, A.M., Jaramillo, D.F., Proctor, M., Kumm, J., Hillenmeyer, M.E., Davis, R.W., Nislow, C., Giaever, G. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics. 2005;169:1915–1925. doi: 10.1534/genetics.104.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dez, C., Tollervey, D. Ribosome synthesis meets the cell cycle. Curr. Opin. Microbiol. 2004;7:631–637. doi: 10.1016/j.mib.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Du, Y.C., Stillman, B. Yph1p, an ORC-interacting protein: Potential links between cell proliferation control, DNA replication, and ribosome biogenesis. Cell. 2002;109:835–848. doi: 10.1016/s0092-8674(02)00773-0. [DOI] [PubMed] [Google Scholar]
- Eppens, N.A., Faber, A.W., Rondaij, M., Jahangir, R.S., van Hemert, S., Vos, J.C., Venema, J., Raue, H.A. Deletions in the S1 domain of Rrp5p cause processing at a novel site in ITS1 of yeast pre-rRNA that depends on Rex4p. Nucleic Acids Res. 2002;30:4222–4231. doi: 10.1093/nar/gkf538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppens, N.A., Rensen, S., Granneman, S., Raue, H.A., Venema, J. The roles of Rrp5p in the synthesis of yeast 18S and 5.8S rRNA can be functionally and physically separated. RNA. 1999;5:779–793. doi: 10.1017/s1355838299990313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, A.W., Vos, J.C., Vos, H.R., Ghazal, G., Elela, S.A., Raue, H.A. The RNA catabolic enzymes Rex4p, Rnt1p, and Dbr1p show genetic interaction with trans-acting factors involved in processing of ITS1 in Saccharomyces cerevisiae pre-rRNA. RNA. 2004;10:1946–1956. doi: 10.1261/rna.7155904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, A.W., Vos, H.R., Vos, J.C., Raue, H.A. 5′-End formation of yeast 5.8SL rRNA is an endonucleolytic event. Biochem. Biophys. Res. Commun. 2006;345:796–802. doi: 10.1016/j.bbrc.2006.04.166. [DOI] [PubMed] [Google Scholar]
- Fatica, A., Cronshaw, A.D., Dlakic, M., Tollervey, D. Ssf1p prevents premature processing of an early pre-60S ribosomal particle. Mol. Cell. 2002;9:341–351. doi: 10.1016/s1097-2765(02)00458-6. [DOI] [PubMed] [Google Scholar]
- Fromont-Racine, M., Senger, B., Saveanu, C., Fasiolo, F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. doi: 10.1016/s0378-1119(03)00629-2. [DOI] [PubMed] [Google Scholar]
- Gallagher, J.E., Dunbar, D.A., Granneman, S., Mitchell, B.M., Osheim, Y., Beyer, A.L., Baserga, S.J. RNA polymerase I transcription and pre-rRNA processing are linked by specific SSU processome components. Genes & Dev. 2004;18:2506–2517. doi: 10.1101/gad.1226604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, A.C., Bosche, M., Krause, R., Grandi, P., Marzioch, M., Bauer, A., Schultz, J., Rick, J.M., Michon, A.M., Cruciat, C.M., et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- Gavin, A.C., Aloy, P., Grandi, P., Krause, R., Boesche, M., Marzioch, M., Rau, C., Jensen, L.J., Bastuck, S., Dumpelfeld, B., et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Gelperin, D., Horton, L., Beckman, J., Hensold, J., Lemmon, S.K. Bms1p, a novel GTP-binding protein, and the related Tsr1p are required for distinct steps of 40S ribosome biogenesis in yeast. RNA. 2001;7:1268–1283. doi: 10.1017/s1355838201013073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami, S., Huh, W.K., Bower, K., Howson, R.W., Belle, A., Dephoure, N., O'Shea, E.K., Weissman, J.S. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Giaever, G., Chu, A.M., Ni, L., Connelly, C., Riles, L., Veronneau, S., Dow, S., Lucau-Danila, A., Anderson, K., Andre, B., et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Grandi, P., Rybin, V., Bassler, J., Petfalski, E., Strauss, D., Marzioch, M., Schafer, T., Kuster, B., Tschochner, H., Tollervey, D., et al. 90S pre-ribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol. Cell. 2002;10:105–115. doi: 10.1016/s1097-2765(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Granneman, S., Baserga, S.J. Ribosome biogenesis: Of knobs and RNA processing. Exp. Cell Res. 2004;296:43–50. doi: 10.1016/j.yexcr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Henry, Y., Wood, H., Morrissey, J.P., Petfalski, E., Kearsey, S., Tollervey, D. The 5′ end of yeast 5.8S rRNA is generated by exonucleases from an upstream cleavage site. EMBO J. 1994;13:2452–2463. doi: 10.1002/j.1460-2075.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R., Tollervey, D., Hurt, E.C. A U3 snoRNP protein with homology to splicing factor PRP4 and G beta domains is required for ribosomal RNA processing. EMBO J. 1993;12:2549–2558. doi: 10.1002/j.1460-2075.1993.tb05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser, A., Bogengruber, E., Hallegger, M., Doppler, E., Lepperdinger, G., Jantsch, M., Breitenbach, M., Kreil, G. Brix from xenopus laevis and brx1p from yeast define a new family of proteins involved in the biogenesis of large ribosomal subunits. Biol. Chem. 2001;382:1637–1647. doi: 10.1515/BC.2001.199. [DOI] [PubMed] [Google Scholar]
- Killian, A., Le Meur, N., Sesboue, R., Bourguignon, J., Bougeard, G., Gautherot, J., Bastard, C., Frebourg, T., Flaman, J.M. Inactivation of the RRB1–Pescadillo pathway involved in ribosome biogenesis induces chromosomal instability. Oncogene. 2004;23:8597–8602. doi: 10.1038/sj.onc.1207845. [DOI] [PubMed] [Google Scholar]
- Lafontaine, D.L., Tollervey, D. Nop58p is a common component of the box C+D snoRNPs that is required for snoRNA stability. RNA. 1999;5:455–467. doi: 10.1017/s135583829998192x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.J., Baserga, S.J. Functional separation of pre-rRNA processing steps revealed by truncation of the U3 small nucleolar ribonucleoprotein component, Mpp10. Proc. Natl. Acad. Sci. 1997;94:13536–13541. doi: 10.1073/pnas.94.25.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger-Silvestre, I., Caffrey, J.M., Dawaliby, R., Alvarez-Arias, D.A., Gas, N., Bertolone, S.J., Gleizes, P.E., Ellis, S.R. Specific role for yeast homologs of the diamond blackfan anemia-associated Rps19 protein in ribosome synthesis. J. Biol. Chem. 2005;280:38177–38185. doi: 10.1074/jbc.M506916200. [DOI] [PubMed] [Google Scholar]
- Milkereit, P., Strauss, D., Bassler, J., Gadal, O., Kuhn, H., Schutz, S., Gas, N., Lechner, J., Hurt, E., Tschochner, H. A Noc complex specifically involved in the formation and nuclear export of ribosomal 40 S subunits. J. Biol. Chem. 2003;278:4072–4081. doi: 10.1074/jbc.M208898200. [DOI] [PubMed] [Google Scholar]
- Ooi, S.L., Shoemaker, D.D., Boeke, J.D. DNA helicase gene interaction network defined using synthetic lethality analyzed by microarray. Nat. Genet. 2003;35:277–286. doi: 10.1038/ng1258. [DOI] [PubMed] [Google Scholar]
- Pestov, D.G., Stockelman, M.G., Strezoska, Z., Lau, L.F. ERB1, the yeast homolog of mammalian Bop1, is an essential gene required for maturation of the 25S and 5.8S ribosomal RNAs. Nucleic Acids Res. 2001;29:3621–3630. doi: 10.1093/nar/29.17.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raué, H.A. Pre-ribosomal RNA processing and assembly in Saccharomyces cerevsiae . In: Olson M.O., editor. The nucleolus. Kluwer Academic/Plenum Publishers; New York: 2004. pp. 199–222. [Google Scholar]
- Schafer, T., Strauss, D., Petfalski, E., Tollervey, D., Hurt, E. The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes. EMBO J. 2003;22:1370–1380. doi: 10.1093/emboj/cdg121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz, L.M., Scharfe, C., Deutschbauer, A.M., Mokranjac, D., Herman, Z.S., Jones, T., Chu, A.M., Giaever, G., Prokisch, H., Oefner, P.J., et al. Systematic screen for human disease genes in yeast. Nat. Genet. 2002;31:400–404. doi: 10.1038/ng929. [DOI] [PubMed] [Google Scholar]
- Vanrobays, E., Gelugne, J.P., Gleizes, P.E., Caizergues-Ferrer, M. Late cytoplasmic maturation of the small ribosomal subunit requires RIO proteins in Saccharomyces cerevisiae . Mol. Cell. Biol. 2003;23:2083–2095. doi: 10.1128/MCB.23.6.2083-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema, J., Tollervey, D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae . Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]
- Venema, J., Tollervey, D. RRP5 is required for formation of both 18S and 5.8S rRNA in yeast. EMBO J. 1996;15:5701–5714. [PMC free article] [PubMed] [Google Scholar]
- Venema, J., Tollervey, D. Ribosome synthesis in Saccharomyces cerevisiae . Annu. Rev. Genet. 1999;33:261–311. doi: 10.1146/annurev.genet.33.1.261. [DOI] [PubMed] [Google Scholar]
- Venema, J., Planta, R.J., Raue, H.A. In vivo mutational analysis of ribosomal RNA in Saccharomyces cerevisiae . Methods Mol. Biol. 1998;77:257–270. doi: 10.1385/0-89603-397-X:257. [DOI] [PubMed] [Google Scholar]
- Vos, H.R., Bax, R., Faber, A.W., Vos, J.C., Raue, H.A. U3 snoRNP and Rrp5p associate independently with Saccharomyces cerevisiae 35S pre-rRNA, but Rrp5p is essential for association of Rok1p. Nucleic Acids Res. 2004a;32:5827–5833. doi: 10.1093/nar/gkh904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, H.R., Faber, A.W., de Gier, M.D., Vos, J.C., Raue, H.A. Deletion of the three distal S1 motifs of Saccharomyces cerevisiae Rrp5p abolishes pre-rRNA processing at site A(2) without reducing the production of functional 40S subunits. Eukaryot. Cell. 2004b;3:1504–1512. doi: 10.1128/EC.3.6.1504-1512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner, K.A., Baserga, S.J. The sigma(70)-like motif: A eukaryotic RNA binding domain unique to a superfamily of proteins required for ribosome biogenesis. Mol. Cell. 2002;9:329–339. doi: 10.1016/s1097-2765(02)00438-0. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Yu, Z., Fu, X., Liang, C. Noc3p, a bHLH protein, plays an integral role in the initiation of DNA replication in budding yeast. Cell. 2002;109:849–860. doi: 10.1016/s0092-8674(02)00805-x. [DOI] [PubMed] [Google Scholar]