Abstract
A 13-year-old dog was presented with clinical signs of anemia, vomiting, weight loss, and progressive abdominal distension. Abdominal ultrasonography and radiography revealed a large mass, which was removed surgically. Cytologic and histologic evaluation of the mass revealed a mixture of fat and hematopoietic tissue, consistent with a splenic myelolipoma.
Résumé
Présentation inhabituelle d’un myélolipome splénique chez un chien. Un chien âgé de 13 ans a été présenté avec des signes cliniques d’anémie, de vomissement, de perte de poids et de distension abdominale progressive. L’échographie et la radiologie abdominale ont révélé une importante masse qui a été excisée. L’évaluation cytologique et histologique de la masse a révélé un mélange de tissus graisseux et hématopoïétique, compatible avec un myélolipome splénique.
(Traduit par Docteur André Blouin)
A 13-year-old, 9.09 kg, spayed female, Cardigan Welsh corgi was presented to the Small Animal Hospital at Purdue University (PUVTH) with a 4-month history of progressive abdominal distension and weight loss. In the last 4 d before presentation, the dog was anorexic and vomiting once or twice per day. The referring veterinarian also noticed submandibular lymph node enlargement and a midabdominal mass by palpation, ultrasonography, and radiography. The dog’s vaccinations were up-to-date and she was on helminth prophylaxis. The dog’s condition was deteriorating and she was referred to the PUVTH for evaluation.
Case description
Physical examination revealed a normal temperature (37.8°C), heart rate (80 beats/min), and respiration rate (40 breaths/min). The submandibular lymph nodes were mildly enlarged. Examination of the teeth revealed gingivitis and dental tartar. Abdominal palpation revealed a nonpainful, large, cranial abdominal mass. A complete blood (cell) count (CBC) and serum biochemical profile was performed. The CBC revealed a mild normocytic, normochromic, nonregenerative anemia (PCV 0.35 L/L; reference range, 0.37 to 0.55 L/L), and lymphopenia. The biochemical profile showed a minimal elevation in alanine aminotransferase (ALT) (118 IU/L; reference range, 3 to 69 IU/L) and alkaline phosphatase (ALP) (183 IU/L; reference range, 20 to 157 IU/L).
A large radio-dense mass in the mid-abdomen was observed on abdominal radiographs. The mass was compressing and displacing the stomach craniad and the small intestine dorsocaudad. No other abnormalities were noticed on abdominal radiographs. Thoracic radiographs showed no evidence of metastasis to the lungs. Abdominal ultrasonography showed an ill-defined, mixed textured intrabdominal mass, contiguous with the tail of the spleen. The mass had several hypoechoic zones, was closely adhered to the mesenteric fat, and was displacing the duodenum to the right. The remainder of the spleen contained several hyperchoic nodules. The liver echotexture was mottled with an ill-defined nodular pattern; however, the overall echotexture was similar to that of the adjacent falciform fat. Intra-abdominal lymph nodes were normal. No obvious abnormalities were noted in the pancreas, stomach, and intestine.
Ultrasonographically guided aspirates using a 20-gauge, 3.8-cm (1.5-in) needle attached to a 6-mL sterile syringe were taken from the abdominal mass, the splenic nodules, and the liver. Cytologic evaluation of the aspirates from the abdominal mass revealed a marked extramedullary hematopoiesis, associated with normal fat and delicate spindle cell stromal tissue. Aspirates from the splenic nodules were of high cellularity, composed of heterogonous lymphoid cells. The lymphoid cells consisted primarily of small lymphocytes and mildly elevated numbers of intermediate, large, and reactive lymphocytes. Based on these findings, the splenic nodules were diagnosed as nodular hyperplasia. Differential diagnoses for the splenic mass were splenic myelolipoma, extramedullary hematopoiesis (EMH), hematoma, hemangioma, and hemangiosarcoma. Cytologic examination of the liver aspirates revealed mild to moderate vacuolar hepatopathy, consistent with either hydropic degeneration or glycogen accumulation.
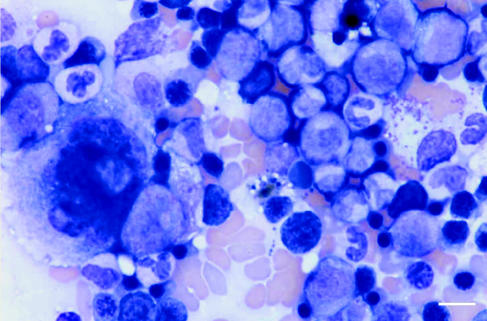
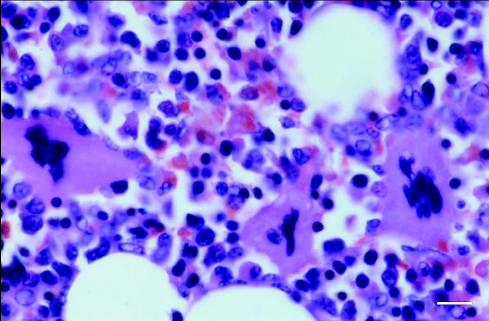
A midline exploratory laparatomy was performed under inhalational general anesthesia. Preoperatively, the dog was administered cefazolin (Kefzol; Eli Lilly, Indianapolis, Indiana, USA), 20 mg/kg bodyweight (BW), IV. Small amounts of hemorrhagic abdominal fluid were noticed. A large, 8 cm × 8 cm, soft, hemorrhagic mass was found attached to the head of the spleen and there were multiple nodules associated with the rest of the spleen. A total splenectomy was performed, and the spleen and multiple hepatic biopsies were submitted for cytologic and histopathologic evaluation. Cytologic examination of the splenic mass revealed benign-appearing adipose tissue associated with large numbers of hematopoietic cells. The hematopoietic cells were composed of all stages of cytomorphologically normal myeloid, erythroid, and megakaryocytic precursors (Figure 1). Low numbers of heterogeneous lymphoid cells were seen. The lymphoid cells comprised primarily small lymphocytes, low numbers of reactive lymphocytes, and few plasma cells. Few capillary structures were seen. Histopathologic examination of the splenic mass revealed a large, relatively well-delineated, nonencapsulated mass. The mass was composed of histomorphologically normal marrow hematopoietic tissue and adipose tissue (Figure 2). Based on these findings, the mass was diagnosed as a splenic myelolipoma.
Figure 1.
Cytologic preparation of splenic myelolipoma formed by myeloid precursors, few erythroid precursors, and megakaryocytes. Wright’s stain. Bar = 10 μm
Figure 2.
Myeloid, erythroid, and megakaryocytic precursors with interspersed adipose tissue from a splenic myelolipoma. Hematoxylin and eosin stain. Bar = 10 μm
Cytologic examination of the liver nodules revealed mild to moderate vacuolar hepatopathy, consistent with either hydropic degeneration or glycogen accumulation, secondary to possible mild cholestasis, mild extramedullary hematopoiesis, and mild lymphocytic inflammation. Histopathologic examination of the hepatic biopsies revealed mild, diffuse, nonspecific, vacuolar hepatopathy, consistent with either glycogen accumulation or potential mild hydropic degeneration.
The dog recovered uneventfully from anesthesia. She was administered morphine sulfate, 0.3 mg/kg, BW, IM, q4h for 2 d for postoperative pain management, discharged from the hospital 2 d following surgery, and prescribed carprofen (Rimadyl; Pfizer, Massachusetts, USA), 25 mg, PO, q12h for 5 d.
Five weeks following surgery, the dog was presented to the PUVTH for a recheck. The owner reported that the dog was doing well, had slightly regained weight, and had stopped vomiting. Abdominal radiographs and ultrasonographs revealed no abnormalities. A CBC and serum chemical profiles were within normal limits, including liver enzymes. A dental examination and complete treatment was performed at this time.
Discussion
Myelolipomas are rare, benign, biochemically nonfunctioning tumors composed of a mixture of mature adipose tissue and normal hematopoietic cells (1–2). Myelolipomas have been reported in humans (1), nonhuman primates (3,4), domestic and exotic cats (5–7), dogs (8–13), birds (14–16), and other exotic species (17). The vast majority of myelolipomas in humans occur within the adrenal glands, but several extra-adrenal myelolipomas have been reported (1–3). These tumors are typically a few millimeters to a few centimeters in size, encapsulated, and solitary. To the best of our knowledge, only 5 giant extra-adrenal myelolipomas have been reported in human patients, all of them perirenal (18). In dogs, only 8 cases of myelolipomas (6 in the spleen, 1 in the adrenal glands, and 1 in the spinal cord) have been reported (8–13). All these cases, and this one, have been in aged dogs (> 10 y) (Table 1). As in humans, myelolipomas in animals are usually diagnosed incidentally at necropsy without previous clinical signs of illness related to the tumor, except in the case of the dog with spinal myelolipoma causing acute paraplegia (9). The case reported herein is the first of splenic myelolipoma in a dog associated with splenic nodular hyperplasia and clinical and biochemical alterations including weight loss, abdominal distension, vomiting, anemia, elevated ALT, and ALP activity.
Table 1.
Review of the literature of canine myelolipomas
| Reference | Number of cases | Age(years) | Sex | Tumor location | Clinical signs |
|---|---|---|---|---|---|
| 13 | 2 | 12 | Female | Spleen | Incidental finding |
| 8 | 1 | Advanced | Female | Spleen | Incidental finding |
| 11 | 2 | NS | NS | Spleen | NSa |
| 10 | 1 | 16 | Female | Spleen | Incidental finding |
| 9 | 1 | 11 | Male | Spinal canal | CNSb |
| 12 | 1 | 11 | Female | Adrenal gland | Incidental finding |
a NS: Not specified
b CNS: Central nervous signs
Several theories regarding the etiology of adrenal myelolipomas have been presented (1–3). The most widely accepted one is that myelolipomas are due to a metaplastic change in the reticuloendothelial cells of blood capillaries in response to various stimuli, including necrosis, infection, or stress (1). This tumor should not be confused with extramedullary hematopoiesis found in other conditions, such as hemoglobinopathies, myeloproliferative disorders, and osseous diseases. These conditions can be ruled out by cytologic and histologic evaluation of the tumor, the absence of anemia and hypoxia, and a lack of bone marrow hyperplasia (1–3). The diagnosis of splenic myelolipoma is based on the cytological and histological findings of hematopoietic cells along with all stages of normal myeloid, erythroid, and megakaryocytic precursor cells, admixed with fat tissue.
Hepatic degeneration may result from hepatotoxic insults or chronic anemia. The hepatobiliary sensitive enzymes ALP and ALT are elevated in cases of cholestasis and severe, acute and diffuse hepatocellular injury, respectively.
Acknowledgments
We thank the internists, radiologists, pathologists, students and staff at the Purdue University Small Animal Teaching Hospital who played a role in the diagnosis and treatment of this patient. CVJ
References
- 1.Benchekroun A, Jira H, Ghadouane M, Kasmaoui EH, Zannoud M, Faik M. Adrenal myelolipoma. A case report. Ann Urol (Paris) 2002;36:95–8. [PubMed] [Google Scholar]
- 2.Lamont JP, Leiberman ZH, Stephens JS. Giant adrenal myelolipoma. Am Surg. 2002;68:392–394. [PubMed] [Google Scholar]
- 3.Porter BF, Goens SD, Brasky KM, Hubbard GB. A case report of hepatocellular carcinoma and focal nodular hyperplasia with a myelolipoma in two chimpanzees and a review of spontaneous hepatobiliary tumors in non-human primates. J Med Primatol. 2004;33:38–47. doi: 10.1111/j.1600-0684.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 4.Heard DJ, Fox EL, Fox J, Neuwirth L, Raskin R. Antemortem diagnosis of myelolipoma-associated hepatomegaly in a Goeldi’s monkey (Callimico goeldii) J Zoo Wildl Med. 1996;27:266–270. [Google Scholar]
- 5.Walzer C, Hittmair K, Walzer-Wagner C. Ultrasonographic identification and characterization of splenic nodular lipomatosis or myelolipomas in cheetahs (Acinonyx jubatus) Vet Radiol Ultrasound. 1996;37:289–292. [Google Scholar]
- 6.McCaw DL, da Silva Curiel JM, Shaw DP. Hepatic myelolipomas in a cat. J Am Vet Med Assoc. 1990;197:243–244. [PubMed] [Google Scholar]
- 7.Sander CH, Langham RF. Myelolipoma of the spleen in a cat. J Am Vet Med Assoc. 1972;160:1101–1103. [PubMed] [Google Scholar]
- 8.King JM. Splenic nodular hyperplasia and myelolipoma in a dog. Vet Med. 1993;88:104–105. [Google Scholar]
- 9.Newman SJ, Inzana K, Chickering W. Extradural myelolipoma in a dog. J Diagn Vet Invest. 2000;12:71–74. doi: 10.1177/104063870001200115. [DOI] [PubMed] [Google Scholar]
- 10.Prater MR, Bender H, Sponenberg P. Intra-abdominal mass aspirate from an aged dog. Vet Clin Pathol. 1998;27:54–65. doi: 10.1111/j.1939-165x.1998.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 11.Spangler WL, Culbertson MR, Kass PH. Primary mesenchymal (non-angiomatous/nonlymphomatous) neoplasm occurring in the canine spleen: Anatomic classification, immunohistochemistry, and mitotic activity correlated with patient survival. Vet Pathol. 1994;31:37–47. doi: 10.1177/030098589403100105. [DOI] [PubMed] [Google Scholar]
- 12.Tursi M, Iussich S, Prunotto M, Buracco P. Adrenal myelolipoma in a dog. Vet Pathol. 2005;42:232–235. doi: 10.1354/vp.42-2-232. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer Ma, Stair EL. Splenic myelolipomas in two dogs. Vet Pathol. 1983;20:637–638. doi: 10.1177/030098588302000517. [DOI] [PubMed] [Google Scholar]
- 14.Andreasen JR, Andreasen CB, Latimer KS, Oliphant JL. Thoracoabdominal myelolipomas and carcinoma in a lovebird (Agapornis sp.) J Vet Diagn Invest. 1995;7:271–272. doi: 10.1177/104063879500700221. [DOI] [PubMed] [Google Scholar]
- 15.Latimer KS, Pakich PM. Subcutaneous and hepatic myelolipoma in four exotic birds. Vet Pathol. 1995;32:84–87. doi: 10.1177/030098589503200117. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki K, Kinoshita H, Kurasho H, Narama I. Cutanous myelolipoma in a peach-faced lovebird (Agaporins roseicollis) Avian Pathol. 1996;25:131–134. doi: 10.1080/03079459608419126. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Fox JG, Erdman SE. Multiple splenic myelolipomas in a ferret (Mustela putorius furo) Lab Anim Sci. 1996;46:101–103. [PubMed] [Google Scholar]
- 18.Amin MB, Tickoo SK, Schultz D, Amin MB. Myelolipoma of the renal sinus: An unusual site for a rare extra-adrenal lesion. Arch Pathol Lab Med. 1999;123:631–634. doi: 10.5858/1999-123-0631-MOTRS. [DOI] [PubMed] [Google Scholar]
- 19.Settakorn J, Sirivanichai C, Rangdaeng S, Chaiwun B. Fine-needle aspiration cytology of adrenal myelolipoma: Case report and review of the literature. Diagn Cytopathol. 1999;21:409–412. doi: 10.1002/(sici)1097-0339(199912)21:6<409::aid-dc9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]