Abstract
An important goal of vaccination is to achieve long-term survival of functional memory T cells. Using a MHC-compatible adoptive transfer system, we show here that a short, 3-day IL-4 but not IL-2 or IL-12 exposure during in vitro T cell receptor stimulation of naive CD8+ T cells induced long-lasting in vivo memory. Such long-term memory CD8+ T cells expressed antigen-specific cytotoxicity and the potential for IFN-γ and IL-4 production. Our results support the concept that functional T cell longevity can be regulated by cytokines during initial antigen encounter and provide a rational foundation for vaccine development. They also may have implications in formulating optimal therapeutic regimens of ex vivo expanded autologous cancer- and HIV-specific CD8+ T cells. In addition, the availability of large numbers of memory CD8+ T cells generated through our high-efficiency system should facilitate progress in the molecular dissection of CD8+ T cell memory development.
The study of CD8+ T cell immunological memory can be divided into the induction phase, during which antigen is encountered, and the subsequent maintenance phase. Although maintenance mechanisms have been studied intensely (1–5), little information exists on how interactions encountered during the induction phase may direct activated T cells toward diametrically opposite fates of apoptosis vs. memory. Because of the many well documented examples of cytokine influence of cell fate determination, particularly T helper 1 (Th1) and Th2 development as the result of IL-12 and IL-4 presence during primary antigen encounter (6–8), we hypothesized a role for cytokines on long-term memory development during primary stimulation of naive CD8+ T cells. Here, we present supporting data for this hypothesis in that naive CD8+ T cells activated in vitro in the presence IL-4 but not IL-2 or IL-12 were able to develop into functional long-term memory cells upon transfer into MHC-compatible congenic hosts in the absence of antigenic stimulation.
Materials and Methods
Mice.
The MHC I-restricted 2C T cell receptor (TCR)-transgenic mice (9) and the C57BL/10ScN (B10) mice were maintained as described previously (10). The derivation of B10.Thy-1aCD8a (B10.TL) congenic strain was as described previously (11). In some experiments, 2C transgenic (H-2bThy-1bCD8b) males were crossed with B10.TL (H-2bThy-1aCD8a) females and offspring were screened to obtain 2C TCR-expressing F1 mice.
T Cell Isolation.
CD8+ T cells obtained by panning (12) were stained with fluorescein isothiocyanate (F)-anti-CD8 (53–6.7; ref. 13), PE-anti-CD4 (GK1.5; ref. 14), Cy5-anti-CD44 (IM7; ref. 15), and TR-anti-CD62L (Mel-14; ref. 16) and subjected to cell sorting (FACStar+; Becton Dickinson). CD8+CD4−CD44lowCD62Lhi phenotype (Fig. 1c) was the sorting criteria for naive CD8+ T cells. The purity of sorted cells was always 99%. Donor memory cells from B10.TL hosts were isolated by staining Ig− spleen cells (12) with F-anti-CD8.2 (clone 2.43; ref. 17), followed by cell sorting of CD8.2+ cells.
Figure 1.
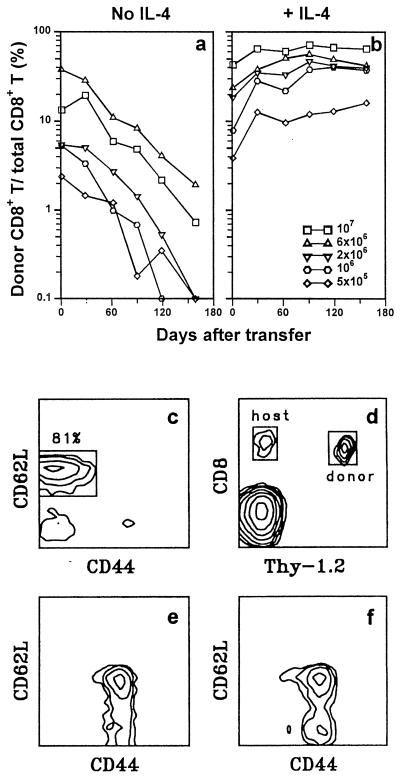
Induction of long-term CD8+ T cell survival by short-term IL-4 exposure during TCR stimulation. Donor/total CD8+ T cell ratios were determined as a function of time for individual B10.TL hosts (400R-irradiated) that had received indicated numbers of 2C CD8+ T cells activated in culture by B10.A peritoneal cells without IL-4 addition (a) or 2C CD8+ T cells activated in culture by B10.A peritoneal cells + IL-4 (b). Each line trace represents data derived from a single B10.TL host over time. (c) Criteria for naive CD8+ T cells. Correlated CD44/CD62L expression is shown for 2C tg CD8+4− T cells, with a box marking naive cells. CD8+CD4−CD44lowCD62Lhi was the criteria for sorting naive CD8+ T cells. (d) PBLs from a B10.TL mouse that received 8 × 106 activated 2C CD8+ T cells 109 days previously were stained with F-anti-Thy-1.2 (30H12) + Cy5-anti-CD8 (53–6.7); regions of donor and host CD8+ cells are marked. (e) 2C naive CD8+ T cells (4 × 106) activated by B10.A B cell blasts + IL-4 were transferred into a B10.TL host. Day 168 posttransfer, host spleen cells were stained with F-anti-CD8.2 + Cy5-anti-CD44 + TR-anti-CD62L; CD44/CD62L-correlated expression was restricted to CD8.2+ donor cells. (f) 2C naive CD8+ T cells (10 × 106) activated by B10.A peritoneal cells + IL-4 were transferred into a B10.TL host. Day 98 posttransfer, donor cell CD44/CD62L-correlated expression was performed as in e.
In Vitro Activated Donor Cells for Adoptive Transfer.
Naive 2C CD8+ T cells (1–2 × 105 cells/ml) were stimulated for 3 days by B10.A peritoneal cells (anti-Thy-1 + C-treated, 750R-irradiated; 1–1.5 × 105 cells/ml) or CD40L-activated B cell blasts (mitomycin-treated; 5 × 105 cells/ml; ref. 18), with or without added rIL-4 (PeproTech, Rocky Hill, NJ; expressed in amounts equivalent to an IL-4 standard provided by William E. Paul, National Institutes of Health), rIL-2 (Biogen; expressed as National Cancer Institute Biological Response Modifiers Program equivalent using IL-2-responsive CTLL cell bioassay), or 2 ng/ml IL-12 (PharMingen). Exogenously added IL-2 and IL-4 were always 0.5 ng/ml and 1.5 ng/ml (3,000 units/ml), respectively. The amount of IL-2 and IL-12 used was based on their respective abilities to maximally stimulate T cell proliferation and induce Tc1 effector generation as monitored by IFN-γ production. 2C CD8+ T cells mount excellent proliferative responses to allostimulation without added cytokine, presumably because of their ability to produce IL-2 (10). The activated cells were washed and rested for 2 days at 2 × 105 cells/ml in the presence of rIL-2 and 1 μg/ml anti-Ld mAb (19) to block possible antigen carryover. Indicated numbers of rested CD8+ T cells were injected i.v. into B10.TL hosts. In certain experiments, naive 2C CD8+ T cells were stimulated with syngeneic macrophages + SL8 (0.5 μg/ml SIYRYYGL; ref. 20), followed by a 2-day rest in IL-2-containing medium. Polyclonal activation of naive CD8+ T cells were induced by (i) 100 ng/ml 500A.A2 anti-CD3 mAb (21), with or without 100 ng/ml of anti-CD28 mAb (22), in the presence of syngeneic peritoneal cells (anti-Thy-1 + C-treated, 750R-irradiated; 1–1.5 × 105 cells/ml), or (ii) phorbol 12-myristate 13-acetate (PMA) (2 ng/ml) + ionomycin (0.15 μM) without accessory cells, at cell concentrations identical to the activation of 2C CD8+ T cells. Polyclonally activated cells similarly were rested and injected into B10.TL hosts as 2C CD8+ T cells.
Effector Cell Generation.
Naive and memory 2C effectors were generated by a 3-day allostimulation culture with or without added IL-4, followed by a 2-day rest in IL-2-containing culture as described in the preceding paragraph.
Flow Cytometric Analysis of Adoptively Transferred Donor Cells.
Blood from B10.TL hosts (tail artery puncture) was treated with ACK buffer (0.83% NH4CL/0.1% KHCO3) to lyse RBCs. For Figs. 1 and 2, peripheral blood lymphocytes (PBLs) were stained with F-anti-Thy-1.2 (30H12; ref. 13) + Cy5-anti-CD8 (53–6.7) and analyzed by flow cytometry. Frequencies of donor (Thy-1.2+CD8+) and host (Thy-1.2−CD8+) T cells were determined from 50,000 live cells (see Fig. 1d). Donor CD8+ T cells were expressed as the percentage of total (donor + host) CD8+ T cells. Donor/total CD8+ ratios of ≤0.1% were plotted as 0.1% and constituted experiment end point. For Fig. 3, PBLs were stained with F-anti-Thy-1.2 (30H12) + Cy5-anti-Thy-1.1 (LS693; courtesy of Irv Weissman, Stanford University), and the relative percentages of Thy1.1+Thy-1.2+ and Thy-1.1−Thy-1.2+ cells are shown.
Figure 2.
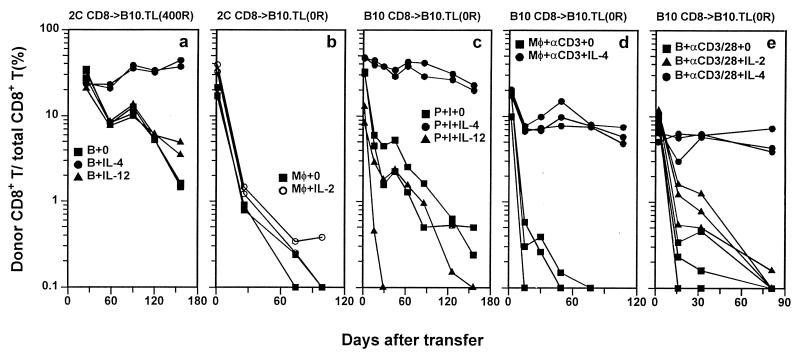
IL-4-induced long-term CD8+ T cell survival in alloantigen-, anti-CD3-, and PMA + ionomycin-induced activation systems. Naive CD8+ T cells were activated in the presence of indicated cytokines and transferred into B10.TL congenic hosts, and donor/total CD8+ T cell ratios were determined as in Fig. 1. Donor CD8+ T cells were 2C naive CD8+ T cells activated by B10.A B cell blasts (a), 2C naive CD8+ T cells activated by B10 macrophages + 0.5 μg/ml SL8 (SIYRYYGL) peptide (b), B10 naive CD8+ T cells activated by PMA + ionomycin without added accessory cells (c); B10 naive CD8+ T cells activated by anti-CD3 mAb + syngeneic peritoneal macrophages (d), or B10 naive CD8+ T cells activated by combined anti-CD3/anti-CD28 mAbs + syngeneic B cell blasts (e). Donor cells identified by F-anti-Thy-1.2 were 98% CD8+, except for PMA + ionomycin-activated donor CD8+ T cells, most of which had lost CD8 expression (23) but remained Thy-1.2+. In this case, Thy-1.2+ alone was used as the criteria to score cells of donor origin. The number of cells transferred into each B10.TL host was 5 × 106 for a, c, d, and e and 6 × 106 for b. B10.TL recipients were unirradiated for b, c, d, and e and 400R-irradiated for a.
Figure 3.
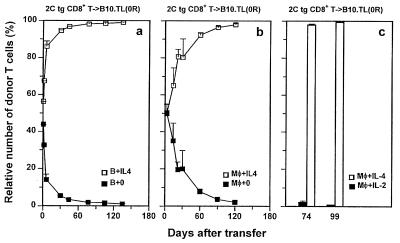
Failure for long-term survival not resulting from immunological rejection. (a) 2C CD8+ T cells from [B10 (H-2bThy-1bCD8b) × B10.TL (H-2bThy-1aCD8a)]F1 and from B10 genetic backgrounds were activated in separate cultures by B10.A B cell blasts with or without IL-4 addition, respectively. Each of three unirradiated B10.TL recipients was transferred with a mixture containing equal numbers (4 × 106 each) of the two differentially primed donor CD8+ populations. Staining PBL with F-anti-Thy-1.2 (30H12) + Cy5-anti-Thy-1.1 (LS693) allowed unambiguous detection of the relative percentage of the two types of donor cells (total donor cells as 100%), with Thy-1.1+Thy-1.2+ and Thy1.1−Thy-1.2+ phenotypes representing donor cells that were primed originally with or without IL-4, respectively. Because of the Thy-1.2− nature of host B10.TL T cells, they were clearly distinguishable from Thy-1.2+ donor CD8+ T cells. (b) 2C CD8+ T cells from B10 and (B10 × B10.TL)F1 genetic backgrounds were activated in separate cultures by B10.A peritoneal macrophages, with or without IL-4 addition, respectively. Each of four unirradiated B10.TL recipients was transferred with a mixture containing equal numbers (4 × 106 each) of the two differentially primed donor CD8+ populations. The relative percentage of IL-4-treated and untreated donor 2C CD8+ T cells was determined identically as in a, except that Thy1.1−Thy-1.2+ and Thy-1.1+Thy-1.2+ phenotypes represented donor cells that originally were primed with or without IL-4, respectively. (c) 2C CD8+ T cells from [B10 × B10.TL] F1 and from B10 genetic backgrounds were activated by B10 macrophages + 0.5 μg/ml SL8 peptide in separate cultures supplemented with IL-4 and IL-2 addition, respectively. Each of three unirradiated B10.TL recipients was transferred with a mixture containing equal numbers (3 × 106 each) of the two differentially primed donor CD8+ populations. The relative percentage of IL-4- and IL-2-treated donor 2C CD8+ T cells was determined identically as in a. Donor/total CD8+ ratios also were determined by staining with F-anti-Thy-1.2 + Cy5-anti-CD8 and consistent results with those presented in Figs. 1 and 2 were obtained (data not shown).
Cytokine Gene Activation.
Culture supernatant was assayed for IL-4 by bioassay (24) and for IFN-γ by ELISA (PharMingen). Total RNA was extracted from indicated cells and reverse-transcribed into cDNA as described (25). For Fig. 4, 103 cell-equivalent cDNA was subjected to PCR amplification by using IL-4- and IFN-γ-specific primers (26). IFN-γ PCR was 35 cycles (94°C, 30 s; 56°C, 45 s; 72°C, 20 s). IL-4 PCR was 38 cycles, followed by 10 additional cycles using 10% of first-round PCR products (94°C, 30 s; 56°C, 45 s; 72°C, 40 s). For Fig. 5, 102 cell-equivalent cDNA was subjected to nested PCR by using IFN-γ-specific primers. First-round PCR (20 cycles; 94°C, 30 s; 56°C, 45 s; 72°C, 20 s) was performed by using external primers: forward, 5′-tgaacgctacacactgcatcttgg-3′; reverse, 5′-cgactccttttccgcttcctgag-3′. Second-round PCR contained a mixture of 5 μl of 1:1,000-diluted first-round PCR product and 5 μl of competitor PQRS DNA (26) at indicated concentrations. The cycling conditions of the second-round PCR was identical to that of first-round PCR, except that the cycle number was increased to 35 and that previously described internal IFN-γ-specific primers were used (26).
Figure 4.
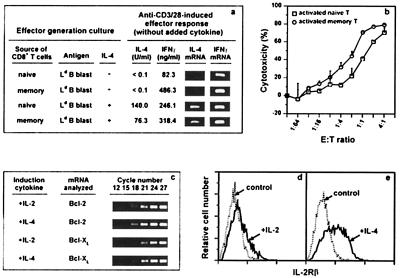
Ex vivo activity of IL-4-induced long-term CD8+ memory T cells. (a) Memory cells were from a B10.TL host 179 days after receiving 107 2C CD8+ T cells activated by B10.A peritoneal cells + IL-4. Naive and memory effectors were generated with or without IL-4 as described in Materials and Methods and stimulated (105 cells/well per ml) in wells coated with 10 μg/ml each of anti-CD3 + anti-CD28. At 24 h poststimulation, IL-4 and IFN-γ gene activation was analyzed according to Materials and Methods. All effectors cultured in wells not coated with anti-CD3/CD28 did not produce detectable IFN-γ or IL-4. (b) Memory CD8+ T cells were from a B10.TL host 168 days after receiving 4 × 106 2C CD8+ T cells activated by B10.A peritoneal cells + IL-4. Sorted naive and memory CD8+ T cells were stimulated by B10.A B cell blasts. On day 3, activated cells were recovered, counted, and assayed for CTL activity at indicated effector-to-target cell ratios, using Ld-bearing P815 target cells (41). Complete blocking of cytotoxicity by 30-5-7 anti-Ld mAb (19) confirmed the 2C TCR recognition specificity (data not shown). B10.A B cell blasts (5 × 104 cells/0.1 ml) stimulated naive and memory CD8+ T cells (104 cells/0.1 ml) to incorporate 20,726 cpm and 18,185 cpm of [3H]thymidine (<100 cpm for responding cells to which no B10.A B cell blasts were added). (c) Donor cells were isolated from B10.TL hosts 7 days after receiving 5 × 106 2C CD8+ T cells activated either by B10.A B cell blasts + IL-2 or by B10.A B cell blasts + IL-4. Duplicate samples were subjected to nested bcl-2 and bcl-xl reverse transcription–PCR (RT-PCR) amplification as described in Materials and Methods. Representative results from one of the duplicate samples taken at indicated cycle number of the second-round PCR are shown. Identical β-actin RT-PCR products for the two donor cell groups were found (data not shown). (d and e) Spleen cells from the same hosts as in c were examined for IL-2Rβ expression by flow cytometric analysis after staining with F-anti-CD8.2 + biotin-anti-IL-2Rβ (PharMingen; detected by TR-streptavidin). IL-2Rβ expression (solid lines) and control staining (anti-IL-2Rβ mAb omitted; dotted lines) among donor CD8+ T cells are shown (d, IL-2-activated donor cells; e, IL-4-activated donor cells).
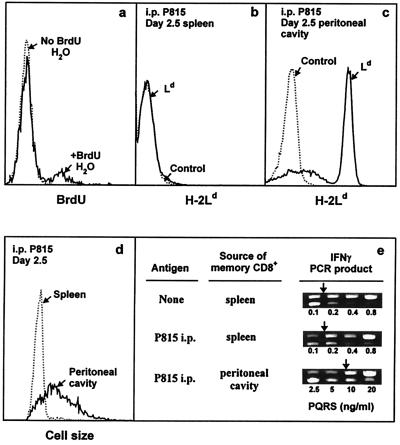
Figure 5.
In vivo activity of IL-4-induced long-term CD8+ memory T cells. (a) Each of two B10.TL hosts was transferred with 107 2C CD8+ T cells activated by B10.A B cell blasts + IL-4 as described in Materials and Methods. On day 88 postadoptive transfer, one host was given BrdUrd drinking water (1 mg/ml BrdUrd/2.5% glucose) and the other was given regular drinking water. On day 9, spleen cells were stained with Cy5-anti-Thy-1.2, followed sequentially by paraformaldehyde (0.5%) and acid (3 M HCl/0.5% Tween 20, 20 min) fixation, neutralization, F-anti-BrdUrd staining, and dual-parameter flow cytometric analysis. Histograms of BrdUrd incorporation by donor (Thy-1.2+) memory cells are shown. There were 12.1% BrdUrd+ cells among memory CD8+ T cells residing in hosts given BrdUrd drinking water (similar levels of BrdUrd labeling were observed for two similarly treated B10.TL hosts; data not shown). Negligible BrdUrd+ cells were found in the adoptive host given regular drinking water. (b– e) Each of two B10.TL mice was transferred with 3 × 106 Ld-reactive 2C CD8+ T cells activated by B10.A B cell blasts + IL-4. On day 277 postadoptive transfer, one host received an i.p. injection of 107 Ld-bearing P815 mastocytoma cells whereas the other did not. On day 2.5 post-P815 stimulation, cells recovered from the spleen and peritoneal cavity were analyzed. First, they were stained with F-anti-Ld to identify P815 cells in the spleen (b) and the peritoneal cavity (c). Second, they were stained with F-anti-Ld + Cy5-anti-Thy-1.2. Light-scatter histograms of donor (Thy-1.2+Ld-) cells from the spleen and peritoneal cavity are shown (d). Similarly identified donor spleen cells from the control host (no P815 challenge) had a nearly superimposable light-scatter distribution to the one shown for donor spleen cells from the P815-challenged host and was only marginally higher than the light-scatter distribution of freshly isolated naive (CD44lowCD62Lhi) CD8+ T cells (data not shown). Total recovered 2C donor cells from the P815-challenged mouse: spleen, 0.4 × 106; peritoneal cavity, 0.26 × 106. Total recovered 2C donor cells from P815-unchallenged mouse: spleen, 0.23 × 106; peritoneal cavity, not found. Third, they were stained as in c, and Thy-1.2+Ld-donor cells were isolated by cell sorting and analyzed for IFN-γ mRNA by nested competitive PCR (e). The concentrations of PQRS competitor DNA are as shown. The PCR products are: higher band, PQRS competitor; lower band, wild-type cDNA. Arrows mark equivalent amounts of wild-type and competitor PCR products.
Bcl-2 and Bcl-xl.
Total RNA (2 × 103 cell-equivalent amounts) was first subjected to a 25-cycle reverse transcription–PCR amplification by using external primers (Bcl-2: forward, 5′-aggattgtggccttctttgag-3′, and reverse, 5′-agacagccaggagaaatcaaac-3′; Bcl-xl: forward, 5′-gagctttgagcaggtagtg-3′, and reverse, 5′-ttgttcccgtagagatcc-3′). First PCR products (5%) were used for a second 35-cycle PCR by using nested (internal) primers (Bcl-2: forward, 5′-atgtgtgtggagagcgtcaac-3′, and reverse, 5′-tcagagacagccaggagaaatc-3′; Bcl-xl: forward, 5′-tgagcaggtagtgaatgaac-3′, and reverse, 5′-tccacaaaagtgtcccag-3′). Both rounds of PCR used identical conditions (94°C, 30 s; 58°C, 45 s; 72°C, 40 s). Fidelity of the 184-bp bcl-2 and the 210-bp bcl-xl PCR products were verified by DNA sequencing.
Results and Discussion
Purified naive CD44lowCD62LhiCD8+ T cells from Ld-alloreactive 2C TCR transgenic mice on B10 background (H-2bThy-1bCD8b) were isolated (sorting criteria shown in Fig. 1c), activated in vitro by Ld-bearing B10.A stimulators for 3 days with or without exogenously supplied test cytokines, then rested for 2 days in IL-2-containing medium. The cells then were transferred into histocompatible B10.TL congenic hosts. Staining with F-anti-Thy-1.2 + Cy5-anti-framework CD8 allowed quantitation of donor (Thy-1.2+) and host (Thy-1.2−) CD8+ T cells (Fig. 1d). Using donor/total CD8+ T cell ratio as a relative index, the decay of donor cells activated in cultures without cytokine addition was dose-dependent and approximated first-order kinetics whether B10.A macrophages (Fig. 1a) or B cell blasts (Fig. 2a) were used as stimulator cells. In marked contrast, IL-4 addition during the 3-day activation culture of 2C CD8+ T cells resulted in long-term survival with not a decrease but a slight increase in donor/total CD8+ ratios over the 5-month observation period (Figs. 1b and 2a). Although the optimal induction of long-term CD8+ T cell survival required 1.5 ng/ml IL-4 (the standard dose used for all experiments reported here), significant survival-potentiating activity was observed for the lowest concentration of 0.25 ng/ml IL-4 we have tested so far (data not shown). In an experiment identical in design to Fig. 1b, we found a total of 8.1 × 106, 5.0 × 106, and 2.8 × 106 donor cells per spleen from hosts that received 5 months previously 10 × 106, 6 × 106, and 4 × 106 donor cells (activated by B10.A macrophages + IL-4), respectively. Because cells present in the spleen represent only a fraction of the total of the entire mouse and donor cells found in the spleen approached the number of adoptively transferred cells, substantial expansion of donor cells must have taken place. This is consistent with the observed rise in donor/total CD8+ T cell ratios, especially in early time points, for adoptively transferred 2C CD8+ T cells activated in the presence of IL-4 (Figs. 1b and 2a). Virtually all (98%) of Thy-1.2+ donor cells coexpressed the 2C transgenic TCR as detected by 1B2 mAb, and their in vivo expansion most likely involved TCR-independent pathways because (i) 2C CD8+ T cells cannot be stimulated by B10.TL (H-2b) APC to enter cell division and (ii) activation-associated CD25 expression and TCR down-regulation were not found for memory CD8+ T cells in adoptive hosts (data not shown).
Addition of IL-12, a potent inducer of Th1 differentiation, to activation cultures did not significantly alter the rapid decay rate of donor cells (Fig. 2a). IL-4-mediated induction of long-term memory CD8+ T cell survival was not due to activation of γc chain alone because addition of IL-2, a known γc activator (27), failed to enhance donor cell survival (Fig. 2b). This result offers a possible reason for the limited success of IL-2-based immunotherapy (28, 29) and provides an impetus to examine potential therapeutic values of IL-4-grown antigen-specific T cells. The uniformly CD44hi phenotype of memory cells generated in our system (Fig. 1 e and f) agrees well with previously published results (30). In addition, the mostly CD62Lhi expression is consistent with the reported finding of long-term, virus-specific memory CTLp within the CD62Lhi rather than CD62Llow compartment (31).
To extend the positive IL-4 effect on long-term 2C tg CD8+ T cell memory development to nontransgenic mice, B10 naive CD8+ T cells were stimulated with PMA + ionomycin, with or without added IL-4, and transferred into unirradiated B10.TL hosts. Again, IL-4 but not IL-12 presence during the 3-day activation phase induced long-term memory CD8+ T cell survival (Fig. 2c). Three additional points can also be made. (i) Because B10 CD8+ T cells were activated without accessory cells, IL-4 acted on CD8+ T cells directly to promote long-term memory development. (ii) Enhanced memory development occurred in unirradiated hosts, ruling out radiation-induced effects as major contributory factors. (iii) PMA and ionomycin, being pharmacological agents with well defined half-lives in vivo, are unlikely to remain cell-associated for the duration of our experiment, thus ruling out their carryover as the cause of “chronic” stimulation, which, in turn, can lead to long-term survival (32). However, activation induced by PMA + ionomycin may be significantly different from TCR stimulation. Therefore, B10 naive CD8+ T cells were activated by anti-CD3 mAb in the presence of syngeneic macrophages. Once more, IL-4 presence during TCR stimulation of CD8+ T cells resulted in highly enhanced long-term survival (Fig. 2d). To address CD28 involvement, B10 naive CD8+ T cells activated by anti-CD3 + anti-CD28 were transferred into unirradiated B10.TL hosts. Without cytokine addition, CD3/CD28 coengagement yielded poor survival kinetics (Fig. 2e). Again, IL-4 but not IL-2 addition during naive CD8+ T cell activation resulted in highly enhanced long-term survival (Fig. 2e).
Because the number of CD8+ T cell divisions has been positively correlated with memory development (33), it is possible that the IL-4-potentiated CD8 memory development we have observed is a result of its growth-promoting activity, resulting in more rapid cell growth and, therefore, more cell divisions. This possibility is unlikely because addition of IL-2 to our 3-day TCR stimulation phase always yielded slightly more cells than cultures receiving IL-4 (data not shown), yet IL-4 but not IL-2 presence induced long-term survival.
Because our congenic transfer system uses donor cells that differ from the host at Thy-1 and CD8 alleles, a possible reason for the poor survival of CD8+ T cells activated without IL-4 addition is that they are more efficient than cells activated in the presence of IL-4 at inducing Thy-1- and/or CD8-specific host rejection responses. To address this possibility, 2C tg CD8+ T cells from [B10 (H-2bThy-1bCD8b) × B10.TL (H-2bThy-1aCD8a)]F1 and B10 backgrounds were activated separately by B10.A B cell blasts with or without added IL-4, respectively. Equal numbers of these two activated cell populations were mixed and transferred into the same unirradiated B10.TL recipients. Relative donor cell survival was monitored by simultaneous Thy-1.1 and Thy-1.2 staining. The finding of similar numbers of 2C CD8+ T cells activated with or without IL-4 on day 1 posttransfer (Fig. 3a) is consistent with similar homing properties. The relative presence of CD8+ T cells activated in the presence of IL-4 rapidly increased over those activated without IL-4 such that 98% of donor cells were derived from CD8+ T cells activated in the presence of IL-4 by day 60 postadoptive transfer. Similar results were obtained in another experiment in which B10.A peritoneal macrophages were used as stimulator cells (Fig. 3b). Relative cell survival of naive CD8+ T cells activated in the presence of exogenously added IL-2 and IL-4 also was performed (Fig. 3c). Clearly, CD8+ T cells exposed to IL-4 displayed strikingly enhanced survival over those exposed to IL-2. These results rule out immunological rejection as the cause of rapid decay of CD8+ T cells activated without IL-4 addition. In addition, the enhanced survival of CD8+ T cells briefly exposed to IL-4 during TCR stimulation is an acquired intrinsic property that displays little influence on cells not similarly potentiated.
Because high-level IFN-γ production is widely considered a unique property of memory CD8+ T cells (34–40), its induction was compared for 2C naive and memory effectors. With effectors generated in the absence of added IL-4, CD3/CD28 stimulation induced a much more potent 486.3-ng/ml IFN-γ production by memory than the 82.3 ng/ml by naive effectors (Fig. 4a). Both naive and memory effectors generated under IL-4 influence produced high levels of 246.1 ng/ml and 318.4 ng/ml IFN-γ, respectively, upon CD3/CD28 stimulation (Fig. 4a). The 25-fold higher IFN-γ production by freshly explanted 2C memory than naive CD8+ T cells in response to B10.A B cell stimulation (data not shown) is also consistent with the expected high-level IFN-γ production associated with memory CD8+ T cells (34–40).
Results we present here have shown that IL-4 priming potently induced long-term CD8+ T cell memory development. Because IL-4 priming also is known to induce Tc2 differentiation (42), we next examined IL-4 production by naive and memory effectors. Although CD3/CD28 stimulation did not induce IL-4 production (assessed by bioassay and reverse transcription–PCR) by naive and memory effectors generated in cultures without added IL-4 (Fig. 4a), it did activate IL-4-primed 2C naive and memory effectors to produce 140.0 units/ml and 76.3 units/ml IL-4, respectively (Fig. 4a). The 140.0 units/ml of IL-4 produced by IL-4-primed naive effectors corresponds to 70 pg/ml, a seemingly low amount. However, this response was derived from 104 effectors and translates to ≈0.7 ng/ml when responding cells are normalized to 106 cells/ml. This level is comparable to similarly normalized ≈1.4 ng/ml reported for anti-H-Y TCR-transgenic Tc2 effectors (43).
Cytotoxicity next was examined for naive and memory effectors generated by stimulation with B10.A B cells (without IL-4 addition). Memory effectors were ≈3-fold more efficient in killing of Ld-bearing target cells than naive effectors (Fig. 4b), a result that similarly has been seen in two other experiments (data not shown). The longevity of memory CD8+ T cells and its heightened potency in cytolytic effector function may be responsible for the observed antitumor response induced by IL-4-transfected cancer cells (44). Similar proliferation responses were found for naive and memory CD8+ T cells stimulated by B10.A B cell blasts (see legend for Fig. 4b). This appears to contrast results of deficient in vitro proliferation by 2C memory CD8+ T cells reported recently by Cho et al. (38). One potential contributing factor for this apparent difference may be the use by Cho et al. of lymphocyte-deficient RAG-null hosts whereas our studies used hosts that contain normal numbers of lymphocytes.
Whether IL-4-mediated potentiation of CD8+ T cell survival also is seen in vivo is unknown. In this regard, a recent paper reported enhanced memory CD8+ T cell generation and maintenance in IL-4-null mice (45). Although IL-4 may play no in vivo role in CD8 memory generation, it is equally possible that both IL-4-dependent and independent pathways exist.
Our initial effort in addressing the mechanism of IL-4-mediated long-term memory development focused on bcl-2/bcl-xl (46, 47) and IL-2Rβ (1). The finding of nearly identical bcl-2/bcl-xl expression in donor cells activated in IL-4 or in IL-2 (Fig. 4c) makes its role in memory development unlikely. Similar levels of Fas and FasL expression were observed for freshly isolated naive and memory CD8+ T cells and for naive and memory CD8+ T cells that had been subjected to a 6-h stimulation by plate-bound anti-CD3 + CD28 (data not shown). On the other hand, IL-2Rβ expression was positively correlated with long-term memory development (Fig. 4 d and e). IL-4 therefore may mediate enhanced long-term CD8+ memory T cell survival by activating genes that lead to constitutive IL-2Rβ expression. Engagement of IL-2Rβ by IL-15 then maintains a stable pool of long-lived memory cells (1). The involvement of Jak-1, Jak-3, Stat-6, IRS-1/2, or other signaling molecules (48, 49) in IL-4-induced long-term CD8 memory requires further experimentation.
In vivo activity of CD8 memory T cells also was studied. First, addition of BrdUrd in drinking water for 9 days resulted in BrdUrd incorporation in 12.1% of memory CD8+ T cells (Fig. 5a), consistent with the established rapid turnover nature of CD8+ memory T cells (50). Because high-level IFN-γ is regarded as an important function of memory CD8+ T cells (34–40), its response to i.p. injected antigen (Ld)-bearing P815 cells was studied. Memory CD8+ T cell activation was examined at 2.5 days post-P815 injection because in vivo generated 2C CD8 memory T cells previously had been shown to infiltrate the peritoneal cavity by this time whereas their naive counterparts take much longer (5 days) before they migrate into the peritoneal cavity (51). Nearly all memory CD8+ T cells from the peritoneal cavity displayed increased cell size (Fig. 5d). In addition, they expressed ≈50-fold more IFN-γ than control 2C memory cells from the spleen of a host not given P815 cells (Fig. 5e). In marked contrast, memory CD8+ T cells from the spleen of the same P815-exposed mouse were small in size and expressed IFN-γ comparable to control memory CD8+ T cells from an unimmunized host (Fig. 5 d and e). The failure of i.p. injected P815 cells to migrate to the spleen (Fig. 5 b and c) offers a likely reason for the lack of memory CD8+ T cell response there. The low endogenous IFN-γ expression by freshly isolated spleen memory CD8+ T cells from mice not exposed to P815 cells (Fig. 5e) is consistent with previously published results (4, 40).
Besides adding another item to a long list of diverse IL-4 functions (52, 53), results presented in this report support the notion that functional T cell longevity can be regulated by cytokines during initial antigen encounter and provide a foundation on which rational and possibly CTL-specific vaccine development can be approached. These potentials are made more relevant in view of the critical role played by CD8+ T cells in combating cancer and virus infection and the relative paucity of effective antivirus and anticancer drugs.
Acknowledgments
We thank Drs. William E. Paul and Shyr-te Ju for critically reading this manuscript. This work was supported by grants from Academia Sinica and National Science Council (NSC87-2311-B-001-110).
Abbreviations
- TCR
T cell receptor
- B10
C57BL/10ScN mouse strain
- B10.TL
B10 congenic strain that expresses Thy-1a and CD8a alleles
- F
fluorescein isothiocyanate
- PMA
phorbol 12-myristate 13-acetate
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060026497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060026497
References
- 1.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 2.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 3.Mullbacher A. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanchot C, Lemonnier F A, Perarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 5.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 6.Betz M, Fox B S. J Immunol. 1990;145:1046–1052. [PubMed] [Google Scholar]
- 7.LeGros G, Ben-Sasson S Z, Seder R, Finkelman F D, Paul W E. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 9.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russell J H, Loh D Y. Nature (London) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen F-L, Kung J T. J Immunol. 1996;156:2036–2044. [PubMed] [Google Scholar]
- 11.Chang J F, Thomas C A, III, Kung J T. J Immunol. 1991;147:851–859. [PubMed] [Google Scholar]
- 12.Fichtner A T, Anderson S, Mage M G, Sharrow S O, Thomas C A, Kung J T. J Immunol. 1987;138:2024–2033. [PubMed] [Google Scholar]
- 13.Ledbetter J A, Herzenberg L A. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 14.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 15.Trowbridge I S, Lesley J, Schulte R, Hyman R, Trotter J. Immunogenetics. 1982;15:299–312. doi: 10.1007/BF00364338. [DOI] [PubMed] [Google Scholar]
- 16.Gallatin W M, Weissman I L, Butcher E C. Nature (London) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 17.Sarmiento M, Glasebrook A L, Fitch F W. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 18.Wakatsuki Y, Neurath M F, Max E E, Strober W. J Exp Med. 1994;179:1099–1108. doi: 10.1084/jem.179.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lie W R, Myers N B, Gorka J, Rubocki R J, Connolly J M, Hansen T H. Nature (London) 1990;344:439–441. doi: 10.1038/344439a0. [DOI] [PubMed] [Google Scholar]
- 20.Ukada K, Wiesmuller K-H, Kienle S, Jung G, Walden P. J Immunol. 1996;157:670–678. [PubMed] [Google Scholar]
- 21.Havran W L, Poenie M, Kimura J, Tsien R, Weiss A, Allison J P. Nature (London) 1987;330:170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- 22.Gross J A, Callas E, Allison J P. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 23.Erard F, Wild M T, Garcia-Sanz J A, Le Gros G. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 24.Hu-Li J, Ohara J, Watson C, Tsang W, Paul W E. J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 25.Chen Y-T, Chen F-L, Kung J T. J Immunol. 1999;163:4747–4753. [PubMed] [Google Scholar]
- 26.Reiner S L, Zheng S, Corry D B, Locksley R M. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 27.Nakarai T, Robertson M J, Streuli M, Wu Z, Ciardelli T L, Smith K A, Ritz J. J Exp Med. 1994;180:241–251. doi: 10.1084/jem.180.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg S A, Yang J C, Topalian S L, Schwartzentruber D J, Weber J S, Parkinson D R, Seipp C A, Einhorn J H, White D E. J Am Med Assoc. 1994;271:907–913. [PubMed] [Google Scholar]
- 29.Rosenberg S A, Yang J C, White D E, Steinberg S M. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budd R C, Cerottini J C, Horvath C, Bron C, Pedrazzini T, Howe R C, MacDonald H R. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 31.Tripp R A, Hou S, Doherty P C. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 32.Beverley P C. Immunol Today. 1990;11:203–205. doi: 10.1016/0167-5699(90)90083-l. [DOI] [PubMed] [Google Scholar]
- 33.Opferman J T, Ober B T, Ashton-Rickardt P G. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 34.Budd R C, Cerottini J-C, MacDonald H R. J Immunol. 1987;138:3583–3586. [PubMed] [Google Scholar]
- 35.Sanders M E, Makgoba M W, Sharrow S O, Stephany D, Springer T A, Young H A, Shaw S. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 36.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho B K, Wang C Y, Sugawa S, Eisen H, Chen J. Proc Natl Acad Sci USA. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann C, Prevost-Blondel A, Blaser C, Pircher H. Eur J Immunol. 1999;29:284–290. doi: 10.1002/(SICI)1521-4141(199901)29:01<284::AID-IMMU284>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann M F, Barner M, Viola A, Kopf M. Eur J Immunol. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Matzinger P. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 42.Seder R A, Boulay J L, Finkelman F, Barbier S, Ben-Sasson S Z, Le Gros G, Paul W E. J Immunol. 1992;148:1652–1656. [PubMed] [Google Scholar]
- 43.Croft M, Carter L, Swain S L, Dutton R W. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golumbek P T, Lazenby A J, Levitsky H I, Jaffee L M, Karasuyama H, Baker M, Pardoll D M. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 45.Villacres M, Bergmann C C. J Immunol. 1999;162:2663–2670. [PubMed] [Google Scholar]
- 46.Akbar A N, Salmon M, Savill J, Janossy G. Immunol Today. 1993;14:526–532. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- 47.Nunez G, Merino R, Grillot D, Gonzalez-Garcia M. Immunol Today. 1994;15:582–588. doi: 10.1016/0167-5699(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 48.Keegan A D, Nelms K, Wang L M, Pierce J H, Paul W E. Immunol Today. 1994;15:423–432. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 49.Chen E H, Gadina M, Galon J, Chen M, O'Shea J J. Immunol Today. 1998;19:338–341. doi: 10.1016/s0167-5699(98)01295-x. [DOI] [PubMed] [Google Scholar]
- 50.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 51.Kedl R M, Mescher M F. J Immunol. 1998;161:674–683. [PubMed] [Google Scholar]
- 52.Paul W E, Ohara J. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- 53.Paul W E, Seder R A. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]