Abstract
Animal studies and preliminary results in humans suggest that lower extremity and myocardial ischemia can be attenuated by treatment with angiogenic cytokines. The resident population of endothelial cells that is competent to respond to an available level of angiogenic growth factors, however, may potentially limit the extent to which cytokine supplementation enhances tissue neovascularization. Accordingly, we transplanted human endothelial progenitor cells (hEPCs) to athymic nude mice with hindlimb ischemia. Blood flow recovery and capillary density in the ischemic hindlimb were markedly improved, and the rate of limb loss was significantly reduced. Ex vivo expanded hEPCs may thus have utility as a “supply-side” strategy for therapeutic neovascularization.
The development of blood vessels in response to tissue ischemia constitutes a natural host defense intended to maintain tissue perfusion required for physiologic organ function. Under certain circumstances, however, including advanced age, diabetes, and hypercholesterolemia, such native angiogenesis is impaired (1–4). In each of these circumstances, animal studies have disclosed a reduction in endogenous expression of vascular endothelial growth factor (VEGF); enhanced neovascularization in response to VEGF supplementation suggests that VEGF expression is indeed a critical determinant of postnatal blood vessel development.
Endogenous cytokine expression, however, is not the only factor contributing to impaired neovascularization. Older, diabetic, and hypercholesterolemic animals—like human subjects (5–12)—also exhibit evidence of age-related endothelial dysfunction. Although endothelial dysfunction does not necessarily preclude a favorable response to cytokine replacement therapy, indices of limb perfusion fail to reach ultimate levels recorded in wild-type animals, reflecting limitations imposed by a less-responsive endothelial cell (EC) substrate (1–4).
We therefore considered a strategy that would provide a robust source of viable ECs to supplement EC mobilization from preexisting blood vessels, according to the classic paradigm of angiogenesis developed by Folkman and colleagues (13). Experiments performed in various adult animal species (14–17) and more recently in human adults (C.K., unpublished data) have isolated from peripheral blood a circulating population of endothelial progenitor cells (EPCs) derived from bone marrow. These cells, shown to participate in physiologic as well as pathologic neovascularization, share with hematopoietic stem cells (HSCs) a common ancestral precursor—the putative hemangioblast (14, 16, 18–21). HSCs have been shown to be present in circulating blood, in quantities sufficient to permit their use as an alternative to bone marrow transplantation (22–25). We therefore inferred that EPCs, as related descendents, might be isolated from peripheral blood in quantities sufficient to permit their harvest, and, after ex vivo expansion, be administered systemically for the purpose of enhancing neovascularization. Indeed, the experiments performed to test this hypothesis have disclosed that not only is neovascularization of the ischemic hindlimb augmented, but that such enhanced perfusion is sufficient to increase the chance of successful limb salvage.
Materials and Methods
Cell Culture.
Total hPBMCs were isolated from blood of human volunteers by density gradient centrifugation with Histopaque-1077 (Sigma) (14). Cells were plated on culture dishes coated with human fibronectin (Sigma) and maintained in EC basal medium-2 (EBM-2) (Clonetics, San Diego). The media was supplemented with EGM-2 MV SingleQuots containing 5% FBS, human VEGF-1, human fibroblast growth factor-2 (FGF-2), human epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and ascorbic acid. After 4 days in culture, nonadherent cells were removed by washing with PBS, new media was applied, and the culture was maintained through days 7–10. Human umbilical vein ECs were prepared as previously described (26), and human microvascular ECs (HMVECs) from adult dermis were purchased from Cascade Biologics (Portland, OR).
Cellular Staining.
Fluorescent chemical detection of EPCs was performed on attached hPBMCs after 7 days in culture. Direct fluorescent staining was used to detect dual binding of FITC-labeled Ulex europaeus agglutinin (UEA)-1 (Sigma) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI)-labeled acetylated low density lipoprotein (acLDL; Biomedical Technologies, Stoughton, MA). Cells were first incubated with acLDL at 37°C and later fixed with 1% paraformaldehyde for 10 min. After washes, the cells were reacted with UEA-1 (10 μg/ml) for 1 h. After the staining, samples were viewed with an inverted fluorescent microscope (Nikon). Cells demonstrating double-positive fluorescence were identified as differentiating EPCs. Cultured human umbilical vein ECs and NIH 3T3 cells served as positive and negative controls, respectively.
Fluorescence-Activated Cell Sorting.
Fluorescence-activated cell sorting (FACS) detection of EPCs was performed on attached hPBMCs after 7 days in culture. Mononuclear cells were detached with the minimal use of trypsin and/or PBS with 1 mM EDTA followed by repeated gentle flushing through a pipette tip. Cells (2 × 105) were incubated for 30 min at 4°C with the monoclonal antibodies against kinase insert domain-containing receptor (KDR) (Sigma), recognizing the extracellular domain, and against human vascular endothelium (VE)-cadherin (BV-9; generous gift from E. Dejana, Istituto di Ricerche Farmacologiche “Mario Negri,” Milan, Italy), the biotinylated hCD62E (E-selectin) (PharMingen, San Diego, CA), the FITC-conjugated hCD3 (Becton Dickinson), hCD51/61 (αvβ3), hCD86 (PharMingen), hCD83 (Immunotech, Marseille, France), hCD68 (Dako), and the phycoerythrin-conjugated hCD31, hCD34, hCD14, and hCD19 (Becton Dickinson). Isotype-identical antibodies served as controls (PharMingen). For analysis of KDR and VE-cadherin, the cells were further incubated with a biotinylated anti-mouse IgG (H + L) antibody made in horse (Vector Laboratories) and with FITC-conjugated streptavidin (Caltag, South San Francisco, CA). After treatment, the cells were fixed in 1% paraformaldehyde. Quantitative FACS was performed on a FACStar flow cytometer (Becton Dickinson). Histograms of cell number vs. logarithmic fluorescence intensity were recorded for 10,000–20,000 cells per sample.
EPC Transplantation Animal Model.
All procedures were performed in accordance with the St. Elizabeth's Institutional Animal Care and Use Committee. Athymic nude mice (The Jackson Laboratory) age 8–10 wk and weighing 17–22 g were anesthetized with 160 mg/kg pentobarbital i.p. for operative resection of one femoral artery, and subsequently for laser Doppler perfusion imaging. Immediately before sacrifice, mice were injected with an overdose of pentobarbital.
The impact of hEPC administration on therapeutic neovascularization was investigated in a murine model of hindlimb ischemia (2, 27, 28). One day after operative excision of one femoral artery, athymic nude mice (n = 17), in which angiogenesis is characteristically impaired (4), received an intracardiac injection of 5 × 105 culture-expanded EPCs. Two control groups were identically injected with either human microvascular ECs (HMVECs, n = 12), harvested at 80–90% confluence, or media from the culture plates used for hEPC ex vivo expansion (n = 14).
For the study of EPC tracking, cells were marked with the fluorescent carbocyanine DiI dye (Molecular Probes). Before cellular transplantation, cells in suspension were washed with PBS and incubated with DiI at a concentration of 2.5 μg/ml PBS for 5 min at 37°C and 15 min at 4°C. After two washing steps in PBS, the cells were resuspended in EBM-2 medium. Mice received DiI-labeled EPCs in a total concentration of 5 × 105 cells, injected as described above. At 30 min before sacrifice, a subgroup of mice received an intracardiac injection of either 50 μg of Bandeiraea simplicifolia lectin I (BS I; Vector Laboratories) or UEA-1 (Sigma).
Physiological Assessment of Transplanted Animals.
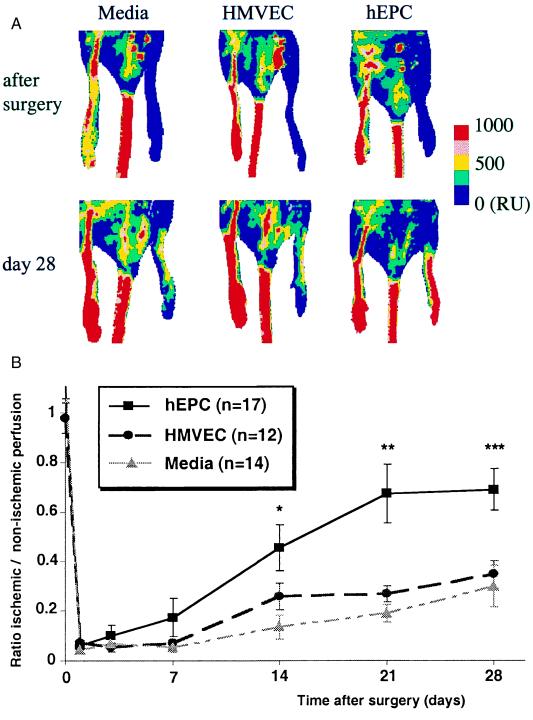
Laser Doppler perfusion imaging (Moor Instrument, Wilmington, DE) (29, 30) was used to record serial blood flow measurements over the course of 4 wk postoperatively, as previously described (1, 2, 4, 27, 28). In these digital color-coded images (Fig. 3A), red hue indicates regions with maximum perfusion; medium perfusion values are shown in yellow; lowest perfusion values are represented as blue. Panel on right displays absolute values in readable units (RU).
Figure 3.
(A) Representative results of laser Doppler perfusion imaging (LDPI) performed immediately after development of hindlimb ischemia and again 28 days later. Examination of hindlimb perfusion by LDPI disclosed profound improvement in the limb perfusion within 28 days after hEPC transplantation. (B) Quantitative analysis of perfusion recovery measured by LDPI. At 3 days postoperatively, limb perfusion was severely reduced in all three groups. Over the subsequent 28 days, however, substantial blood flow recovery in mice receiving hEPCs returned perfusion of the ischemic hindlimb to levels that were similar to those recorded in the contralateral nonischemic hindlimb. In contrast, limb perfusion remained markedly depressed in mice receiving either HMVECs or culture media.
Histologic Assessment of Transplanted Animals.
Tissue sections from the lower calf muscles of ischemic and healthy limbs were harvested on days 3, 7, 14, and 28. Tissue from other organs and the healthy hindlimb were also examined for incorporation of hEPCs. For immunohistochemistry, tissues were embedded in OCT compound (Miles Scientific, Elkhardt, IN) and snap frozen in liquid nitrogen. Frozen sections of 6-μm thickness were mounted on silane-coated glass slides, air-dried for 1 h, and counterstained with biotinylated antibodies to UEA-1, mouse and human CD31 (PECAM-1; Dako), and conjugated with FITC-streptavidin (Caltag), as previously described (14). Sections from other organs, including liver and spleen, were also examined for incorporation of hEPCs. Before direct immunofluorescent examination, slides were covered with Vectashield mounting medium for fluorescence microscopy (Vector Laboratories). The extent of neovascularization was assessed by measuring capillary density in light microscopic sections of muscles retrieved from ischemic mouse hindlimbs. The entire infrapatellar segment of each limb was examined. Sections were stained for alkaline phosphatase with indoxyl-tetrazolium and counterstained with eosin to detect capillary ECs as previously described (31). At each time point, 20 fields (×40 magnification) were counted for each of 3 animals.
Statistical Analysis.
All data are presented as mean ± SEM. Intergroup comparisons were performed by paired Student's t test or ANOVA. The comparative incidence of limb salvage was evaluated by χ2. Probability values of P < 0.05 were interpreted to denote statistical significance.
Results
Characterization of Human EPCs Expanded ex Vivo.
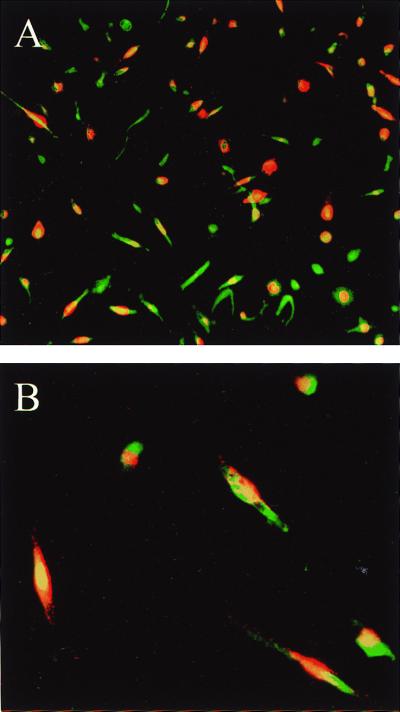
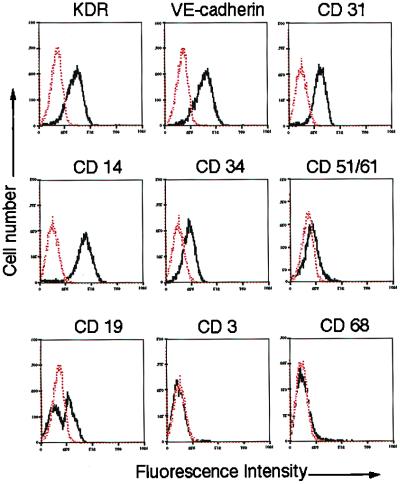
Total hPBMCs isolated and cultured for 7–10 days resulted in a spindle-shaped, EC-like morphology (14). These differentiating cells could be shown to endocytose acLDL and bind UEA-1, consistent with endothelial lineage cells (Fig. 1). FACS after 1 wk in culture disclosed that the vast majority of the cells not only assumed morphologic but also qualitative properties of ECs. Among 88.5 ± 4.6% antigenically defined cells, >70% of adherent cells expressed EC-specific antigens, including VEGF receptor-2 [VEGFR-2 (KDR), 77 ± 4.1%], VE-cadherin (78.1 ± 8.2%), and CD31 (72 ± 8.8%) (Fig. 2). Positive immunostaining for CD14 was identified in 90.7 ± 1.8% of the cells. The cell surface adhesion molecule CD34 and the integrin αvβ3 were expressed by 28.6 ± 9.5% and 11.7 ± 2.2% of the cells, respectively. Further characterization excluded significant contamination by hematopoietic lineage cells, such as T lymphocytes (CD3, 2.6 ± 1.8%) or macrophages.
Figure 1.
In vitro differentiation of peripheral mononuclear cell subpopulation into EPCs. Endocytosis of acLDL (red fluorescence) and binding to UEA (green fluorescence) identified EPCs. (A) ×10 magnification; (B) ×40 magnification.
Figure 2.
Ex vivo expansion of endothelial progenitor cells. After 7 days in culture, among 85% antigenically identified cells, >70% expressed endothelial-specific antigens KDR, VE-cadherin, and CD31, whereas contamination by hematopoietic lineage cells was not significant.
Physiological Assessment of EPC Transplantation.
The impact of hEPC administration on therapeutic neovascularization was investigated in a murine model of hindlimb ischemia (2, 27, 28). One day after operative excision of one femoral artery, athymic nude mice (n = 17), in which angiogenesis is characteristically impaired (4), received an intracardiac injection of 5 × 105 culture-expanded EPCs. Two control groups were identically injected with either HMVECs (n = 12) or media from the culture plates used for hEPC ex vivo expansion (n = 14). Serial examination of hindlimb perfusion by laser Doppler perfusion imaging (1, 2, 4, 27, 28) performed at days 3, 7, 14, 21, and 28 disclosed profound differences in the limb perfusion within 28 days after induction of limb ischemia (Fig. 3A). At 3 days postoperatively, limb perfusion was severely reduced in all three groups. Over the subsequent 28 days, however, substantial blood flow recovery in mice receiving hEPCs returned perfusion of the ischemic hindlimb to levels that were similar to those recorded in the contralateral nonischemic hindlimb. In contrast, limb perfusion remained markedly depressed in mice receiving either HMVECs or culture media. By day 28, the ratio of ischemic/normal blood flow in hEPC-transplanted mice had improved to 0.69 ± 0.08 vs. 0.27 ± 0.08 and 0.34 ± 0.05 in mice receiving HMVECs and culture media, respectively (P < 0.003) (Fig. 3B). Such improvement in hindlimb perfusion in this animal model has been previously shown to reflect neovascularization based on morphometric analyses of capillary density and EC proliferative activity (27).
Histologic Assessment of EPC Transplantation.
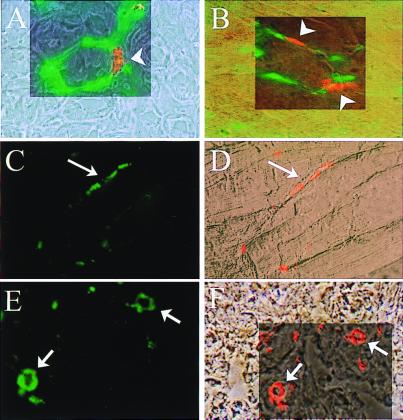
To confirm the homing and incorporation of administered hEPCs to sites of neovascularization, hEPCs labeled with fluorescent carbocyanine DiI dye were administered systemically by means of intracardiac injection after surgical excision of one femoral artery. Tissue sections harvested from the ischemic hindlimb were then inspected for fluorescent-labeled EPCs. Mouse endothelium was visualized by FITC-conjugated BS-1 injected premortem and against murine CD31 (PECAM) immunostaining. FITC-conjugated UEA-1 and human CD31 (Fig. 4) identified human EPCs in mouse ischemic hindlimb tissues. Colocalization of UEA-1 and DiI fluorescence documented that the injected population of labeled hEPCs incorporated into neovascular foci (Fig. 4). Time-course studies demonstrated that peak hEPC incorporation was achieved within 3–7 days postadministration of hEPCs (data not shown). Review of 16 sections retrieved from the ischemic limbs of 4 animals in which DiI-labeled hEPCs were administered identified labeled hEPCs in up to 56 ± 4.7% (mean ± SEM, range 30–80%) of vessels in a ×10 field. Similarly, up to 2.1 ± 0.4 (mean ± SEM, range 0.5–5.1) labeled hEPCs were incorporated into vessels in a ×10 field. Other than in ischemic tissue and very rarely in the spleen, hEPCs were detected neither in other organs nor the contralateral nonischemic hindlimb.
Figure 4.
Heterologous hEPCs incorporate into sites of neovascularization. Phase contrast photomicrographs show anatomic localization of DiI-labeled hEPCs incorporated (red, arrowheads) into foci of neovascularization in ischemic mouse muscle stained with BS1-lectin specific for mouse EC (green) (A) and stained against mouse CD31 (PECAM) (B). Human-specific EC marker UEA-I lectin (C) and human CD31 identified transplanted hEPCs as endothelial lineage cells (green, arrows) (E). (D and F) Equivalent phase contrast pictures show anatomic localization of DiI-labeled hEPCs (red) in mouse muscle.
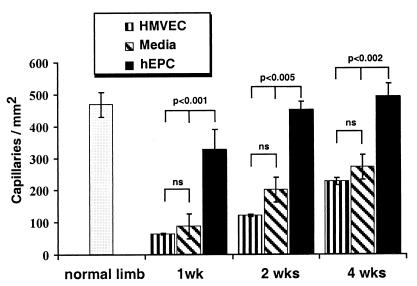
Histologic evaluation of skeletal muscle sections retrieved from the ischemic hindlimbs of mice killed at days 7, 14, and 28 showed that capillary density, an index of neovascularization, was markedly increased in hEPC-transplanted mice. As shown in Fig. 5, a statistically significant increase in capillary density was observed as early as day 7 in mice receiving hEPCs vs. control media (327 ± 61 vs. 87 ± 13 capillaries/mm2, P < 0.01). This difference remained significant through days 14 (452 ± 26 vs. 201 ± 32 capillaries/mm2, P = 0.0003) and 28 (492 ± 41 vs. 278 ± 13 capillaries/mm2, P < 0.002). Capillary density in the hindlimbs of hEPC-transplanted mice was similarly superior to mice receiving HMVECs (capillaries/mm2 at day 7 = 63 ± 2.5, P < 0.01; day 14 = 120.6 ± 4.4, P < 0.0001; day 28 = 226 ± 11.2, P = 0.0003). At no time point did capillary density in mice receiving HMVECs differ significantly from that measured in mice treated with control culture media.
Figure 5.
Histologic evaluation of neovascularization in ischemic hindlimb. Capillary density was significantly increased in mice receiving hEPC vs. culture media at 1 wk (P < 0.001 hEPC vs. culture media; P < 0.01 hEPC vs. HMVEC); at 2 wk (P < 0.0005 hEPC vs. culture media; P < 0.0001 hEPC vs. HMVEC); and at 4 wk postoperatively (P < 0.002 hEPC vs. culture media; P < 0.0003 hEPC vs. HMVEC). There were no statistically significant differences in capillary density between groups receiving culture media and HMVEC at the observed time points.
Tissue Salvage Achieved by EPC Transplantation.
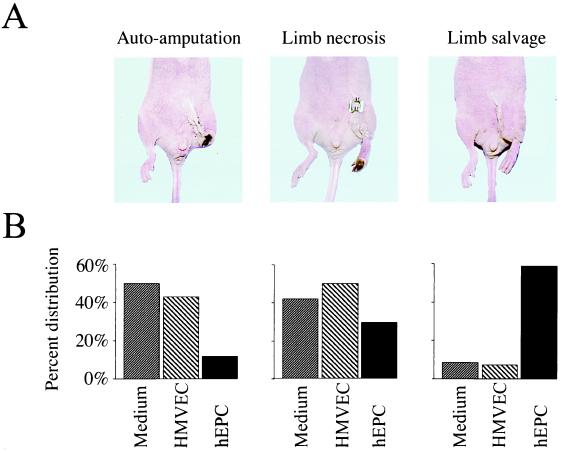
Enhanced neovascularization in mice transplanted with hEPCs led to important biological consequences. Because hindlimb neovascularization is inherently impaired in the athymic nude mouse, these mice typically develop extensive limb necrosis, often leading to autoamputation of the ischemic limb (4); rarely does the limb survive the entire 28-day study period intact (Fig. 6). Indeed, among mice in which induction of hindlimb ischemia was followed by administration of HMVECs, limb salvage was limited to 1 (8.3%) of 12 animals, whereas the remainder developed extensive forefoot necrosis (n = 5, 41.7%), leading in 6 (50%) to spontaneous amputation. Likewise, a preserved limb was observed in only 1 (7.1%) of 14 mice treated with culture media, whereas foot necrosis and/or autoamputation developed in 7 (50%) and 6 (42.9%) mice, respectively.
Figure 6.
Administration of hEPCs leads to reduced limb loss and increased limb salvage. (A) Representative macroscopic photographs of mice showing three different outcomes observed in the study. (Left) Autoamputation, characterized by loss of the ischemic hindlimb. (Middle) Severe foot necrosis. (Right) Most favorable outcome, complete salvage of ischemic hindlimb with intact function. (B) Percent distribution of above outcomes among mice receiving control media, HMVECs, and hEPCs. The differences in outcome were statistically significant (hEPC vs. HMVEC, P = 0.006; hEPC vs. culture media, P = 0.003, HMVEC vs. culture media, P = NS).
In contrast, hEPC transplantation was associated with successful limb salvage in 10 (58.8%) of 17 animals (Fig. 6). Foot necrosis was limited to five (29.4%) mice, and only two (11.8%) experienced spontaneous limb amputation. The difference in outcome between the hEPC-treated mice and both control groups was statistically significant (for hEPC vs. HMVEC, P = 0.006; for hEPC vs. control media, P = 0.003). The outcomes in mice receiving HMVECs vs. culture media were similar (P = 0.9).
Discussion
The present study indicates that ex vivo cell therapy, consisting of culture-expanded EPC transplantation, may be used to successfully promote neovascularization of ischemic tissues. These findings provide evidence that exogenously administered EPCs augment naturally impaired neovascularization in an animal model of experimentally induced limb ischemia. Not only did heterologous cell transplantation improve neovascularization and blood flow recovery, but important biological consequences—notably limb necrosis and autoamputation—were reduced by 50% in comparison with two different types of controls.
Cell transplantation in this case is predicated on ex vivo expansion of EPCs isolated from hPBMC population of healthy adult human subjects. Incubation with endothelial mitogens, including VEGF, bFGF, IGF, and EGF, for 7–10 days resulted in 80- to 90-fold expansion of cells expressing the EC-specific antigens KDR, CD31, and VE-cadherin. This calculation is based on data from our laboratory (our unpublished observations) that indicate that approximately 0.05% or 5 × 102 hEPCs can be isolated from 1 × 106 hPBMCs of human subjects. The ex vivo culture strategy allows expansion of the population of hEPCs to 4–5 × 104 cells per 1 × 106 hPBMCs, yielding an 80- to 90-fold increase in the original number of harvested cells. Previous analyses of embryonic neovascularization suggest that coexpression of flk-1 (KDR) and VE-cadherin denote the point of divergence of ECs from hematopoietic lineages (32). Moreover, a combination of monoclonal antibodies prepared against flk-1/KDR, VE-cadherin, CD31, Tie-1, and Tie-2 have been interpreted to define most intermediate stages during differentiation of embryonic stem cell-derived ECs (21, 32–34). The capacity to take up acLDL as well as UEA-1 further characterize ECs (19).
Cultured EPCs, as opposed to freshly isolated CD34 antigen-positive (CD34+) EPCs (14), were used in these experiments for three reasons. First, the number of EPCs obtained by ex vivo expansion (3.5 × 104 from 1 ml whole blood) exceed the number of CD34+ cells that can be freshly isolated (0.5 × 104/ml blood). Second, the purity and quality of EPCs in a cultured population are superior to that of freshly isolated CD34+ cells; as CD34+ was originally described as the prototypical antigen expressed by both HSCs and endothelial lineage cells, hematopoietic cells may contaminate freshly isolated CD34+ cells. Indeed, pilot studies demonstrated that the extent of neovascularization achieved after transplantation of freshly isolated CD34+ cells was inferior to culture-expanded EPCs. Third, for therapeutic strategies designed to employ transplanted cells that constitutively express pro- or anti-angiogenic factors, gene transfer of EPCs is facilitated by the use of culture-committed vs. less-differentiated CD34+ EPCs (T.A., unpublished data).
Under the described conditions, contamination by other cell lines, including lymphocytes, macrophages, and dendritic cells, was minimized as indicated by limited to absent expression of CD3, CD19, CD68, CD83, and CD86. Incubation of similar mononuclear cell cultures with other cytokines, such as granulocyte–macrophage colony-stimulating factor (GM-CSF) or tumor necrosis factor (TNF)-α, has been reported to favor isolation of dendritic cells (35, 36); in contrast, VEGF appears to inhibit dendritic cell maturation from CD34+ precursors (37, 38). As cytokine composition of the culture media may influence in vitro mononuclear cell differentiation (W.M.K.-M., unpublished data), the cytokine mixture used here for EPCs, containing VEGF, bFGF, IGF, and EGF, appears to preferentially promote endothelial lineage differentiation.
Isolation of circulating mononuclear cells for ex vivo EPC expansion was carried out by using peripheral blood from human donors. Isolation of circulating EPCs from human subjects thus appears realistic for harvesting EPCs for therapeutic neovascularization in future clinical applications. The feasibility of retrieving cells from peripheral blood has been previously established (39). Augmented mobilization of bone marrow-derived EPCs may be achieved by using several cytokines, including GM-CSF (15), similar to the approach used in preparation for stem-cell transplants (40, 41). The potential value of this approach is that it supplies substrate, i.e., a source population of robust ECs, that may complement current strategies of ligand administration for patients in whom depleted and/or dysfunctional ECs preclude an optimal response to cytokine supplements.
Acknowledgments
We thank Elisabetta Dejana for generously providing the VE-cadherin antibody. We also thank M. Neely for secretarial assistance and C. Milliken for technical assistance. C.K. is the recipient of a grant from the Cologne Fortune program of the Medical Faculty, University of Cologne, Germany. This work is supported in part by National Institutes of Health Grants HL53354, HL57516, and HL60911, the Peter Lewis Foundation, and the E. L. Wiegand Foundation.
Abbreviations
- VEGF
vascular endothelial growth factor
- EC
endothelial cell
- EPC
endothelial progenitor cell
- PBMC
peripheral blood mononuclear cell
- h
human
- FGF
fibroblast growth factor
- EGF
endothelial growth factor
- IGF
insulin-like growth factor
- HSC
hematopoietic stem cell
- HMVEC
human microvascular EC
- VE
vascular endothelium
- acLDL
acetylated low density lipoprotein
- UEA
Ulex europaeus agglutinin
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
- FACS
fluorescence-activated cell sorting
- KDR
kinase insert domain-containing receptor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070046397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070046397
References
- 1.Rivard A, Fabre J-E, Silver M, Chen D, Murohara T, Kearney M, Magner M, Isner J M. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner J M. Am J Pathol. 1999;154:355–364. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Belle E, Rivard A, Chen D, Silver M, Bunting S, Ferrara N, Symes J F, Bauters C, Isner J M. Circulation. 1997;96:2667–2674. doi: 10.1161/01.cir.96.8.2667. [DOI] [PubMed] [Google Scholar]
- 4.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner J M. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan A, More R S, Mullins P A, Taylor G, Petch M C, Schofield P M. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- 6.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo B, Sudano I, Salvetti A. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 7.Gerhard M, Roddy M-A, Creager S J, Creager M A. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 8.Johnstone M D, Creager S J, Scales K M, Cusco J A, Lee B K, Creager M A. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 9.Cosentino F, Luscher T F. J Cardiovasc Pharmacol. 1998;32:S54–S61. [PubMed] [Google Scholar]
- 10.Tschudi M R, Barton M, Bersinger N A, Moreau P, Cosentino F, Noll G, Malinski T, Luscher T F. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drexler H, Zeiher A M, Meinzer K, Just H. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 12.Luscher T F, Tshuci M R. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. In: Cancer Medicine. Holland J F, Frei E III, Bast R C Jr, Kute D W, Morton D L, Weichselbaum R R, editors. Philadelphia: Lea & Febiger; 1993. pp. 153–170. [Google Scholar]
- 14.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner J M. Science. 1997;275:965–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner J M, Asahara T. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearney M, Magner M, Isner J M. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Rafii S, Wu M H-D, Wijelath E S, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage L R, et al. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 18.Risau W, Flamme I. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 19.Rafii S, Shapiro F, Rimarachin J, Nachman R L, Ferris B, Weksler B, Moore M A S, Asch A S. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- 20.Noden D M. In: The Development of the Vascular System. Feinberg R N, Sherer G K, Auerbach R, editors. Basel: Karger; 1991. pp. 1–24. [Google Scholar]
- 21.Choi K, Kennedy M, Kazarov A, Papadimitriou J C, Keller G. Development (Cambridge, UK) 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 22.Majolino I, Cavallaro A M, Scime R. Bone Marrow Transplant. 1996;2:171–174. [PubMed] [Google Scholar]
- 23.Schmitz N, Bacigalupo A, Labopin M, Jamolino I, Laport J P, Brinch L, Cook G, Deliliers G L, Lange A, Rozman C, et al. Br J Haematol. 1996;95:715–723. doi: 10.1046/j.1365-2141.1996.d01-1958.x. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler B L, Kanz L. Curr Opin Hematol. 1998;5:434–440. doi: 10.1097/00062752-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Russel N, Gratwohl A, Schmitz N. Br J Haematol. 1996;93:747–753. doi: 10.1046/j.1365-2141.1996.d01-1712.x. [DOI] [PubMed] [Google Scholar]
- 26.Brogi E, Schatteman G, Wu T, Kim E A, Varticovski L, Keyt B, Isner J M. J Clin Invest. 1996;97:469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couffinhal T, Silver M, Zheng L P, Kearney M, Witzenbichler B, Isner J M. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 28.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes J F, Fishman M C, et al. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardell K, Jakobsson A, Nilsson G E. IEEE Trans Biomed Eng. 1993;40:309–316. doi: 10.1109/10.222322. [DOI] [PubMed] [Google Scholar]
- 30.Linden M, Sirsjo A, Lindbom L, Nilsson G, Gidlof A. Am J Physiol. 1995;269:H1496–H1500. doi: 10.1152/ajpheart.1995.269.4.H1496. [DOI] [PubMed] [Google Scholar]
- 31.Takeshita S, Tsurumi Y, Couffinhal T, Asahara T, Bauters C, Symes J F, Ferrara N, Isner J M. Lab Invest. 1996;75:487–502. [PubMed] [Google Scholar]
- 32.Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Development (Cambridge, UK) 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 33.Vittet D, Prandini M H, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- 34.Yamaguchi T P, Dumont D J, Conlon R A, Breitman M L, Rossant J. Development (Cambridge, UK) 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 35.Reid C, Stackpoole A, Meager A, Tikerpae J. J Immunol. 1992;149:2681–2688. [PubMed] [Google Scholar]
- 36.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. Nature (London) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 37.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone D P. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 38.Gabrilovich K I, Chen H L, Girgis K R, Cunningham H T, Meny G M, Nadaf S, Kavanaugh D, Carbone D P. Nat Med. 1996;10:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 39.Brugger W, Heimfeld S, Berenson R J, Mertelsmann R, Kanz L. N Engl J Med. 1995;333:283–287. doi: 10.1056/NEJM199508033330503. [DOI] [PubMed] [Google Scholar]
- 40.Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni A M. Blood. 1989;74:1905–1914. [PubMed] [Google Scholar]
- 41.Duhrsen U, Villeval J L, Boyd J, Knnourakis G K, Morstyn G, Metcalf D. Blood. 1988;72:2074–2082. [PubMed] [Google Scholar]