Abstract
Compared to normal human tissues, many common human cancers, including carcinoma of the colon, prostate, ovary, breast, and endometrium, express high levels of fatty acid synthase (FAS, EC 2.3.1.85), the primary enzyme responsible for the synthesis of fatty acids. This differential expression of FAS between normal tissues and cancer has led to the notion that FAS is a target for anticancer drug development. Recent studies with C75, an inhibitor of fatty acid synthesis, have shown significant antitumor activity with concomitant inhibition of fatty acid synthesis in tumor tissue and normal liver. Importantly, histopathological analysis of normal tissues after C75 treatment showed no adverse effects on proliferating cellular compartments, such as bone marrow, gastrointestinal tract, skin, or lymphoid tissues. In this study, we describe the de novo synthesis of C75 based on the known mechanism of action of cerulenin and the theoretical reaction intermediates of the β-ketoacyl synthase moiety of FAS. In addition, we demonstrate that C75 is a synthetic, chemically stable inhibitor of FAS. C75 inhibits purified mammalian FAS with characteristics of a slow-binding inhibitor and also inhibits fatty acid synthesis in human cancer cells. Treatment of human breast cancer cells with [5-3H]C75 demonstrates that C75 reacts preferentially with FAS in whole cells. Therefore, we have shown that the primary mechanism of the antitumor activity of C75 is likely mediated through its interaction with, and inhibition of, FAS. This development will enable the in vivo study of FAS inhibition in human cancer and other metabolic diseases.
Fatty acid synthesis is common to most organisms. In well-nourished humans, however, the fatty acid synthetic pathway is down-regulated because of sufficiently high levels of dietary fat (1). Although normal tissues have low levels of fatty acid synthesis, a number of recent studies have demonstrated surprisingly high levels of fatty acid synthase expression (FAS, EC 2.3.1.85) in a wide variety of human malignancies and their precursor lesions, including carcinoma of the colon (2), prostate (3, 4), ovary (5), endometrium (6), and breast (7–9). Ex vivo measurements in tumor tissue have revealed high levels of both FAS and fatty acid synthesis, indicating that the entire genetic program is highly active, consisting of some 25 enzymes from hexokinase to FAS (2, 10). This differential expression of FAS between normal tissues and cancer has led to the notion that FAS is a target for anticancer drug development.
The widespread expression of FAS in human cancer and its association with aggressive disease in breast (5, 9, 11), prostate (3, 4), and ovarian cancer (5) suggests that fatty acid synthesis provides an advantage for tumor growth. This is in marked contrast to its role as an anabolic energy storage pathway in liver and adipose tissue. Treatment of cancer cells in vitro with cerulenin, a covalent inactivator of the β-ketoacyl synthase reaction on FAS, led to cell death by means of apoptosis, demonstrating that cancer cells with highly active fatty acid synthesis require a functional pathway (12). Because of its chemical instability, however, cerulenin had limited activity in vivo.
To study systemic anticancer effects of FAS inhibition in vivo, a chemically stable type I FAS inhibitor was required. In this study, we describe the de novo synthesis of a synthetic, chemically stable inhibitor of mammalian FAS, C75, based on the known mechanism of action of cerulenin and the theoretical reaction intermediates of the β-ketoacyl synthase moiety of FAS. We further show that C75 binds to and inhibits mammalian FAS and inhibits fatty acid synthesis in human cancer cells. Recent studies have shown C75 to have significant in vivo antitumor activity against human breast cancer xenografts (13). Thus, the development of C75 should enable extensive in vivo study of FAS inhibition in human cancer and other diseases associated with dysfunctional fatty acid synthesis activity.
Materials and Methods
Synthesis of C75 and Related α-Methylene-γ-Butyrolactones.
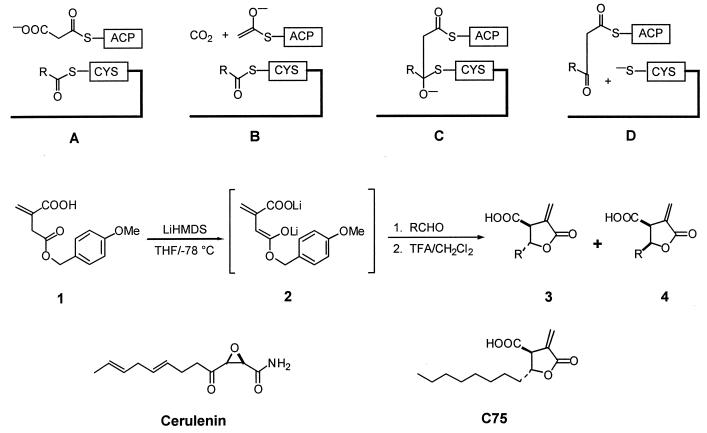
Lithiumhexamethyldisilyl amide (LiHMDS 1 M in THF; 40 ml, 40 mmol) was added to a solution of p-methoxybenzyl itaconate (1, 20 mmol) dissolved in dry tetrahydrofuran (THF, 200 ml) at −78°C. After 1 h, the aldehyde (20 mmol in 10 ml THF) was added at low temperature and stirred for 3–4 h. The reaction was quenched by the addition of cold 6N sulfuric acid (20 ml), and the products were extracted into ether. The organic solution was dried over anhydrous magnesium sulfate and evaporated to a gummy solid, which was dissolved in methylene chloride (100–125 ml) and treated with trifluoroacetic acid (1.5 ml) at room temperature for 10–12 h. The products were partitioned into aqueous sodium bicarbonate, reacidified, and extracted again into ether. Drying and removal of the solvent as before gave the lactones a mixture of trans- and cis-diastereomers (3 and 4) as a crystalline solid. These were separated by flash column chromatography on silica gel by using ethyl acetate:hexanes:acetic acid 30:70:1 as eluent, and individually crystallized from boiling hexanes. The following analytical data were collected for C75 (3, r = n − C8H17) and are representative: trans-isomer (1, see Fig. 1 3, r = n − C8H17): 25–30% isolated yield, mp 76–77°C; IR (film) 3000–3400, 2924, 2852, 1743, 1717, 1660, 1621, 1460 cm−1; 1H NMR (CDCl3) δ 0.84 (t, 3H, J = 6.8 Hz), 1.2–1.8 (m, 14H), 3.59 (dt, 1H, J = 2.8, 5.6, 12.8 Hz), 4.77 (qapp, 1H, J = 6, 12.8 Hz), 6.0 (d, 1H, J = 2.8 Hz), 6.4 (d, 1H, J = 3.2 Hz); 13C NMR (CDCl3) δ 14.0, 22.6, 24.7, 29.12, 29.14, 29.3, 31.7, 35.1, 49.4, 78.7, 125.9, 132.2, 168.1, 174.5; exact mass calculated for 254.1518, found 254.1514.
Figure 1.
Proposed mechanism of chain elongation in fatty acid biosynthesis and the synthesis of 3-carboxy-4-alkyl-2-methylenebutyrolactones. (A) Malonate, bound as its thioester to ACP, enters the active site where the elongating fatty acid chain is covalently linked to a reactive cysteine residue. (B) Decarboxylation of the malonyl-ACP results in a reactive enolate anion. (C) Enolate anion attacks the acyl-ACP in a thioester Claisen condensation to form the transiently generated tetrahedral oxyanion. (D) This rapidly decomposes to generate the resting state of KAS and the homologated β-ketoacyl intermediate bound to ACP. 1, Deprotonation of p-methoxybenzyl itaconate at low temperature produces the dianion 2, which underwent aldol reaction with a series of aldehydes, RCHO, to give on strongly acidic workup a mixture of γ-lactones 3 and 4. Separation on silica gel and crystallization afforded the pure diastereomers. Note the structural differences and similarities between cerulenin [(2S, 3R) 2,3-epoxy-4-oxo-7E, 10E-dodecadienamide] and C75. Although C75 lacks the highly reactive epoxide moiety, it retains the 8-carbon lipid tail without the two double bonds.
Synthesis of [5-3H]C75.
[1-3H]Nonanal was obtained by treatment of unlabeled aldehyde (1.42 g, 10.0 mmol) with sodium borohydride (0.18 g, 0.5 mmol) in absolute ethanol followed by sodium [3H]borohydride (Amersham Pharmacia; 500 mCi, 15 mCi/mmol). After 18 h at room temperature, the reduction was completed by the addition of excess sodium borohydride (0.4 g, 11 mmol). After filtration through a short column of silica gel, the tritiated alcohol was oxidized to [1-3H]nonanal with pyridinium chlorochromate (1.5 equiv.) in methylene chloride, and the product was purified by silica gel chromatography in 72% overall yield. [5-3H]C75 was obtained by reaction of the [1-3H]nonanal as above.
Growth Inhibition of α-Methylene-γ-Butyrolactones and Cerulenin in SKBR3 Breast Cancer Cells and Normal Human Skin Fibroblasts.
Both SKBR3 cells and fibroblasts were maintained in RPMI medium 1640 with 10% FBS under standard culture conditions. Cells were plated in 96-well plates at a density of 5,000 cells per well. After overnight growth, compounds solubilized in DMSO were added at concentrations ranging from 0.08 to 40 μg/ml in a volume of 100 μl with a maximum DMSO concentration of 1.25%. Vehicle controls were included for each plate. In addition, cis and trans isomers of C75 were tested against SKBR3 cells solubilized in DMSO or RPMI. Each concentration was plated in quadruplicate. After 72 h of incubation with drug at 37°C, plates were stained with crystal violet (0.2% in 12% ethanol), and absorbance was measured at 490 nm. ID50 (dose resulting in 50% growth inhibition) was computed by using linear regression (excel). Error determinations were computed from 95% confidence intervals of the regression model.
Purification of FAS and FAS Enzyme Assay.
FAS was purified from rat liver without enzyme or protease inhibitors by using stepwise polyethylene glycol and ammonium sulfate precipitations, gel filtration, and anion exchange chromatography as previously described (14). FAS was 95% pure as estimated from SDS/PAGE with Coomassie blue staining. FAS activity was measured by spectrophotometrically monitoring oxidation of NADPH at 340 nm as described (15). Briefly, 6.25 μg FAS, 50 μl of 1 M K2PO4 (pH 7.6) at 25°C, 25 nm of acetyl-CoA and 75 nm of NADPH in 0.5 ml reaction volume were monitored at 340 nm in a heated chamber spectrophotometer at 37°C for 3 min to measure background NADPH oxidation. After the addition of 27 nm of malonyl-CoA, the reaction was assayed for an additional 3 min to determine FAS-dependent oxidation of NADPH. One A340 unit is equivalent to 80.5 nm of NADPH oxidized. To detect slow-binding inhibition, FAS, C75, water, and K2PO4 buffer were incubated at 37°C for increasing incubation times from 0 to 30 min before commencing the standard FAS assay. Controls consisted of DMSO vehicle without drug.
[5-3H]C75 Binds to FAS in Human Cancer Cells.
To demonstrate [5-3H]C75 binding to FAS in a whole cell lysate, 1 × 106 SKBR3 cells plated in 60-mm dishes and cultured as above were incubated with [5-3H]C75 at 10 μg/ml for 4 h. After washing in PBS, cells were solubilized in Laemmli's buffer, resolved with 5% SDS/PAGE, and visualized by using fluorography with En3Hance (New England Nuclear), per manufacturer's instructions.
To confirm that [5-3H]C75 bound to FAS, FAS was immunoprecipitated from SKBR3 cells. A total of 5 × 106 SKBR3 cells were treated with [5-3H]C75 at 10 μg/ml for 4 h. Cells were then lysed and immunoprecipitated by using 20 μg polyclonal rabbit anti-human FAS with protein-A agarose (Roche Molecular Biochemicals) according to the manufacturer's protocol. Immunoprecipitated proteins were resolved with 5% SDS/PAGE and fluorography as above.
Measurement of Fatty Acid Synthesis in HL60 Cells.
HL60 human promyelocytic leukemia cells were grown in serum- and fatty acid-free medium as previously described (16). C75 (10 μg/ml in DMSO) or vehicle alone was added to cultures of 5 × 105 cells. After 2 h, cultures were labeled in triplicate with 1.0 μCi of [U-14C]acetate for 2 h, and total lipids were extracted with chloroform/methanol as previously described (16, 17). Labeled lipids were subjected to TLC (Analtech) in hexane/diethyl ether/acetic acid 90:10:1, which separates the following lipid classes: cholesterol, cholesterol ester, triglycerides, phospholipids, and free fatty acids; 14C-labeled standards were run for each of the lipid classes. After chromatography, labeled lipid classes were identified on a Molecular Dynamics Storm PhosphorImager and quantitated with imagequant software.
Results
Design and Synthesis of C75 and Preparation of [5-3H]C75.
The iterative two-carbon chain extension characteristic of fatty acid biosynthesis takes place at the β-ketoacyl synthase (KAS) domain of FAS (18, 19). Malonate, bound as its thioester to acyl carrier protein (ACP), enters the active site where the elongating fatty acid chain is covalently linked to a reactive cysteine residue (Fig. 1A). Decarboxylation of the malonyl–ACP takes place to give a reactive enolate anion (Fig. 1B), which attacks the acyl-ACP in a thioester Claisen condensation. The transiently generated tetrahedral oxyanion (Fig. 1C) rapidly decomposes to generate the resting state of KAS and the homologated β-ketoacyl intermediate bound to ACP (Fig. 1D).
The design of inhibitors of FAS was predicated on both an understanding of the mechanism of fatty acid biosynthesis, in particular that of the critical β-ketoacyl synthase step (18) and the well-studied inactivation of FAS by cerulenin (20). We have conducted simple molecular modeling exercises and structure-based searches based on the mechanism outlined in Fig. 1 A–D. A number of potential inhibitors of the reaction were identified. Among these, α-methylene-γ-butyrolactones having hydrocarbon side chains were chosen for further study. Several natural products of this general type are known, notably methylenolactocystin (3, r = n − C5H11), which was shown earlier to have activity against Gram-positive bacteria and prolong the survival of mice inoculated with Ehrlich carcinoma (21). Although the mechanisms of action for either of these activities were unknown, these observations prompted us to survey several side chain lengths to test their antitumor and FAS inhibitory behavior.
Although total syntheses of methylenolactocystin have been reported, we sought a procedurally simpler approach to rapidly assemble alkyl variants of this structural family. A modification of the reaction developed by Carlson and Oyler (22) achieved this goal and is set out in Fig. 1. Deprotonation of p-methoxybenzyl itaconate (1) at low temperature gave the dianion 2, which underwent aldol reaction with a series of aldehydes, RCHO, to give on strongly acidic workup a mixture of γ-lactones 3 and 4. Separation on silica gel and crystallization afforded the pure diastereomers. The results obtained for a representative selection of these compounds are shown in Table 1. One of these, C75 (Table 1, r = n − C8H17), was prepared in tritium-labeled form. Nonanal was reduced by the “sandwich” procedure with sodium [3H]borohydride (23) and oxidized to [1-3H]nonanal, which was elaborated as above to give [5-3H]C75.
Table 1.
In vitro growth inhibition of compounds against SKBR3 human breast cancer cells
| Compound | Alkyl side chain (r =) | SKBR3 cells ID50, μg/ml | JW fibroblasts ID50, μg/ml |
|---|---|---|---|
| Cerulenin | —C8H13 (1–4 trans diene) | 3.3 ± 0.2 | 7.2 ± 3.1 |
| C83 | —C13H27 | 3.9 ± 0.1 | 10.6 ± 0.3 |
| C81 | —C11H23 | 4.8 ± 0.2 | 29.0 ± 5.0 |
| C77 | —C9H19 | 5.2 ± 0.3 | 12.8 ± 1.2 |
| C75* | —C8H17 | 5.0 ± 0.1 | 21.6 ± 1.4 |
| C49 | —C7H15 | 4.8 ± 0.5 | 21.7 ± 0.5 |
| C73 | —C6H13 | 8.4 ± 0.2 | 12.4 ± 0.8 |
| C271 | —C8H13 (1–4 trans diene) | 26.3 ± 2.4 | N.T. |
| DMSO control | N.T. | N.T. |
N.T., no toxicity identified; C75, racemic mixture of cis and trans isomers.
α-Methylene-γ-Butyrolactones Selectively Inhibit Growth of Human Cancer Cells in Vitro.
The family of α-methylene-γ-butyrolactones was screened for selective growth inhibition based on differential ID50 between SKBR3 cells, a human breast cancer cell line with high levels of fatty acid synthesis (24, 25), and normal skin fibroblasts. Table 1 summarizes the structures and growth inhibition obtained for a representative selection of the α-methylene-γ-butyrolactones with varying alkyl side chains. An alkyl side chain length of seven or eight carbon atoms gave optimum selective growth inhibition and aqueous solubility.
Initially, a racemic mixture of cis and trans C75 isomers dissolved in DMSO and diluted into buffer displayed selective toxicity against SKBR3 cells (Table 1). Although the cis isomer was less soluble in aqueous solutions, the trans isomer of C75 was soluble in RPMI culture medium and showed activity against SKBR3 cells (ID50 3.2 +/− 0.2) similar to cerulenin (Table 1). The reason for the increased activity of trans-C75 in aqueous vehicle has not been determined. Interestingly, adding the double bonds to C75 in the same location of the alkyl side chain as cerulenin (Table 1, C271) markedly reduced growth inhibitory activity.
C75 Inhibits Purified Mammalian FAS.
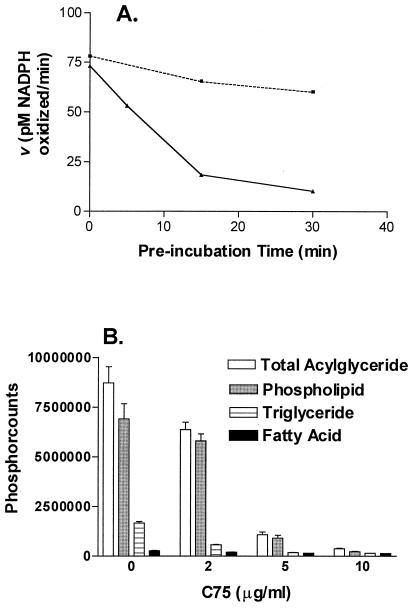
C75 exhibits characteristics of a slow-binding inhibitor of FAS. Slow-binding inhibitors have been defined as compounds in which equilibrium among enzyme, inhibitor, and enzyme–inhibitor complex occurs on a scale of seconds to minutes and include a number of commonly prescribed drugs (26). Under conditions of the standard assay for FAS activity, enzyme and inhibitor are preincubated for approximately 3 min at 37°C, before initiation of the enzyme reaction by the addition of malonyl-CoA (16). C75 is inactive in this standard assay. When 37°C preincubation times were increased, there was a directly proportional inactivation of FAS activity, consistent with slow-binding inhibition, up to 83% after 30 min (Fig. 2A). Interestingly, C83 (Table 1, r = n − C13H17), a compound with an alkyl side chain five carbons longer than C75, is active in the standard assay, suggesting that alkyl side chain length influences enzyme-inhibitor binding (data not shown).
Figure 2.
C75 inhibits FAS and fatty acid synthesis. (A) C75 has characteristics of a slow-binding inhibitor of type I FAS. With increasing preincubation time of FAS and C75, there is greater inhibition of FAS activity (solid line) compared with control (dotted line). (B) C75 inhibits fatty acid synthesis in HL60 cells. With increasing doses of C75, [U-14C]acetate incorporation into acylglycerides is inhibited by 80%. Incorporation into the free fatty acid pool is reduced by about 50%.
C75 Inhibits Fatty Acid Synthesis in Vitro and in Vivo.
HL60 human promyelocytic leukemia cells proliferate in serum and fatty acid-free culture media and as such provide a convenient in vitro model for the study of fatty acid synthesis in cancer cells (16). In this environment, the cells are solely dependent on endogenous fatty acid synthesis for the production of storage, structural, and signaling lipids. As shown in Fig. 2B, after 4 h exposure to drug at 5 μg/ml, [U-14C]acetate incorporation into phospholipids and triglycerides was inhibited by 87% and 89%, respectively. The transfer of label into the free fatty acid pool was also reduced by 44%. In HL60 cells grown in 10% FBS, fatty acid synthesis was down-regulated, but the fractional inhibition by C75 was similar to serum-free conditions (27). C75 treatment of HCT116 human colon cancer cells (27) and MCF7 and SKBR3 human breast carcinoma cells grown with 10% FBS showed similar magnitudes of pathway inhibition (data not shown).
C75 Binds to Human FAS.
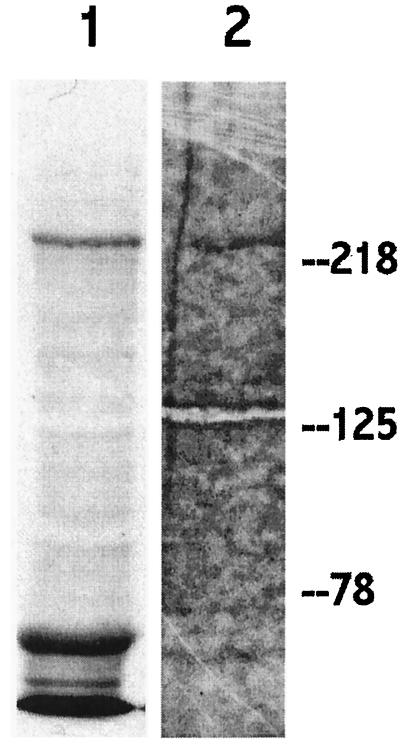
Although C75 inhibits purified mammalian FAS and inhibits fatty acid synthesis, we used [3H]C75 to examine its interaction with FAS in whole cells. SKBr3 cells are ideal for these studies as they express the highest per-cell amounts of FAS yet described in human established cancer cell lines. After a 4-h exposure of SKBr3 cells to [3H]C75, a band of approximately 250 kDa is labeled from a whole cell lysate (Fig. 3, lane 1). Immunoprecipitation with anti-human FAS from similarly labeled cells, identifies the 250-kDa protein as FAS (Fig. 3, lane 2). In a parallel experiment, the 250-kDa band was confirmed as FAS by an immunoblot with polyclonal rabbit anti-human FAS antibodies (data not shown). The additional 60-kDa band labeled in lane 1may represent [3H]C75 binding to (i) albumin from the culture medium, (ii) an FAS fragment, or (iii) binding to an unknown cellular target.
Figure 3.
C75 labels FAS. SDS/PAGE and fluorography of SKBr3 cell lysate after 4 h exposure of cells to 3H-C75 [5 μg/ml]. Lane 1, whole cell lysate. Lane 2, FAS immunoprecipitation. Note the labeling of the approximately 250-kDa band, which represents human FAS.
Discussion
C75 treatment of MCF-7 human breast cancer xenografts has shown significant anti-tumor activity with concomitant inhibition of fatty acid synthesis in tumor tissue and normal liver (13). Importantly, histopathological analysis of normal tissues after C75 treatment showed no adverse effects on proliferating cellular compartments, such as bone marrow, gastrointestinal tract, skin, or lymphoid tissues. Transient reversible weight loss was the only toxicity noted.
C75 treatment of human cancer cells in vitro led to rapid inhibition of fatty acid synthesis, followed by inhibition of DNA replication culminating in apoptosis (27). Further in vitro and in vivo mechanistic studies have shown that high levels of malonyl-CoA resulting from C75 treatment is likely the mediator of apoptosis in cancer cells (13). Although these biological data strongly suggested that C75 action occurred by means of inhibition of FAS, this study provides direct evidence of C75 interaction with human FAS.
The synthetic strategy used for FAS inhibitor design led to a family of α-methylene-γ-butyrolactones with anti-tumor activity. Structurally, they all lack the reactive epoxide present on cerulenin, enhancing chemical stability and specificity (Fig. 1). Moreover, C75 synthesis was accomplished in two steps resulting in high yields of a pure crystalline stereoisomer with aqueous solubility.
C75 demonstrated kinetics consistent with slow binding inhibition of purified mammalian FAS by C75 (Fig. 2A) in which 50% inhibition occurred within 10 min. Slow-binding inhibition is a desirable pharmacological characteristic because, in contrast to classical inhibitors, buildup of substrates following enzyme inhibition is less likely to reverse the inhibition because of isomerization of the enzyme–inhibitor complex (26). Slow-binding inhibitors include many important drugs, such as methotrexate, allopurinol, and acyclovir (26).
Reflecting its inhibition of purified FAS, C75 is an effective inhibitor of fatty acid synthesis both in vitro and in vivo. C75 significantly inhibited radiolabeled acetate incorporation into phospholipids and triglycerides in HL60 cells (Fig. 2B). Because these biochemical measurements took place within 4 h of drug exposure, they represented fatty acid synthesis pathway inhibition in viable cells before the onset of early apoptotic events as measured by flow cytometry (27).
In whole cell lysates of SKBr3 human breast cancer cells treated with [3H]C75, labeled C75 bound to FAS with a high degree of specificity. In addition to FAS, an additional 60-kDa protein was labeled, which represents binding to serum proteins from the culture medium, a FAS fragment, or an additional unknown cellular target. In addition to specificity, these data demonstrate that C75 binds tightly to FAS surviving highly denaturing conditions. This tight-binding may represent a covalent interaction with FAS, which is often characteristic of slow-binding inhibitors.
In summary, we have described the design and synthesis of a chemically stable inhibitor of mammalian FAS, C75. This compound afforded the first demonstration of systemic inhibition of fatty acid synthesis resulting in specific anti-tumor cytotoxicity. The synthetic strategy, which led to these compounds, will likely yield other inhibitors, other type I and type II fatty acid synthases. Further development of C75 should enable extensive study of fatty acid synthesis inhibition in human cancer and other diseases associated with dysfunctional fatty acid synthesis activity.
Acknowledgments
We thank Fawn Wood and Wan Fang Han for technical assistance. This work was supported in part by grants from the Department of the Army, National Institutes of Health, American Chemical Society, Cope Scholar Award, and the Raynam Research Fund.
Abbreviations
- FAS
fatty acid synthase
- KAS
β-ketoacyl synthase
- ACP
acyl carrier protein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050582897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050582897
References
- 1.Weiss L, Hoffman G, Schreiber R, Andres H, Fuchs E, Korber E, Kolb H. Biol Chem. 1986;367:905–912. doi: 10.1515/bchm3.1986.367.2.905. [DOI] [PubMed] [Google Scholar]
- 2.Rashid A, Pizer E S, Moga M, Milgraum L Z, Zahurak M, Pasternack G R, Kuhajda F P, Hamilton S R. Am J Pathol. 1997;150:201–208. [PMC free article] [PubMed] [Google Scholar]
- 3.Shurbaji M S, Kalbfleisch J H, Thurmond T S. Hum Pathol. 1996;27:917–921. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- 4.Epstein J I, CarMichael M, Partin A W. Urology. 1995;45:81–86. doi: 10.1016/s0090-4295(95)96904-7. [DOI] [PubMed] [Google Scholar]
- 5.Gansler T S, Hardman W, III, Hunt D A, Schaffel S, Henninger R A. Hum Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 6.Pizer E, Lax S, Kuhajda F, Pasternack G, Kurman R. Cancer. 1998;83:528–537. [PubMed] [Google Scholar]
- 7.Alo P L, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Cancer. 1996;77:474–482. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Alo P L, Visca P, Trombetta G, Mangoni A, Lenti L, Monaco S, Botti C, Serpieri D E, Di Tondo U. Tumori. 1999;85:35–40. doi: 10.1177/030089169908500108. [DOI] [PubMed] [Google Scholar]
- 9.Milgraum L Z, Witters L A, Pasternack G R, Kuhajda F P. Clin Cancer Res. 1997;3:2115–2120. [PubMed] [Google Scholar]
- 10.Goodridge A G. Fatty Acid Synthesis in Eucaryotes. Amsterdam: Elsevier Science; 1991. [Google Scholar]
- 11.Jensen V, Ladekarl M, Holm-Nielsen P, Melsen F, Soerensen F B. J Pathol. 1995;176:343–352. doi: 10.1002/path.1711760405. [DOI] [PubMed] [Google Scholar]
- 12.Pizer E S, Jackisch C, Wood F D, Pasternack G R, Davidson N E, Kuhajda F. Cancer Res. 1996;56:2745–2747. [PubMed] [Google Scholar]
- 13.Pizer E S, Thupari J, Han W F, Pinn M L, Chrest F J, Frehywot G L, Townsend C A, Kuhajda F K. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 14.Linn T. Arch Biochem Biophys. 1981;209:613–619. doi: 10.1016/0003-9861(81)90320-9. [DOI] [PubMed] [Google Scholar]
- 15.Dils R, Carey E M. Methods Enzymol. 1975;35:74–83. doi: 10.1016/0076-6879(75)35140-9. [DOI] [PubMed] [Google Scholar]
- 16.Pizer E S, Wood F D, Pasternack G R, Kuhajda F P. Cancer Res. 1996;1996:745–751. [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane S. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Wakil S. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 19.Smith S. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 20.Funabashi H, Kawaguchi A, Tomoda H, Omura S, Okuda S, Iwasaki S. J Biochem. 1989;105:751–755. doi: 10.1093/oxfordjournals.jbchem.a122739. [DOI] [PubMed] [Google Scholar]
- 21.Park B, Nakagawa M, Hirota A, Nakayama M. J Antibiot. 1988;41:751–758. doi: 10.7164/antibiotics.41.751. [DOI] [PubMed] [Google Scholar]
- 22.Carlson R, Oyler A. J Org Chem. 1976;41:4065–4069. [Google Scholar]
- 23.Krol W J, Basak A, Salowe S P, Townsend C A. J Am Chem Soc. 1989;111:7625–7626. [Google Scholar]
- 24.Kuhajda F P, Jenner K, Wood F D, Hennigar R A, Jacobs L B, Dick J D, Pasternack G R. Proc Natl Acad Sci USA. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson B J, Smith S. Pediatr Res. 1985;19:139–143. doi: 10.1203/00006450-198501000-00036. [DOI] [PubMed] [Google Scholar]
- 26.Morrison J, Walsh C. In: Advances In Enzymology. Meister A, editor. Vol. 61. New York: Wiley; 1988. pp. 201–301. [DOI] [PubMed] [Google Scholar]
- 27.Pizer E S, Chrest F J, DiGiuseppe J A, Han W F. Cancer Res. 1998;58:4611–4615. [PubMed] [Google Scholar]