Abstract
Using biochemical and imaging approaches we examined the post-endocytotic fate of the complex formed by human choriogonadotropin (hCG) and a constitutively active mutant of the human lutropin receptor (hLHR-L457R) found in a boy with precocious puberty and Leydig cell hyperplasia.
Following internalization, some of the complex formed by the hLHR-wt and hCG recycles to the cell surface and some is found in lysosomes where the hormone is degraded. In contrast, the complex formed by the hLHR-L457R and hCG is not routed to the lysosomes, most of it is recycled to the cell surface and hormone degradation is barely detectable. For both, hLHR-wt and -L457R, there is an hCG-induced loss of cell surface receptors that accompanies internalization but this loss cannot be prevented by leupeptin.
The removal of recycling motifs of the hLHR by truncation of the C-terminal tail at residue 682 greatly enhances the lysosomal accumulation of the hormone-receptor complexes formed by the hLHR-wt or the L457R mutant, the degradation of the internalized hormone and the loss of cell surface receptors. The degradation of the hormone internalized by these mutants as well as the loss of cell surface receptors is largely prevented by leupeptin.
These results highlight a previously unrecognized complexity in the post-endocytotic trafficking of the hLHR and document a clear difference between the properties of the constitutively active mutant and the agonist-activated hLHR-wt. This lack of lysosomal degradation of the L457R mutant could contribute to its constitutive activity by prolonging the duration of signaling.
Introduction
Although much is now known about the pathways by which G protein-coupled receptors (GPCRs) are internalized (reviewed in refs. 1, 2-6), less is known about the sorting of the internalized receptors to a recycling or a degradation pathway (reviewed in refs. 3, 5, 6-9). A better understanding of the structural features of GPCRs and the cellular proteins that participate in their post-endocytotic sorting is needed because receptor internalization has important functional implications. For example, recycling of internalized GPCRs is thought to be involved in the resensitization of cellular responses (4, 5, 7) and is necessary for preserving cellular responsiveness by maintaining a relatively constant density of receptors at the cell surface (10-12). Conversely, lysosomal degradation of internalized GPCRs is thought to be involved in the acute termination of signaling (13-15) and to contribute to a more prolonged attenuation of signaling because it results in a net loss of cell surface receptors (10-12).
In a series of recent studies we have shown that following hCG-induced internalization, a portion of the hCG-human LHR (hLHR) complex is recycled to the cell surface where it releases the bound hormone and can mediate additional rounds of hCG binding and internalization (16-19). We have also identified two distinct structural motifs present in the C-terminal tail of the hLHR and a protein with a PDZ-type I domain (GIPC) that participate in the recycling of this receptor (16-19). Lastly, we have shown that the recycling of the hLHR is important in maintaining a relatively constant level of cell surface receptors and cellular responsiveness (12, 18, 19).
In addition to laboratory-designed mutations there are a growing number of naturally occurring mutations of the hLHR gene associated with reproductive disorders (reviewed in refs. 20, 21). Some of these are loss-of-function mutations that prevent agonist-induced activation, whereas others are gain-of-function mutations that result in constitutive activity. Although the hormone binding and activation properties of these mutants are generally well characterized (reviewed in refs. 20, 21), less is known about their internalization (22-25) or their intracellular trafficking after internalization (26). With this in mind we examined the post-endocytotic trafficking of the hLHR-L457R mutant, a well characterized constitutively active mutant of the hLHR gene associated with Leydig cell hyperplasia and precocious puberty (23, 24, 27-29). We report here that the post-endocytotic fate of this constitutively active mutant of the hLHR differs from that of the hLHR-wt.
Results
The hLHR-L457R does not route the internalized hCG to the lysosomes.
293T cells were transiently transfected with the hLHR-wt, a well characterized constitutively active mutant (hLHR-L457R, see refs. 23, 24, 27, 28, 29), a truncated version of the hLHR at residue 682 (hLHR-t682) that routes most of the internalized receptor to a lysosomal degradation pathway because it removes recycling motifs present in the C-terminal tail of the (18, 19) and a new construct that incorporates both of these mutations (hLHR-L457R/t682).
The binding and signaling properties of these different mutants have been previously characterized under conditions where the density of receptors at the cell surface is the same. This can be done by manipulating the amounts of plasmid used for transfections and it is necessary because receptor density can affect the magnitude and/or sensitivity of hormonal responses (30). The functional properties of hLHR-t682 are similar to those of the hLHR-wt (12). The constitutive activity of the L457R mutant is very similar to the maximal activity observed when the hLHR-wt is activated by hCG (23, 24, 27-29). In addition, the high constitutive activity of the L457R mutant is not further increased upon stimulation with hCG in spite of the fact that it binds hCG with the same affinity as the wild-type receptor (23, 24, 27-29). The functional properties of hLHR-L457R/t682 are very similar to those of hLHR-L457R (data not shown). In contrast, the trafficking properties of the LHR and mutants thereof are independent of receptor density (31-34). Therefore all experiments presented below were done using cells transfected with identical amounts of plasmid for each receptor construct.
The fate of the internalized 125I-hCG was examined in cells that were allowed to bind and internalize 125I-hCG (1 nM, enough to occupy ∼ 25% of the surface receptors, c.f. Fig 2) for 20 min and then, after removal of the surface-bound hormone, they were allowed to process the internalized hormone for 2 h. A 20 min internalization period was chosen because it roughly corresponds to the half-life of internalization of hCG mediated by the hLHR-wt (32, 34). These experiments were done in the absence or presence of leupeptin to allow or prevent the lysosomal degradation of the internalized hormone, respectively (10, 35), and in the presence of an excess of hCG in the medium to prevent rebinding of any hormone that had recycled back to the surface (18, 33). Table 1 shows that in the absence of leupeptin ∼20% of the 125I-hCG internalized by the hLHR-wt is degraded and released. The rest of the 125I-hCG remains in intracellular compartments (∼25%), or is recycled back to the surface and it remains bound to the receptor (∼34%) or released to the medium in an undegraded form (∼21%). As expected (19), cells expressing the hLHR-t682 display an increase in the amount of degraded 125I-hCG (∼44%) and a decrease in the amount of recycled hormone. Cells expressing the hLHR-L457R mutant degrade only a small amount of the internalized 125I-hCG (∼3%) and most of the hormone remains intracellularly (50%) or is recycled to the surface. In contrast to cells expressing any of the other constructs, however, most of the 125I-hCG recycled by the hHLR-L457R mutant remains receptor bound (∼42%) and only a small amount is released back into the medium (∼5%). A t682 truncation of the L457R increases hormone degradation and decreases recycling.
Figure 2.
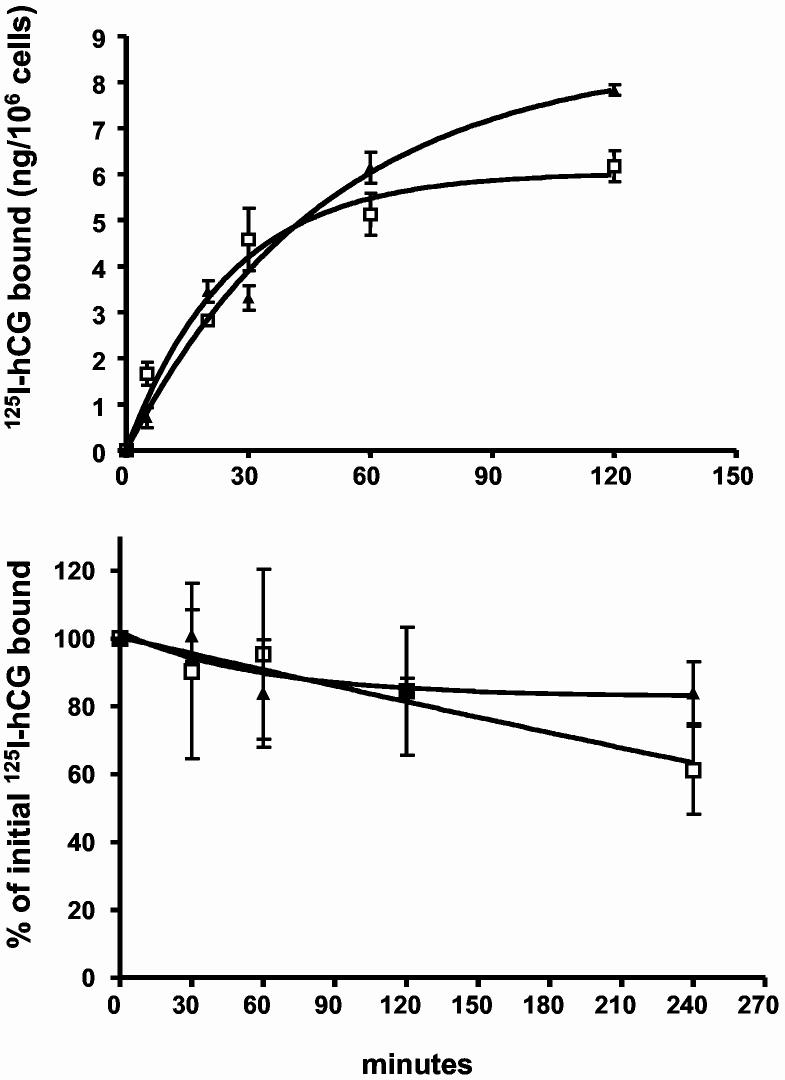
Kinetics of association and dissociation of 125I-hCG to and from cells expressing the myc-hLHR-wt or myc-hLHR-L457R. 293T cells were transiently co-transfected with Dynamin-K44A (to prevent internalization) and either the myc-hLHR-wt (□) or myc-hLHR L457R (▲) as previously described (24). In the top panel the cells were incubated with 125I-hCG (2.5 nM, Kd = 1-3 nM) at 37°C and the amount of hormone bound was determined at the times indicated. In the bottom panel the cells were preincubated with 125I-hCG (2.5 nM, Kd = 1-3 nM) for 1h at 37°C and then washed to remove the free hormone. The cells were placed back in medium without hormone (t = 0) and incubated at 37°C. The amount of hormone that remained bound to the cells was determined at the times indicated and expressed as % of the amount of hormone bound at t = 0. Each point is the mean ± SEM of 3 independent experiments.
Table 1.
Fate of the 125I-hCG internalized by 293T cells expressing the myc-hLHR-wt or mutants thereof
| 125I-hCG (% of initial cpm internalized) | |||||
|---|---|---|---|---|---|
| hLHR | Leupeptin | Intracellular | Recycled | Degraded | |
| Surface- bound | Released undegraded | ||||
| Wt | - | 25 ± 1 | 34 ± 1 | 21 ± 3 | 20 ± 3 |
| + | 30 ± 1 | 32 ± 2 | 30 ± 3 | 8 ± 2 | |
| t682 | - | 27 ± 1 | 14 ± 1 | 15 ± 1 | 44 ± 2 |
| + | 61 ± 1 | 14 ± 1 | 19 ± 1 | 6 ± 1 | |
| L457R | - | 50 ± 2 | 42 ± 4 | 5 ± 1 | 3 ± 1 |
| + | 46 ± 1 | 46 ± 1 | 6 ± 1 | 2 ± 1 | |
| L457R/t682 | - | 57 ± 3 | 9 ± 1 | 5 ± 1 | 29 ± 3 |
| + | 83 ± 1 | 13 ± 1 | 3 ± 1 | 1 ± 1 | |
The results presented in Table 1 also show that leupeptin readily inhibits the degradation of 125I-hCG mediated by the hLHR-wt, -t682 and -L457R/t682 mutants. This inhibition is reflected as a decrease in the amount of degraded 125I-hCG released (see hLHR-wt) and/or a decrease in the amount of degraded 125I-hCG released as well as an increase in the amount of 125I-hCG that remains intracellularly (see hLHR-t682 and -L457R/t682). Leupeptin had virtually no effect on the processing of the 125I-hCG internalized by hLHR-L457R.
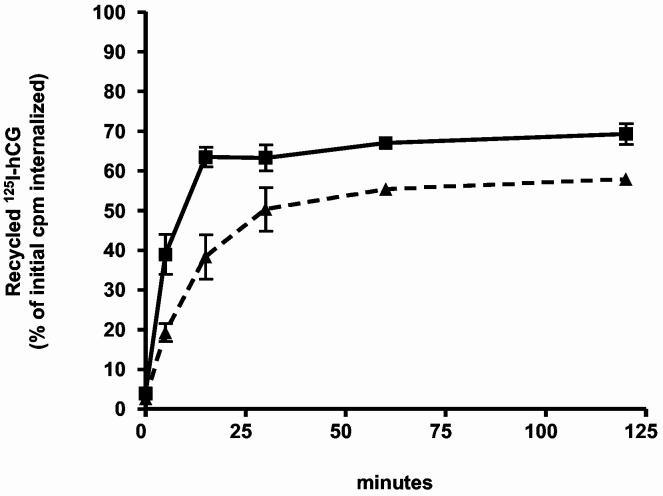
Since these experiments were done using a single time point we also compared the time courses involved in the processing of the 125I-hCG internalized by cells expressing these mutants. In addition to the differences shown in Table 1 the time course experiments show a delay in the rate of recycling of the internalized 125I-hCG in cells expressing hLHR-L457R when compared to that observed in cells expressing the hLHR-wt (Figure 1). This difference was not obvious from the experiments presented in Table 1 because at the end of a 2 hour period the extent of recycling in cells expressing either construct is similar (Figure 1 and Table 1). Using a somewhat different protocol others (26) have shown that another constitutively active mutant of the hLHR (D578H) also recycles slower than the hLHR-wt.
Figure 1.
Recycling of the internalized 125I-hCG in cells transfected with the myc-hLHR-wt or myc-hLHR-L457R. 293T cells were transiently transfected with the hLHR-wt (▲) or -L457R (■) and incubated with 125I-hCG (1 nM, Kd = 1-3 nM) for 20 min at 37°C. At this time the free and bound hormone were removed and the cells were further incubated at 37°C for 2 h in medium containing an excess of non-radioactive hCG. The amount of recycled 125I-hCG (surface bound + intact hormone released, see Table 1) was then measured at the times indicated as described in Materials and Methods. Results are expressed as a percent of the total intracellular 125I-hCG radioactivity present at the end of the binding and internalization period (defined as t = 0 in the figure) and they represent the mean ± range of two independent transfections. At t = 20 min the amount of internalized radioactivity varied between 30,000 and 60,000 cpm/well.
The lack of dissociation of the recycled hCG/hLHR-L457R complex shown in Table 1 is also surprising because this mutant binds hCG with the same affinity as the hLHR-wt (27). In an attempt to understand this observation we measured association and dissociation kinetics of 125I-hCG to cells expressing the hLHR-wt and the L457R mutant. The data are presented in Figure 2 show that these parameters are the same for both receptors.
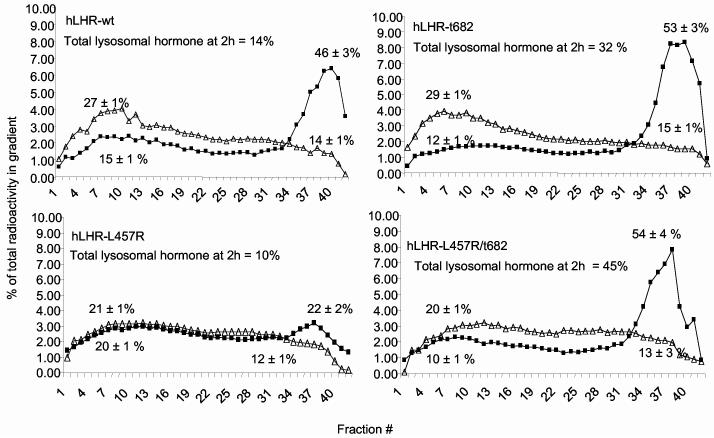
The location of the 125I-hCG internalized by the different receptors was ascertained by Percoll gradient centrifugation (10, 35). To enhance our ability to detect 125I-hCG in the lysosomes only cells incubated with leupeptin were analyzed at the end of the binding and internalization period (defined as t = 0) and at the end of the 2 h incubation period (t = 2 h) used to allow for processing of the internalized hormone (Figure 3). Regardless of the receptor used, 20-30% and 12-15% of the radioactivity present in the gradient at t = 0 migrates in the endosomal and lysosomal fractions, respectively. At the end of the 2 h processing period the fraction of the gradient radioactivity present in endosomes declines to 10-15% and the fraction of radioactivity present in the lysosomes increases to 46-54% in cells transfected with the hLHR-wt, hLHR-t682 or hLHR-L457R/t682 (Figure 3). In contrast, there is little or no change in the distribution of radioactivity in cells transfected with the hLHR-L457R mutant (Figure 3).
Figure 3.
Analysis of the distribution of the internalized 125I-hCG present in 293T cells expressing the myc-hLHR wt or mutants thereof. 293T cells were transiently transfected with the indicated constructs and incubated with 125I-hCG (1 nM, Kd = 1-3 nM) and leupeptin for 20 min at 37°C. At this time (defined as t = 0) the free and bound hormone were removed and some of the cells were processed immediately (open triangles) and the rest were placed back in medium containing leupeptin and an excess of non-radioactive hCG and incubated at 37°C for another 2 h (black squares) to allow them to process the internalized hormone. At the end of this incubation the cells were washed again with a neutral and acidic buffer as described above prior to further processing. The cells were collected, homogenized, and analyzed on Percoll gradients as described in Materials and Methods. As shown previously (35) the plasma membranes and endosomes band towards the top (fractions 4-15) whereas the lysosomes band towards the bottom (fractions 33-42) of these gradients. The radioactivity banding towards the top of the gradient is considered to be in the endosomal only because the surface bound 125I-hCG was released prior to analysis (see above and Materials and Methods). Each point is the average of 2 to 8 experiments and the results are plotted as the radioactivity present in each fraction expressed as a % of the total radioactivity present in the gradient. The total amount of radioactivity present in each gradient was 60,000-90,000cpm. The numbers shown on top of each of the endosomal or lysosomal peaks represent the amount radioactivity present in these peaks expressed as % of the total radioactivity present in the gradient (mean ± range or SEM of 2-8 experiments). The total lysosomal hormone at t = 2 h was calculated as discussed in the text.
Although the fraction of intracellular radioactivity that accumulates in the lysosomes is similar for the hLHR-wt, -t682 and -L457R/t682 mutants, the total accumulation of radioactivity in the lysosomes of cells expressing either of the two mutants is higher than that of cells expressing the hLHR-wt because of the higher amount of intracellular radioactivity found in cells expressing the mutants (Table 1). When this distribution is taken into account it can be readily estimated that in cells expressing the hLHR-wt only ∼14% of the internalized 125I-hCG is present in the lysosomes at the end of the 2 h incubation (30% of the hormone remains intracellularly × 46% of the intracellular hormone present in the lysosomes). In contrast, in cells expressing hLHR-t682 or hLHR-L457R/t682, ∼32% and ∼45% of the internalized 125I-hCG, respectively, is present in the lysosomes. The same calculations revealed that in cells expressing hLHR-L457R only 10% of the 125I-hCG is present in the lysosomes.
Since it was previously shown that the hCG-LHR-wt complex remains associated after internalization the fate of the internalized 125I-hCG can be used as a convenient and quantitative method to follow the fate of the internalized receptor (10, 35-37). This is documented again in Table 2 where we measured the fraction of the 125I-hCG radioactivity present in the lysosomal fraction that was free or receptor bound. Since the hLHR-wt and mutants are tagged with the myc-epitope, any radioactivity immunoprecipitated by the 9E10 antibody has to be receptor bound rather than free hormone (10, 35). Table 2 shows that at the end of the 2 h processing period, 20-40% of the 125I-hCG present in the lysosomal fraction of cells transfected with the hLHR-wt or any of the mutants can be immunoprecipitated with the 9E10 antibody. Since the efficiency of immunoprecipitation is ∼40% (see Materials and Methods and the legend to Table 2) we estimated that 50-100% of the radioactivity present in the lysosomes represents 125I-hCG bound to the receptor.
Table 2.
Receptor-bound 125I-hCG in the lysosomes of cells expressing the myc-hLHR-wt or mutants thereof
| Receptor | Receptor-bound 125I-hCG (% of total cpm present in lysosomes) |
|---|---|
| hLHR-wt | 29 ± 6 |
| hLHR-t682 | 22 ± 6 |
| hLHR-L457R | 29 ± 1 |
| hLHR-L457R/t682 | 41 ± 6 |
We conclude that the hCG-receptor complex internalized by the hLHR-L457R mutant is not routed to the lysosomes. Instead it seems to continuously cycle between the endosomes and the cell surface. Our data also show that similarly to the hLHR-wt, truncation of the C-terminal tail of the L457R mutant allows for a greater accumulation of the hormone receptor complex in the lysosomes.
The internalized hLHR-L457R is not routed to the lysosomes.
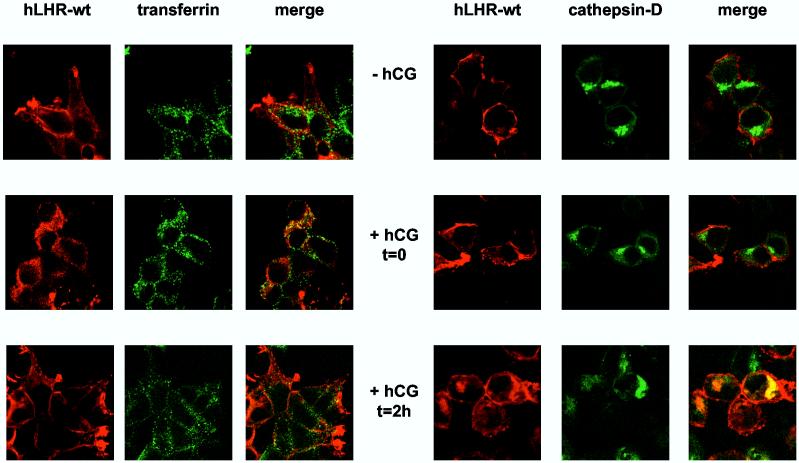
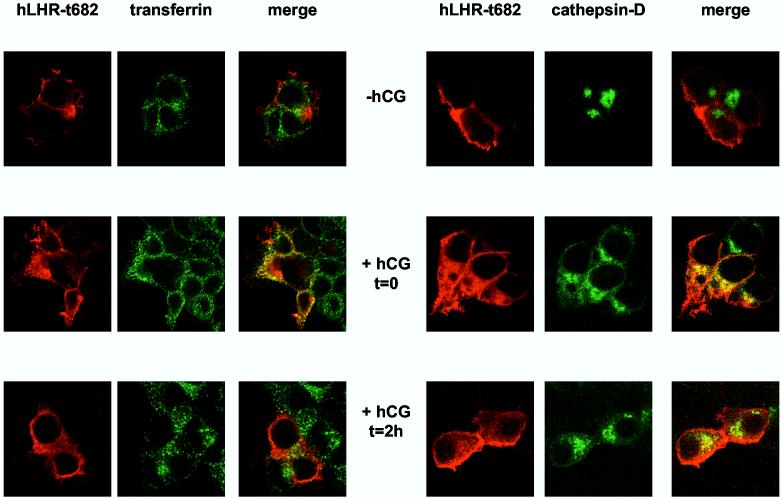
The trafficking of the receptors was ascertained by confocal imaging in 293T cells cotransfected with a GFP-cathepsin D fusion protein (a lysosomal marker) or in cells incubated for 2.5 min with Alexa488-conjugated transferrin to label early endosomes. The top panels of Figures 4A and B show that, in the absence of hCG, the hLHR-wt and hLHR-t682 localize mostly to the cell surface. As shown previously (19) the hCG-induced internalization of the hLHR-wt and hLHR-t682 can be clearly demonstrated after a 20 min incubation with hCG when both receptors localize in early endosomes (middle panels in Figures 4A and B). During this short incubation the hLHR-t682 can be found in the lysosomes of ∼60% of cells (Figure 4B, middle panels) but the hLHR-wt cannot be found in the lysosomes of any cell (Figure 4A, middle panels). Once the cells are allowed to process the internalized hCG for an additional 2 hours then the internalized hLHR-wt or the hLHRt682 can be found in early endosomes and lysosomes (Figure 4A and B, lower panels). However the percentage of cells showing the hLHR-wt in lysosomes is 50-60% whereas ∼90% of cells expressing the hLHR-t682 show a lysosomal accumulation. The increased localization of the hLHR-t682 in lysosomes is in general agreement with the results on the fate of the 125I-hCG measured on Percoll gradients (Figure 3). The only difference is at t = 0 where the imaging reveals the presence of hLHR-t682 and absence of hLHR-wt in lysosomes whereas the Percoll gradients do not show a lysosomal accumulation of 125I-hCG in cells expressing hLHR-t682 (Figure 3).
Figure 4.
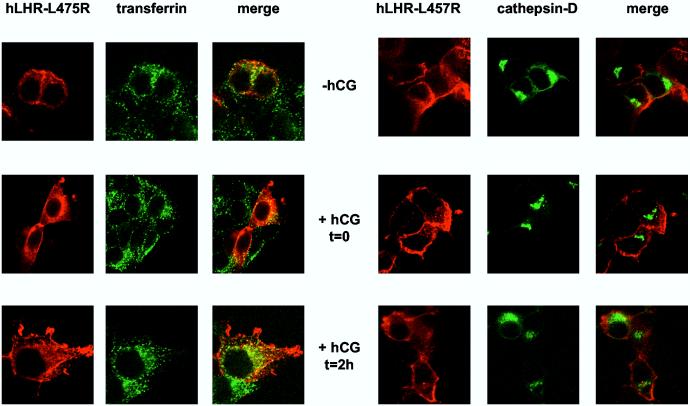
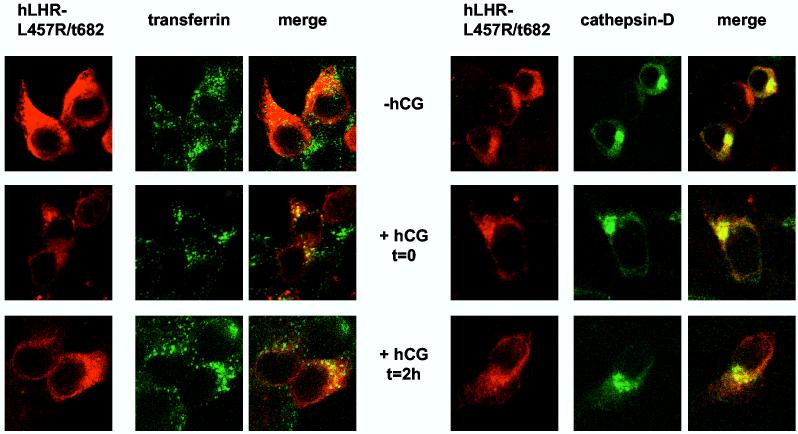
Confocal imaging of the myc-hLHR-wt and mutants thereof 293T cells were transiently transfected with the indicated myc-hLHR constructs without (left panels) or with cathepsin D-GFP (right panels). The transfected cells were washed and incubated without (-hCG) or hCG (2.5 nM, Kd = 1-3 nM) for 20 min at 37°C (+ hCG, t = 0) and further processed as described in Materials and Methods. Another group of cells was incubated with hCG (52 nM) at 37°C for 20 min and then, after removal of the receptor-bound hormone they were further incubated without hCG for another 2 h at 37°C (+hCG, t = 2h) to allow for processing of the internalized hormone. The cells transfected with the receptor only (left panels) were also incubated for 2.5 min with Alexa488 conjugated-transferrin and then treated with an isotonic acid buffer to release the surface-bound transferrin while leaving the internalized transferrin in the early endosomes. The receptors (in red) were visualized using an anti-myc monoclonal antibody (9E10) and a CY5™-conjugated anti-mouse antibody. Alexa488 conjugated transferrin and cathepsin D-GFP are shown in green and colocalized compartment are shown in yellow. The cells were observed and analyzed using a Zeiss confocal microscope.
Figures 4C and D show the imaging results obtained with the hLHR-L457R and hLHR-L457R/t682 mutants. Even in the absence of hCG some of the hLHR-L457R is intracellular and located in early endosomes but not in lysosomes (top panels of Figure 4C). This is not unexpected, of course, because of the constitutive activity of this mutant (23, 24, 27-29). Stimulation of these cells with hCG (middle panels in Figure 4C) and further processing of the bound hormone (lower panels in Figure 4C) do not appear to alter this distribution and the hLHR-L457R does not localize in the lysosomes. The majority of the hLHR-L457R/t682 mutant is also intracellular in the absence of hCG stimulation. Most of this intracellular hLHR-L457R/t682 does not co-localize with endosomes or lysosomes because it is the intracellular LHR precursor present in the endoplasmic reticulum (see Figure 6 below). Some of the intracellular hLHR-L457R/t682, however, localizes in endosomes and lysosomes even in the absence of hormone (top panels of Figure 4D). Again, stimulation of the cells with hCG (middle panels in Figure 4D) and further processing of the bound hormone (lower panels in Figure 4D) do not appear to alter this distribution. These results are also in general agreement with the Percoll gradient data (Figure 3) which show no lysosomal accumulation of the internalized 125I-hCG in cells expressing the hLHR-L457R mutant and substantial lysosomal accumulation of 125I-hCG in cells expressing the hLHR-L457R/t682 mutant.
Figure 6.
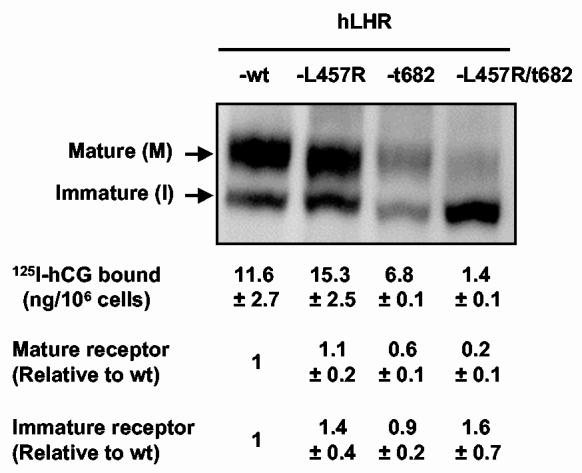
Expression of the myc-hLHR-wt and mutants thereof 293T cells were transiently transfected with the indicated constructs, lysed and immunoprecipitated with the 9E10 antibody. The mature, cell surface (M) and immature intracellular precursor (I) of the hLHR were visualized on western blots using the 9E10 antibody coupled to horseradish peroxidase as described in Materials and Methods. The relevant portions of a representative blot are shown. The amounts of mature and immature receptor were quantitated by densitometry and are expressed relative to the amounts detected in cells expressing the hLHR-wt (mean ± SEM of 3 independent transfections). In other experiments the binding of 125I-hCG to the surface of cells transiently transfected with each of the constructs shown was measured as described in Materials and Methods. The results of these binding assays are shown below the respective receptor lanes and they also show the mean ± SEM of three independent transfections.
The fate of the hLHR-wt and mutants was also ascertained using a quantitative method in which the density of the receptor at the cell surface was measured by immunoprecipitation of the receptors from biotinylated cells (17, 19). In the first series of experiments we examined the levels of cell surface receptors under the same experimental conditions used above: a 20 min binding and internalization period (defined as t = 0) followed by the removal of the surface bound hormone and a 2 h incubation (t = 2 h) to allow the cells to process the internalized hormone-receptor complex. Since we wanted to detect changes in receptor density that may occur because of the degradation of the internalized receptor (17, 19) these experiments were done using a saturating concentration of hormone to maximize receptor occupancy and in the absence of leupeptin to allow for lysosomal degradation to proceed. Figure 5 shows that a small loss (∼20%) of cell surface receptors detected during the 2 h processing period in the cells expressing the hLHR-wt and a greater loss (∼40%) in the cells expressing the hLHR-t682 (19). This is consistent with the larger accumulation of the hormone receptor complex in the lysosomes of cells expressing hLHR-t682 when compared to the hLHR-wt. Surprisingly however, the loss of cell surface receptors detected in cells expressing the hLHR-L457R mutant that does not accumulate in lysosomes is relatively high (∼34%). The hLHR-L457R/t682 mutant behaved as expected from its higher lysosomal accumulation with a pronounced loss (∼80%) of cell surface receptors.
Figure 5.
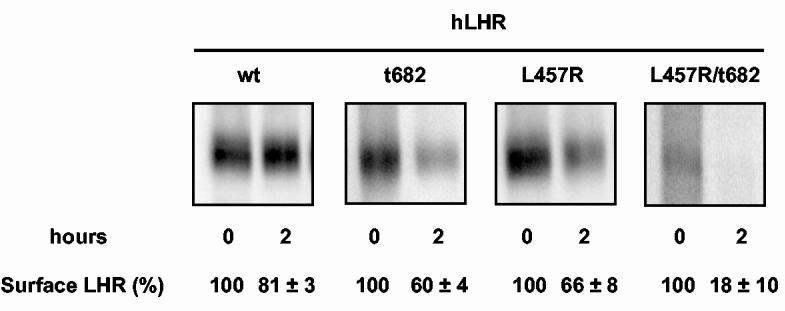
Short-term agonist induced degradation of the cell-surface myc-hLHR-wt and mutants thereof. Transiently transfected 293T cells were biotinylated and incubated with a saturating concentration of hCG (25 nM, Kd = 1-3 nM) for 20 min at 37°C. The cells were then washed to remove the free and bound hormone and either lysed immediately (t = 0) or after an additional 2 h incubation at 37°C (t = 2 h). Lysates were immunoprecipitated with the anti-myc antibody (9E10) and the surface receptors were visualized and quantitated on western blots using streptavidin covalently coupled to horseradish peroxidase as described in Materials and Methods. The relevant portions of a representative blot are shown. The numbers under each lane represent the quantitation of receptor levels (mean ± SEM of 5 to 8 independent transfections) expressed as a percent of the amount of cell surface receptors present at t = 0.
In a series of related experiments, the density of cell surface receptors was measured in cells incubated with a saturating concentration of hCG and in the absence or presence of leupeptin for a much longer period of time (6 h). Under these conditions the cells continuously bind and internalize the hormone thus allowing us to determine more pronounced changes in the density of cell surface receptors that would occur after many rounds of internalization and processing of the internalized hormone-receptor complex. The data presented in Table 3 show that under long-term hCG stimulation the density of cell surface receptors in cells expressing the hLHR-wt or hLHR-t682 decrease to ∼70% and ∼20% of control, respectively. Leupeptin inhibits the decrease in hLHR-t682 but it has no effect on the loss of hLHR-wt. The loss of cell surface hLHR-L457R is similar or slightly more pronounced than that detected in cells expressing the hLHR-wt whereas the loss of hLHR-L457R/t682 is similar to that of the hLHR-t682. Leupeptin has no effect on the loss of hLHR-L457R but it inhibits the loss of hLHR-L457R/t682.
Table 3.
Long-term agonist-induced degradation of the cell-surface myc-hLHR-wt and mutants thereof.
| Receptor | Leupeptin | Residual cell surface receptors (% of initial) |
|---|---|---|
| hLHR-wt | - | 71 ± 3 |
| + | 77 ± 7 | |
| hLHR-t682 | - | 20 ± 1 |
| + | 66 ± 8 | |
| hLHR-L457R | - | 63 ± 5 |
| + | 57 ± 14 | |
| hLHR-L457R/t682 | - | 34 ± 8 |
| + | 52 ± 7 |
We also attempted to document the recycling of the internalized receptor with the aid of disulfide-linked biotin (38). This strategy proved difficult to implement with the hLHR, however. We are not sure of the reason for this but we suspect it is related to the large and disulfide-rich extracellular domain of the hLHR (20).
Lastly it should be noted that the biotinylation experiments (see Figure 5) indicated that, when identical amounts of plasmid are transfected, the density of cell surface receptors in cells expressing hLHR-t682 and particularly the hLHR-L457R/t682 mutant is much lower than that of cells expressing the hLHR-wt or hLHR-L457R. This observation was confirmed by 125I-hCG binding assays, which determine only the receptor at the cell surface, and by western blots which determine the relative levels of the mature cell surface receptor and its immature intracellular precursor (reviewed in ref. 20). These data are presented in Figure 6 and show that, when identical amounts of plasmid are transfected, the density of cell surface receptors in cells expressing hLHR-t682 and hLHR-L457R/t682 is lower than the density of cell surface receptors in cells transfected hLHR-wt or hLHR-L457R. A similar result was obtained when the relative density of cell surface receptors was ascertained using Western blots (Figure 6). Lastly, the Western blots presented in Figure 6 also show that whereas cells expressing the hLHR-wt, hLHR-t682 or hLHR-L457R express similar amounts of the mature and intracellular receptors, those expressing the hLHR-L457R/t682 mutant express mostly the intracellular precursor. These results also explain why there is a substantial amount of intracellular receptor that does not co-localize with endosomal or lysosomal markers in cells expressing the hLHR-L457R/t682 mutant incubated without hormone (Figure 4D, upper panels). This intracellular receptor is likely to represent the immature form of the hLHR which predominates in cells expressing this mutant (Figure 6) and localizes to the endoplasmic reticulum (20). Many other mutations of the hLHR have been previously shown to impair the maturation and/or transport of the LHR precursor to the cell surface (reviewed in ref. (20)
Collectively the biochemical and imaging data show an hCG-dependent internalization as well as endosomal and some lysosomal accumulation of the internalized hLHR-wt. This lysosomal accumulation is enhanced in hLHR-t682 and the loss of cell surface receptors becomes more pronounced. Interestingly our data also show that some of the hLHR-L457R localizes to early endosomes even in the absence of hormone and that this mutant does not accumulate in lysosomes even after hCG addition. The hLHR-L457R/t682 mutant is also internalized constitutively and after hCG stimulation but it accumulates in lysosomes.
Discussion
Together with our previous results (17-19, 35) and data from other GPCRs (39, 40) the results presented here are consistent with the notion that the internalized hCG/hLHR-wt complex recycles by default. Since the process of recycling cannot be fully efficient a portion of the internalized complex is transported to the lysosomes. This results in the release of hormone degradation products in the medium and the net loss of a small amount of cell surface receptors. The degradation of the internalized hormone clearly occurs in lysosomes because it is sensitive to leupeptin (this paper) and other lysosomal inhibitors (41). On the other hand, since the loss of cell surface hLHR-wt that ensues is not sensitive to leupeptin it appears that in spite of its lysosomal accumulation, the internalized hLHR-wt is degraded elsewhere.
We have previously shown that two residues present in the C-terminal tail of the hLHR, Leu683 and the C-terminal Cys699 are essential for recycling (18, 19). Cys699 is essential for binding GIPC, a PDZ-domain protein that mediates recycling (18). The reason why Leu683 is needed for recycling is unclear but this residue may be involved in the formation of an internal PDZ ligand that binds another (as yet unidentified) PDZ-domain protein that also mediates recycling (42). The removal of this Leu683/Cys699 motif (by truncation of the C-terminal tail of the hLHR at residue 682) interferes with recycling and a higher proportion of the internalized hCG/hLHR-t682 accumulates in lysosomes and follows a lysosomal degradation pathway. The involvement of the lysosomes in the degradation of the internalized hLHR-t682 and its associated hormone can be clearly documented in this case because the loss of cell surface receptors and the degradation of the internalized hormone are both sensitive to leupeptin.
The results presented here with the hLHR-L457R highlight a previously unrecognized complexity in the post-endocytic trafficking of the hLHR. The hLHR-L457R mutant has a high degree of constitutive activity in all signaling assays tested, it binds hCG with the same affinity as the hLHR-wt and its activity cannot be further increased upon hCG binding (23, 24, 27-29). The finding that the L457R mutant accumulates in early endosomes even in the absence of hormone stimulation is not unexpected because internalization is one of the consequences of activation and this mutant is constitutively active. Surprisingly, however, the hLHR-L457R that is internalized constitutively or after hormone stimulation does not traffic to lysosomes. The internalized hLHR-L457R can be detected only in endosomes and there is little (if any degradation) of the internalized hCG. In addition, recycling of the hCG internalized by the hLHR-L457R mutant is slower and most of the hormone that recycles back to the cell surface remains bound to the receptor. As shown herein the lack of dissociation of the hCG/hLHR-L457R complex cannot be explained by a slower rate of dissociation of the hormone but it may be explained by the faster rate of internalization of hCG mediated by this mutant (23) which would result in a shorter residency time of the recycled complex at the cell surface. In fact it appears that this complex enters a futile cycle of recycling and internalization.
Since internalized proteins traverse different endosomal compartments (43) and the hCG internalized by the hLHR-L457R recycles slower a possible explanation for these results is that the hCG/hLHR-wt and hCG/hLHR-L457R traffic through different endosomal compartments. For example the hCG/hLHR-wt complex could follow a fast recycling pathway from early endosomes whereas the hCG/hLHR-L457R could follow a slower recycling pathway involving either the recycling and/or the late endosomes. We can localize both receptors to early endosomes but we have not yet attempted to localize them to recycling or late endosomes. We do know, however, that the recycling of the L457R mutant depends on the same recycling motifs present in the hLHR-wt because a truncation at residue 682 allows for a higher lysosomal accumulation and degradation of both hCG/receptor complexes as well as a more pronounced decline in the density of cell surface receptors.
Since recycling requires an association of the LHR with GIPC (18) and at least one other protein (see above) it is possible that the stability of these recycling complexes is enhanced by the state of activation of the receptor. If this is the case the recycling complexes formed with the hLHR-L457R would be more stable than those formed by the hLHR-wt because the former is irreversibly activated by the receptor mutation whereas the latter is reversibly activated by hormone binding. We already know that the co-immunoprecipitation of GIPC with the hLHR-wt is not enhanced by hCG stimulation (18) but this assay is unlikely to detected subtle changes in affinity that could influence the rate of dissociation of this complex. Moreover, since GIPC is not the only protein that participates in the recycling of the hLHR it is also possible that activation of the receptor influences the association of the receptor with this other protein. Alternatively, the stability of the recycling complexes formed by hLHR-L457R could still be caused by the mutation and could be unrelated to receptor activation. Although the L457R mutation is on a residue present in transmembrane helix 3 of the hLHR (27) it is not difficult to envision how a mutation in this region could affect the conformation of the C-terminal tail. In fact another activating mutation of the hLHR (D578H in transmembrane helix 6) was recently shown to impair the palmitoylation of one or more cysteine residues present in the juxtamembrane region of its C-terminal tail (26). Also there are several examples of mutations in the transmembrane regions of the LHR that affect the conformation of the extracellular domain, as judged by changes in hormone binding affinity (44) or specificity (45).
Clearly more experiments are needed to determine if the internalized hLHR-wt and -L457R traffic through the same endosomal compartments and if they form stable or unstable complexes with GIPC and/or other recycling proteins.
Our data also show that the hCG-induced loss of cell surface receptors that occurs during endocytosis is similar in cells expressing the hLHR-wt or hLHR-L457R and in both cases this process is insensitive to leupeptin. The lack of effect of leupeptin on the loss of the L457R mutant agrees with the finding that this mutant does not accumulate in lysosomes. On the other hand, the lack of effect of leupeptin on the hLHR-wt is unexpected because we can detect the hCG/hLHR-wt complex in lysosomes and the degradation of the hormone internalized by this receptor is sensitive to leupeptin. Likewise, the similarity in the magnitude of the hormone-induced loss of the hLHR-wt and L457R mutant from the cell surface is surprising because only the hLHR-wt accumulates in lysosomes. Lastly, truncation of the hLHR-wt or the L457R mutant at residue 682 promotes not only the lysosomal accumulation of these mutants and the ligand but also a greater extent of ligand degradation and loss of cell surface receptors, which are sensitive to leupeptin. Clearly then these receptor mutants follow a lysosomal degradation pathway.
In summary the results presented here extend the characterization of the post-endocytotic trafficking of the hLHR and they highlight a previously unrecognized complexity in this process. Although the reasons for the differential fate of the hCG-activated internalized hLHR-wt and the free or hCG-activated hLHR-L457R are not fully understood these experiments are important because they document a clear difference between the behavior of a constitutively active mutant of the hLHR (hLHR-L457R) and the agonist activated hLHR-wt. Most (or all) of the other functional properties of the constitutively active mutants of the hLHR are basically indistinguishable from those of the agonist-activated hLHR-wt (23, 24, 27-29). It should be noted that the different trafficking of the hLHR-L457R is not unique to this constitutively active mutant. More limited studies published earlier (26) with another naturally occurring constitutively active mutant of the hLHR (hLHR-D578H) show that this mutant also mediates a slower rate of recycling and degradation of the internalized hCG. If some hormonal signals originate from endosomes then this pathway could contribute to the constitutive activity of the mutants. Alternatively, the lack of lysosomal accumulation could simply prolong the duration of the signals generated by these mutants.
Materials and Methods
Plasmids and cells
The preparation and characterization of expression vectors for the myc-hLHR-wt and the mutants (in pcDNA3.1) have been described (19, 23, 30, 46). The expression vector for human cathepsin D fused with the green fluorescent protein was kindly provided by Dr. Jonathan A. Backer (Dept of Molecular Pharmacology, Albert Einstein College of Medicine of Yeshiva University).
Human embryonic kidney 293T cells were maintained in DMEM containing 10 mM HEPES, 10% newborn calf-serum and 50 μg/ml gentamicin, pH 7.4 (Growth medium). Cells were plated in gelatin-coated 35mm wells or 100mm dishes and transiently transfected with 0.5 μg or 5 μg of plasmid DNA, respectively, using the calcium phosphate method of Chen and Okayama (47) when 70-80% confluent. After an overnight incubation with the transfection mixture, the cells were washed and used 24 h later.
125I-hCG binding assays
Transiently transfected cells were washed and placed in 1 ml of Binding medium (RPMI-1640 medium pH 7.4 without NaHCO3 but containing 20 mM Hepes, 50 μg/ml gentamicin and 1 mg/ml bovine serum albumin). The cells were incubated with a concentration of 125I-hCG similar to the Kd (2.5 nM, Kd = 1-3 nM, see ref. 16, 30) overnight at 4°C. At the end of this incubation the cells were washed 2 times with cold binding medium to remove the free hormone, solubilized with 100 μl of 0.5 N NaOH and collected with a cotton swab to measure the bound 125I-hCG using a γ counter. Three wells were used for each receptor construct tested. The first two wells received 125I-hCG only and were used to measure the total 125I-hCG bound. The third well received 125I-hCG and 50 IU/ml of crude hCG and was used to correct for the non specific binding.
For the experiments shown in Figure 2 the cells were co-transfected with Dynamin-K44A to prevent internalization of the bound hormone (24). Binding was then determined as described above.
Fate of the internalized hormone
Transiently transfected cells were washed and then allowed to bind and internalize 125I-hCG (1 nM, Kd = 1-3 nM) for 20 min at 37°C in Assay medium (RPMI 1640 medium with 20 mM Hepes, 50 μg/ml gentamicin and 1 mg/ml bovine serum albumin, pH7.4) without or with 200 μM leupeptin (to inhibit lysosomal proteolysis, see refs. 10, 35) as indicated in the figures and tables. The cells were placed on ice and washed to remove the free hormone. The cell surface bound 125I-hCG was then released by a brief exposure (3-4 min) of the cells to an isotonic pH 3 buffer (48). This was defined as t = 0 min in the figures, and the cells (which now contain only internalized 125I-hCG) were incubated for an additional 2 h at 37C in assay medium without or with 200 μM leupeptin and an excess of crude hCG (50 IU/ml) to prevent rebinding of any intact hormone released into the medium (33). At the end of this second incubation (defined as t = 2 h in the figures and tables), the medium was saved and the cells were washed with cold medium. They were briefly exposed again to the isotonic pH 3 buffer to release and measure any internalized hormone that had recycled back to the cell surface. The acid-stripped cells were solubilized with NaOH to measure residual radioactivity that remained internalized. Finally, the saved medium was precipitated with 10% trichloracetic acid to determine the amount of degraded and undegraded 125I-hCG released (10, 33, 35).
In other experiments the cells were treated as above but at t = 20 or 120 min they were collected and homogenized to determine the location of the internalized radioactivity (10, 35). To do this the cells were washed twice with cold homogenization buffer (0.25 M sucrose, 10 mM Hepes, 1 mM EDTA pH 7.4 containing 40μl/ml of protease inhibitor cocktail (Complete protease inhibitor cocktail EDTA free, Roche Molecular Biochemical, Indianapolis, IN) and scraped into 2 ml of this buffer. The cell suspension was centrifuged for 15 min at 800×g, the supernatant was discarded and the cells resuspended in 2 ml of cold homogenization buffer and lysed by forcing them through a 21-gauge needle 10 times. The cells were centrifuged again at 800×g for 10 min and the supernatant was kept. The pellets were homogenized and centrifuged again. The two supernatants were combined and a 2 ml aliquot was thoroughly mixed with 8 ml of a Percoll solution (with a density of 1.046g/ml) prepared in homogenization buffer. These mixtures were then centrifuged at 33,000×g for 20 min (70.1 Ti rotor in a L7-80 ultracentrifuge, Beckman Coulter, Inc., Palo Alto, CA) at 4C. The content of the gradients were then collected from the top (250 μl/fraction), and the fractions were assayed for radioactivity with the aid of a γ counter. Under these conditions the plasma membranes and endosomes band together towards the top of the gradient whereas the lysosomes band towards the bottom of the gradient (10, 35).
Analysis of receptor-bound 125I-hCG
After Percoll gradient centrifugation, the fractions containing the lysosomes (fractions 33-42) were pooled. Each pool received 200 μl of protease inhibitor cocktail and 500 μl of 10% NP-40 prepared in 20 mM hepes, 0.15 M NaCl pH7.4. The volume was adjusted to 5 ml with homogenization buffer, and the samples were rotated 45 min at 4°C. The detergent extracts were then centrifuged at 100, 000×g for 1 h at 4°C to remove the Percoll and insoluble material.
Duplicate aliquots of the supernatants were used to determine the total amount of 125I-hCG present, and duplicate aliquots (500 μl) were mixed with an antibody to the myc epitope (9E10) that was previously bound to protein G agarose. Prebinding of the 9E10 antibody to the protein G-sepharose was accomplished by mixing 50 μl of a 25 fold dilution of 9E10 antibody (ascites fluid from the 9E10 hybridoma cell line) with 30 μl of protein G agarose (50% slurry in 0.1% NP-40, 20 mM Hepes, 0.15 M NaCl and 1 mM EDTA pH7.4) overnight at 4C. The bound antibody was recovered by centrifugation at 4C and the agarose beads were washed twice with the same solution before using. The beads were then mixed with the solubilized cell samples and the mixture was rotated overnight at 4°C. The beads were then recovered by centrifugation (4°C) and washed twice with 0.1% NP-40, 20 mM Hepes, 0.15M NaCl pH7.4. Finally the beads were counted in a γ counter to determine the amount of 125I-hCG that was bound to the immunoprecipitated receptor (35).
We immunoprecipitated the 125I-hCG-receptor complex present at the cell surface of 293T cells transiently transfected with the myc-hLHR wt as a positive control and the non-tagged hLHR-wt as a negative control. This was done by immunoprecipitation of detergent lysates of transfected cells that had been allowed to bind 2.5 nM (Kd = 1-3 nM) 125I-hCG for 4 h at 4°C (35). Under these conditions the amount of solubilized 125I-hCG immunoprecipitated from myc-hLHR-wt or hLHR-wt transfected cells was 39 ± 1 and 5 ± 1 %, respectively. Since 125I-hCG binding to cells incubated at 4C occurs only at the cell surface (48) the percentage of hormone immunoprecipitated from myc-hLHR-wt and hLHR-wt transfected cells is a good measure of the efficiency and specificity of immunoprecipitation, respectively.
Loss of cell surface receptors
Transiently transfected cells were washed twice with PBS (137 mM NaCl, 2.7 mM KCl, 1.4 mM KH2PO4, 4.3 mM Na2HPO4, pH 8) and then the surface proteins were labeled by incubation of the cells with a 0.5 mg/ml of freshly prepared Biotin (EZ-Link TM sulfo-NHS-LC-Biotin, Pierce, IL) for 30 min at room temperature. At the end of this incubation the cells were washed once with DMEM containing 20 mM Hepes, 10% newborn calf serum, 50 μg/ml gentamicin and incubated for 5 min in 1 ml of this medium to quench the unreacted biotin.
In some experiments the loss of cell surface receptors was ascertained using the same experimental conditions used for determining the fate of the internalized hormone (see above). For these experiments the biotinylated cells were washed twice with assay medium (see above) and incubated in 1 ml of warm assay medium with a saturating concentration of hCG (25 nM, Kd = 1-3 nM) for 20 min at 37C. At this point (t = 0 in the figures) the cells were placed on ice, washed twice with wash medium (see above) to remove the free hormone and then treated with an isotonic pH 3 buffer to release the bound hormone. The cells which now contain only internalized hCG were incubated for an additional 2 h at 37C in assay medium, washed and treated with the pH 3 isotonic buffer again to release any internalized hormone that had recycled to the surface (t = 2 h in the figures). The cells were then lysed in 1ml of RIPA buffer as previously described (23) and aliquots (∼ 500 μl) of the lysates containing 0.5 mg of protein were then used to immunopreciptiate the myc-tagged receptors using the 9E10 antibody as described above and elsewhere (23). The immunoprecipitates were resolved on SDS gels and electrophoretically transferred to polyvinylidene difluoride membranes as described elsewhere (49). The receptors were revealed using streptavidin conjugated to horseradish peroxidase (Vector Laboratories Inc, CA) at a dilution of 1:5,000 in blocking solution. The proteins were visualized and quantitated using the Super Signal West FEMTO Maximum Sensitivity system (Pierce, IL) and a Kodak digital imaging system as described elsewhere (23). The image capture system alerts us when image saturation occurs and prevents the quantitative analysis of saturated images.
In other experiments the loss of surface receptors was ascertained after a long incubation with the hormone that allows for several rounds of endocytosis (18, 19). For these experiments the biotinylated cells were incubated in medium without or with a saturating concentration of hCG (25 nM, Kd = 1-3 nM) and with or without leupeptin (200 μM) for 6 h at 37°C. At the end of this incubation the cells were placed on ice, lysed, immunoprecipitated and analyzed as described above.
Receptor immunoprecipitation and western blots
Cell lysates were prepared as described earlier (23). Aliquots containing 0.5 mg of the solubilized protein were immunoprecipitated using the anti-myc 9E10 antibody prebound on protein G agarose beads, resolved on SDS gels and transferred to membranes as described above. The receptors were visualized by western blots using an anti-myc 9E10 antibody conjugated to horseradish preroxidase (Roche Applied Science, IN) used at a 1:1,000 dilution during a 1 h incubation at room temperature. The immune complexes were finally visualized and quantitated as described above.
Confocal Microscopy
Confocal microscopy experiments were performed basically as described previously (17, 19, 24) but using experimental conditions that mimic those used above to follow the fate of the internalized hormone. Briefly, 293T cells were plated in eight-chamber coverslip culture vessels coated with polylysine (Biocoat polylysine slides, Becton-Dickinson). They were transfected (in a total volume of 400 μl) with 100 ng of the receptor expression vectors alone or together with 10 ng of the cathepsin D-GFP expression vector as described above. In order to determine the localization of the receptor in hCG stimulated cells two sets of cells transfected with the receptor alone (groups A and B) or with the receptor and catepsin D-GFP (groups C and D) were incubated for 20 min in assay medium containing 2.5 nM hCG (Kd = 1-3 nM). At the end of this incubation one of the sets of cells transfected with the receptor alone (group A) was also incubated with 20 μg/ml of Alexa 488-conjugated transferrin (Molecular probes) for 2.5 min to label the early endosomes (50) whereas another group co-transfected with the receptor and cathepsin D-GFP (group C) was incubated for 2.5 min without transferrin. These two groups of cells (groups A and C) were washed twice with wash medium then subjected to a brief exposure (3-4 min) to an isotonic pH 3 buffer to remove any surface bound transferrin and hCG and then washed two more times with PBS pH7.4 and processed for microscopy as described below. These cells are labeled as (+ hCG, t =0 min) and they are the same as the t = 0 min cells in the assays used to determine the fate of the internalized hormone (see above). Group A allows for co-localization of the receptor and transferrin (an early endosomal marker) whereas group C allows for co-localization of the receptor and cathepsin D-GFP (a lysosomal marker).
Groups B and D were incubated for 20 min with hCG as described above, and washed twice with wash medium prior to a brief exposure (3-4 min) to an isotonic pH 3 buffer to remove any surface bound hCG and then washed two more times with assay medium. These cells were further incubated in assay medium without hormone for 2 h to allow for processing of the internalized hormone. Group B was then incubated with 20 μg/ml of Alexa 488-conjugated transferrin (Molecular Probes) for 2.5 min whereas group D was incubated for 2.5 min without transferrin. At the end of this incubation groups B and D were washed twice with wash medium then subjected to a brief exposure (3-4 min) to an isotonic pH 3 buffer to remove any surface bound transferrin and hCG and then washed two more times with PBS pH 7.4 and processed for microscopy as described below. These cells are labeled as (+ hCG, t = 2h) in the figures and they correspond to the t = 2 h cells in the assays used to determine the fate of the internalized hormone (see above). Group B allows for co-localization of the receptor and transferrin (an early endosomal marker) whereas group D allows for co-localization of the receptor and cathepsin D-GFP (a lysosomal marker).
In order to localize the receptor in cells incubated without hCG, two additional groups of cells transfected with the receptor alone (group E) or together with the cathepsin D-GFP plasmid (group F) were incubated for 20 min in assay medium without hCG. Those in group E also received 20 μg/ml Alexa 488-conjugated transferrin for the last 2.5 min of this incubation. Finally they were washed twice with wash medium then subjected to a brief exposure (3-4 min) to an isotonic pH 3 glycine buffer to remove the surface bound transferrin and washed two more times with PBS pH 7.4. Those cells were then treated for microscopy and are labeled-hCG in the figures. Group E allows for co-localization of the unoccupied receptor and transferrin (an early endosomal marker) whereas group F allows for co-localization of the unoccupied receptor and cathepsin D-GFP (a lysosomal marker).
To visualize the myc-hLHR, the cells were washed 3 times in PBS pH 7.4 and fixed in 4% paraformaldehyde (in PBS) for 30 min at room temperature, washed again in PBS and permeabilized for 1 min in 1% Trixon ×-100 in PBS. The cells were then incubated in 5% bovine serum albumin in PBS for 1 hour and then incubated for 1 hour at room temperature with a 1:100 dilution of the myc monoclonal antibody (9E10, see above) dissolved in PBS containing 5 mg/ml bovine serum albumin. After washing three times the cells were incubated for another hour at room temperature with a 1:1,000 dilution of Cy™5-conjugated sheep anti-mouse IgG (Jackson Immunoresearch Laboratories). Finally, the cells were washed four times, dried and mounted using Prolong antifade (Molecular probes). The Cy™5 labeled myc-hLHR receptors and the Alexa488-conjugated transferrin or the cathepsin D-GFP were visualized with a Zeiss confocal microscope. An oil 63× objective was used and the iris opening was 2 to 2.2 for each color filter.
For each probe, 5 to 10 fields were observed and 5 to 10 pictures of each field were taken. This allows us to estimate the number of cells showing co-localization of the receptor with the endosomes and the lysosomes.
Hormones and supplies
The 9E10 hybridoma cell line was obtained from the American Type Culture Collection. These cells were used by Covance to prepare an ascites fluid containing the 9E10 antibody. Human kidney 293T cells are a derivative of 293 cells that express the SV40T antigen (51) and were provided to us by Doctor Marlene Hosey (Northwestern University, Chicago, IL). Purified hCG (CR-127, ∼ 13000 IU/mg) was purchased from Dr A. Parlow and the National Hormone and Pituitary Agency and purified recombinant hCG was generously provided by Ares-Serono (Randolph, MA). 125I-hCG was prepared as described elsewhere (52). Partially purified hCG (∼300 IU/mg) was purchased from Sigma, and it was used only for the determination of nonspecific binding. Cell culture plasticware and medium were obtained from Corning and Invitrogen, respectively. All others chemicals were obtained from commonly used suppliers.
293T cells were transiently transfected with the indicated constructs and incubated with 125I-hCG (1 nM, Kd = 1-3 nM) for 20 min at 37°C in the absence or presence of leupeptin as indicated. At this time the cells were washed to remove the free and bound hormone and placed back in medium containing an excess of non-radioactive hCG without or with leupeptin at 37°C for 2 h as indicated. The fate of the internalized 125I-hCG was then measured as described in Materials and Methods.
Results are expressed as a percent of the total intracellular 125I-hCG radioactivity present at the end of the binding and internalization period and they represent the mean ± SEM of three to six independent transfections. At this point the amount of internalized radioactivit1 y varied between 30,000 and 60,000 cpm/well.
293T cells were transiently transfected with the indicated constructs and incubated with 125I-hCG (1 nM, Kd = 1-3 nM) and leupeptin for 20 min at 37°C. At this time the cells were washed to remove the free and bound hormone and placed back in medium with leupeptin and an excess of hCG at 37°C for 2 h. At the end of this incubation the cells were collected, homogenized, and analyzed on Percoll gradients as described in Figure 2. A pool of the fractions containing the lysosomes was solubilized with detergent and immunoprecipitated with an antibody (9E10) to the myc-epitope. The immunoprecipitated radioactivity was expressed as % of the total radioactivity found in the pooled fractions.
Each of the samples used for immunoprecipitation contained 2,000-5,000 cpm. Each value shows the mean and SEM of 3-4 independent transfections. Control samples in which equivalent amounts of free 125I-hCG were immunoprecipitated as described above revealed that only about 0.5% of the free 125I-hCG was immunoprecipitated. In contrast, when cells transiently transfected with myc-hLHR-wt were incubated with 125I-hCG for 4 h at 4°C followed by detergent solubilization, about 40% of the solubilized radioactivity could be immunoprecipitated using the method described above.
293T cells were transiently transfected with the indicated constructs. The cells were biotinylated and incubated at 37°C in the presence of a saturating concentration of hCG (25 nM, Kd = 1-3 nM) for 6 h in the presence or absence of leupeptin (200 μM) as indicated. The cells were lysed and the lysates immunoprecipitated with the 9E10 antibody. The amount of surface receptor was visualized and quantitated on western blots using streptavidin covalently coupled to horseradish peroxidase as described in Materials and Methods and is expressed as % of the receptor present at t = 0.
Footnotes
Supported by a grant from the National Cancer Institute (CA-40629).
References
- 1.Pierce KL, Lefkowitz RJ. Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nature Reviews Neuroscience. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 2.Miller WE, Lefkowitz RJ. Expanding roles for β-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 4.Perry SJ, Lefkowitz RJ. Arresting developments in heptahelical receptor signaling and regulation. Trends Cell Biol. 2002;12:130–138. doi: 10.1016/s0962-8924(01)02239-5. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin A, von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 6.Marchese A, Chen C, Kim Y-M, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28:369–376. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- 7.Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- 8.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 9.Hicke L. A new ticket for entry into budding vesicles-ubiquitin. Cell. 2001;106:527–530. doi: 10.1016/s0092-8674(01)00485-8. [DOI] [PubMed] [Google Scholar]
- 10.Ascoli M. Lysosomal accumulation of the hormone-receptor complex during receptor-mediated endocytosis of human choriogonadotropin. J Cell Biol. 1984;99:1242–1250. doi: 10.1083/jcb.99.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Segaloff DL, Ascoli M. Lutropin/choriogonadotropin down-regulates its receptor by both receptor mediated endocytosis and a cAMP-dependent reduction in receptor mRNA. J Biol Chem. 1991;266:780–785. [PubMed] [Google Scholar]
- 12.Bhaskaran RS, Ascoli M. The post-endocytotic fate of the gonadotropin receptors is an important determinant of the desensitization of gonadotropin responses. J Mol Endocrinol. 2005;34:447–457. doi: 10.1677/jme.1.01745. [DOI] [PubMed] [Google Scholar]
- 13.Trejo J, Hammes SR, Coughlin SR. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci (USA) 1998;95:13698–13702. doi: 10.1073/pnas.95.23.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trejo J, Coughlin SR. The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J Biol Chem. 1999;274:2216–2224. doi: 10.1074/jbc.274.4.2216. [DOI] [PubMed] [Google Scholar]
- 15.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and Intracellular Trafficking Pathways of the Endothelin Receptors. J Biol Chem. 2000;275:17596–17604. doi: 10.1074/jbc.M000142200. [DOI] [PubMed] [Google Scholar]
- 16.Kishi M, Liu X, Hirakawa T, Reczek D, Bretscher A, Ascoli M. Identification of two distinct structural motifs that, when added to the C-terminal tail of the rat lutropin receptor, redirect the internalized hormone-receptor complex from a degradation to a recycling pathway. Mol Endocrinol. 2001;15:1624–1635. doi: 10.1210/mend.15.9.0698. [DOI] [PubMed] [Google Scholar]
- 17.Galet C, Min L, Narayanan R, Kishi M, Weigel N,L, Ascoli M. Identification of a transferable two amino acid motif (GT) present in the C-terminal tail of the human lutropin receptor that redirects internalized G protein-coupled receptors from a degradation to a recycling pathway. Mol Endocrinol. 2003;17:411–422. doi: 10.1210/me.2002-0161. [DOI] [PubMed] [Google Scholar]
- 18.Hirakawa T, Galet C, Kishi M, Ascoli M. GIPC binds to the human lutropin receptor (LHR) through an unusual PDZ domain binding motif and it regulates the sorting of the internalized human choriogonadotropin (hCG) and the density of cell surface LHR. J Biol Chem. 2003;278:49348–49357. doi: 10.1074/jbc.M306557200. [DOI] [PubMed] [Google Scholar]
- 19.Galet C, Hirakawa T, Ascoli M. The postendocytotic trafficking of the hLHR is mediated by a transferable motif consisting of the C-terminal cysteine and an upstream leucine. Mol Endocrinol. 2004;18:434–446. doi: 10.1210/me.2003-0293. [DOI] [PubMed] [Google Scholar]
- 20.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor. A 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 21.Themmen APN. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130:263–274. doi: 10.1530/rep.1.00663. [DOI] [PubMed] [Google Scholar]
- 22.Min K-S, Liu X, Fabritz J, Jaquette J, Abell AN, Ascoli M. Mutations that induce constitutive activation and mutations that impair signal transduction modulate the basal and/or agonist-stimulated internalization of the lutropin/choriogonadotropin receptor. J Biol Chem. 1998;273:34911–34919. doi: 10.1074/jbc.273.52.34911. [DOI] [PubMed] [Google Scholar]
- 23.Min L, Ascoli M. Effect of activating and inactivating mutations on the phosphorylation and trafficking of the human lutropin/choriogonadotropin receptor. Mol Endocrinol. 2000;14:1797–1810. doi: 10.1210/mend.14.11.0555. [DOI] [PubMed] [Google Scholar]
- 24.Min L, Galet C, Ascoli M. The association of arrestin-3 with the human lutropin/choriogonadotropin receptor depends mostly on receptor activation rather than on receptor phosphorylation. J Biol Chem. 2002;277:702–710. doi: 10.1074/jbc.M106082200. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury FA, Menon KMJ. Evidence that constitutively active luteinizing hormone/human chorionic gonadotropin receptors are rapidly internalized. Biochemistry. 1999;38:8703–8712. doi: 10.1021/bi990169t. [DOI] [PubMed] [Google Scholar]
- 26.Munshi UM, Clouser CL, Peegel H, Menon KMJ. Evidence that Palmitoylation of Carboxyl Terminus Cysteine Residues of the Human Luteinizing Hormone Receptor Regulates Postendocytic Processing. Mol Endocrinol. 2005;19:749–758. doi: 10.1210/me.2004-0335. [DOI] [PubMed] [Google Scholar]
- 27.Latronico AC, Abell AN, Arnhold IJP, Liu X, Lins TSS, Brito VN, Bilerbeck AE, Segaloff DL, Mendonica BB. A unique constitutively activating mutation in third transmbembrane helix of luteinizing hormone receptor causes sporadic male gonadotropin-independent precocious puberty. J Clin Endocrinol Metab. 1998;83:2435–2440. doi: 10.1210/jcem.83.7.4968. [DOI] [PubMed] [Google Scholar]
- 28.Shinozaki H, Fanelli F, Liu X, Butterbrodt J, Nakamura K, Segaloff DL. Pleiotropic effects of substitutions of a highly conserved leucine in transmembrane helix III of the human lutropin/choriogonadotropin receptor with respect to constitutive activation and hormone responsiveness. Mol Endocrinol. 2001;15:972–984. doi: 10.1210/mend.15.6.0661. [DOI] [PubMed] [Google Scholar]
- 29.Hirakawa T, Ascoli M. A constitutively active somatic mutation of the human lutropin receptor found in Leydig cell tumors activates the same families of G proteins as germ line mutations associated with Leydig cell hyperplasia. Endocrinology. 2003;144:3872–3878. doi: 10.1210/en.2003-0365. [DOI] [PubMed] [Google Scholar]
- 30.Hirakawa T, Galet C, Ascoli M. MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR) A novel experimental paradigm to study the functional properties of the hLHR. Endocrinology. 2002;143:1026–1035. doi: 10.1210/endo.143.3.8702. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Lazari MFM, Li S, Korgaonkar C, Ascoli M. Role of the rate of internalization of the agonist-receptor complex on the agonist-induced down-regulation of the lutropin/choriogonadotropin receptor. Mol Endocrinol. 1999;13:1295–1304. doi: 10.1210/mend.13.8.0331. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Liu X, Ascoli M. The rate of internalization of the gonadotropin receptors is greatly affected by the origin of the extracellular domain. J Biol Chem. 1999;274:25426–25432. doi: 10.1074/jbc.274.36.25426. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Ascoli M. A dileucine-based motif in the C-terminal tail of the lutropin/choriogonadotropin receptor inhibits endocytosis of the agonist-receptor complex. Mol Pharmacol. 1999;56:728–736. [PubMed] [Google Scholar]
- 34.Nakamura K, Liu X, Ascoli M. Seven non-contiguous intracellular residues of the lutropin/choriogonadotropin receptor dictate the rate of agonist-induced internalization and its sensitivity to non-visual arrestins. J Biol Chem. 2000;275:241–247. doi: 10.1074/jbc.275.1.241. [DOI] [PubMed] [Google Scholar]
- 35.Kishi M, Ascoli M. The C-terminal tail of the rat lutropin/choriogonadotropin receptor independently modulates hCG-induced internalization of the cell surface receptor and the lysosomal targeting of the internalized hCG-receptor complex. Mol Endocrinol. 2000;14:926–936. doi: 10.1210/mend.14.6.0475. [DOI] [PubMed] [Google Scholar]
- 36.Ghinea N, Vuhai MT, Groyer-Picard M-T, Houllier A, Schoëvaërt D, Milgrom E. Pathways of internalization of the hCG/LH receptor: immunoelectron microscopic studies in Leydig cells and transfected L cells. J Cell Biol. 1992;118:1347–1358. doi: 10.1083/jcb.118.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baratti-Elbaz C, Chinea N, Lahuna O, Loosfelt H, Pichon C, Milgrom E. Internalization and recycling pathways of the thyrotropin receptor. Mol Endocrinol. 1999;13:1751–1765. doi: 10.1210/mend.13.10.0360. [DOI] [PubMed] [Google Scholar]
- 38.Graeve L, Drickamer K, Rodriguez-Boulan E. Polarized endocytosis by Madin-Darby canine kidney cells transfected with functional chicken liver glycoprotein receptor. J Cell Biol. 1989;109:2809–2816. doi: 10.1083/jcb.109.6.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gage RM, Kim K-A, Cao TT, von Zastrow M. A Transplantable Sorting Signal That Is Sufficient to Mediate Rapid Recycling of G Protein-coupled Receptors. J Biol Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- 40.Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- 41.Ascoli M, Puett D. Inhibition of the degradation of receptor-bound human choriogonadotropin by lysosomotropic agents, protease inhibitors, and metabolic inhibitors. J Biol Chem. 1978;253:7832–7838. [PubMed] [Google Scholar]
- 42.Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, Dahl SG, Attramadal H. Subtype-Specific Sorting of the ETA Endothelin Receptor by a Novel Endocytic Recycling Signal for G Protein-Coupled Receptors. Mol Pharmacol. 2005;67:1581–1590. doi: 10.1124/mol.104.007013. [DOI] [PubMed] [Google Scholar]
- 43.Gaborik Z, Hunyady L. Intracellular trafficking of hormone receptors. Trends Endocrinol Metabol. 2004;15:286–293. doi: 10.1016/j.tem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Quintana J, Wang H, Ascoli M. The regulation of the binding affinity of the luteinizing hormone/choriogonadotropin receptor by sodium ions is mediated by a highly conserved aspartate located in the second transmembrane domain of G protein-coupled receptors. Mol Endocrinol. 1993;7:767–775. doi: 10.1210/mend.7.6.8395653. [DOI] [PubMed] [Google Scholar]
- 45.Costagliola S, Urizar E, Mendive F, Vassart G. Specificity and promiscuity of gonadotropin receptors. Reproduction. 2005;130:275–281. doi: 10.1530/rep.1.00662. [DOI] [PubMed] [Google Scholar]
- 46.Fabritz J, Ryan S, Ascoli M. Transfected cells express mostly the intracellular precursor of the lutropin/choriogonadotropin receptor but this precursor binds choriogonadotropin with high affinity. Biochemistry. 1998;37:664–672. doi: 10.1021/bi972355+. [DOI] [PubMed] [Google Scholar]
- 47.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ascoli M. Internalization and degradation of receptor-bound human choriogonadotropin in Leydig tumor cells. Fate of the hormone subunits. J Biol Chem. 1982;257:13306–13311. [PubMed] [Google Scholar]
- 49.Quintana J, Hipkin RW, Ascoli M. A polyclonal antibody to a synthetic peptide derived from the rat FSH receptor reveals the recombinant receptor as a 74 kDa protein. Endocrinology. 1993;133:2098–2104. doi: 10.1210/endo.133.5.8404659. [DOI] [PubMed] [Google Scholar]
- 50.Sheff DR, Daro EA, Hull M, Mellman I. The Receptor Recycling Pathway Contains Two Distinct Populations of Early Endosomes with Different Sorting Functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margolskee R, McHenry-Rinde B, Horn R. Panning transfected cells for electrophysiological studies. Biotechniques. 1993;15:906–911. [PubMed] [Google Scholar]
- 52.Ascoli M, Puett D. Gonadotropin binding and stimulation of steroidogenesis in Leydig tumor cells. Proc Natl Acad Sci (USA) 1978;75:99–102. doi: 10.1073/pnas.75.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]