Abstract
We examined demographic, microbiologic, and clinical data from patients presenting during 1988, 1998, and 2004 flood-associated diarrheal epidemics at a diarrhea treatment hospital in Dhaka, Bangladesh. Compared with non-flood periods, individuals presenting during flood-associated epidemics were older, more severely dehydrated, and of lower socioeconomic status. During flood-associated epidemics, Vibrio cholerae was the most commonly identified cause of diarrhea, and the only diarrheal pathogen whose incidence proportionally increased in each epidemic compared with seasonally matched periods. Rotavirus was the second most frequently identified flood-associated pathogen, although the proportion of cases caused by rotavirus infection decreased during floods compared with matched periods. Other causes of diarrhea did not proportionally change, although more patients per day presented with enterotoxigenic Escherichia coli, Shigella, and Salmonella species-associated diarrhea during floods compared with matched periods. Our findings suggest that cholera is the predominant cause of flood-associated diarrheal epidemics in Dhaka, but that other organisms spread by the fecal-oral route also contribute.
INTRODUCTION
Bangladesh is a riverine country, which is prone to severe flooding. Recently, there were extensive floods during the monsoons of 1988, 1998, and 2004. During these periods, 25-50% of Bangladesh was submerged, resulting in the destruction of infrastructure, contamination of water, and epidemics of diarrheal illness.1
Flooding has been shown to cause epidemics of water-borne and vector-borne disease.2 Water-borne outbreaks of diarrheal illness after floods are thought to result primarily from contamination of water caused by disruption of water purification and sewage disposal systems. However, it has been hypothesized that the secondary effects of flooding, including crowding and subsequent fecal-oral spread of gastrointestinal pathogens, may also contribute to spread of diarrheal diseases.3,4
Flood-related diarrheal epidemics cause significant morbidity and mortality in Bangladesh and throughout the world.5 During the 1988 flood in Bangladesh, diarrheal disease was responsible for 35% of all flood-related illness and 27% of 154 flood-related deaths in a population of more than 45,000 patients in rural Bangladesh.6 During the 1998 flood period, 25% of 3,109 people surveyed in two rural areas of Bangladesh reported diarrheal illness.4
Despite the public health consequences of flood-related diarrheal epidemics, few studies have characterized the microbiologic characteristics of these events in Bangladesh. One study examined 2,400 stool samples of patients with diarrheal illness in rural Bangladesh in 1988. Vibrio cholerae was cultured from 40% of patients, but the results did not discriminate between the flood period and the cumulative 9 months studied.7 In 1998, Tanabe and others8 examined 76 patient stool samples during a post-flood diarrheal epidemic in the Chandpur district of southeastern Bangladesh. They found V. cholerae in 50% of patients; V. cholerae non-O1/non-O139, non-cholera vibrios, and enteropathogenic Escherichia coli infection were also detected, but at much lower frequencies. However, the small sample size and lack of comparison with other periods limit the generalizability of this study. Qadri and others9 recently reported the results of a focused evaluation for enterotoxigenic E. coli (ETEC) during a 6-week period of the first Dhaka flood of 2004; they found that patients with ETEC infection represented a significant fraction of those presenting to a diarrheal treatment facility in Dhaka. In particular, ETEC accounted for 18% of cases, whereas V. cholerae infection accounted for 22% of cases.
An enhanced understanding of the epidemiology of flood-related diarrheal epidemics would help clinicians and public health officials prepare for future events. We therefore examined the demographic and microbiologic features that characterized recent flood-related diarrheal epidemics in Dhaka, Bangladesh.
MATERIALS AND METHODS
Study site
The International Center for Diarrheal Disease Research, Bangladesh (ICDDR,B) is located in the densely populated city of Dhaka (population estimate 11.6 million in 2003).10 The city is surrounded by the Buriganga, Lakhya, Turag, and Tongi Khal rivers, and during floods, numerous areas of the city are inundated. The ICDDR,B treats approximately 100,000 children and adults with diarrheal illness each year, in inpatient and outpatient facilities; therapeutic interventions include administration of oral rehydration solution (ORS), intravenous fluids, and antibiotics. Between 1987 and 1989, there were an estimated 71,000 patient visits/yr; between 1997 and 1999, there were 130,000 patient visits/yr; and between 2003 and 2004, there were 102,000 patient visits/yr.
Surveillance system
A surveillance system was established at the ICDDR,B in 1979 to systematically sample children and adults with diarrheal illness.11 Between 1980 and 1995, every 25th patient presenting to the ICDDR,B was enrolled. Since 1996, every 50th patient has been enrolled. The patient and family members are interviewed by health workers who collect demographic, socioeconomic, and clinical data. A physician documents the clinical condition, including dehydration status, and a stool or rectal swab sample is collected for microbiologic evaluation. All demographic, microbiologic, treatment, and outcome data are systematically recorded.
Microbiological evaluation
As part of the microbiological surveillance system, stool and rectal swab samples were evaluated for a range of microorganisms. A list of microorganisms screened for during each time period of study is listed in Table 1. In all time periods, stool was routinely evaluated for V. cholerae, Shigella species, Salmonella species, Enta moeba histolytica/dispar, and Giardia lamblia. Rectal swabs or stool samples were plated directly on salmonella-shigella (SS), taurocholate-tellurite-gelatin, and MacConkey agars. Specimens were also enriched in alkaline peptone water and plated on taurocholate-tellurite-gelatin agar. Plates were examined for salmonella, shigella, and vibrios by standard methods. V. cholerae- like colonies were further characterized and classified by serogroup, biotype, and serotype. Non-lactose fermenting colonies from MacConkey and SS agars were screened on Kligler iron agar slants and urea-motility-indole medium. Shigella isolates were biochemically identified and serologically grouped using commercially available antisera (Burroughs Wellcome, Research Park, NC). Campy-BAP agar was used to screen for C. jejuni (BBL Microbiology Systems, Cockeysville, MD). ETEC was detected through specific gene amplification in pools of five colonies of E. coli recovered from stool. Rotavirus antigen (group A- specific VP6 protein) was identified by enzyme-linked immunosorbent assay (Dakoplatts, Copenhagen, The Netherlands).12 Fresh stool specimens were tested for parasites by direct smear microscopy without concentration techniques.
TABLE 1.
Intestinal pathogens screened for in the ICDDR,B surveillance system, by year
| 1987-1989 | 1997-1999 | 2003-2004 | |
|---|---|---|---|
| V. cholerae | + | + | + |
| Shigella species | + | + | + |
| Salmonella species | + | + | + |
| E. histolytica * | + | + | + |
| G. lamblia | + | + | + |
| Rotavirus | + | + | |
| ETEC | + | ||
| C. jejuni | + |
Screening method used was unable to distinguish between E. histolytica and E. dispar.
Definition of epidemic and flood periods
We defined the onset of an epidemic as the first of 3 consecutive days during which patient visits per day exceeded the 90th percentile of visits per day for the year before and the year after the year of flooding. The 90th percentile for visits per day was 275 in 1987 and 1989, 450 in 1997 and 1999, and 350 in 2003 (data not available for 2005). Similarly, we defined cessation of an epidemic as the day when the visits per day fell below the 90th percentile for 3 consecutive days.
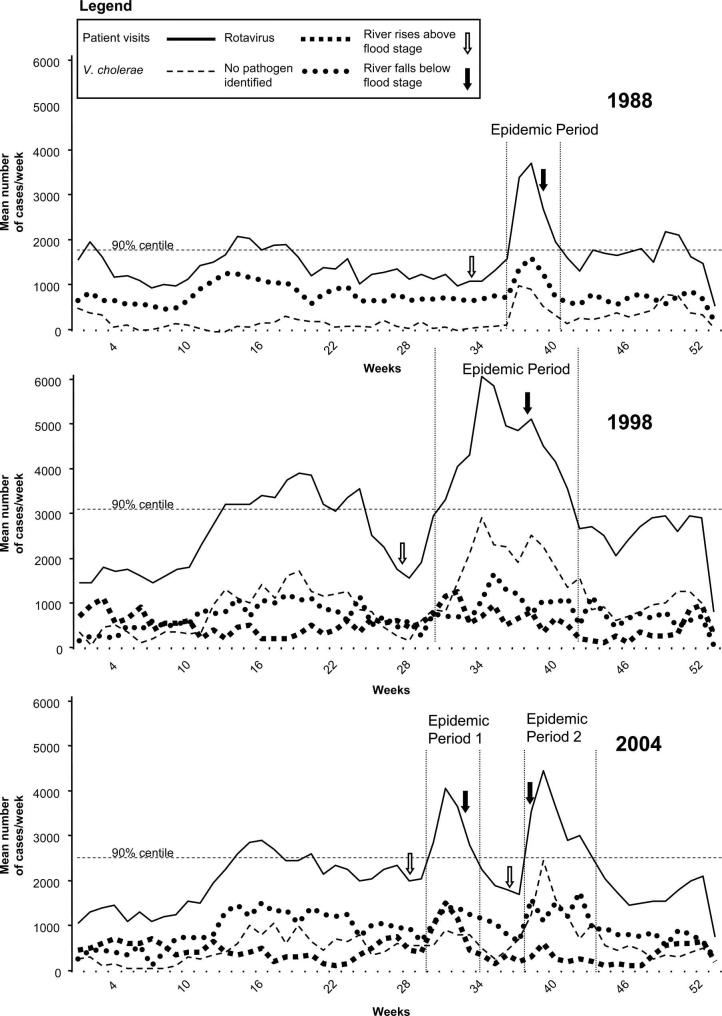
A flood period was defined from the earliest date that any of the rivers surrounding Dhaka exceeded the predetermined flood stage through the latest date that any of the river levels fell below the flood stage. An individual river’s flood stage was defined by the Flood Forecasting Watch Center, Bangladesh.13 Using this definition, there were four flood-related diarrheal epidemics: September 6 to October 13, 1988; July 25 to October 13, 1998; July 20 to August 21, 2004; and September 16 to October 24, 2004 (Figure 1). The comparator non-flood periods were defined as the matching dates in the flanking years. For example, the periods of September 6 to October 13 in 1987 and 1989 were used as the comparator non-flood periods for the 1988 epidemic, and microbiological data from the corresponding surveillance system periods were obtained. This definition was chosen to account for seasonal variation in diarrheal pathogens.
FIGURE 1.
Mean cases per week of patient visits and selected enteric pathogens at the Dhaka Hospital of the ICDDR,B during 1988, 1998, and 2004. All values are estimated cases per week based on surveillance data representing 4% (1987-1989) and 2% (1997-2004) of total cases. River level data was only available from May 1 (week 18) through October 15 (week 42) for each year studied. These periods correspond to highest river stages. Horizontal dashed line represents 90th percentile of mean number cases/week for non-flood years for each flood year. Vertical dashed lines represent epidemic periods.
Statistical methods
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) Version 12.0. Differences in proportion of cases were assessed by Pearson’s χ2 analysis. Associations were made by calculation of the odds ratio (OR) with 95% confidence intervals (CIs). Comparison between two means was performed using the independent sample t test; when data were not normally distributed, the Mann-Whitney U test was used. Statistical significance was defined as a two-tailed P < 0.05.
RESULTS
River levels and the timing of diarrheal epidemics
Figure 1 shows the relationship between river levels and diarrheal epidemics for the flood periods of 1988, 1998, and 2004. River levels remained above flood stage for a mean of 26.3 days during flood periods. There were 29 flood days in 1988, 39 in 1998, 31 in the first 2004 flood, and 6 in the second 2004 flood. We observed a median of 8.5 days (range, 3-13 days) from the time the rivers reached flood stage until the beginning of epidemics, and a median of 17.5 days (range, 8-36 days) for the epidemics to end after falling of the river levels below flood stage. In the 1998 and the second 2004 floods, there was a longer lag from the end of flooding until the end of the diarrheal epidemic (22 and 36 days, respectively) compared with the 1988 and the first 2004 floods (13 and 8 days, respectively).
Patient characteristics
Patient characteristics for flood-related epidemics and comparator non-flood periods are summarized in Table 2. For the years analyzed, a total of 20,395 patients were enrolled into the surveillance system, representing a systematic sample of approximately 800,000 patient visits with a mean of 275 ± 129 (SD) patient visits/d to the ICDDR,B. Two thousand two hundred twenty-nine patients were surveyed during the flood-related epidemics: 493 in 1988, 1,050 in 1998, and 686 in 2004 (304 in the first epidemic and 382 in the second epidemic). Extrapolating from this, approximately 100,000 patients presented to the ICDDR,B during these four analyzed flood-related epidemics with 542 ± 153 mean visits/d compared with 249 ± 69 mean visits/d in matched non-flood periods (P < 0.001).
TABLE 2.
Patient characteristics for pooled flood-related epidemics and non-flood periods
| Flood* (N = 1,983) N (%) | Non-flood* (N = 1,466) N (%) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Male sex | 1104 (56) | 856 (59) | 0.89 (0.79-1.02) | 0.10 |
| Age (years) | ||||
| < 2 | 690 (35) | 695 (46) | 0.59 (0.52-0.68) | < 0.001 |
| 2-4 | 246 (12) | 133 (9) | 1.32 (1.06-1.66) | 0.002 |
| 5-14 | 238 (12) | 137 (9) | 1.32 (1.05-1.65) | 0.01 |
| > 15 | 806 (41) | 498 (34) | 1.33 (1.16-1.53) | < 0.001 |
| Before arrival | ||||
| ORS | 1771 (91) | 1243 (87) | 1.50 (1.20-1.87) | < 0.001 |
| Antibiotics | 248 (21) | 208 (23) | 0.89 (0.72-1.09) | 0.27 |
| Father uneducated† | 1097 (59) | 765 (53) | 1.15 (1.00-1.32) | 0.05 |
| Z score < -2‡ | ||||
| Ht-for-age | 336 (35) | 302 (36) | 0.96 (0.79-1.17) | 0.69 |
| Wt-for-age | 519 (54) | 456 (54) | 0.99 (0.83-1.19) | 0.94 |
| Wt-for-ht | 263 (27) | 209 (25) | 1.14 (0.93-1.41) | 0.22 |
| Diarrhea | ||||
| Watery | 1867 (95) | 1337 (92) | 1.75 (1.32-2.31) | < 0.001 |
| Bloody | 56 (3) | 68 (5) | 0.60 (0.42-0.86) | 0.005 |
| Fever | 75 (4) | 86 (6) | 0.63 (0.46-0.87) | 0.004 |
| Severe dehydration | 612 (31) | 203 (14) | 2.78 (2.33-3.32) | < 0.001 |
| Intravenous rehydration | 856 (44) | 366 (25) | 2.32 (1.99-2.69) | < 0.001 |
| Death | 6 (< 1) | 3 (< 1) | 1.48 (0.37-5.93) | 0.58 |
Values in these columns have been adjusted to equalize weight of the 4% population screened in 1987-1989 compared with the 2% screened in all other years.
Patient’s father had no formal education.
Z scores were calculated as the percentage of the median-for-age from the US National Center for Health Statistics using EpiInfo and only for patients ≤ 5 years of age, using admission weight.
As shown in Table 2, patients presenting during pooled flood-related epidemics were older than patients presenting during non-flood periods. Compared with patients in non-flood periods, those presenting during flood-associated epidemics used more ORS before presentation, but were still more likely to present with severe dehydration and to require intravenous rehydration than patients in the comparator periods. To determine whether differences in dehydration status were caused by an increased incidence of V. cholerae during flood-associated epidemics, we stratified the analysis for cholera versus non-cholera patients. This showed that patients with V. cholerae (OR = 1.63; 95% CI, 1.23-2.14; P = 0.001) and those without microbiologically identifiable V. cholerae infection (OR = 2.75; 95% CI, 2.11-3.59; P < 0.001) were both more likely to have severe dehydration during flood-related epidemics than during non-flood periods. Patients who presented during flood-associated epidemics were also more likely to have a father without formal education, a marker of lower socioeconomic status. However, there was no significant difference in the anthropometric markers of nutritional status in children in the surveillance population during flood-associated epidemics. Analysis of demographic features for individual flood and control periods disclosed findings similar to those observed for aggregate flood and aggregate control periods (data not shown).
Pathogens
Vibrio cholerae. Over the 8 discontinuous years of observation included in this analysis, a total of 4,427 positive stool cultures for V. cholerae were obtained by the ICDDR,B surveillance system, representing 191,000 cholera cases. The proportion of V. cholerae infection increased during all of the flood-related epidemics compared with non-flood periods (Table 3). In both the 1998 and first 2004 flood-associated epidemics, there was an approximate doubling in the proportion of patients with V. cholerae infection compared with the seasonally matched control period (1998: 42% from 20%, P ≤ 0.001; 2004 first epidemic: 23% from 11%, P ≤ 0.001). In the 1988 and second 2004 epidemics, there was a lower but still significant increase in the proportion of patients with V. cholerae compared with seasonally matched periods (1988: 25% from 15%, P ≤ 0.01; 2004 second epidemic: 41% from 32%, P = 0.03).
TABLE 3.
Mean cases per day of enteric pathogens in pooled flood-related epidemics and non-flood periods*
| Pooled periods |
||||
|---|---|---|---|---|
| Flood cases/day (%) | Non-flood cases/day (%) | P value of proportions | P value of mean cases/day | |
| Patient visits | 542 (100) | 249 (100) | < 0.001 | |
| V. cholerae | 200 (37) | 49 (20) | < 0.001 | < 0.001 |
| Shigella sp. | 29 (5) | 16 (6) | 0.66 | < 0.001 |
| Salmonella sp. | 11 (2) | 4 (2) | 0.90 | 0.001 |
| E. histolytica† | 10 (2) | 4 (2) | 0.96 | 0.008 |
| G. lamblia | 12 (2) | 5 (2) | 0.94 | 0.002 |
| Rotavirus‡ | 96 (17) | 68 (26) | 0.002 | 0.004 |
| Other pathogens§ | 64 (12) | 52 (21) | 0.001 | 0.13 |
| No pathogen identified¶ | 139 (26) | 67 (27) | 0.77 | < 0.001 |
All values estimated cases/day based on surveillance data representing 4% (1987-1989) and 2% (1997-2004) of total cases.
Screening method was unable to distinguish E. histolytica from E. dispar.
Rotavirus was only screened for after 1989.
Other potential pathogens screened for during different periods are listed in Table 1. Totals may add up to > 100% because of co-infection.
Cases where no pathogen was identified: variability existed in pathogen screening during time periods studied (see Table 1 for details).
Although the O1 serogroup and El Tor biotype of V. cholerae predominated in all periods studied, we did observe differences in the predominating serotype of V. cholerae between flood-related epidemics. The proportion of V. cholerae O1 cases caused by the Ogawa serotype was 54% in 1988, 96% in 1998, 28% in the first 2004 epidemic, and 20% in the second 2004 epidemic. However, the proportion of patients infected with each serotype reflected the proportions circulating in the 3 months before each flood-associated epidemic (data not shown).
V. cholerae
of the O139 serogroup was not present before 1992. However, during the three later epidemics, the O139 serogroup was found in 2% of patients during pooled flood-related epidemics and in 9% of patients during comparator non-flood periods.
Rotavirus
As shown in Table 3, the overall proportion of patients infected with rotavirus decreased during flood-related epidemics compared with matched non-flood periods (26% versus 17%, non-flood versus flood; P = 0.002). The decrease in proportion of patients with rotavirus was significant for the 1998 and the second 2004 epidemic. However, despite this, rotavirus was the second most frequently identified cause of diarrhea during all flood periods (when considered in aggregate), and the single most commonly identified cause of diarrhea during the first 2004 epidemic, accounting for 30% of all cases of diarrhea among individuals presenting to the ICDDR,B during that epidemic. Notably, rotavirus was not screened for during the 1988 epidemic, which shared a number of epidemiologic features with the first 2004 epidemic, including the predominance of children less than 2 years of age, the shorter duration of the epidemic, and the lower proportion of V. cholerae isolates.
Shigella and Salmonella species
Although there was an increase in the mean cases/day of shigella-related infections during flood-related epidemics (16 versus 29; non-flood versus flood; P < 0.001), there was no proportional change in Shigella cases when considering non-flood versus flood periods (6% versus 5%, non-flood versus flood; P = 0.66). Shigella flexneri was the predominant species during both flood-related epidemics and non-flood periods. Similarly, there was an overall increase in mean cases per day of Salmonella species compared with corresponding non-flood periods (4 versus 11, non-flood versus flood; P = 0.001), although there was no proportional change in Salmonella cases (2% versus 2%, non-flood versus flood; P = 0.90).
Other pathogens
E. histolytica/dispar and G. lamblia were screened for throughout the entire surveillance period. Comparing flood to non-flood periods, there was no proportional change in these pathogens, although there was a significant increase in mean cases per day. Between 1997 and 1999, ETEC and C. jejuni were additionally assessed for by the surveillance system. During this period, flood-related ETEC cases proportionally decreased compared with non-flood periods (18% versus 9%, non-flood versus flood; P < 0.001). C. jejuni cases did not proportionally change, although there was a significant increase in mean cases per day during the 1998 epidemic compared with corresponding non-flood periods (33 versus 78, non-flood versus flood; P < 0.001).
No pathogen identified
Patients presenting to the ICD-DR,B with diarrhea and no identifiable organism comprised between 23% and 51% per year of the surveillance population. The number of cases in which no pathogen was identified is confounded by the variability in the screening tests used during the different periods. However, when considering each flood period and matched control period, we found a proportional decrease in cases with no specific identified pathogen during flood periods, perhaps reflecting the increased proportion of diagnosed V. cholerae- associated cases.
Individual versus aggregate microbiological analysis
Analysis of microbiological results for individual flood and control periods disclosed findings similar to those observed for aggregate flood and aggregate control periods (data not shown).
Outcomes
Despite high patient volume and increase in severe dehydration during the flood-related epidemics, patients presenting to the ICDDR,B during these epidemics had no statistically significant difference in the low in-hospital case-fatality rates that were observed for patients presenting during flood-associated epidemics and corresponding non-flood periods (three fatalities per 1,000 patient visits during flood-associated epidemics versus two fatalities per 1,000 patient visits in non-flood periods; P = 0.58).
DISCUSSION
Our data show that V. cholerae was the primary pathogen in four major flood-associated diarrheal epidemics that occurred in Dhaka, Bangladesh, from 1988 to 2004. In the Bay of Bengal, where cholera is endemic, Sack and others14 previously showed a seasonality of cholera, with regular out-breaks of cholera occurring in spring and autumn (premonsoon and post-monsoon, respectively) simultaneously in various areas of Bangladesh. In addition to the yearly epidemic cycles of transmission, there is also significant interannual variability in the incidence of cholera. Variation in the incidence of cholera over these annual and inter-annual cycles may be related to a number of variables. These include climatic factors such as rainfall, surface water temperatures, surface water salinity, and El Nino Southern Oscillation activity,15,16 host factors including population-level immunity,15 and microbiologic factors such as the presence of toxigenic environmental isolates and phage populations.16-18
In addition to the dynamic pattern of endemic disease, certain conditions also predispose to large-scale epidemics of cholera. Naive populations are particularly susceptible to such outbreaks. For instance, an outbreak among approximately 500,000 refugees in Goma, Zaire, in July 1994 resulted in more than 35,000 cases of cholera over a 1-month period.19,20 Although V. cholerae is a classic example of a water-borne pathogen, it can also spread by direct fecal-oral transmission, and V. cholerae organisms acquire a transient hyper-infectious phenotype after passage through the human intestine21,22; such hyperinfectivity may contribute to the epidemic spread of V. cholerae.23 We observed that, during each flood-associated epidemic, the distribution of serotypes of V. cholerae reflected the distribution in the 3 months immediately preceding each epidemic period, perhaps reflecting host-mediated amplification of circulating strains of V. cholerae rather than emergence of new environmental isolates. The proportional decrease in cholera caused by V. cholerae O139 during flood compared with non-flood periods may reflect amplification of the predominant circulating strain of V. cholerae in the community when a flood occurs, which has historically been V. cholerae O1.
Along with a proportional increase in cholera cases during flood periods, there was a proportional decrease in cases of rotavirus. Despite this, rotavirus was, overall, the second most frequently identified pathogen during epidemics and was the most commonly identified pathogen in the first 2004 epidemic. In addition, the absolute number of patients presenting per day with rotavirus infection during two of three examined flood periods increased compared with seasonally matched non-flood periods.
Although evaluation for ETEC was not part of the routine surveillance system, it was part of the surveillance system from 1997 to 1999. During the 1998 flood, ETEC was the fourth most commonly identified pathogen in diarrheal stools (after V. cholerae, rotavirus, and Campylobacter) and was proportionally decreased compared with seasonally matched periods (18% versus 9%, non-flood versus flood; P ≤ 0.001). In contrast, during the first 2004 epidemic, Qadri and others9 found a significant contribution of ETEC to overall cases (18% compared with 22% for cholera cases). No seasonally matched control data were available for this period.
Although our data clearly support the predominance of V. cholerae as the cause of flood-associated diarrheal epidemics and suggest contributions of other causes of secretory diarrhea such as rotavirus and possibly ETEC, it is important to realize that there was also an increase in the absolute number of patients per day presenting with diarrhea caused by Shigella species, Salmonella species, C. jejuni, G. lamblia, and E. histolytica during some flood-associated epidemics. Taken cumulatively, this increase suggests a generalized increase in fecal-oral transmission in post-flood epidemics. In addition, many of these latter pathogens require treatment other than rehydration alone, and preparation for future flood-related epidemics should include maintaining access to appropriate antimicrobial agents for a variety of pathogens.
Regardless of the infecting pathogen, patients presenting to the ICDDR,B during flood-associated epidemics were more severely dehydrated than those presenting during non-flood periods. This may be caused by an increased in the number of cases of secretory diarrhea or to the inherent difficulty of seeking medical attention during a flood situation. Patients of lower socioeconomic status were also more likely to present for medical attention during flood-related epidemics, suggesting that such individuals may be inordinately affected by floods because of inferior sanitation, living in flood-prone areas, or having less access to home treatments such as ORS. Despite this, there was no significant increase in the in-hospital case-fatality rates during flood-associated epidemics.
In conclusion, we observed that V. cholerae played a primary role in four distinct flood-related diarrheal epidemics in Dhaka. Other pathogens that cause secretory diarrhea, particularly ETEC and rotavirus, also contributed significantly to flood-related epidemics. Despite a proportional decrease in the cases of rotavirus-associated diarrhea, cholera and rotavirus together accounted for the majority of cases of diarrhea among individuals presenting to the ICDDR,B during all flood periods examined. There was also more limited data to suggest that ETEC and other pathogens contribute significantly to flood-related epidemics. Our results may be helpful to public health officials to prepare for future flood-related epidemics in Bangladesh. Possible preparations may include stocking adequate supplies of ORS, intravenous fluids, anti-biotics, and, perhaps, targeted use of cholera vaccines to prevent or interrupt transmission of V. cholerae.24,25
Footnotes
Financial support: This work was supported by funding support from the ICDDR,B: Centre for Health and Population Research and NIH Grants K01-TW07144 (R.C.L.), U01-AI58935 (S.B.C.), and AI40725 (E.T.R.). J. H. is an NICHD fellow of the Pediatric Scientist Development Program (NICHD Grant K12-HD00850).
Authors’ addresses: Brian Schwartz, UCSF-Mt. Zion Hospital, Box 1945, Room 532W, 1600 Divisadero Street, San Francisco, CA 94115, E-mail: bschwartz@medicine.ucsf.edu. Jason Harris, Regina LaRocque, Stephen Calderwood, and Edward Ryan, Division of Infectious Diseases, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, E-mail: jbharris@partner.org, rclarocque@partners.org, scalderwood@partner.org, etryan@partners.org. Ashraful Khan, David Sack, Mohammad Malek, Abu Faruque, Firdausi Qadri, and Stephen Luby, International Centre for Diarrhoeal Disease Research (ICDDRB), GPO Box 128, Mohakhali, Dhaka 1000, Bangladesh, E-mail: ashrafk@icddrb.org; dsack@icddrb.org, malekd05@yahoo.com, gfaruque@icddrb.org, sluby@icddrb.org.
REFERENCES
- 1.Rashid SF. The urban poor in Dhaka City: their struggles and coping strategies during the floods of 1998. Disasters. 2000;24:240–253. doi: 10.1111/1467-7717.00145. [DOI] [PubMed] [Google Scholar]
- 2.Kondo H, Seo N, Yasuda T, Hasizume M, Koido Y, Ninomiya N, Yamamoto Y. Post-flood epidemics of infectious disease in Mozambique. Prehosp Disast Med. 2002;17:126–133. doi: 10.1017/s1049023x00000340. [DOI] [PubMed] [Google Scholar]
- 3.Aghababian RV, Teuscher J. Infectious disease following major disasters. Ann Emerg Med. 1992;21:362–367. doi: 10.1016/s0196-0644(05)82651-4. [DOI] [PubMed] [Google Scholar]
- 4.Kunii O, Nakamura S, Abdur R, Wakai S. The impact on health and risk factors of the diarrhoea epidemics in the 1998 Bangladesh floods. Public Health. 2002;116:68–74. doi: 10.1038/sj.ph.1900828. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Cholera. Wkly Epidemiol Rec. 2003;79:281–288. [PubMed] [Google Scholar]
- 6.Siddique AK, Baqui AH, Eusof A, Zaman K. 1998 Floods in Bangladesh: pattern of illness and causes of death. J Diarrhoeal Dis Res. 1991;9:310–314. [PubMed] [Google Scholar]
- 7.Siddique AK, Islam Q, Akram K, Mazumder Y, Mitra A, Eusof A. Cholera epidemic and natural disasters; where is the link. Trop Geogr Med. 1989;41:377–382. [PubMed] [Google Scholar]
- 8.Tanabe K, Nakamura S, Kunii O. Bacteriological survey of diarrheal epidemics in the 1998 Bangladesh floods. J Jap Assoc Infect Dis. 1999;73:918–922. doi: 10.11150/kansenshogakuzasshi1970.73.918. [DOI] [PubMed] [Google Scholar]
- 9.Qadri F, Khan AI, Faruque ASG, Begum Y, Chowdury F, Nair GB, Salam MA, Sack DA, Svennerholm A. Enterotoxigenic Escherichia coli together with V. cholerae O1 was a major cause of acute watery diarrhea during the epidemic caused by flooding in Dhaka, Bangladesh during the summer of 2004. Emerg Infect Dis. 2005;11:1104–1107. doi: 10.3201/eid1107.041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat World population prospects: the 2004 revision and world urbanization prospects: the 2003 revision. 2004 Available at http://esa.un.org/unpp. Last accessed June 1, 2005.
- 11.Stoll B, Glass R, Huq MI, Khan MU, Holt J, Banu H. Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. BMJ. 1982;285:1185–1188. doi: 10.1136/bmj.285.6349.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unicomb LE, Kilgore PE, Faruque SG, Hamadani JD, Fuchs GJ, Albert MJ, Glass RI. Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatr Infect Dis J. 1997;16:947–951. doi: 10.1097/00006454-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Flood Forecasting Watch Center Bangladesh Water levels and hydrographs for 1988, 1998. 2004 Available at www.ffwc.net. Last accessed June 1, 2005.
- 14.Sack RB, Siddique AK, Longini IM, Jr, Nizam A, Yunus M, Islam MS, Morris JG, Jr, Ali A, Huq A, Nair GB, Qadri F, Faruque SM, Sack DA, Colwell RR. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J Infect Dis. 2003;187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- 15.Koelle K, Rodo X, Pascal M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 16.Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A, Morris JG, Jr, Khan MN, Siddique AK, Yunus M, Albert MJ, Sack DA, Colwell RR. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faruque SM, Islam MJ, Ahmad QS, Faruque AS, Sack DA, Nair GB, Mekalanos JJ. Self-limiting nature of seasonal cholera epidemics: Role of host-mediated amplification of phage. Proc Natl Acad Sci U S A. 2005;102:6119–6124. doi: 10.1073/pnas.0502069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faruque SM, Naser IB, Islam MJ, Faruque AS, Ghosh AN, Nair GB, Sack DA, Mekalanos JJ. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci U S A. 2005;102:1702–1707. doi: 10.1073/pnas.0408992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Public Health Impact of Rwandan Refugee Crisis What happened in Goma, Zaire, in July, 1994? Goma Epidemiology Group. Lancet. 1995;345:339–344. [PubMed] [Google Scholar]
- 20.Siddique AK, Salam A, Islam MS, Akram K, Majumdar RN, Zaman K, Fronczak N, Laston S. Why treatment centres failed to prevent cholera deaths among Rwandan refugees in Goma, Zaire. Lancet. 1995;345:359–361. doi: 10.1016/s0140-6736(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 21.Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. Hostinduced epidemic spread of the cholera bacterium. Nature. 2002;417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam A, LaRocque RC, Harris JB, Vanderspurt C, Ryan ET, Qadri F, Calderwood SB. Hyperinfectivity of human-passaged Vibrio cholerae can be modeled by growth in the infant mouse. Infect Immunol. 2005;73:6674–6679. doi: 10.1128/IAI.73.10.6674-6679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartley DM, Morris JG, Smith DL. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006;3:63–68. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calain P, Chaine JP, Johnson E, Hawley ML, O’Leary MJ, Oshitani H, Chaignat CL. Can oral cholera vaccination play a role in controlling a cholera outbreak? Vaccine. 2004;22:2444–2451. doi: 10.1016/j.vaccine.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 25.Lucas M, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, Ali M, Ansaruzzman M, Amos J, Macuamule A, Cavailler P, Guerin PJ, Mahoudeau C, Kahozi-Sangwa P, Chaignat CL, Barreto A, Songane FF, Clemens JD. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005;352:757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]