Abstract
The struggle among social classes or castes is well known in humans. Here, we show that caste inequality similarly affects societies of ants, bees and wasps, where castes are morphologically distinct and workers have greatly reduced reproductive potential compared with queens. In social insects, an individual normally has no control over its own fate, whether queen or worker, as this is socially determined during rearing. Here, for the first time, we quantify a strategy for overcoming social control. In the stingless bee Schwarziana quadripunctata, some individuals reared in worker cells avoid a worker fate by developing into fully functional dwarf queens.
Keywords: caste conflict, social insects, stingless bees, Schwarziana quadripunctata
1. Introduction
The caste system of social insects is both a defining feature of eusociality (reproductive division of labour) and a major evolutionary puzzle: why do most individuals develop as workers rather than queens, altruistically reducing their opportunity for direct reproduction? A partial solution is kinship (Hamilton 1964; Bourke & Franks 1995). By rearing relatives, workers indirectly propagate copies of their own genes. However, recent research (Wenseleers et al. 2003, 2004b,c; Wenseleers & Ratnieks 2004) suggests that the intermediate levels of relatedness found in insect societies are insufficient to explain the extreme altruism observed. Instead, a worker's fate frequently appears enforced (Bourke & Ratnieks 1999; Wenseleers et al. 2003; Wenseleers & Ratnieks 2004). In the advanced eusocial Hymenoptera, queens are generally much larger than workers and caste is normally determined by nutrition (Wilson 1971; Michener 1974; Wheeler 1986; Bourke & Franks 1995). Hence, the adult workers can force immature females to develop as workers simply by limiting their food intake (Bourke & Ratnieks 1999; Beekman & Ratnieks 2003; Wenseleers et al. 2003, 2004b; Wenseleers & Ratnieks 2004; Ratnieks & Wenseleers 2005).
Although the theory is clear, how can one prove that larvae are forced to develop as workers against their own interests? One prediction is that strategies for evading social control should occur (Bourke & Ratnieks 1999). For example, females could develop as ‘dwarf’ queens, even when fed on an amount of food normally only sufficient to become a worker (Bourke & Ratnieks 1999). Dwarf queens have been reported in some ants and stingless bees (Engels & Imperatriz-Fonseca 1990; Imperatriz-Fonseca & Zucchi 1995; Heinze 1998; Rüppell et al. 2001). However, in none of these cases has it been tested whether dwarf queens are produced as a consequence of caste conflict. Here, we present such a test. In the stingless bee Schwarziana quadripunctata, normal queens are reared from larger, specialized queen cells constructed near the comb periphery, but females in worker cells also sometimes develop into dwarf queens (Camargo 1974; Ribeiro & Alves 2001; figure 1). We show that the dwarf queens and workers have virtually identical weights, implying that individual females can develop into queens despite lower worker levels of nutrition. Furthermore, we show that dwarf queens frequently head colonies and so are reproductively competent. These results strongly support the existence of underlying conflict over caste fate in insect societies (Bourke & Ratnieks 1999; Wenseleers et al. 2003) and support the prediction that evading a low-status worker fate can have significant direct fitness benefits (Bourke & Ratnieks 1999; Wenseleers et al. 2004b).
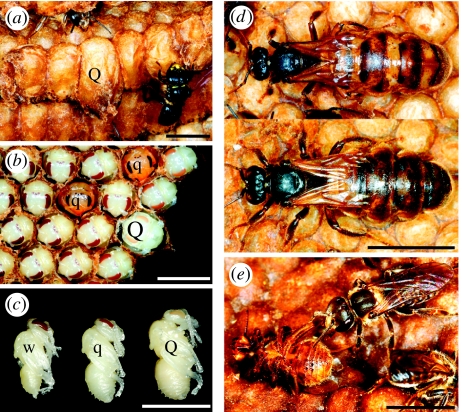
Figure 1.
Evasion of socially controlled caste development in the stingless bee, S. quadripunctata. (a) Queens are normally reared from larger, specialized queen cells (Q) that are provisioned with a greater quantity of food. The smaller cells in the comb are used to rear workers and males. (b) Here, the comb in (a) has been uncapped to reveal a queen pupa in the queen cell, and worker pupae in most of the smaller cells. However, two of these contain dwarf queens (q) which can be recognized by their smaller heads and eyes and their lack of pollen baskets. (c) The dwarf queens (q) are approximately the same size as workers (w), but are much smaller than normal queens (Q). This shows that the dwarf queens successfully evade an intended worker fate. (d) Like normal queens (bottom), the dwarf queens (top) can successfully head colonies. (e) Workers often discriminate against the dwarf queens. Here, a worker (right) kills a dwarf queen (left) that has recently emerged from her cell. (Scale bars, 5 mm.)
2. Methods
(a) Biology of Schwarziana quadripunctata
S. quadripunctata is an underground-nesting stingless bee (Apidae: Meliponinae: Trigonini; Camargo 1974). Colonies are perennial, founded by swarms and typically have a single once-mated queen and several thousand workers (Camargo 1974; Tóth et al. 2003). Broods are reared individually in cells that are mass-provisioned with food immediately before the egg is laid and then sealed (figure 1).
(b) Collection and maintenance of colonies
Over a 6-year period (1998–2004), 54 colonies of S. quadripunctata were collected in the states of São Paulo and Paraná in southeast and southern Brazil (Cunha: 23°05′ S 44°55′ W, Cotia: 23°39′ S 46°47′ W, Prudentópolis: 25°13′ S 50°59′ W). Colonies were kept in free-flying observation boxes (Ribeiro & Alves 2001) at the University of São Paulo (23°33′ S 46°43′ W).
(c) Production of normal and dwarf queens
Dwarf queen, normal queen and worker production were measured in 19 colonies from January 2003 to January 2004. This was carried out by collecting 90 combs containing mature pupae, removing the cell cappings and counting the workers, dwarf queens, males (all reared in small cells) and normal queens (reared in special larger cells) present in each comb (figure 1). In total, 14 216 sealed cells were inspected, out of which 11 574 (81.4%) contained queen or worker broods.
(d) Testing self-determination of caste fate
To test whether females in worker cells can develop into dwarf queens, independent of the amount of provisions, we compared the dry weights of dwarf queen, worker and normal queen pupae. If caste were self-determined, we predicted no difference in the weight of dwarf queens and workers. The remainder of the brood were returned to the colony. The positions of cells containing dwarf queens were mapped onto hexagonal grids, and the distance to the comb edge divided by comb diameter, in number of cells, was calculated. This allowed us to test whether cells containing dwarf queens occurred in random positions.
(e) Fitness benefits of becoming a dwarf queen
To determine the fitness benefits of becoming a dwarf queen, we estimated the proportion of colonies that were headed by dwarf versus normal queens and the fecundity of dwarf and normal queens, as measured by their ovary weight. To determine the proportion of colonies headed by dwarf queens, we measured the head width (HW) and intertegular distance (ITD) of the queens heading each of the 54 colonies (Ribeiro & Alves 2001). Discriminant analysis of the dimensions of dwarf and normal queen pupae allowed us to classify each mother queen unambiguously as either a dwarf or a normal queen (queens could be classified with an average confidence of 99%). The ovary weights of dwarf and normal queens were estimated as their fresh weight minus the baseline (fresh) weight of dwarf and normal queen pupae: 25.86 (n = 42) and 49.34 mg (n = 9), respectively.
3. Results
(a) Rate of production of normal and dwarf queens
Out of 11 574 female brood examined from 90 combs and 19 colonies, 11 495 were workers, 11 normal-sized queens and 68 dwarf queens. Normal-sized queens were always reared from queen cells constructed near the comb periphery, while dwarf queens were all reared in worker cells (figure 1). Queen cells never gave rise to workers. Even though only 0.6% (68/11 563) of females reared in worker cells were dwarf queens, this represented the majority, 86%, of all queens reared. Dwarf queens were found in most (15 out of 19) colonies. Normal-sized queens were found in nine colonies, and each of these also produced dwarf queens. That no dwarf or normal queens were found in some colonies is probably owing to sampling error.
(b) Evidence for self-determination of caste fate
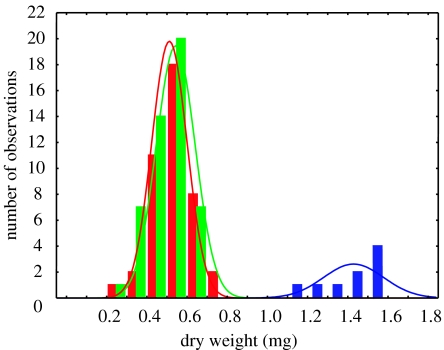
Dwarf queens had a dry weight (, s.d. = 0.96, n = 42) not significantly (t =1.62, p =0.11) different from that of workers (, s.d. = 0.87, n = 49), whereas normal queen pupae were more than twice as heavy (, s.d. = 1.49, n = 9, t = 22.47, p < 1.10−26; figure 2). This demonstrates that immature Schwarziana females can develop into queens independent of the amount of provisions in their cell. The locations of the dwarf queens in the combs were not significantly different from a simulated, uniform random distribution (, p = 0.99), and the mean proportional distance to the comb edge, 0.51, was not significantly different from the expected mean, 0.5, if all cells were equally likely to yield dwarf queens (one-sample t-test: t = 0.13, p = 0.90).
Figure 2.
Dry weights of worker (red), dwarf queen (green) and normal queen (blue) pupae.
(c) Fitness benefits of becoming a dwarf queen
Discriminant analysis of queen pupae showed that dwarf queens had a HW (in mm) < 4.33 − 1.09 × ITD (in mm). Using this formula, dwarf queens were heading 12 (22%) out of the 54 colonies inspected (figure 1). Hence, dwarf queens gain significant fitness benefits. However, the proportion of dwarf queens heading colonies (22%) was significantly lower than the proportion reared (86%, Fisher's exact test, p<1.10−13). Dwarf queens also had a lower fresh weight (, s.d. = 16.75, n = 12) than normal queens (, s.d. =17.06, n = 42, t = −9.91, p<1.10−12) and a reduced fecundity, since the ovary weight of dwarf queens (, s.d. =16.75, n = 12) was lower than that of normal queens (, s.d.=17.06, n = 42, t = −5.69, p < 1.10−6).
4. Discussion
All results support the hypothesis that dwarf queens in S. quadripunctata are produced as part of an adaptive strategy for evading a worker fate (Bourke & Ratnieks 1999). Most surprising was the rate at which this occurred: 86% of all the queens reared were dwarf queens. That dwarf queens were reared in excess of normal queens is as predicted by evolutionary theory, which shows that individual females should develop into queens at a rate greater than that favoured by the colony as a whole (Bourke & Ratnieks 1999; Ratnieks 2001; Wenseleers et al. 2003, 2004b; Wenseleers & Ratnieks 2004). In fact, stingless bees require new queens to be produced only occasionally, to replace a failing mother queen or to head a daughter swarm (Engels & Imperatriz-Fonseca 1990). Hence, any overproduction of dwarf queens is expected to come at a cost to the colony as a whole (Wenseleers et al. 2003, 2004b). Excess queen production owing to individual interests needs self-determination so that females in worker cells can become either a queen or a worker. Self-determination and choice are supported by the nearly identical weights of dwarf queens and workers (figure 2). Dwarf queens were also produced from random locations inside the comb. This indicates that dwarf queens are not reared in some special type of cell in a particular region of the comb, as is the case for normal queens.
Our data also reject other alternative hypotheses. For example, the non-overlap of the weights of dwarf and normal queen (figure 2) means that dwarf queens are not produced from worker cells that are accidentally overprovisioned (Camargo 1974; Nogueira-Ferreira et al. 2000; Ribeiro et al. 2003). Most importantly, our data show that dwarf queens are not developmental mistakes (Engels & Imperatriz-Fonseca 1990), as they were reproductively functional. On the other hand, the proportion of dwarf queens heading colonies (22%) was significantly lower than the rate at which they were reared (86%). This may be a result of workers discriminating against dwarf queens. In fact, Imperatriz-Fonseca (1990) reported that whereas large queens are usually confined in special ‘queen prison’ cells for later use, dwarf queens are frequently eliminated by the workers (figure 1). Judging from their lower ovary weight, dwarf queens were also less fecund than were normal queens. Hence, dwarf queens do not have the full reproductive potential of normal queens. Theory predicts that such costs should reduce the proportion of females that become queens (Wenseleers et al. 2004b), thereby explaining why only 0.6% of all females in worker cells became dwarf queens (Wenseleers et al. 2004b). In this respect, S. quadripunctata contrast with Melipona stingless bees. Melipona do not rear queens in specialized queen cells. Instead, all queens and workers (including males) develop in identical cells (Engels & Imperatriz-Fonseca 1990). Consequently, in Melipona, queens reared in worker cells do not compete with larger queens reared in special cells. In line with theoretical expectation (Wenseleers et al. 2003, 2004b; Wenseleers & Ratnieks 2004), a larger proportion (5–14%) of Melipona females develop into queens, and as in Schwarziana, the excess is killed (Wenseleers et al. 2004a). Another difference is that although the females that develop as queens are being selfish in Melipona, they do not really have to ‘beat’ the caste system because social food control, resulting in different-sized queens and workers, is absent. It is possible, however, that similar cheating strategies occur in other species, as small queens also occur in some ants and some other trigonini stingless bees (Bourke & Ratnieks 1999; Wenseleers et al. 2003). In ants, however, the small ‘inter-caste’ queens that occur in some species are significantly larger than workers and their production is probably part of a dispersal polymorphism (Heinze 1998; Rüppell et al. 2001). In Schwarziana, at least, this cannot be the case, since all colonies are swarm-founded (Engels & Imperatriz-Fonseca 1990).
By showing that the dwarf queens gain significant fitness benefits, our study is the first to conclusively demonstrate that social insects can evade a socially imposed worker-caste fate. As individualistic humans, we may well applaud the high status that these erstwhile lower class individuals achieve. Indeed, equal opportunity is a goal towards which all modern, democratic societies strive. However, insect societies are intrinsically unequal, and a move towards greater opportunity, as occurs in S. quadripunctata, requires active strategies for evading social control.
Footnotes
Present address: Wissenschaftskolleg zu Berlin (Institute for Advanced Study), Wallotstrasse 19, D-14193 Berlin, Germany.
References
- Beekman M, Ratnieks F.L.W. Power over reproduction in social Hymenoptera. Phil. Trans. R. Soc. B. 2003;358:1741–1753. doi: 10.1098/rstb.2002.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke A.F.G, Franks N.R. Princeton University Press; Princeton, NJ: 1995. Social evolution in ants. [Google Scholar]
- Bourke A.F.G, Ratnieks F.L.W. Kin conflict over caste determination in social Hymenoptera. Behav. Ecol. Sociobiol. 1999;46:287–297. [Google Scholar]
- Camargo J.M.F. Notas sobre a morfologia e biologia de Plebeia (Schwarziana) quadripunctata quadripunctata (Hymenoptera, Apidae, Meliponinae) Studia Entomologica. 1974;17:433–470. [Google Scholar]
- Engels W, Imperatriz-Fonseca V.L. Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees. In: Engels W, editor. Social insects. An evolutionary approach to castes and reproduction. Springer; Berlin, Heidelberg: 1990. pp. 167–230. [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. I & II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Heinze J. Intercastes, intermorphs, and ergatoid queens: who is who in ant reproduction? Insectes Soc. 1998;45:113–124. [Google Scholar]
- Imperatriz-Fonseca V.L. Swarming activity in Schwarziana quadripunctata (Apidae, Meliponinae) In: Veeresh G.K, Mallik B, Viraktamath C.A, editors. Social insects and the environment. IBH Publishing Co.; Oxford and Bombay, India: 1990. pp. 744–745. [Google Scholar]
- Imperatriz-Fonseca V.L, Zucchi R. Virgin queens in stingless bee (Apidae, Meliponinae) colonies: a review. Apidologie. 1995;26:231–244. [Google Scholar]
- Michener C.D. Harvard University Press; Cambridge, MA: 1974. The social behavior of the bees. [Google Scholar]
- Nogueira-Ferreira F.H, Baio M.V, Noll F.B, Tidon-Sklorz R, Zucchi R. Morphometric study in Schwarziana quadripunctata with emphasis on virgin queens (Hymenoptera, Apidae, Meliponinae) Sociobiology. 2000;35:99–108. [Google Scholar]
- Ratnieks F.L.W. Heirs and spares: caste conflict and excess queen production in Melipona bees. Behav. Ecol. Sociobiol. 2001;50:467–473. [Google Scholar]
- Ratnieks F.L.W, Wenseleers T. Policing insect societies. Science. 2005;307:54–56. doi: 10.1126/science.1106934. [DOI] [PubMed] [Google Scholar]
- Ribeiro M.F, Alves D.A. Size variation in Schwarziana quadripunctata queens (Hymenoptera, Apidae, Meliponini) Rev. Etol. 2001;3:59–65. [Google Scholar]
- Ribeiro M.d.F, Imperatriz-Fonseca V.L, Filho P.d.S.S. Exceptional high queen production in the brazilian stingless bee Plebeia remota. Stud. Neotrop. Fauna Environ. 2003;38:111–114. [Google Scholar]
- Rüppell O, Heinze J, Hölldobler B. Alternative reproductive tactics in the queen-size-dimorphic ant Leptothorax rugatulus (Emery) and their consequences for genetic population structure. Behav. Ecol. Sociobiol. 2001;50:189–197. [Google Scholar]
- Tóth E, Strassmann J.E, Imperatriz-Fonseca V.L, Queller D.C. Queens, not workers, produce the males in the stingless bee Schwarziana quadripunctata. Anim. Behav. 2003;66:359–368. [Google Scholar]
- Wenseleers T, Ratnieks F.L.W. Tragedy of the commons in Melipona bees. Proc. R. Soc. B. 2004;271(Suppl. 5):S310–S312. doi: 10.1098/rsbl.2003.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenseleers T, Ratnieks F.L.W, Billen J. Caste fate conflict in swarm-founding social Hymenoptera: an inclusive fitness analysis. J. Evol. Biol. 2003;16:647–658. doi: 10.1046/j.1420-9101.2003.00574.x. [DOI] [PubMed] [Google Scholar]
- Wenseleers T, Hart A.G, Ratnieks F.L.W, Quezada-Euán J.J.G. Queen execution and caste conflict in the stingless bee Melipona beecheii. Ethology. 2004;110:725–736. [Google Scholar]
- Wenseleers T, Hart A.G, Ratnieks F.L.W. When resistance is useless: policing and the evolution of reproductive acquiescence in insect societies. Am. Nat. 2004b;164:E154–E167. doi: 10.1086/425223. [DOI] [PubMed] [Google Scholar]
- Wenseleers T, Helanterä H, Hart A.G, Ratnieks F.L.W. Worker reproduction and policing in insect societies: an ESS analysis. J. Evol. Biol. 2004;17:1035–1047. doi: 10.1111/j.1420-9101.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- Wheeler D.E. Developmental and physiological determinants of caste in social hymenoptera: evolutionary implications. Am. Nat. 1986;128:13–34. [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]