Abstract
Fever, like other mechanisms for defence against pathogens, may have positive and negative consequences for host fitness. In ectotherms, fever can be attained through modified behavioural thermoregulation. Here we examine potential costs of behavioural fever by holding adult, gregarious desert locusts at elevated temperatures simulating a range of fever intensities. We found no effect of fever temperatures on primary fitness correlates of survival and fecundity. However, flight capacity and mate competition were reduced, although there was no relation between time spent at fever temperatures and magnitude of the response. While these effects could indicate a direct cost of fever, they are also consistent with a shift towards the solitaria phase state that, in a field context, could be considered an adaptive life-history response to limit the impact of disease. These conflicting interpretations highlight the importance of considering complex defence mechanisms and trade-offs in an appropriate ecological context.
Keywords: behavioural fever, costs of resistance, host–pathogen interactions, locust phase change, entomopathogens
1. Introduction
The insect immune system has recently received much attention from evolutionary ecologists as a means of testing hypotheses relating to the costs of defence against pathogens (Moret 2003; Schmid-Hempel 2003; Schmid-Hempel & Ebert 2003). One component of the immune system that has rarely been considered in this light is fever, although it is a common defence response with apparently well-conserved physiological mechanisms in a diversity of invertebrate and vertebrate taxa (Kozak et al. 2000; Bundey et al. 2003). An increase in an animal's body temperature above the normal set point would be likely to carry costs (Kluger et al. 1998). Indeed, fever can be lethal (notably in mammals), which has led to debate as to whether fever should, in fact, be regarded as an adaptive defence mechanism at all (Banet 1986; Blatteis 1986; Kluger et al. 1998).
In many ectotherms, thermoregulation is achieved by behavioural adjustment in the local environment. When infected, ectotherms may alter thermoregulatory behaviour to shift their normally preferred body temperature to a higher set point in what is termed ‘behavioural fever’ (Kluger et al. 1975; Covert & Reynolds 1977; Watson et al. 1993). Using desert locusts (Schistocerca gregaria (Forskål)) and the fungal pathogen Metarhizium anisopliae var. acridum, we have demonstrated that behavioural fever can be an essential defence response to combat disease (Elliot et al. 2002). In addition, however, we have recently demonstrated that following exposure to fever temperatures, gregaria phase S. gregaria adults produce more solitaria phase state offspring (versus gregaria) than do parents that are not subjected to fever regimes (Elliot et al. 2003). It remains unclear whether this (trans-generational) change in life history can be defined as a cost and what the mechanisms are.
Here, we further explore the effects of fever by manipulating locust body temperatures to investigate the effects of a range of simulated fever conditions on both gross fitness measures (survival and fecundity) and more subtle measures (mate guarding and flight capacity). We focused on uninfected locusts so as to discriminate effects of elevated temperatures from effects of disease. Our hypothesis was that if fever temperatures are costly, then we should see effects on some aspect of fitness, and that these costs should vary depending on the pattern and intensity of exposure to fever temperatures.
2. Materials and methods
(a) Temperature treatments
Populations of S. gregaria were maintained in standard aluminium locust cages, with 40 W light bulbs to allow thermoregulation. After final moult, 0–3-day-old adults were taken from these stock cages and placed in ventilated plastic cages (22×15.5×11.5 cm3), with seven females and five males per cage. There were five blocks each with seven such cages. Blocks were staggered over approximately 5 days so that all locusts were placed in the treatments at a similar physiological age.
The plastic cages were maintained in a climate room on a 9L : 15D cycle. Temperature was set at 20±1 °C in the dark phase (Elliot et al. 2002). The daytime temperature was set at 40 °C, which created a natural gradient within the climate room of approximately 37–44 °C. The room was ‘mapped’ using a digital thermometer to identify 35 positions providing normal thermoregulatory temperatures (37.4–38.9 °C) and 30 positions at elevated fever temperatures (42.0–43.8 °C). Locust cages were placed at normal thermoregulatory temperatures and then a range of fever responses were simulated by moving cages to ‘fever’ positions for either 1 or 5 h per day (in the middle of the light phase) during days 1–10, 11–20 or 1–20 of the adult locust maturation phase, giving six fever treatments. Cages were assigned randomly among the 30 fever positions. Control cages remained at normal thermoregulatory temperature but they were moved randomly among the 35 suitable positions.
(b) Fitness correlates
Following the first phase of the experiment, females from the different treatments were returned to standard locust cages with 10 stock males added to each cage. Food was made available for only 1 in 3 days to induce a degree of stress.
Female deaths were recorded each day and analysed using Kaplan–Meier survival analysis (Spss for Windows v. 6.1). Fecundity was measured by collecting eggs from oviposition cups and monitoring hatching. Hatchling counts were analysed with a Kruskal–Wallis test because irresolvable heteroscedasticity did not permit parametric statistics.
Treatment males were retained in the plastic cages and were maintained in their replicate groups at a normal rearing temperature (30±1 °C) with food provided every third day. Once the females had been removed, five female and five male stock locusts were added to each cage, with treated and stock males differentially marked with Tipp-Ex. Thrice daily, observations were made of how many of each type of male were successfully guarding a female (i.e. sitting on her back), giving paired observations of mate guarding by treated versus stock males. These observations were made for 14 days and were then pooled for sign tests (n=201).
Between 16 and 24 days post-treatment, the ability of the treated male locusts to sustain flight was assessed in a wind tunnel (Seyoum et al. 1994). The locusts were flown in batches of seven (one individual from each treatment), and this was repeated four times using previously untested insects for each replicate cage. The locusts were attached to metal rods using a loop of nylon tied just behind the front legs and over the pronotum. The wind speed was set to 3.9±0.1 m s−1 (Seyoum et al. 1994) and each test run lasted 3.5 h. Every 30 min, locusts were observed for five 1 min intervals to record whether they were flying or not, giving a total of 30 counts for each locust. These data were analysed using linear mixed-effects models in R v. 1.5 (Crawley 2002; p. 659), with time as the primary covariate and block as the grouping factor.
Following the flight experiment, males were kept separately in plastic pots, and at 27 days post-treatment, provision of food was stopped. Mortality was recorded daily and analysed as above.
3. Results
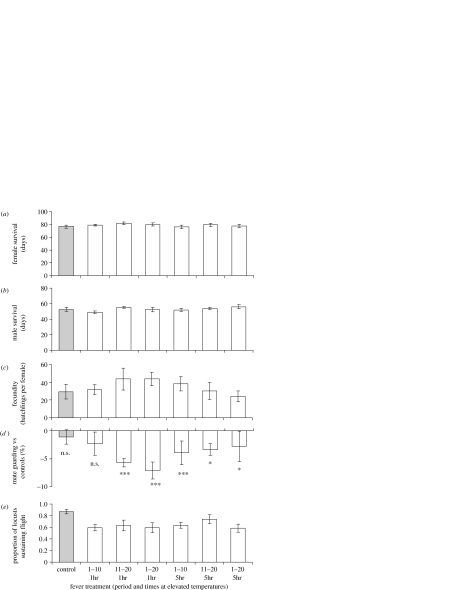
There were no significant differences in mean survival times of either male or female locusts between treatments (Kaplan–Meier survival analyses with log-rank statistics accounting for block 0.00–2.44, p>0.05 (range 0.118–0.955), figure 1a,b). Similarly, there was no effect of treatment on fecundity (Kruskal–Wallis p=0.807, d.f.=6; figure 1c). However, repeated observations of which males were mounted on females revealed an overall tendency for the males from the fever regimes to be less competitive than their untreated counterparts (sign tests, p<0.05, figure 1d). In addition, observations of the ability of males to fly in a wind tunnel showed that control males were 15–25% better than those subjected to fever temperatures in sustaining flight (linear mixed-effects model, p<0.001, F1,8=15.02, figure 1e). However, as with mating competitiveness, there were no differences between fever treatments, indicating no obvious effect of intensity of fever on fitness (linear mixed-effects models, deletion of second 10 days as a factor: p=0.719, F1,14=0.129; deletion of first 10 days as factor: p=0.167, F1,11=1.910; deletion of intensity: p=0.635, F1,8=0.225).
Figure 1.
Fitness correlates (means±s.e.m.) measured from adult desert locusts subjected to simulated fever regimes during sexual maturation. Locusts were kept at 38 °C for 7 h during the day; within this period, treated locusts were elevated to 42 °C for 1 or 5 h per day, on days 1–10, 11–20 or 1–20 of the maturation period. (a) and (b) show average survival times for females and males, respectively; (c) female fecundity; (d) male mating competitiveness (mate guarding against untreated males; sign tests, *p<0.05, ***p<0.001, n.s.=not significant); (e) male ability to sustain flight in a wind tunnel over 3.5 h (control versus all other treatments significantly different (p<0.001), all other comparisons p>0.05).
4. Discussion
Our results revealed no impacts of fever temperatures on survival and fecundity but significant impacts on flight and mating behaviour (figure 1). Interestingly, the effects were detectable with a total of only 10 h of elevated temperature over 10 days and were insensitive to changes in the pattern or intensity of fever.
The increase in metabolic rate and consequent energetic costs associated with fever temperatures can be substantial (e.g. Muchlinski 1985; Sherman & Stephens 1998; Elia 1992). In addition, a common response to heat stress is the production of protective molecular chaperones such as heat shock proteins (Hsps; Hasday & Singh 2002) or trehalose (Singer & Lindquist 1998). Production and breakdown of these chaperones would have an associated cost. Moreover, acclimation to higher temperatures can lead to higher standing titres of Hsps (Buckley & Hofmann 2002), which might explain the one-off threshold effect we observe, rather than any increase in side effects as intensity of fever increases (although this is not the only explanation as studies using other immune stimulation techniques have also reported insensitivity in the response of life-history traits to ‘dose’; Ahmed et al. 2002). Furthermore, fever in locusts has been shown to increase mobilization of haemocytes and anti-pathogen metabolites (Ouedraogo et al. 2003). Thus, we can identify possible physiological or biochemical responses to elevated temperature that might be expressed as costs. However, why these should trade-off against subtle traits such as mating behaviour rather than primary fitness correlates such as fecundity is unclear.
We suggest an alternative (although not necessarily mutually exclusive) hypothesis based on effects of temperature on locust phase state that is, we believe, more compelling. Several studies implicate adipokinetic hormone (AKH) in locust flight performance since it modulates the transport of lipids to the flight muscles. Ayali & Pener (1995) showed that isolation of male locusts from a gregarious stock alters their ability to mobilize lipid reserves for flight (whereas solitary S. gregaria have poorer flight performance than gregarious), which correlated with the strength of the adipokinetic response (Schneider & Dorn 1994). Additionally, recent studies on courtship in S. gregaria have shown the pheromone phenylacetonitrile (PAN) to play a role in modulating the interactions between males. In gregarious locusts, PAN acts to conceal males already mounted on a female and, in so doing, reduces sperm competition and same-sex encounters. Solitary males do not produce PAN and tests indicate that much greater competitive interactions occur where solitary and gregarious males are attempting to copulate or guard a female than when two gregarious males are involved (Seidelmann & Ferenz 2002). These two phase-dependent differences in behaviour are similar to the responses we observe in locusts exposed to fever temperatures. Together with the results of our earlier studies (Elliot et al. 2003), this suggests that regular exposure to fever temperatures can cause a reversion towards the solitaria base state, even where gregarising stimuli from conspecifics are present.
The suggestion that fever affects locust phase state is itself an interesting finding. However, it also complicates the issue of whether the changes in life-history traits we observe represent costs of fever or not. On the one hand, poorer mate guarding and flight potential could represent significant ecological costs, perhaps representing an underlying trade-off between reproduction and immune defence. In this regard, our results add to a growing body of literature indicating that consequences of defence may be expressed through a variety of fitness measures and may be direct, or indirect (Agrawal 1999; Agrawal et al. 2002; Ahmed et al. 2002; Heil 2002; Heil & Baldwin 2002; Karban et al. 1997; Kraaijeveld & Godfray 1997; Moret & Schmid-Hempel 2000; Strauss et al. 2002). On the other hand, given that solitary locusts tend to disaggregate, which will probably reduce density-dependent disease transmission risk and result in oviposition away from gregarious egg beds that could be foci of future infection (Inglis et al. 1998), a shift towards the solitaria phase state may actually represent a facultative change in life history that serves to limit the impact of disease (Moret 2003). Thus, rather than a cost, the life-history changes we observe could be part of an additional defence response triggered by a fever signal. These contrasting hypotheses highlight the need for careful consideration in selecting appropriate life-history traits and interpreting fitness costs given that these depend on both the mechanisms through which responses are mediated, and on the ecological context in which the interactions are played out.
Acknowledgments
The paper forms a part contribution to the EU-funded ESLOCO project and the project entitled ‘Development of biologically based strategies for sustainable control of red locust in Central and Southern Africa’ funded by United Kingdom Department for International Development (DFID). The views expressed are not necessarily those of DFID.
References
- Agrawal A.A. Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology. 1999;80:1713–1723. [Google Scholar]
- Agrawal A.A, Janssen A, Bruin J, Posthumus M.A, Sabelis M.W. An ecological cost of plant defence: attractiveness of bitter cucumber plants to natural enemies of herbivores. Ecol. Lett. 2002;5:377–385. [Google Scholar]
- Ahmed A.M, Baggott S.L, Maingon R, Hurd H. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos. 2002;97:371–377. [Google Scholar]
- Ayali A, Pener M.P. The relations of adipokinetic response and body lipid-content in locusts (Locusta migratoria migratorioides) with special reference to phase polymorphism. J. Insect Physiol. 1995;41:85–89. [Google Scholar]
- Banet M. Fever in mammals: is it beneficial? Yale J. Biol. Med. 1986;59:117–124. [PMC free article] [PubMed] [Google Scholar]
- Blatteis C.M. Fever in mammals: is it beneficial? Yale J. Biol. Med. 1986;59:107–116. [PMC free article] [PubMed] [Google Scholar]
- Buckley B.A, Hofmann G.E. Thermal acclimation changes DNA-binding activity of heat shock factor 1 (HSF1) in the goby Gillichthys mirabilis: implications for plasticity in the heat-shock response in natural populations. J. Exp. Biol. 2002;205:3231–3240. doi: 10.1242/jeb.205.20.3231. [DOI] [PubMed] [Google Scholar]
- Bundey S, Raymond S, Dean P, Roberts S.K, Dillon R.J, Charnley A.K. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Arch. Insect Biochem. 2003;52:183–192. doi: 10.1002/arch.10081. [DOI] [PubMed] [Google Scholar]
- Covert J.B, Reynolds W.W. Survival value of fever in fish. Nature. 1977;267:43–45. doi: 10.1038/267043a0. [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester: 2002. Statistical computing. An introduction to data analysis using S-Plus. [Google Scholar]
- Elia M. Energy expenditure to metabolic rate. In: Kinney J.M, Tucker H.N, editors. Energy metabolism: tissue determinants and cellular corollaries. Raven Press; New York: 1992. pp. 19–59. [Google Scholar]
- Elliot S.L, Blanford S, Thomas M.B. Host–pathogen interactions in a varying environment: temperature, behavioural fever and fitness. Proc. R. Soc. B. 2002;269:1599–1607. doi: 10.1098/rspb.2002.2067. (doi:10.1098/rspb.2002.2067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot S.L, Blanford S, Horton C.M, Thomas M.B. Fever and phenotype: transgenerational effect of disease on desert locust phase state. Ecol. Lett. 2003;6:1–7. [Google Scholar]
- Hasday J.D, Singh I.S. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones. 2002;5:471–480. doi: 10.1379/1466-1268(2000)005<0471:fathsr>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M. Ecological costs of induced resistance. Curr. Opin. Plant Biol. 2002;5:345–350. doi: 10.1016/s1369-5266(02)00267-4. [DOI] [PubMed] [Google Scholar]
- Heil M, Baldwin I.T. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci. 2002;7:61–67. doi: 10.1016/s1360-1385(01)02186-0. [DOI] [PubMed] [Google Scholar]
- Inglis G.D, Johnson D.L, Kawchuk L.M, Goettel M.S. Effect of soil texture and soil sterilization on susceptibility of ovipositing grasshoppers to Beauveria bassiana. J. Invertebr. Pathol. 1998;71:73–81. doi: 10.1006/jipa.1997.4698. [DOI] [PubMed] [Google Scholar]
- Karban R, Agrawal A.A, Mangel M. The benefits of induced defenses against herbivores. Ecology. 1997;78:1351–1355. [Google Scholar]
- Kluger M.J, Ringler D.J, Anver M.R. Role of fever in disease. Science. 1975;188:166–168. [PubMed] [Google Scholar]
- Kluger M.J, Kozak W, Conn C.A, Leon L.R, Soszynski D. Role of fever in disease. Ann. NY Acad. Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- Kozak W, Kluger M.J, Tesfaigsi J, Kozak A, Mayfield K.P, Wachulec M, Dokladny K. Molecular mechanisms of fever and endogenous antipyresis. Ann. NY Acad. Sci. 2000;917:121–134. doi: 10.1111/j.1749-6632.2000.tb05376.x. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- Moret Y. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Oikos. 2003;102:213–216. [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Muchlinski A.E. The energetic cost of the fever response in 3 species of ectothermic vertebrates. Comp. Biochem. Physiol. 1985;81A:577–579. doi: 10.1016/0300-9629(85)91028-x. [DOI] [PubMed] [Google Scholar]
- Ouedraogo R.M, Cusson M, Goettel M.S, Brodeur J. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var. acridum. J. Invertebr. Pathol. 2003;82:103–109. doi: 10.1016/s0022-2011(02)00185-4. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. B. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. (doi:10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. [Google Scholar]
- Schneider M, Dorn Lipid storage and mobilization by flight in relation to phase and age of Schistocerca gregaria females. Insect Biochem. Mol. Biol. 1994;24:883–889. [Google Scholar]
- Seidelmann K, Ferenz H.-J. Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol. 2002;48:991–996. doi: 10.1016/s0022-1910(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Seyoum E, Moore D, Charnley A.K. Reduction in flight activity and food consumption by the desert locust, Schistocerca gregaria, Forskål (Orth., Cyrtacanthacrinae), after infection with Metarhizium flavoviride. J. Appl. Entamol. 1994;118:310–315. [Google Scholar]
- Sherman E, Stephens A. Fever and metabolic rate in the toad Bufo marinus. J. Thermal Biol. 1998;23:49–52. [Google Scholar]
- Singer M.A, Lindquist S. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol. 1998;16:460–468. doi: 10.1016/s0167-7799(98)01251-7. [DOI] [PubMed] [Google Scholar]
- Strauss S.Y, Rudgers J.A, Lau J.A, Irwin R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002;17:278–285. [Google Scholar]
- Watson D.W, Mullens B.A, Petersen J.J. Behavioural fever response of Musca domestica (Diptera: Muscidae) to infection by Entomophthora muscae (Zygomycetes: Entomophthorales) J. Invertebr. Pathol. 1993;61:10–16. [Google Scholar]