Abstract
Several recent experimental studies have provided strong evidence for the ability of birds to manipulate the sex ratio of their offspring prior to laying. Using a captive population of peafowl (Pavo cristatus), we tested experimentally the effects of paternal attractiveness on offspring sex ratio, and related sex ratio deviations to egg-yolk concentrations of testosterone, 17β-estradiol and corticosterone. When females were mated to males whose attractiveness had been experimentally reduced by removing prominent eyespot feathers from their trains, they produced significantly more female offspring, had significantly higher yolk corticosterone concentrations and tended to have lower levels of yolk testosterone than when mated to the same males with their full complement of feathers. Concentrations of 17β-estradiol did not vary consistently with sex ratio biases. These findings add to the small number of studies providing experimental evidence that female birds can control the primary sex ratio of their offspring in response to paternal attractiveness, and highlight the possibility that corticosterone and perhaps testosterone are involved in the sex manipulation process in birds.
Keywords: maternal effect, paternal attractiveness, avian sex ratio, manipulation
1. Introduction
When males and females have an equivalent reproductive value, parents should invest equally in sons and daughters (Fisher 1930). However, in certain circumstances one sex may be more profitable than the other, and by adjusting their sex allocation parents could maximize their fitness (Frank 1990). For example, when the relative fitness returns from sons and daughters vary with some ecological or morphological variable, sex allocation theory would predict that females distribute resources between them in response to that variable (Charnov 1982; Frank 1990). One such cue may be paternal attractiveness, because provided that variance in mating success is greater in males and male sexual attractiveness is heritable, a female mated to an unattractive male will maximize her fitness by investing in daughters (Weatherhead & Robertson 1979).
The peacock's train is a classic example of a male characteristic thought to have evolved by female choice. Previous work has shown the number of eyespots in the train to be an important predictor of male mating success (Petrie et al. 1991), and for an experimental reduction in eyespot number to result in a decline in the number of mates obtained (Petrie & Halliday 1994). Moreover, the degree of train elaboration, which is a heritable trait (M. Petrie, unpublished data), is positively correlated with offspring survival, with a stronger effect in sons than in daughters (Petrie 1994). It thus seems likely that genetic benefits maintain the strong female preference in this species. Therefore, in peafowl all the necessary conditions for an expected effect of paternal attractiveness on offspring sex ratio are met, although establishing the precise mechanism of sex ratio adjustment still presents a major challenge.
2. Material and methods
This experiment was carried out during the summer of 2003 at a peafowl farm in Norfolk, UK. Male and female peafowl, all at least 3 years old and sexually experienced, were selected randomly from the stock population. Each male (n=21) was housed with four females in outdoor wooden pens and supplied with food and water ad libitum. This meant that we knew the paternity, but not maternity of the eggs laid. At the time of capture we measured male and female body weight (to the nearest gram) and the number of eyespot feathers present in each male's train. Because train feathers were occasionally lost naturally, the number of eyespot feathers was recounted half way through the season. Female body weight did not differ significantly between pens (mean±s.d.: 3.63±0.34 kg; one-way ANOVA: F20,63=1.24, p=0.253).
Pens were divided equally into three groups: A, B and C. Prior to the start of egg laying, males in group A had 20 randomly selected eyespot feathers removed from their train (treatment) following the methodology of Petrie & Halliday (1994). The mean (±s.d.) pre-manipulation number of eyespots was 158±11.2, so we reduced the number of eyespots by 12.7%, on average, without affecting any other aspect of train elaboration. This is within the natural range for this population (mean±s.d. (range): 143±16.8 (72−185)) and despite the skew in reproductive success in favour of males with a large number of eyespots, most males manipulated in an identical manner by Petrie & Halliday (1994) still received matings. It is thus reasonable to assume that the males in the present study could also have achieved matings if allowed to breed naturally. Approximately half way through the breeding season (after four weeks), the same 20 feathers were reattached (treatment-control). Each feather had been cut at a 45° angle near its base allowing it to be reattached in exactly the same orientation, and held in place using a thin metal internal support and white duct tape. The join was hidden below the feather line. Males in group C underwent exactly the same procedures as those in group A except that treatment and treatment-control were reversed. Twenty eyespot feathers were removed, then immediately replaced, and finally removed again after four weeks. Males in group B formed an overall control. They were handled in the same way as those in the other two groups, both at the beginning and half way through the breeding season, but no train feathers were removed (see table 1). The complexity of the peacock's train precluded a positive manipulation to increase the number of eyespots.
Table 1.
Experimental manipulations of, mean sex ratios (sr), and proportion of successfully sexed eggs (se) produced by each group during the first and second halves of the breeding season.
| group A (n=7 pens) | group B (n=7 pens) | group C (n=7 pens) | |
|---|---|---|---|
| first half of season | treatment (eyespots removed) | overall control (no manipulation) | treatment-control (eyespots replaced) |
| sr=0.35±0.11a | sr=0.54±0.12 | sr=0.57±0.12 | |
| se=0.81 | se=0.83 | se=0.87 | |
| n=49/60 eggs sexed | n=59/72 eggs sexed | n=60/68 eggs sexed | |
| second half of season | treatment-control (eyespots replaced) | overall control (no manipulation) | treatment (eyespots removed) |
| sr=0.47±0.27 | sr=0.47±0.14 | sr=0.28±0.16a | |
| se=0.91 | se=0.78 | se=0.87 | |
| n=48/54 eggs sexed | n=40/51 eggs sexed | n=39/45 eggs sexed |
The sex ratio differed significantly from parity (p<0.05).
Prior to being sent for incubation, a random sample of 50 eggs taken from all those laid in all pens during the previous week was selected each week for use in this study. A yolk sample (200 mg) was taken by inserting a 25-guage needle through the small end of each egg into the yolk. After sealing the hole with Germolene ‘New Skin’, the sample was weighed and stored at −20 °C until analysis. Ten yolk samples were randomly selected from each treatment group and 10 from each treatment-control group for yolk-hormone analysis. We did not analyse yolk samples from group B. After alcohol extraction, all yolk samples were assayed for testosterone, 17β-estradiol and corticosterone using commercially available enzyme-immunoassay (EIA) kits. Egg collection was stopped when the peacocks began to drop naturally their train feathers (after seven weeks).
Each week the eggs were incubated commercially until hatching. In the hatcher, each egg had an individual compartment so a chick could be reliably assigned to a particular egg. At hatching, blood samples (2–15 μl), taken under licence from the Home Office, were obtained from all chicks and embryonic tissue removed from all unhatched eggs where a visible embryo had developed. Blood samples and embryonic tissue were stored in absolute ethanol at −20 °C until analysis. Genomic DNA was extracted using the salt-based method of Bruford et al. (1998), and embryos sexed using the polymerase chain reaction (PCR) to amplify part of the W-linked avian CHD gene (CHD-W) in females, and its non-W-linked homologue (CHD-Z) in both sexes using primers 2718R and 2550F (Fridolfsson & Ellegren 1999). The sexing and hormone assays were performed blind and in a random order.
Two sex ratios (the number of males/total number of sexed eggs) were calculated for each male, one for each half of the breeding season, and analysed by fitting a generalized linear mixed model (GLMM), using the GLIMMIX macro (binomial errors, logit link function) in SAS v. 8. In the analysis, group (treatment, treatment-control or control) was included as a fixed factor and, where the same male contributed more than one sex ratio to the analysis, male identity was included as a random factor to control for non-independence of the sex ratios. Repeated-measures analyses, which compare changes in sex ratio between halves of the breeding season, were used for all tests. All other data met the assumptions of normality (Ryan–Joiner tests, all p>0.1). Means are presented±s.e. and statistical tests are two-tailed with the significance level set at 5%.
3. Results
Overall, the proportion of sons produced did not differ significantly from parity (0.47; binomial test: n=295 eggs, p=0.352), and while the control group (group B) showed a reduction in sex ratio during the second half of the season, this was not significant (F1,6=1.28, p=0.301; table 1). Moreover, the proportion of males produced by the treatment-control groups did not differ significantly from the control group sex ratios (group A (second half)+group C (first half) versus group B (second half)+group B (first half): F1,7=0.26, p=0.625) or from parity (table 1). However, the sex ratio produced by each treatment group was significantly lower than the sex ratios produced by the control group (group A (first half)+group C (second half) versus group B (first half)+group B (second half): F1,7=6.48, p=0.038; table 1) and comparing within groups, there was a significant reduction in sex ratio during treatment (group A (first half)+group C (second half) versus group A (second half)+group C (first half): F1,13=11.95, p=0.004).
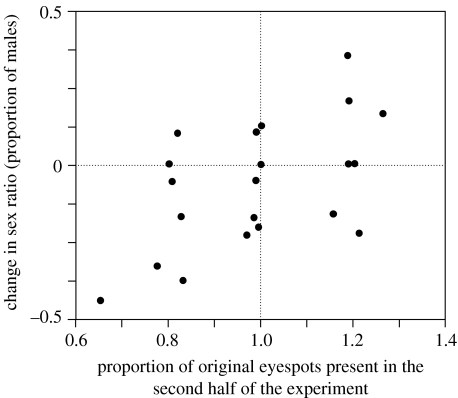
Looking at all pens, the change in sex ratio between the first and second halves of the season was significantly positively related to the proportion of original eyespots present in the second half of the experiment (i.e. a measure of the change in eyespot number; r=0.56, n=21 pens, p=0.009; figure 1), but not to changes in paternal body weight (r=0.12, p=0.608).
Figure 1.
Proportion of eyespots in the first half of the experiment that were present in the second half in relation to changes in the sex ratio of offspring he sired. The three clusterings of data points represent the three groups, A (right), B (centre) and C (left). Each data point represents one pen (n=21).
Even though infertile eggs or eggs in which a visible embryo failed to develop could not be sexed, there was no difference between treatment and treatment-control groups with respect to the proportion of unsexed eggs (F1,13=0.88, p=0.366; table 1) suggesting that the observed biases were present at laying (although care should be taken with this interpretation because the power of this analysis is only 0.27).
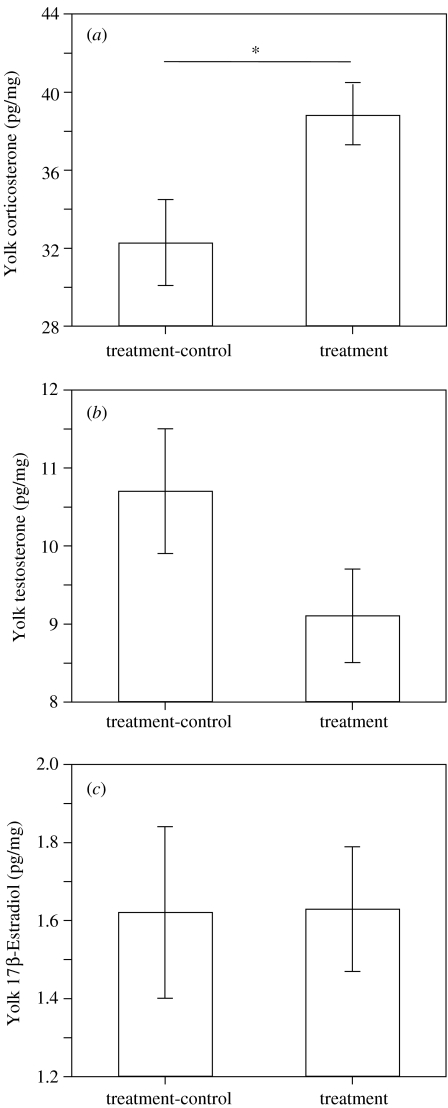
We used two factor ANOVAs to compare yolk hormone levels between eggs laid during treatment and treatment-control (figure 2), and between eggs of different sex. Yolk levels of testosterone and 17β-estradiol were not significantly affected by either treatment (testosterone: F1,36=2.29, p=0.139; 17β-estradiol: F1,36=0.01, p=0.985) or sex (testosterone: F1,36=0.23, p=0.637; 17β-estradiol: F1,36=0.87, p=0.358). However, while there was no effect of sex on yolk levels of corticosterone (F1,36=2.21, p=0.146), eggs produced during treatment contained significantly higher concentrations of corticosterone than those laid during treatment-control (F1,36=7.83, p=0.008).
Figure 2.
Differences in mean (±s.e.) yolk concentrations of (a) corticosterone (b) testosterone and (c) 17β-estradiol from eggs laid during treatment and treatment-control. Asterisk indicates a significant difference ( p<0.05).
4. Discussion
The results of the experiment described here show that when peahens were mated to peacocks whose attractiveness had been experimentally reduced by removing prominent eyespot feathers from their trains, they produced significantly more daughters than when mated to the same males with their full quotient of feathers. Our data strongly suggest that the observed changes in sex ratio were mediated by changes in eyespot number, and judging by the variance in sex ratio explained, this suggests that peahens have a remarkable degree of control over the sex of their offspring. These findings add to the small number of studies providing experimental evidence that female birds can control the primary sex ratio of their offspring in response to paternal attractiveness (Burley 1986; Sheldon et al. 1999). However, the mechanism by which sex ratio deviations are achieved remains unknown.
Several authors have suggested that yolk hormones could be involved in the sex biasing mechanism (e.g. Krackow 1999; Petrie et al. 2001), although we could find no evidence that yolk hormone levels differed between the sexes (cf. Petrie et al. 2001). However, yolk testosterone levels in this study were considerably higher than those detected by Petrie et al. (2001; mean females: 1.4 pg ml−1, mean males: 2.0 pg ml−1; collected on day 10 of incubation), raising the possibility that the previous findings were brought about by selective embryonic utilization of steroids rather than differential maternal allocation. Here, yolk corticosterone levels were significantly elevated in eggs laid during treatment, and hence during times in which the sex ratio was significantly female-biased, while testosterone concentrations tended to be lower. Corticosterone is the major avian glucocorticoid released in response to stress (Harvey et al. 1984) and we might have detected higher corticosterone levels in eggs sired by treatment males because females were unwilling, but unable to avoid mating with males they perceived as less attractive. Chronic exposure to high levels of corticosterone can adversely affect body condition, which raises the possibility that the observed sex ratio biases were the result of decreases in maternal body condition (a common factor linked to sex ratio deviations; see references in Pike & Petrie 2003) mediated by our experimental manipulations, and that maternal plasma corticosterone was reflected in her eggs (Hayward & Wingfield 2001). However, our data cannot test this directly. Testosterone levels may have been varying in response to increases in corticosterone. This is a common finding in endocrine studies of birds and is probably bought about by a suppressive effect of corticosterone on reproductive processes (e.g. Wingfield et al. 1994). However, our data cannot tell whether the relationship between hormones and sex ratio skews is one of cause or effect, or whether corticosterone concentrations vary with some other variable. Further studies are needed to address these questions, and to elucidate the precise mechanism of avian sex ratio adjustment.
Acknowledgments
We are grateful to BBSRC and NERC for financial support, Quinton Spratt for allowing us to work at his farm, and Tom Smulders, Stuart West and an anonymous referee for valuable comments on the manuscript.
Footnotes
Present address: Division of Environmental & Evolutionary Biology, Graham Kerr Building, University of Glasgow, Glasgow G12 8QQ, UK
References
- Bruford M.W, Hanotte O, Brookfield J.F.Y, Burke T. Multilocus and single-locus DNA fingerprinting. In: Hoezel A.R, editor. Molecular genetic analysis of populations: a practical approach. IRL Press; Oxford: 1998. pp. 287–336. [Google Scholar]
- Burley N. Sex-ratio manipulation in color-banded populations of zebra finches. Evolution. 1986;40:1191–1206. doi: 10.1111/j.1558-5646.1986.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Charnov E.L. Parent–offspring conflict over reproductive effort. Am. Nat. 1982;119:736–737. [Google Scholar]
- Fisher R.A. Clarendon Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Frank S.A. Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 1990;21:13–55. [Google Scholar]
- Fridolfsson A.K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. [Google Scholar]
- Harvey S, Phillips J.F, Rees A, Hall T.R. Stress and adrenal function. J. Exp. Zool. 1984;232:633–645. doi: 10.1002/jez.1402320332. [DOI] [PubMed] [Google Scholar]
- Hayward L.S, Wingfield J.C. Laying Japanese Quail transfer corticosterone to egg yolk. Am. Zool. 2001;41:1468–1469. [Google Scholar]
- Krackow S. Avian sex ratio distortions: the myth of maternal control. In: Adams N, Slotow R, editors. Proc. 22nd International Ornithological Congress, Durban, 16–22 August 1998. BirdLife South Africa; Johannesburg: 1999. pp. 425–433. [Google Scholar]
- Petrie M. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature. 1994;371:598–599. [Google Scholar]
- Petrie M, Halliday T. Experimental and natural changes in the peacock's (Pavo cristatus) train can affect mating success. Behav. Ecol. Sociobiol. 1994;35:213–217. [Google Scholar]
- Petrie M, Halliday T, Sanders C. Peahens prefer peacocks with elaborate trains. Anim. Behav. 1991;41:323–331. [Google Scholar]
- Petrie M, Schwabl H, Brande-Lavridsen N, Burke T. Sex differences in avian yolk hormone levels. Nature. 2001;412:489. doi: 10.1038/35087652. [DOI] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Andersson S, Griffith S.C, Örnborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. [Google Scholar]
- Weatherhead P, Robertson R. Offspring quality and the polygyny threshold: the sexy son hypothesis. Am. Nat. 1979;113:201–208. [Google Scholar]
- Wingfield J.C, Suydam R, Hunt K. The adrenocortical responses to stress in snow buntings (Plectrophenax nivalis) and Lapland longspurs (Calcarius lapponicus) at Barrow, Alaska. Comput. Biochem. Physiol. B. 1994;108:299–306. [Google Scholar]