Abstract
House-hunting ants avoid otherwise excellent potential nest sites that contain dead ants which may pose risks from poor hygiene. This discovery adds another category to the exceptionally long list of nest site attributes that ants evaluate. It further indicates the importance of disease as a selection pressure on social insects.
Keywords: decision making, disease, pathogen, social insects, Temnothorax albipennis
1. Introduction
House-hunting by complete social insect colonies provides an opportunity to study links between individual and collective decision-making (Franks et al. 2002). This is important beyond social insects because, for example, our own brains can be considered as societies of neurons, and similar processes might apply (Schall 1999, 2001; Shadlen & Newsome 2001; Fewell 2003; Heekeren et al. 2004). This is not as incredible as it might at first appear. For example, in house-hunting ant colonies, a classic speed–accuracy trade-off has been demonstrated (Franks et al. 2003a) and such trade-offs occur in the decision-making systems of innumerable organisms from solitarily foraging bumble bees (Chittka et al. 2003) to humans (Edwards 1965). One of the reasons for a speed–accuracy trade-off is the compromise needed between gathering more information to make a well-informed decision and the time that this takes. Thus, speed–accuracy trade-offs are all the more likely if diverse information on a variety of very different attributes is required (Franks et al. 2003a,b).
Two house-hunting social insect systems have been investigated in-depth to date: first, new hive selection by swarms of honeybees (Seeley & Buhrman 1999, 2001); second, new nest site selection by colonies of Temnothorax (formerly Leptothorax) albipennis (Franks et al. 2003a,b). In both cases, individual scouts gather and evaluate diverse qualitative and quantitative information on potential nest sites. For example, honeybees assess, among other factors: the volume of the cavity, the size of the entrance, the height of the entrance above the ground, the height of the entrance above the floor of the cavity, and the compass bearing of the entrance (Seeley & Morse 1978; Seeley 1982; Visscher et al. 1985). Temnothorax albipennis workers assess the floor area of a cavity, its darkness, the amount of headroom it offers and the width of the entrance (Franks et al. 2003a). As first shown for the ants, quorum sensing is used to collate individual assessments and form them into a collective decision (Pratt et al. 2002). Recent work strongly suggests a similar phenomenon in honeybees (Seeley & Visscher 2004).
One intriguing potential difference between these systems is associated with the costs and benefits of nest site re-usage. An obvious disadvantage of using a previously occupied nest site is that it may be a source of disease due to lingering pathogens. Hygiene is important to many social insects. For example, when waste, including dead ants, accumulates in leaf cutter ant colonies, there is an increase in ant mortality (Bot et al. 2001), and undertaking behaviour is well documented in other ants (Theraulaz et al. 2002) and honeybees (Frumhoff & Baker 1988; Robinson & Page 1988; Trumbo et al. 1997; Julian & Cahan 1999). Nevertheless, honeybees are known to prefer new nest sites with honeycomb present (Seeley 1982; Visscher et al. 1985). In this case, the benefits of utilizing such a valuable resource might outweigh the costs associated with the risk of pathogen transmission. Here, we test T. albipennis ant colonies to determine whether they avoid potentially good nest sites in which there are dead conspecifics. If they do, this would add an entirely new category of criterion to the long list of house selection attributes that these ants evaluate. In addition, it would further indicate the importance of disease as a major selection pressure on social insect populations that affects both individual and colony-level behaviours (Schmid-Hempel 1998; Hart & Ratnieks 2001, 2002; Hart et al. 2002).
2. Methods
Individual T. albipennis colonies were presented with a choice between two potential new nest sites. All the nests were constructed from cardboard, 76×51 mm and 2 mm thick, sandwiched between two microscope slides of the same length and breadth. The nest cavity was 32×24 mm with a nest entrance 3 mm wide and 5 mm long. We induced colonies to leave their old nest by removing the top slide, exposing the ants and brood. The old nest was destroyed in a large Petri dish, of dimensions 220×220×22 mm, containing the two symetrically arranged potential new nest sites, each 10 cm from the old nest.
Are dead foreign conspecific workers sufficiently repulsive to influence nest site choices? In experiment 1, 15 emigrating colonies had to choose, on two separate occasions, between a nest containing dead ant material from foreign colonies and a nest containing carborundum grains. Carborundum grains were used to control for the avoidance of fragmentary material in potential nest sites (which would occupy space or need to be removed). Grade 16 carborundum is approximately equal in volume to an intact worker and grade 36 to that of a head, thorax or abdomen. Piles of dead ants consisting of an intact worker corpse, a head, a thorax and an abdomen, or piles of carborundum grain equivalents, were made at each of five points around the cavity perimeter (figure 1a,b) before the nest top slide was added. The dead ants used in all 15 emigrations originated from the same foreign conspecific colony. In all the experiments involving dead ants, workers were killed by freezing, stored in a deep freeze for 1.5 h, and then thawed out for 48–72 h prior to use.
Figure 1.
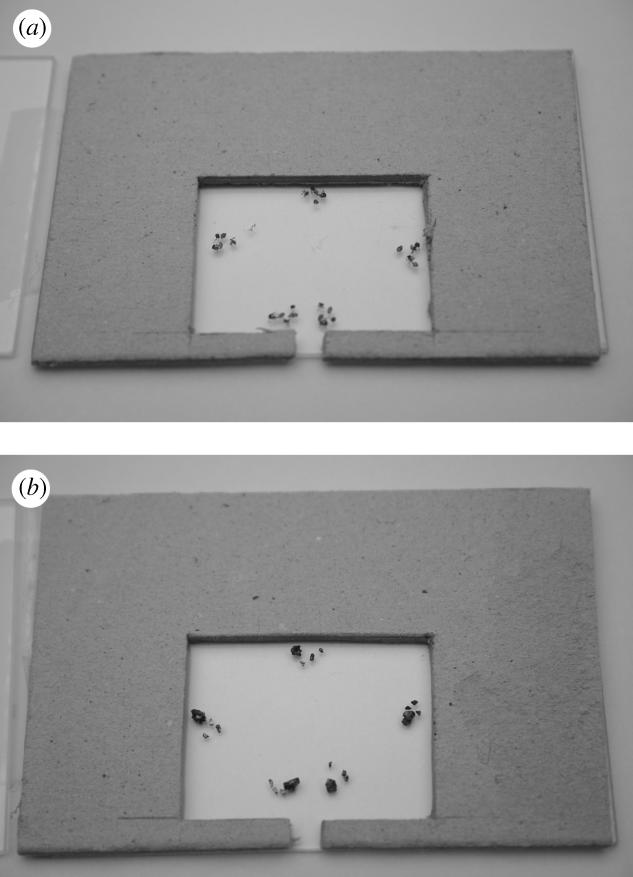
This figure shows the pattern of (a) dead ants and (b) carborundum grains in potential nest sites.
Are dead nest-mates as repulsive as dead foreign conspecifics? To answer this, experiment 2 replicated experiment 1 but used dead nest-mates instead of dead foreign conspecific workers. In experiment 2, 20 emigrating colonies had to choose between a nest containing carborundum, and a nest containing whole corpses and body parts of individual workers previously taken at random from their own colony.
Are dead ants repulsive and/or is carborundum attractive? The ants might exhibit a preference for nests containing carborundum grit because such grit might be useful, for example, to reduce the width of the nest entrance for better defence (Franks et al. 1992). Experiment 3 is thus a further control. In experiment 3, 20 emigrating colonies had to choose between a nest containing carborundum and an empty nest. As above, apart from the nest contents, all new nests were identical. All other aspects of the three choice experiments 1, 2 and 3 are identical to those in Franks et al. (2003b).
3. Results
In experiment 1, 10 of the 15 colonies chose carborundum nests twice, and none chose dead ant nests twice. This is a significant choice of carborundum over dead ants (Wilcoxon statistic 55, n=15, p=0.006). If colonies were choosing at random we would expect them to choose both types of nest at the same frequency. Thus each colony might be expected to choose the nest with carborundum grains once in its two trials and the nest with dead ants once in its two trials. We therefore tested for preferences by asking how many times colonies choose carborundum nests twice against the random expectation of one such choice. In experiment 1, only one colony split, i.e. brood was present in both nests 48 h after the start of the experiment (table 1).
Table 1.
Nest choices of colonies in the three experiments.
| nests containing: | ||||||
|---|---|---|---|---|---|---|
| dead ants | ||||||
| experiment | nest-mate | non-nest-mate | carborundum | empty | split colonies | n |
| 1a | — | 4 | 10 | — | 1 | 15 |
| 1b | — | 0 | 15 | — | 0 | 15 |
| 2 | 0 | — | 20 | — | 0 | 20 |
| 3 | — | — | 10 | 6 | 4 | 20 |
In experiment 2, all 20 colonies choose the nests with carborundum grit rather than their own dead nest-mates and no colonies split (two-tailed cumulative binomial test, p<0.0001; table 1).
In experiment 3, 10 colonies chose the carborundum nest, 6 choose the empty nests and 4 split, indicating that the ants did not have a preference for either nest type (two-tailed cumulative binomial test of 10 versus 6, p=0.4544). Furthermore, splitting by some colonies is indicative of the nests being treated as if they are of similar quality (Franks et al. 2003b; table 1). Experiment 3 therefore suggests that the presence of carborundum grains does not make nests more attractive.
4. Discussion
The results of experiments 1 and 2 suggest that house-hunting ant colonies avoid otherwise ideal nest sites if they contain dead conspecific workers irrespective of their origin (i.e. whether they were nest-mates or non-nest-mates). Our controls clearly showed that it was the presence of dead ants, rather than material per se, that made nest sites unattractive. Furthermore, carborundum grit, a potential building material (Franks et al. 1992), was not attractive to the ants. Franks et al. (in preparation) have shown that this species avoids non-nest-mate conspecific odours, but we have shown that all dead conspecific ants are repulsive. This indicates hygiene as the selection pressure that has favoured such behaviour, rather than foreign dead ants being a cue to the possible presence nearby of living conspecific competitors.
An intriguing comparative test would be to see if honeybees reject cavities, with and without honey comb, in which there are dead bees. The latter might indicate that the previous inhabitants suffered deadly diseases.
Our results add a whole new qualitative category, hygiene awareness, to the nest evaluations of house-hunting ant colonies. Until now, it had been assumed that ant colonies sometimes emigrated to avoid pests and diseases at their old nest site (Hart 2002). We have shown for the first time that ants evaluate hygiene issues before moving to a new nest site. Earlier work has shown that T. albipennis colonies use a weighted additive decision-making strategy, one of the most thorough and time-consuming strategies of all, to evaluate the physical properties of nest sites (Franks et al. 2003b). It will now be interesting to determine whether such evaluations are completely abandoned when the ants find dead conspecifics in potential nest sites. In other words, is tomb evasion an over-riding criterion for abandoning nest-site evaluation? Irrespective of the ranking of hygiene considerations in the nest-choice criteria of these ants, our findings show, for the first time, that disease risk and hygiene issues must be added to the long list of attributes that the ants take into account. This further supports the notion that parasites and pathogens are a major selective force on social insect ecology and behaviour.
Acknowledgments
A.D. thanks the German Science Foundation (DFG, Emmy Noether fellowship) for funding. N.R.F. thanks the EPSRC (GR/S78674/01) for funding.
Footnotes
Present address: GKT School of Medicine, First Floor, Hodgkin Building, Guy's Campus, London SE1 9RT, UK
References
- Bot A.N.M, Currie C.R, Hart A.G, Boomsma J.J. Waste management in leafcutting ants. Ethol. Ecol. Evol. 2001;13:225–237. [Google Scholar]
- Chittka L, Dyer A.G, Bock F, Dornhaus A. Bees trade off foraging speed for accuracy. Nature. 2003;424:388. doi: 10.1038/424388a. [DOI] [PubMed] [Google Scholar]
- Edwards W. Optimal strategies for seeking information: models for statistics, choice reaction times, and human information processing. J. Math. Psychol. 1965;2:312–329. [Google Scholar]
- Fewell J.H. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. [DOI] [PubMed] [Google Scholar]
- Franks N.R, Wilby A, Silverman B.W, Tofts C. Self-organizing nest construction in ants: sophisticated building by blind bulldozing. Anim. Behav. 1992;44:357–375. [Google Scholar]
- Franks N.R, Pratt S.C, Mallon E.B, Britton N.F, Sumpter D.J.T. Information flow, opinion-polling and collective intelligence in house-hunting social insects. Phil. Trans. R. Soc. B. 2002;357:1567–1583. doi: 10.1098/rstb.2002.1066. doi:10.1098/rstb.2002.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R, Dornhaus A, Fitzsimmons J.P, Stevens M. Speed versus accuracy in collective decision-making. Proc. R. Soc. B. 2003;270:2457–2463. doi: 10.1098/rspb.2003.2527. doi:10.1098/rspb.2003.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R, Mallon E.B, Bray H.E, Hamilton M.J, Mischler T.C. Strategies for choosing among alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 2003;65:215–223. [Google Scholar]
- Franks, N. R., Dornhaus, A., Hooper, J., Webb, C., Guillem, R. & Hitchcock, G. In preparation. Competitor avoidance during nest choice by the ant Temnothorax albipennis
- Frumhoff B.C, Baker J. A genetic component to division of labor within honey bee colonies. Nature. 1988;333:358–361. [Google Scholar]
- Hart A.G. Does disease threat cause colony emigrations in the leafcutting ant Atta colombica (Guerin)? Entomol. Monthly Mag. 2002;138:41–42. [Google Scholar]
- Hart A.G, Ratnieks F.L.W. Task partitioning, division of labour and nest compartmentalization collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav. Ecol. Sociobiol. 2001;49:387–392. [Google Scholar]
- Hart A.G, Ratnieks F.L.W. Waste management in the leaf-cutting ant Atta colombica. Behav. Ecol. 2002;13:224–231. [Google Scholar]
- Hart A.G, Bot A.N.M, Brown M.J.F. A colony-level response to disease control in a leaf-cutting ant. Naturwissenschaften. 2002;89:275–277. doi: 10.1007/s00114-002-0316-0. [DOI] [PubMed] [Google Scholar]
- Heekeren H.R, Marrett S, Bandettini P.A, Ungerleider L.G. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Julian G.E, Cahan S. Undertaking specialization in the desert leaf-cutter ant Acromyrmex versicolor. Anim. Behav. 1999;58:437–442. doi: 10.1006/anbe.1999.1184. [DOI] [PubMed] [Google Scholar]
- Pratt S.C, Mallon E.B, Sumpter D.J.T, Franks N.R. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2002;52:117–127. [Google Scholar]
- Robinson G.E, Page R.E. Genetic determination of guarding and undertaking in honeybee colonies. Nature. 1988;333:356–358. [Google Scholar]
- Schall J.D. Weighing the evidence: how the brain makes a decision. Nat. Neurosci. 1999;2:108–109. doi: 10.1038/5663. [DOI] [PubMed] [Google Scholar]
- Schall J.D. Neural basis of deciding, choosing and acting. Nat. Rev. Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Princeton University Press; Princeton, NJ: 1998. Parasites in social insects. [Google Scholar]
- Seeley T.D. How honey-bees find a home. Sci. Am. 1982;247:158–168. [Google Scholar]
- Seeley T.D, Buhrman S. Group decision making in swarms of honeybees. Behav. Ecol. Sociobiol. 1999;45:19–31. [Google Scholar]
- Seeley T.D, Buhrman S.C. Nest-site selection in honeybees: how well do swarms implement the “best-of-N” decision rule? Behav. Ecol. Sociobiol. 2001;49:416–427. [Google Scholar]
- Seeley T.D, Morse R. Nest site selection by the honeybee, Apis mellifera. Insect. Soc. 1978;25:323–337. [Google Scholar]
- Seeley T.D, Visscher P.K. Quorum sensing during nest-site selection by honeybee swarms. Behav. Ecol. Sociobiol. 2004;56:594–601. [Google Scholar]
- Shadlen M.N, Newsome W.T. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophys. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Theraulaz G, Bonabeau E, Sole R.V, Schatz B, Deneubourg J.L. Task partitioning in a ponerine ant. J. Theor. Biol. 2002;215:481–489. doi: 10.1006/jtbi.2001.2518. [DOI] [PubMed] [Google Scholar]
- Trumbo S.T, Huang Z.Y, Robinson G.E. Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behav. Ecol. Sociobiol. 1997;41:151–163. [Google Scholar]
- Visscher P.K, Morse R.A, Seeley T.D. Honeybees choosing a home prefer previously occupied cavities. Insect. Soc. 1985;32:217–220. [Google Scholar]


